Recent advances in transition metal-catalyzed enantioselective hydrogenation of unprotected enamines
Abstract
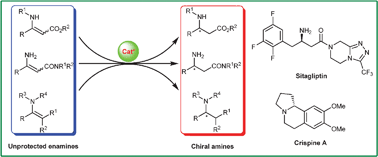
Transition metal-catalyzed enantioselective hydrogenation of enamines is undoubtedly a useful and environment-friendly method for the preparation of optically pure chiral amines and amine derivatives. Over the last few decades, the use of transition metal catalysts containing chiral phosphorus or phosphine–oxazoline ligands attracted much attention for the hydrogenation of unprotected enamines. A number of efficient chiral catalysts have been developed, and some of them have shown high potential for the application in the synthesis of optical chiral amines in both laboratory and industry. This tutorial review focuses on the contributions concerning the transition metal-catalyzed enantioselective hydrogenation of unprotected enamines for the synthesis of chiral amines and amine derivatives.

 Please wait while we load your content...
Please wait while we load your content...