A QM:MM model for the interaction of DNA nucleotides with carbon nanotubes†
Abstract
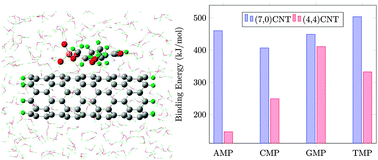
Hybrid materials formed by DNA and carbon nanotubes (CNTs) have shown very interesting properties, but their simulation in solution using quantum mechanical approaches is still a challenge in the computational chemistry community. In this paper, we developed a QM:MM model to study the interactions between charged DNA nucleotides and carbon nanotubes in solution. All four types of DNA nucleotides were taken to interact with two CNTs of similar diameter but different chiralities: (4,4) and (7,0). The nucleotides and CNTs were treated at the QM level, while added water and neutralizing ions were modeled at the MM level. ONIOM simulations were performed at the (M06-2X/6-31G(d):Amber) level for the hybrids, as well as for individually solvated CNTs and nucleotides, which allowed us to evaluate the energy of binding. Our binding energy (BE) values range from 146.60 to 503.43 kJ mol−1, indicating strong physisorption of nucleotides on CNTs. The relatively large BE, compared with past studies on nucleobase–CNT binding in a vacuum, could be due to the larger size of nucleotides compared with nucleobases, the charges on the nucleotides, and the inclusion of solution which causes the release of water molecules upon hybridization.

 Please wait while we load your content...
Please wait while we load your content...