Endowing phenolic formaldehyde resin with sustainability: why and how?
Xinyao
Jiang
 ,
Bing
Zhu
,
Shahid
Hussain
and
Maiyong
Zhu
,
Bing
Zhu
,
Shahid
Hussain
and
Maiyong
Zhu
 *
*
School of Materials Science & Engineering, Jiangsu University, Zhenjiang, 212013, P. R. China. E-mail: maiyongzhu@ujs.edu.cn
First published on 2nd September 2025
Abstract
The past century has witnessed the widespread application of phenolic formaldehyde (PF) resin, the first synthetic polymer material in the world, due to its excellent oil resistance and electrical insulation properties. The market demand for PF resin is expected to continue increasing year by year. To meet the requirements of Sustainable Development Goals, it is necessary to endow PF resin with sustainability. To date, efforts have been made to improve the synthesis process, search for alternative materials, and recycle phenolic resin into other functional materials. This has led to significant achievements in PF resin sustainability. In this review, we provide an in-depth analysis of sustainable strategies for PF resin, focusing on its market consumption, environmental impact, structure, and properties. In addition to recycling waste PF resin, achieving sustainability throughout the entire PF resin lifecycle has become an important focus. The improvement of synthesis technology has increased the lifespan of phenolic resin products and contributed to a reduction of waste PF resin. The addition of scavengers makes PF resin products safer in daily life. The use of more environmentally friendly raw materials, such as biomass, to replace phenol and formaldehyde has been implemented, thus improving the cost and environmental benefits. The application of dynamic covalent bonds makes waste PF resin decompose more easily. Combining these with the latest waste PF resin conversion methods, the sustainability of PF resin is expected to be raised to a higher level.
1. Introduction
Phenolic resin, the first synthetic polymer, was discovered in the late 19th century and has excellent mechanical properties due to its high molecular weight and intermolecular cross-linked structure.1 It is also chemically and thermally stable. The synthesis process can be altered to adapt it to various requirements, and it can function as either a thermoplastic or thermosetting resin depending on the phenol/formaldehyde ratio and catalytic environment. Commercial phenolic resin products are widely used in furniture, construction, and household items, making life easier for many people. Previous work has analyzed phenolic resin hazards and recycling processes, while a previous article discussed phenolic resin synthesis and pollution arising from the main raw materials: phenol and formaldehyde.2As illustrated in Fig. 1, phenolic resins, as important synthetic materials, have experienced continuous production growth in China. By 2020, annual production reached 1.43 million tons, with a production capacity of approximately 1.752 million tons, and consumption is projected to increase to 1.85 million tons by 2025. In China, usage is predominantly in the fields of Phenolic molding compound (22%), wood processing (20%), and friction material (20%), while the global market is mainly focused on Wood adhesive (35%) and phenolic molding compound (15%). The global market was valued at 13 billion USD in 2020 and is expected to exceed 17.9 billion USD by 2027. It is noteworthy that discarded plastics, including hard-to-degrade phenolic resins, are being discharged into the ocean in large quantities, particularly in East Asia, where they enter the marine ecosystem via rivers and coastlines. This has led to a series of ecological crises, such as microplastic pollution and marine life entanglement and ingestion. Conventional landfills and pyrolysis treatments have inherent limitations, necessitating the development of efficient recycling technologies to alleviate environmental pressures.
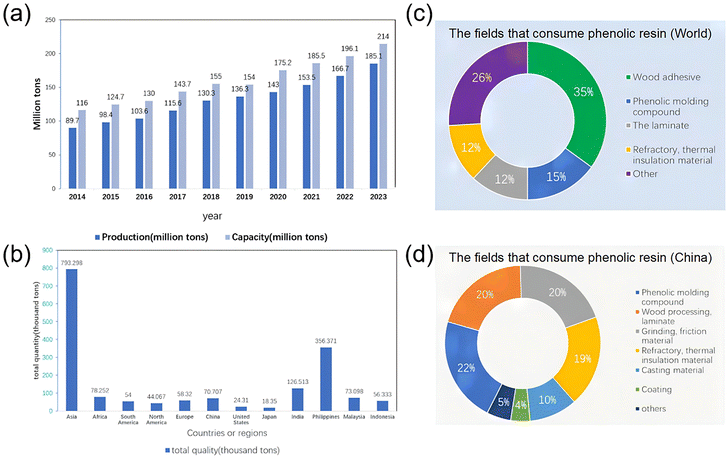 | ||
| Fig. 1 (a) The total production and storage volume of phenolic resins in China from 2014 to 2020. (b) The amount of plastic entering the ocean in the form of waste in various countries and regions. The amounts of phenolic resin consumed by various fields in the world (c) and in China (d). (Reproduced with permission from ref. 2; copyright: 2024 MDPI.) | ||
Traditional resin disposal methods through landfills and incineration have drawbacks, and advanced recycling technologies are needed. However, end-of-life treatment for phenolic resins may not be the best choice, as the development of manufacturing processes, particularly nanotechnology, has expanded phenolic resin applications beyond basic household goods. Nanoscale phenolic resins and other materials of various sizes offer engineering applications, utilizing phenolic resin's mechanical properties, flame retardancy, and rich carbon content.3 This broad platform of functional materials through nanoscale phenolic resin combinations achieves sustainability without being limited to waste phenolic resin recycling (Fig. 2).
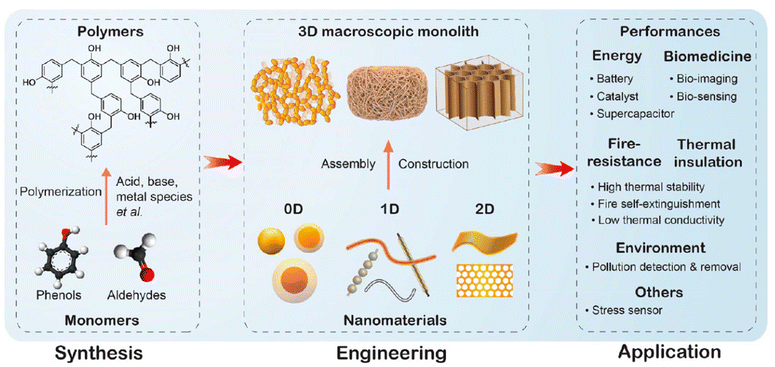 | ||
| Fig. 2 The synthesis, nanoscale processing and assembly of phenolic resins and their macroscopic applications. Reproduced with permission from ref. 3; copyright: 2024 American Chemical Society. | ||
At all stages of the phenolic resin life cycle, there is potential for sustainable improvement. Sustainable changes can be made in raw material selection, synthesis, product development, and waste management. Using environmentally friendly raw materials like biomass can reduce pollution and costs. Advanced synthesis processes can improve resin performance and durability, aiding waste reduction. The application of dynamic covalent bonds and scavengers can enhance convenience and reduce formaldehyde release. Finally, recycling is still an integral part of the sustainability of the resin, and some recent studies on the conversion of phenolic resins will be mentioned in the last section, including depolymerization to small-molecule monomers and conversion to carbon materials.
2. Green synthesis strategies
The phenolic resin production process needs improvement for sustainable development. Current methods use harsh reaction conditions and strong acids or bases, which are harmful to the environment. To achieve green development, it is important to extend the service life of materials and reduce waste. In addition, traditional phenolic resin products and industrial wastewater contain toxic substances that can harm human health and nature. Therefore, removing or capturing these substances during the synthesis process is crucial.2.1. Synthetic system improvement
The first strategy to make PF resin sustainable is improving the synthesis processes, which can be done by modifying the structure of phenolic resin. This modification is aimed at optimizing the properties of phenolic resin. Unmodified phenolic resin has many imperfections that can be improved, such as easy oxidation, decomposition at high temperatures, and poor toughness. PF formation typically involves dehydration between formaldehyde and phenol under alkali or acidic conditions, similar to the Stöber process for silicon alkoxides. However, nanostructured PF resins have gained attention due to their superior biocompatibility for biomedicine and potential for use in nano-carbon materials. Liu et al.4 proposed synthesizing phenolic resin colloidal materials and extended the Stöber method for the preparation of monodisperse resorcinol–formaldehyde (RF) resin polymer spheres.The synthesis process of RF resin colloidal spheres involves adding ammonia, resorcinol, and formaldehyde to a solvent, forming emulsion droplets, and catalyzing the ammonia inside to form uniform spheres, as shown in Fig. 3b. The resulting particles have a spherical morphology and are about 500 nm in size (Fig. 3d). After carbonization, these spheres can be converted to carbon spheres, with higher yields than other PF resins. The carbon spheres maintain a spherical morphology and are around 400 nm in size (Fig. 3f). This synthesis process allows for better control over particle size, size distribution, and other factors affecting performance and applications. While many researchers have synthesized nanostructured phenolic resins, this method offers advantages, such as low cost and suitability for large-scale industrial production.5,6
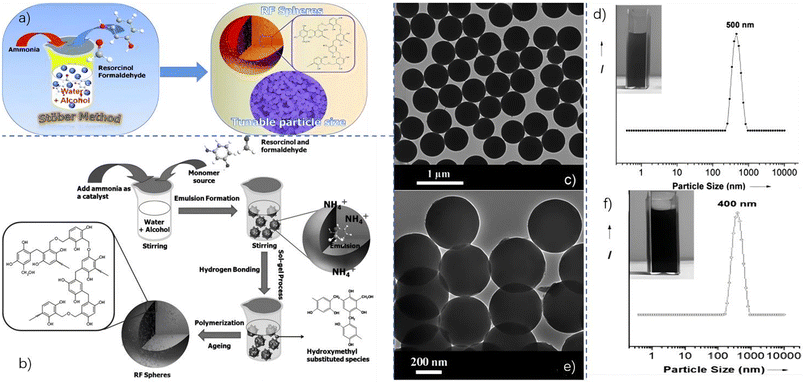 | ||
| Fig. 3 (a) A schematic diagram of extending the Stöber process to synthesize RF resin spheres; (b) the formation of RF resin spheres using the extended Stöber method; (c) a TEM image and (d) DLS plot of the RF resin spheres; and (e) a TEM image and (f) DLS plot of carbon spheres. Reproduced from ref. 4 with permission; copyright: 2011 Wiley-VCH. | ||
Traditional phenolic resins are synthesized under alkaline or acidic conditions, and the quality of the product is reduced when the catalyst is removed.8 Some studies have explored using microwaves to synthesize phenolic resin efficiently without an acidic catalyst, but this method still has limitations.7 Xu et al. synthesized thermosetting phenolic resin and its montmorillonite/silica nanocomposites using a method that takes advantage of the Lewis acidity of the borate ester bond, creating a boron-modified phenolic resin.8 This synthesis process does not require basic or acidic catalysts, which is uncommon. While there are many articles on improving phenolic resin synthesis processes, most of these focus on replacing or improving raw materials rather than changing the synthesis system.
2.2. Composite engineering
As Xu et al.8 noted, most phenolic resin process improvements cannot completely eliminate the use of acidic or alkaline catalysts, suggesting that the green advancement of synthetic systems may have reached a plateau. Therefore, many researchers try to extend the lifecycle of phenolic resin by improving the durability and physical properties of phenolic resin, so as to reduce the frequency of resin waste and achieve the effect of sustainable resin use. In order to improve the application performance of PF resin, compositing with other components is an option. Take the application of resin in tribology as an example: immersing resin in oil is a common method to enhance its tribological properties. Xiong et al.9 reported that oil-containing carbon nanocapsules (Oil@C), as reinforced particles, can improve the tribological properties of solvent-based PF resin. By adding Oil@C with a diameter of 190 nm into PF resin, a shell layer covering the PF resin may be formed. Owing to the presence of Oil@C, the coefficient of friction (COF) of the PF composite exhibits a decrease compared to pristine PF resin, as evidenced by Fig. 4. There is no linear relationship between the COF and the amount of Oil@C. However, there is a critical value of Oil@C content at which the smallest COF value is achieved. As depicted in Fig. 4b, this critical value is experimentally determined to be 5 wt% Oil@C, at which the shell thickness is 12.5 nm and the COF value decreases by 81.9% compared with pristine PF resin (from 0.54 to 0.098). Expectedly, the decrease in COF leads to a prolonged service life, as shown in Fig. 4c. In particular, a greatly prolonged service life can be obtained for the PF composite sample containing 5 wt% Oil@C, from 460 s to 5 h (Fig. 4c). Morphology analysis and transferred film from the friction pairs reveal that the self-lubricating property of PF composites is associated with the released oil, which inhibits the transfer of PF resin and exfoliation of the coating. Unfortunately, the shear strength of the PF composites is observed to decline, and this must be subject to further improvement.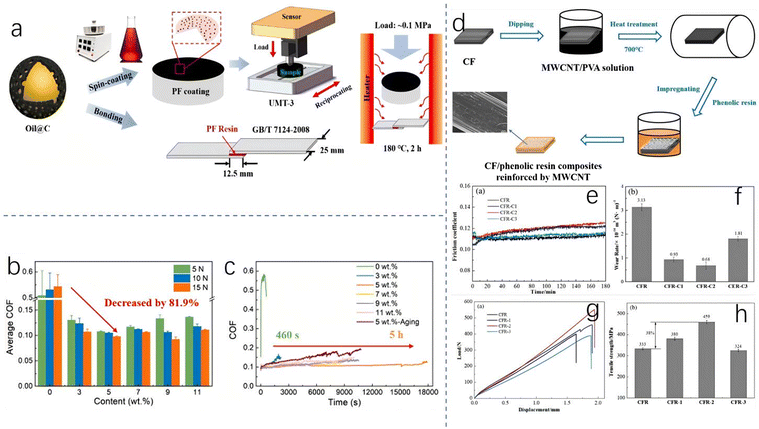 | ||
| Fig. 4 (a) The preparation process of oil-containing carbon nanocapsules (Oil@C); (b) Oil@C content-dependent COF values in a PF resin composite; and (c) a service life comparison among PF resin composites with different Oil@C content levels. Reproduced from ref. 9 with permission; copyright: 2023 Elsevier. (d) The preparation process of MWCNT/carbon fiber fabric multiscale hybrid materials; (e) the dynamic friction coefficients of different samples; (f) the wear rates of different samples; (g) load–displacement curves of different samples; and (h) tensile strengths of different samples. Reproduced from ref. 10 with permission; copyright: 2022 John Wiley & Sons. | ||
Wang et al. developed a multiscale hybrid material using multiwalled carbon nanotubes (MWCNTs) and carbon fiber fabric to enhance the mechanical and tribological properties of phenolic resin composites.10 The materials showed increased dynamic friction coefficients and reduced wear rates compared to an unmodified sample when modified with MWCNTs. The mechanical properties of the composites also improved, withstanding heavier loads. However, while the tensile strength initially increased due to a uniform MWCNT distribution, it then decreased due to agglomeration. Further research is needed to prevent agglomeration and enhance wear resistance.
Researchers have experimented with various lubricants to prolong the service life of phenolic resins in tribology. Table 1 lists some of these lubricants and their effects on phenolic resins. Phenolic resin, as a friction material, has a high friction coefficient and good wear resistance, among other properties. To meet safety, durability and comfort requirements, phenolic resin composites have been widely studied and applied. Numerous literature studies suggest that these composites have greater friction coefficients and wear resistance, allowing for smoother braking, reduced noise and increased service life.
| Solid lubricant | Improved properties | Optimum formula | Ref. |
|---|---|---|---|
| Flake graphite | Friction coefficient; wear rate | Phenolic resin 20%; aramid pulp 22.5%; potassium titanate whiskers (K2Ti6O13) 7.5%; BaSO: 50–40%; solid lubricant 0–10%; flake graphite 7.5% | 11 |
| Polytetrafluoroethylene (PTFE) | Friction coefficient; wear rate | Phenolic resin 20%; aramid pulp 22.5%; potassium titanate whiskers (K2Ti6O13) 7.5%; BaSO4 50–40%; solid lubricant 0–10%; polytetrafluoroethylene (FTFE) 12.5% | 11 |
| Molybdenum disulfide (MoS2) | Friction coefficient; wear rate | Phenolic resin 20%; aramid pulp 22.5%; potassium titanate whiskers (K2Ti6O13) 7.5%; BaSO4 50–40%; solid lubricant 0–10%; molybdenum disulfide (MoS2) 12.5% | 11 |
| PFW | Hydrophobic properties; friction and wear behavior | 30 wt% (a diameter of approximately 3–4 μm) | 12 |
| PTFE | Hydrophobic properties; friction and wear behavior | 30 wt% (average diameter of 5 μm) | 12 |
| FEP | Hydrophobic properties; friction and wear behavior | 30 wt% (average diameter of 15 μm) | 12 |
| Graphene oxide | Thermal stability and tribological properties | PF–graphene composites with graphene oxide content (0.3 wt%) | 13 |
| Graphitic carbon nitride nanosheets (g-C3N4) | Tribological performance | 1 wt% g-C3N4 nanosheets/phenolic coating | 14 |
| Hexagonal prism ZnO | Tribological performance | 1 wt% ZnO micro-disks | 15 |
| Molybdenum dialkyldithiocarbamate (Mo-DTC) | Hardness and anti-wear properties | 1% Mo-DTC content | 16 |
2.3. Pollutant fixation
Scientists are researching methods to reduce the environmental hazards caused by free phenol and formaldehyde in PF resin. One solution is to improve the resin synthesis system, but this has been difficult to achieve. A simpler strategy is adding formaldehyde scavenger chemicals to phenolic resins. These chemicals react with formaldehyde to decrease its content. Numerous chemicals have been found to be effective as scavengers, and the small quantity added does not significantly impact the resin's mechanical properties or product quality.17Table 2 shows the reactivity of many commonly available scavengers toward formaldehyde.| Scavenger | Reaction mechanism | Ref. |
|---|---|---|
| Urea | CO(NH2)2 + CH2O → C2H6N2O2 | 18 |
| Na2SO3 | Na2SO3 + HCHO + H2O → NaSO3CH2OH + NaOH | 17 |
| Melamine | C3H6N6 + CH2O → C4H8N6O | 19 |
| Dicyandiamide | C2H4N4 + 2CH2O → C4H6N4O | 20 |
| Na2S2O5 | Na2S2O5 + H2O → 2NaHSO3 | 21 |
| NaHSO3 + HCHO → NaSO3CH2OH | ||
| Acetoacetate | 2CH3COCH2COOC2H5 + HCHO → CH2(CH3COCH2COOC2H5)2 + H2O | 22 |
| Hydroxylamine hydrochloride | HCHO + NH2OH·HCl → H2O + CH2NOH + HCl | 17 |
| Ammonia | NH4+ + OH− → NH3 + H2O | 21 |
| 4NH3 + 6HCHO → (CH2)6N4 | ||
| Tannin | C6H5OH + HCHO → C6H3(CH2OH)2OH + CH2OH(C6H4)OH | 23 |
| Dicyandiamide | C2H4N4 + 2CH2O → C4H6N4O | 24 |
| Ammonium bisulphite | NH4HSO3 → NH4 + HSO3 | 21 |
| Na + HSO3 + HCHO → NaSO3CH2OH |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the substituted amount of lignin for formaldehyde was 15 wt%, with 15 wt% NaOH and 5 wt% urea at a temperature of 95 °C for 4 h. When Fan et al.28 measured the properties of phenol–urea–formaldehyde co-condensed (PUF) resins, they found that urea was not only useful as a raw material but also had the function of reducing free formaldehyde. As shown in Fig. 5e, there were no peaks at around 80–90 ppm in the 13C NMR spectra of resins, indicating that no free formaldehyde was present in any form, e.g. hemiacetals, hemiformals, or oxymethylenes.
2, the substituted amount of lignin for formaldehyde was 15 wt%, with 15 wt% NaOH and 5 wt% urea at a temperature of 95 °C for 4 h. When Fan et al.28 measured the properties of phenol–urea–formaldehyde co-condensed (PUF) resins, they found that urea was not only useful as a raw material but also had the function of reducing free formaldehyde. As shown in Fig. 5e, there were no peaks at around 80–90 ppm in the 13C NMR spectra of resins, indicating that no free formaldehyde was present in any form, e.g. hemiacetals, hemiformals, or oxymethylenes.
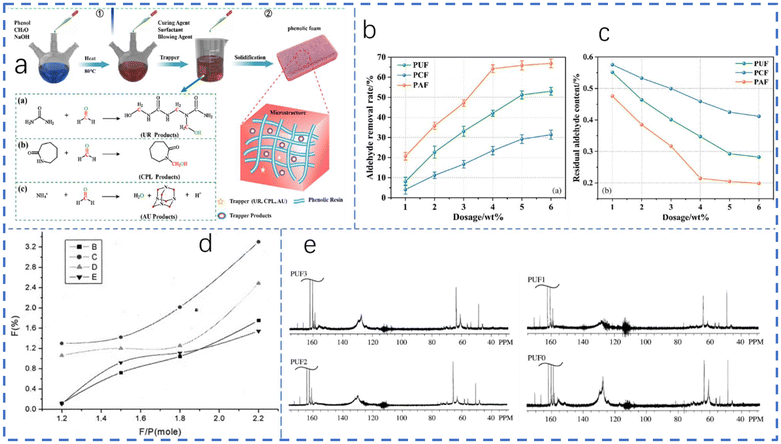 | ||
| Fig. 5 (a) The reaction process and mechanisms between formaldehyde trapping agents and formaldehyde; and (b) aldehyde removal rates and (c) free formaldehyde content levels of phenolic resin; reproduced from ref. 25 with permission; copyright: Wiley Periodicals LLC. (d) Free formaldehyde content in resol under different conditions: (B) 92 °C with urea, (C) 92 °C without urea, (D) 80 °C without urea, and (E) 80 °C with urea; reproduced from ref. 26 with permission; copyright: 2003 Wiley Periodicals, Inc. (e) 13C NMR spectra of PUF co-condensed resins with different urea content levels; reproduced from ref. 28 with permission; copyright: 2009 Koninklijke Brill NV, Leiden. | ||
It is necessary to compare and summarize some specific properties of the products in these experiments, so as to help readers gain an understanding. Ramdugwar et al.17 aimed to reduce the formaldehyde content in a phenolic resin binder, so more attention was paid to the peel strength and thermal stability of the product; the results showed that the addition of a small amount of urea did not affect these properties of the binder. Dong et al.25 used urea to successfully reduce free formaldehyde in phenolic resin; the thermal stability of the product is close to that of unmodified phenolic resin, and the limiting oxygen index is similar, so the product can be used as a fireproof material. Fan et al.28 found that with an increase in the proportion of urea, the curing time and thermal stability of the product were improved, but the water resistance was greatly decreased, and it could be used as structural plywood in reasonable proportions. These phenolic resin products modified with urea show excellent properties that can meet the application conditions.
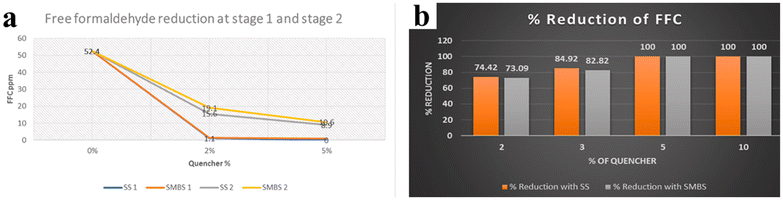 | ||
| Fig. 6 (a) The effects of SS and SMS on the reduction of FFC at different addition stages. (b) The effects of different dosages of SS and SMS on the reduction of FFC. Reproduced from ref. 17 with permission; copyright: 2022 Elsevier. | ||
Various materials have been used as formaldehyde scavengers. Over the past few decades, researchers have used both chemical reagents and natural ingredients as formaldehyde traps. Among the chemical scavengers, urea and sodium sulfite are the focus of this discussion; although other chemical scavengers are not discussed in detail, their reaction mechanisms are summarized in Table 2. Among natural ingredients, starch, cellulose, and tannins are the most common scavengers. Natural ingredients offer unique advantages for sustainable development, prompting extensive research into various natural substances that can reduce formaldehyde emissions. Demir studied the effects of tannins extracted from a kind of acorn on the removal of formaldehyde.30 As a renewable resource with a phenolic structure, tannin has high potential in wood-adhesive industries. Some researchers have pointed out that tannins are highly reactive with formaldehyde, which can accelerate the curing reaction and reduce formaldehyde emissions; some tannins can even enhance the mechanical properties of the product.31,32 Demir gives the mechanism of this reaction based on the research of Gonultas et al.33 They pointed out that valerian tannins contain a lot of gallic and ellagic tannins; these tannins contain polyflavonoids, which easily react with formaldehyde. Fig. 7a intuitively reflects the results of the overall experiment. With an increase in the tannin ratio, the formaldehyde emission from the product gradually decreases but, at the same time, the mechanical properties of the product also decline (Fig. 7b). In other words, in the experiment of Demir, although it has been confirmed that this tannin can reduce formaldehyde emissions, it will inevitably have adverse effects on the mechanical properties of the product, such as the bonding strength. Similar results have been found in other studies. Dukarska et al. used pine needles as a formaldehyde scavenger in the production of urea–formaldehyde resin sheets.34 As pine needles contain a large amount of tannin and other phenolic compounds, the reaction mechanism is roughly the same as for tannin. Dukarska et al. point out that the scavenging ability of pine needles is affected by various factors, such as species and habitat.
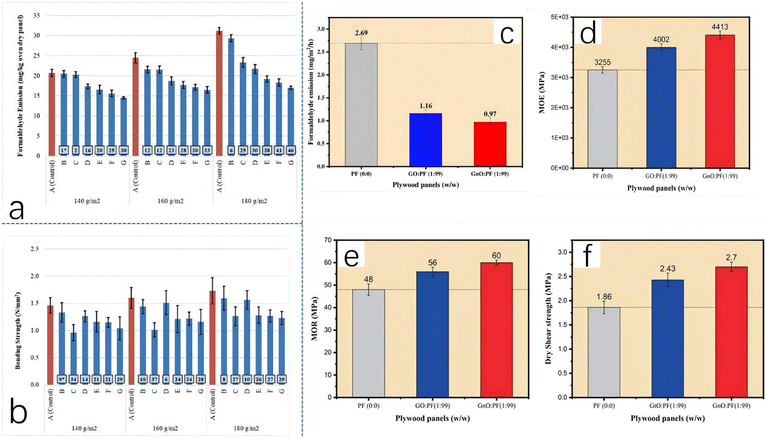 | ||
| Fig. 7 (a) Formaldehyde emission from different samples and (b) bonding strengths of different samples; reproduced from ref. 30 with permission; copyright: 2023 Elsevier Ltd. (c) Bonding strengths of samples containing GnO; (d) modulus of elasticity of samples containing GnO; (e) modulus of rupture of samples containing GnO; and (f) dry shear strengths of samples containing GnO; reproduced from ref. 35 with permission; copyright: 2023 Wiley. | ||
With the continuous development of carbon materials by scientists, some researchers have used carbon materials to remove free formaldehyde. Benhamou et al. added graphene oxide (GnO) to phenolic resin adhesive, reducing the release of free formaldehyde and successfully improving the mechanical properties and water resistance of the product.35 When it comes to how graphene oxide can reduce formaldehyde emissions, the researchers attribute this to a chemical bridge that is formed between the –OH and –COOH groups of GnO and hydroxymethyl-phenol groups. As seen in Fig. 7c, after the addition of graphene oxide, the formaldehyde emission from phenolic resin was greatly reduced, from the original 2.69 mg m−2 h−1 to 0.96 mg m−2 h−1. At the same time, the bond strength of GnO–PF resin is better, reaching 5.9 MPa (for PF this is 4.57 MPa). Fig. 7d, e, and f also clearly show the improvement of the modulus of elasticity, modulus of rupture and dry shear strength.
2.4. Substitutes for phenols and formaldehydes
The above discussion focused on improving the synthesis processes of phenolic resin products to increase their service life and reduce the content of toxic formaldehyde and phenol. Importantly for sustainable development, current production relies on nonrenewable petroleum resources. Therefore, researchers are exploring alternatives to phenol and formaldehyde, including biomass, a sustainable natural source that can be transformed into various chemicals. A recent study found that using biobased compounds to partially replace phenol in the production of polymeric adhesives significantly reduces carbon emissions and improves the resin performance. Additionally, biomass-derived raw materials are cost-effective compared to petroleum-based chemicals. Lignin, a component of biomass, has a similar macromolecular structure to phenol compounds and can replace phenols in various applications. Like phenol, lignin can undergo polycondensation reactions with formaldehyde under alkali conditions, enabling the production of phenolic resin.As shown in Fig. 8a, lignin, possessing three common states (H, G, S) shares a similar molecular structure to phenol compounds. There are several reactive sites in both the benzene ring and other side groups (Fig. 8b), enabling the feasibility of undergoing polycondensation with other molecules. When lignin meets formaldehyde, three types of reactions may occur under alkali conditions: the Lederer-Manasse reaction, Tollens reaction, and Prins reaction, as illustrated in Fig. 8c–e.
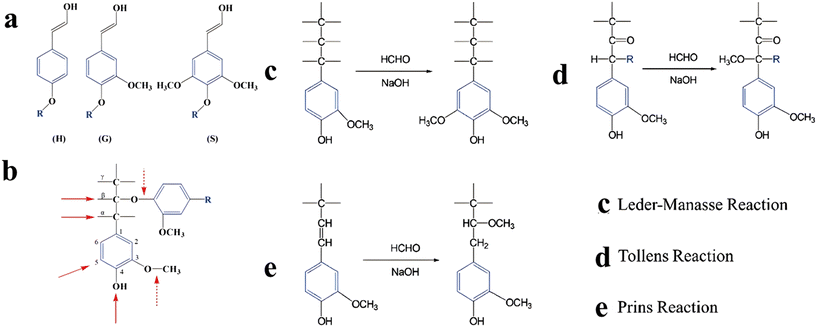 | ||
| Fig. 8 (a) The basic unit structure of lignin (R: point of linkage with other subunits of lignin) and (b) active positions on the phenolic lignin structure. (c–e) Types of lignin hydroxymethylation reaction. Reproduced from ref. 36 with permission; copyright: 2022 Wiley-VCH Gmb. | ||
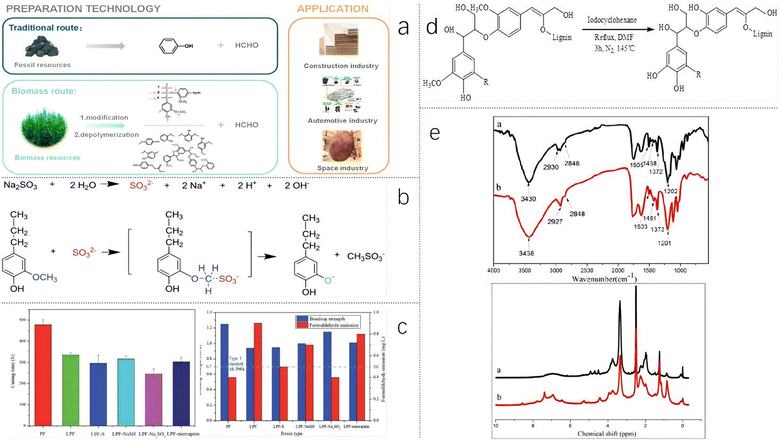 | ||
| Fig. 9 (a) Two routes to obtain phenolics and their applications. Reproduced from ref. 88 with permission; copyright: 2021 ACS. (b) The lignin demethylation reaction, and (c) bond strengths, formaldehyde emission and gel times of different samples; reproduced from ref. 37 with permission; copyright: 2016 The Royal Society of Chemistry. (d) The demethylation route of WSAL with iodocyclohexane, and (e) FTIR spectra and 1H NMR spectra of WSAL (a) and D-WSAL (b); reproduced from ref. 38 with permission; copyright: 2016 MDPI. | ||
Song et al. prepared a lignin-based phenolic resin adhesive (D-LPF) by replacing 60 wt% of phenol with lignin38 and demethylated wheat straw alkali lignin (WSAL) to enhance its reactivity. However, unlike Li et al., they used iodocyclohexane (ICH) as a demethylation reagent. The reaction process is shown in Fig. 9d. From the FTIR spectra and 1H NMR spectra of WSAL (marked a in Fig. 9e) and D-WSAL (marked b in Fig. 9e), the characterization results are the same as the theory. The methoxy group peaks decrease, and the hydroxyl group peaks increase. In the FTIR spectra, the O–H peak area (around 3430 cm−1) of D-WSAL is bigger than that of WSAL. The 1H NMR spectra showed that compared with WSAL the methoxy content (4.00–3.50 ppm) of D-WSAL decreases, while the phenol hydroxyl (2.40–2.17 ppm) content increases. Finally, the properties of samples are measured to assess their suitability for practical applications. The gel time of D-WSAL (150 °C) is 245 s and that of PF (150 °C) is 531 s. The bonding strengths of PF and D-WSAL are similar. The most prominent feature is that the free phenol content of D-WSAL is greatly reduced (from 3.01% to 0.92%) (Fig. 9e).
Yang et al. also explored environmentally friendly approaches to phenolic resin production. Finally, they developed a strategy to use decomposed monomers of lignin, rather than phenol, for resin synthesis39 (Fig. 10a). This unique strategy substituted monomers for phenol almost completely. In the depolymerization of oxidative lignin, researchers confirmed that vanillin, syringaldehyde and methyl vanillate were the main products.40 In addition, this experiment also builds on other experiments focused on synthesizing biobased resins from these monomers.41 To avoid formaldehyde, vanillin's ester and aldehyde groups are reduced to introduce hydroxymethyl groups, and vanillyl alcohol and syringic alcohol are polymerized to form a biobased phenolic resin under acidic conditions. In order to reduce ester and aldehyde groups, LiAlH4 is used as a reducing agent,42 and the reduction reaction is shown in Fig. 10b. As Fig. 10c shows, the polymerization of vanillyl alcohol and syringic alcohol is very similar. From the experimental results, the resin obtained by this method has some advantages in terms of thermal properties compared with novolak phenolic resins, but it has some shortcomings in mechanical properties. Using syringic alcohol as a substitute, the resin (PR-SA-t18) obtained after 18 h of reaction has the best thermal properties (Fig. 10d and e). The glass transition temperature is 115 °C, and the degradation temperature (340 °C) is higher than that of phenolic resin (234 °C). These findings indicate that the thermal properties of this resin are superior to those of novolak phenolic resins. However, the mechanical properties of all the samples were unsatisfactory, and even the best-performing samples had lower adhesion strengths than commercial phenolic resins (Fig. 10f).
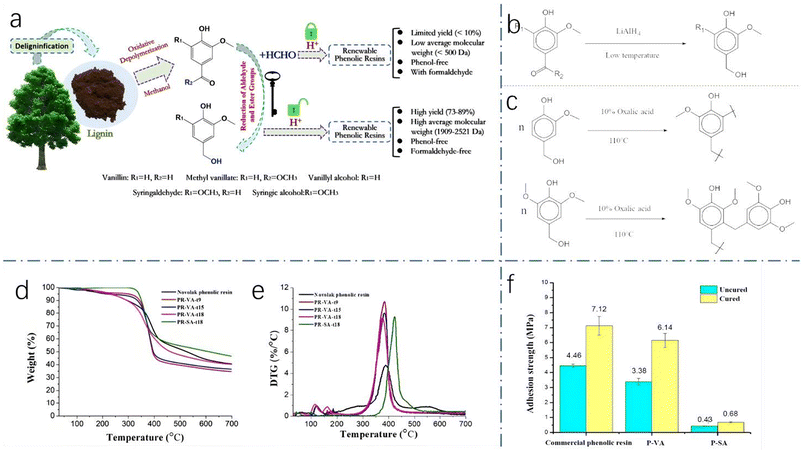 | ||
| Fig. 10 (a) The synthetic process for biobased phenolic resin; (b) the reduction of lignin monomers by LiALH4; (c) the synthesis routes from vanillyl alcohol and syringic alcohol; (d) TG curves of phenolic resin samples; (e) DTG curves of samples; and (f) adhesion strengths of samples; reproduced from ref. 39 with permission; copyright: 2021 American Chemical Society. | ||
Phenolic derivatives are often used to replace phenol in PF resin due to their simple structures, low toxicity, and availability. Aminophenol is commonly used as a substitute for phenol as it efficiently reduces free formaldehyde content. Aminophenol reacts with formaldehyde to form hemiaminals, which then react with triazines to form imines.43 There is an equilibrium between imines and triazines, and the imines react with phenolic compounds to form benzylamines. The reactions of triazines with phenol or aminophenol form benzoxazines. The thermal stability of the resin increases with the degree of polymer crosslinking. 2-Aminophenol-modified resin is highly crosslinked and does not degrade at high temperatures, indicating improved thermal stability. The mechanical properties of the modified resin may decrease slightly compared to the unmodified resin. Formaldehyde can also be replaced by simple aromatic aldehydes like benzaldehyde, terephthalaldehyde, and 4,4-dihydroxybenzaldehyde.44 The phenolic ring forms a methylene bridge with the aldehyde, which then condenses with another phenolic ring to remove water. Most of the resins obtained are brittle; however, the addition of terephthalaldehyde (TPA) enhances their thermomechanical properties, bringing their performance closer to that of traditional phenolic resin. Using other substances to replace formaldehyde can improve the properties of phenolic resin and reduce pollution.
Most alternative materials are just theoretical and lack practical application. However, some commercial materials can be used as phenol substitutes, and some results have been achieved. One such alternative is cashew nut shell liquid (CNSL), a versatile renewable resource used in industry.45 CNSL is a liquid found in cashew shells that contains phenolic substances structurally similar to phenol. These can be used to prepare phenolic resin with better ductility.46 Da silva et al.47 synthesized a kind of phenolic resin by the reaction of CNSL and paraformaldehyde with oxalic acid as a catalyst. The essence of this reaction is the electrophilic addition of phenol at the ortho-position or para-position. In oxalic acid, an acidic medium, phenol undergoes nucleophilic attack by other phenol molecules, resulting in compounds linked by methylene bridges. This process repeats, ultimately yielding a new type of phenolic resin (Fig. 11d).
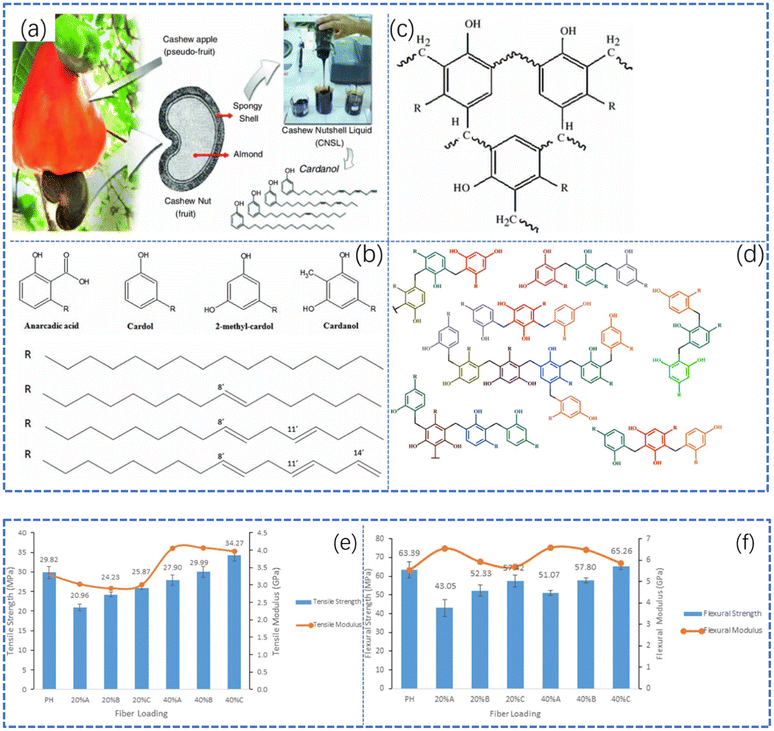 | ||
| Fig. 11 (a) The extraction of cashew nut shell liquid. Reproduced from ref. 45 with permission; copyright: American Coatings Association & Oil and Colour Chemists’ Association 2013. (b) Major components of CNSL. Reproduced from ref. 46 with permission; copyright: Taylor & Francis Group, LLC. (c) The crosslinked structure of CNSL-formaldehyde resin. Reproduced from ref. 45 with permission; copyright: American Coatings Association & Oil and Colour Chemists’ Association 2013. (d) Possible structures formed via the condensation of CNSL phenolic units. Reproduced from ref. 47 with permission; copyright: 2023 Wiley Periodicals LLC. (e) Tensile strength and modulus of phenolic resin samples and (f) flexural strength and modulus of phenolic resin samples; reproduced from ref. 48 with permission; copyright: Springer Nature 2021. | ||
CNSL increases the thermal decomposition temperature of resin products, improving their thermal resistance and reducing formaldehyde emissions. A study by Loganathan et al. tested the mechanical properties of phenolic resin made from CNSL, finding that it had excellent tensile and flexural strength, comparable to reinforced resin.48 Since the 1980s, phenolic resin synthesized from CNSL has been widely used due to its superior thermal and mechanical properties, as well as its ability to reduce phenol usage. This demonstrates that CNSL is a highly effective green commercial material. Research has shown that replacing phenol in phenolic resin with lignin or other phenols decreases the mechanical properties of the resulting products, even if their thermal properties improve.37 To mitigate this, researchers have explored using other substances to replace formaldehyde in phenolic resin raw materials. One such substance is glucose. The synthesis of phenolic–glucose resin involves Friedel–Crafts condensation between phenol and glucose under strongly acid conditions. The process produces hydroxymethylfurfural and carboxylic acids, which react with phenol to generate resin. This environmentally friendly process can produce phenolic–glucose resin with similar thermal properties to phenolic–formaldehyde resin, indicating its potential as a substitute.
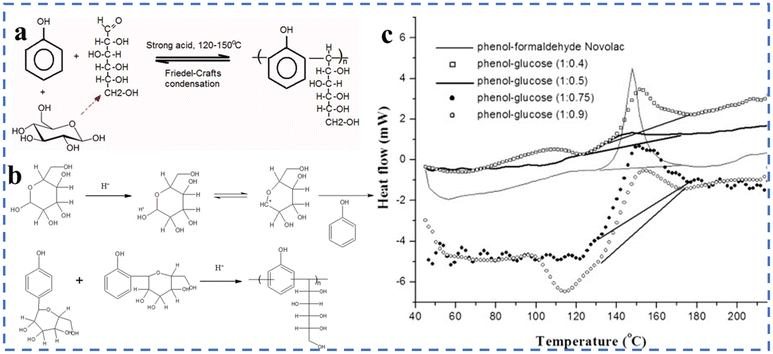 | ||
| Fig. 12 (a) A schematic illustration of the synthesis of phenolic–glucose resin through Friedel–Crafts condensation. (b) A plausible mechanism for the formation of phenolic–glucose resin. (c) DSC curves of various phenolic resins. Reproduced from ref. 49 with permission; copyright: 2010 Wiley. | ||
Granado et al. replaced formaldehyde in the synthetic material with terephthalaldehyde (TPA).50 They chose TPA as the substituent rather than other nontoxic aromatic dialdehydes. The reason for this is that TPA showed the best reactivity. The reaction process of terephthalic acid and phenolic substances is shown in Fig. 13a. For the curing behaviors of these different formulations, the cross-linkage kinetics can provide some perception of this behavior (Fig. 13b). The E1 energy of phenol–TPA is around 78 ± 1 kJ mol−1. The activation energy for the condensation of phenol on methyl groups is equivalent to this value, which indicated the condensation mechanism of phenol–TPA is the same as the reaction of phenol and formaldehyde. Meanwhile, the E1 energies of resorcinol–TPA and guaiacol–TPA are lower than for phenol–TPA. This may indicate that the more substituents, the lower the activation energy required for the reaction. The reaction viscosity increases dramatically during the curing process of thermosetting resins, due to the importance of diffusion. Therefore, the activation energy of reaction II may determine the ability to spread.51 The E2 energy of guaiacol–TPA is higher than the other samples, which means guaiacol is hard to diffuse into TPA. From the crosslinking kinetic analysis, we can not only intuitively judge the size of the activation energy of the reactions but also better understand the crosslinking mechanisms. To study the thermal properties of every sample, thermal gravimetric analysis was conducted. From Fig. 13c, tyrosol–TPA has the lowest thermal performance, while resorcinol–TPA and guaiacol–TPA have satisfactory thermal performance, comparable to commercial phenolic resin, making them rare biological materials with such excellent thermal properties.
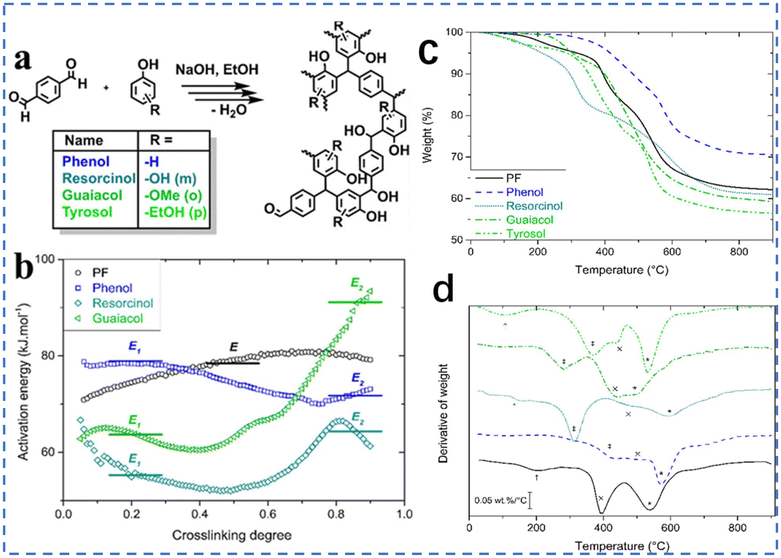 | ||
| Fig. 13 (a) The reactions between TPA and different green phenols. (b) Apparent activation energies throughout the cross-linking process. (c) TGA thermograms of fully cured resoles. (d) Derivative curves highlighting changes in degradation mechanisms. Reproduced from ref. 50 with permission; copyright: 2019 American Chemical Society. | ||
3. Establishing dynamic covalent bonds
Researchers have proposed dynamic covalent bonds as a way to recycle phenolic resin sustainably. These bonds are chemical bonds that can break and reform under specific conditions, providing various physical and chemical properties. The cross-linked network in phenolic resins is difficult to recover, so researchers are exploring the possibility of bonds that can break down under certain conditions. While non-covalent interactions have been successful in some fields, they are less heat-resistant than covalent-bonding-based thermosetting resins.52 Therefore, dynamic covalent bonds are more suitable for applications requiring high heat resistance.Dynamic covalent networks (DCNs) or covalent adaptive networks (CANs) can form repeating units that bind or dissociate following certain pathways. Walter Alabiso used this concept to explain differences in smart polymer chemistry and properties.53 DCN construction helps with phenolic resin recycling, an important step towards sustainable development. Many researchers have used dynamic covalent bonds to address phenolic resin recycling, with innovative methods compared to traditional ones. This section introduces four phenolic resins with dynamic covalent bonds.
3.1. Introducing dynamic acetal bonds
The cross-linked structure of phenolic resin makes it difficult to recycle, so researchers have added new structures to resins that are easier to break, creating reversible structures. Chen et al.54 designed a new dynamic thermosetting material (PRT) based on this idea, using linear phenolic resin and tri(ethylene glycol)divinyl ether (TEGDVE). The material has dynamic acetal bonds that can be adjusted to change the mechanical properties or improve the degradation ability. The ratio of phenolic resin to TEGDVE depends on the molar ratio of phenolic hydroxyl to vinyl.In order to synthesize reversible and hydrolysable acetal bonds, Markovnikov addition of –OH and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO– is used for reference (Fig. 14b). FTIR analysis of PRTs was carried out after production for observation and comparison (Fig. 14c). A decrease in the peak strength of C
CHO– is used for reference (Fig. 14b). FTIR analysis of PRTs was carried out after production for observation and comparison (Fig. 14c). A decrease in the peak strength of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –OH was found. After about 2 h, the peak of C–H from the acetal structure appeared at around 1350 cm−1. When the reaction went on for 24 h, there was no peak at around 3300 cm−1. In short, the phenolic structures and crosslinking points provide the main support structure of the network, resulting in high mechanical strength, while flexible structural units containing reversible acetal connections provide high elongation at break. In order to study the thermal stability and influence of materials, thermogravimetric analysis (TGA) of PRTs was carried out. The analysis results are clear and easy to understand. The increase of weak acetal bonds will reduce the thermal stability, while the high crosslinking point can increase the thermal stability. In the stress–strain diagram (Fig. 14d), the sample with the highest tensile strength is PRT-1:0.9; the value is up to 50 MPa. This value is slightly lower than that of phenolic resin (60–80 MPa). From the DSC curves of PRTs (Fig. 14e), the results showed that the Tg value of PRT-1:0.5 is 67.44 °C (for PF is 68.31 °C). From these two aspects, we can see that the phenolic resin synthesized via dynamic acetal bonding can show similar properties to phenolic resin upon changing the formula. Some degradable materials based on labile bonds have poor chemical stability, but PRTs have excellent stability under strongly alkaline and acidic conditions, which is also a prominent advantage.
C and –OH was found. After about 2 h, the peak of C–H from the acetal structure appeared at around 1350 cm−1. When the reaction went on for 24 h, there was no peak at around 3300 cm−1. In short, the phenolic structures and crosslinking points provide the main support structure of the network, resulting in high mechanical strength, while flexible structural units containing reversible acetal connections provide high elongation at break. In order to study the thermal stability and influence of materials, thermogravimetric analysis (TGA) of PRTs was carried out. The analysis results are clear and easy to understand. The increase of weak acetal bonds will reduce the thermal stability, while the high crosslinking point can increase the thermal stability. In the stress–strain diagram (Fig. 14d), the sample with the highest tensile strength is PRT-1:0.9; the value is up to 50 MPa. This value is slightly lower than that of phenolic resin (60–80 MPa). From the DSC curves of PRTs (Fig. 14e), the results showed that the Tg value of PRT-1:0.5 is 67.44 °C (for PF is 68.31 °C). From these two aspects, we can see that the phenolic resin synthesized via dynamic acetal bonding can show similar properties to phenolic resin upon changing the formula. Some degradable materials based on labile bonds have poor chemical stability, but PRTs have excellent stability under strongly alkaline and acidic conditions, which is also a prominent advantage.
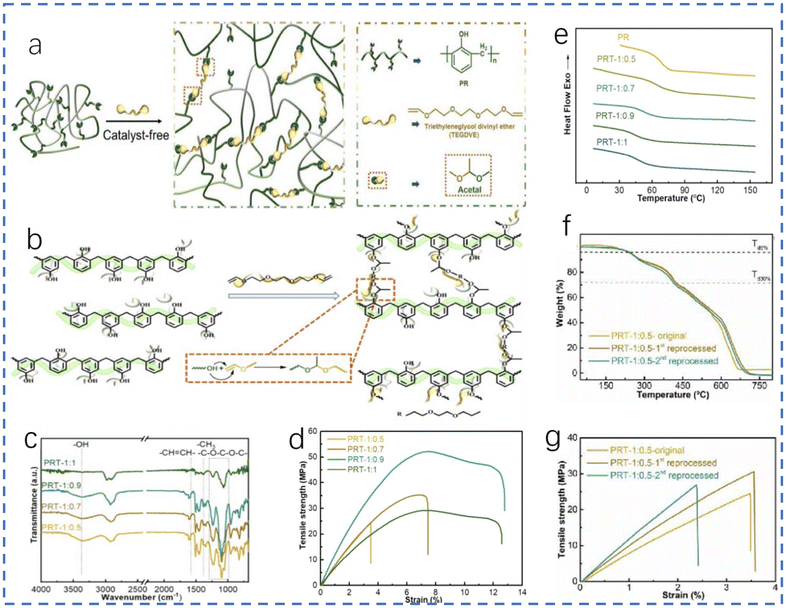 | ||
| Fig. 14 (a) Synthetic routes to PRTs; (b) Markovnikov addition of PR and TEGDVE; (c) FTIR spectra of PRTs; (d) tensile stress–strain curves of PRTs; (e) DSC curves of PR and PRTs; (f) TGA curves of original and reprocessed PRTs; and (g) tensile stress–strain curves of original and reprocessed PRTs; reproduced with permission from ref. 54; copyright: 2022 Elsevier. | ||
In terms of reprocessing, researchers found that the reprocessing conditions were more moderate than for other dynamic phenolic networks,55 which is attributed to the mobility of segments. In the process of reprocessing, the diffusion and randomization of chain segments play a dominant role in healing. After healing, the mechanical properties of PRTs are measured again and compared with the original PR. From the synthetic materials, PRTs can be decomposed into linear phenolic resin, triethylene glycol and acetaldehyde. In order to verify the decomposed products, the products were characterized by observing the FTIR and H NMR spectra. Fig. 14f and g show the mechanical and thermal properties of regenerated PRTs. The results show that the tensile strength of the recycled materials experiences only a slight decrease after the first recovery, but declines significantly with the second and subsequent recoveries. There was only a slight decrease in thermodynamic performance. This is certainly beneficial for regeneration.
3.2. Introducing B–O bonds
Scientists are exploring the introduction of B–O bonds into phenolic resin to enhance its characteristics.56 This bond can be easily formed due to the Lewis acidity of boron atoms, which react with Lewis bases to form complexes. This bond also has greater bond energy than C–C, increasing the thermal and mechanical stability of plastics. Initially, B–O bonds were used in organic frameworks and polymer synthesis, later leading to the development of traditional crosslinked polymer synthesis through the introduction of boronic esters. The bond's dynamic reversibility can be regulated based on pH and the amount of alcohol. Polymer synthesis mechanisms based on boronic esters involve hydrolysis and re-esterification (dissociative exchange) and transesterification (associative exchange). Most reactions use a combination of these two mechanisms (Fig. 15).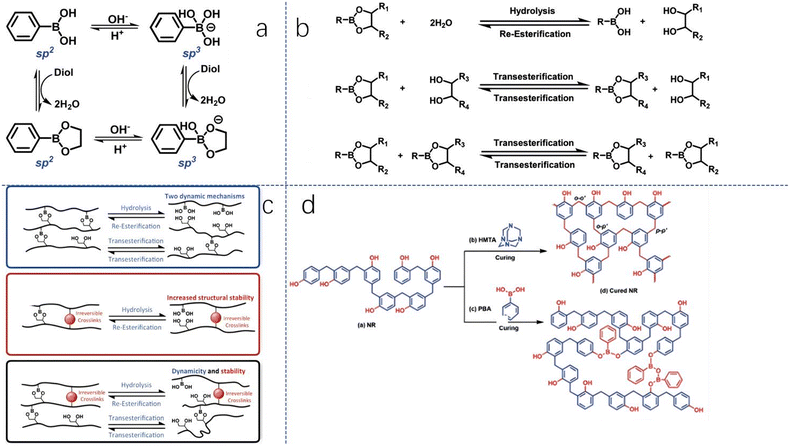 | ||
| Fig. 15 (a) The reaction characteristics of boronic acid; (b) the three exchange reaction mechanisms of boronic ester-based polymers, (c) introducing traditional covalent bonds into boronic ester networks, and (d) the curing process and cured structures of NR/HMTA and PBNR. Reproduced with permission from ref. 55; copyright: 2021 The Royal Society of Chemistry and the Chinese Chemical Society. | ||
In recent years, researchers have studied the recycling of materials prepared with boronic ester linkages. In 2015, Guan et al.57 synthesized two telechelic diboronic ester small molecules, which, after recycling, showed similar properties to the original samples. In 2017, Leibler58 introduced dioxaborolanes into several polymers, including PMMA and PS, which can be processed multiple times. In 2018, a dynamic cross-linked phenolic resin was produced using PBA and novolac resin, showing excellent mechanical properties and easy decomposition in ethanol.59 Zhang et al. used two methods to improve the stability of this polymer: adjusting the ratio of methylene to boron ester bonds, and replacing the borate bonds with nitrogen-coordinating cyclic boronic diester linkages. These new bonds improved the water resistance and thermal stability compared to common boronic esters.
Scientists have emphasized the importance of recycling polymers with boronic ester linkages, but hydrolysis and alcoholysis have limited their use. To control this, two methods have been developed: using traditional, irreversible covalent bonds instead of dynamic ones; and designing a unique boronic ester linkage. The first method, verified by the experiments of Torkelson et al.60 and Sumerlin et al.,61 improves material stability by introducing a proportion of irreversible bonds. Yoshie et al.62 introduced a tetrahedral boronic ester with B–N coordination into a polymer, enhancing its mechanical properties among others. Ding, using B–N coordination bonds, achieved similar results.
3.3. Introducing dynamic urethane bonds
The recovery of phenolic resins has puzzled researchers for years, and the use of dynamic covalent bonds is one method being explored to achieve this. Some researchers have studied urethane bonds, which are structurally similar to borate bonds. Urethane bonds can form blocked isocyanates that can decompose at high temperatures or in the presence of a catalyst.63 Some researchers have discovered a way to develop catalyst-free dynamic networks by adjusting the steric effects of the related covalent bonds.64 However, most dynamic cross-linked thermosetting materials based on this technology cannot meet application standards in terms of mechanical strength and thermal properties. Liu et al. developed a method to form reversible urethane bonds based on the synthesis of steric phenol hydroxyls and isocyanates. These materials, called TDNRs, can change mechanical properties upon adjusting the ratio of raw materials. TDNRs exhibit tensile strength up to 55 MPa and a glass transition temperature up to 200 °C, and these mechanical properties remain almost unchanged after several recycling cycles (Fig. 16). The material is easy to prepare, has low cost and a simple process and has the potential to become a recyclable polymer.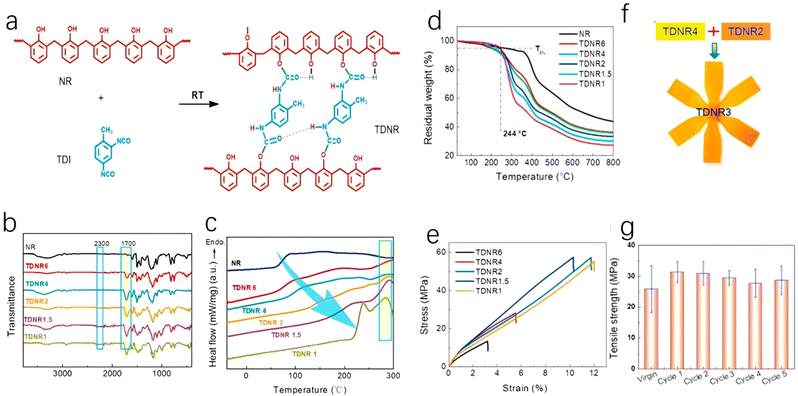 | ||
| Fig. 16 (a) Possible states of TDNR crosslinking; (b) FTIR spectra of pristine NR and the as-synthesized TDNRs with different amounts of TDI; (c) DSC curves of TDNR samples with different amounts of TDI; (d) the thermal stability of TDNRs under a nitrogen atmosphere; (e) tensile properties of TDNRs; (f) the pathway for the acquisition of a TDNR3 sample; and (g) the tensile strength of TDNR4 after recycling and re-molding processes; reproduced with permission from ref. 65; copyright: 2021 Elsevier. | ||
Fourier-transform infrared spectra analysis shows a strong peak at 1710 cm−1 for all TDNR samples, indicating the formation of a hydrogen bond corresponding to the polyurethane bond and a hydrogen bond formed between the carbonyl group and imine. The glass transition temperature (Tg) is also an important parameter; from the results, the Tg values of all experimental samples were higher than that of the original phenolic varnish resin, and it can be seen that the change in Tg is related to the –OH/–NCO molar ratio. After mechanical experiments, it was found that most phenolic resins with the correct molar ratio showed excellent mechanical performance, with tensile strengths higher than 55 MPa when the molar ratio between the hydroxyl group and isocyanate group was set at 1 and 1.5. The tensile strength is close to that of phenolic resin, but the Young's modulus is only half, which may be suitable for application in some fields.
Urethane bonds in TDNRs are sensitive to heat and need to maintain thermal stability. At 200 °C, the TGA curves of TDNRs show a significant downward trend, and they begin to decompose at about 244 °C. This may be due to four possible thermal degradation reactions. However, when ethyl carbamate bonds are made with excessive aromatic hydroxyl groups and isocyanates, the bonds mainly undergo reverse reactions. This causes weight loss due to water and TDI volatilization. The fracturing of urethane bonds leads to polymer degradation. The experiment showed that urethane bond reverse reactions occur easily below 200 °C, with almost no decomposition loss. TDNRs can be recycled at high temperatures, breaking urethane bonds into oligomers and DTI. Further disassembly or synthesis can happen at temperatures below 200 °C. Researchers also found a new TDNR with performance between TDNR4 and TDNR2 by mixing TDNR2 and TDNR4; this process can produce phenolic resins quickly without meeting the performance requirements of most other dynamic phenolic resins.
The introduction of dynamic covalent bonds in phenolic resins makes them easy to recycle. Although a new method, it holds considerable potential for further exploration. Resins containing these bonds perform well in recycling but exhibit lower thermal stability and mechanical properties compared to traditional phenolic resin. Some bonds are also susceptible to hydrolysis and alcoholysis, which limits practical applications. While scientists have proposed solutions, these often require customization for each specific case. In summary, dynamic covalent bonding plays a significant role in the synthesis and recovery of greener phenolic resins, but further research is necessary to fully realize their practical application.
4. Recycling of PF-resin
As mentioned above, numerous studies have focused on improving the sustainability, environmental friendliness and recyclability of PF-resin. However, in terms of the huge and diverse PF-resin market, it is difficult for a universal and cheap modification scheme to be created, accepted by governments and put to industrial use in the near future. Therefore, an unavoidable problem in the area of plastic recycling will be discussed in this section: how to release and transform the residual value of PF-resin waste as much as possible under artificial conditions. In contrast to the previously discussed modifications of PF-resin, the chemical treatment approaches in this section directly convert PF-resin into other valuable chemical raw materials and products.Further conversion is conducted using the purified PF-resin samples obtained through physical recovery. This section discusses chemical and mechanochemical methods used to treat waste PF-resin to release its residual value as much as possible, including but not limited to depolymerizing the resin to obtain small-molecule chemical products and using its rich carbon content for conversion into functional carbon materials.
4.1. Depolymerization
Depolymerization is the breaking down of a polymer into its most basic monomers: in the case of phenolic resins, a series of phenols and their homologues. Due to the breaking of molecular bonds involved, the difficulty of depolymerizing thermoset polymers with a lot of crosslinking is obviously greater than for thermoplastic polymers.In the modern electronics industry, thermosetting resins are used in packaging materials and adhesives for printed circuit boards (PCBs) and integrated circuits (ICs). Unlike the dispersion of plastic parts, this type of assembly links the resin and metal parts closely, making it difficult to separate them using mechanical means. Therefore, a process is needed to decompose the resin, presenting the product in a solid–liquid separation state. Supercritical water (SCW), a fluid whose temperature and pressure exceed the critical state, has unique physical properties and is used in various applications, like natural product extraction and polymer recovery.66 The depolymerization of organic matter in supercritical water can be regarded as a chain reaction involving free radicals and hydroxyl free radicals (Fig. 17). This chain reaction can be initiated by the reaction between oxygen and organic matter. The depolymerization of other thermosetting resins may provide ideas for the recovery of phenolic resins, and we discuss the product distribution of mainstream thermosetting resins, including phenolic resins, that can be treated with SCW. Tagaya et al.67 treated phenolic resin with supercritical water, depolymerizing it to phenol and its derivatives. Li et al.68 polymerized epoxy resin from ICs via supercritical hydrolysis, obtaining hydrocarbons from H2O and CO2. However, high temperatures and pressures were still required. Due to high energy consumption, researchers turned to other methods, like using organic solvents instead of water, to achieve lower critical temperatures and pressures. Additionally, the organic parts of PCBs and ICs often have brominated flame retardants (BFRs), which can cause secondary pollution during recycling if not treated. Chen et al.69 used NaOH to create an alkaline environment that removes BFRs and reduces energy requirements. The alkaline substances help break down the resin's physical structure and advance the debromination reaction. Ultimately, the degree of debromination is 95.11% under subcritical water conditions, reducing energy demand.
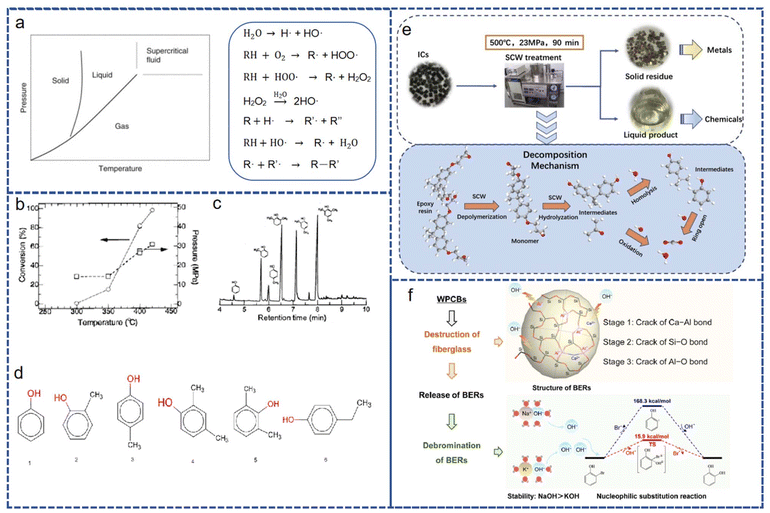 | ||
| Fig. 17 (a) The temperature and pressure limits of supercritical fluids and reactions of free radicals of organic matter in supercritical water. (b) The curve of phenolic resin conversion with temperature and pressure. (c) The variation of product components with reaction time. (d) The main products of phenolic resin treated with SCW. (e) The product distribution of epoxy resins in ICs after SCW treatment. (f) The debromination and decomposition of epoxy resins with brominated flame retardants in subcritical water assisted by an alkaline environment. (a) and (e) reproduced with permission from ref. 67; copyright: 2019 Elsevier. (b)–(d) reproduced with permission from ref. 66; copyright: 2000 American Chemical Society. (f) Reproduced with permission from ref. 68; copyright: 2020 Elsevier. | ||
Supercritical fluid processes have limited large-scale application due to the high-temperature and -pressure requirements and potential secondary environmental pollution from recycling. Researchers are exploring alternative methods for thermosetting resin degradation, such as mechanical energy ball milling and biodegradation, which have achieved good results without the problems of liquid-phase reactions. For phenolic resins with BFRs, debromination is a primary task to curb pollution.70 Pyrolysis is a common method to treat BFR-containing products, but it produces brominated products unacceptable for green recycling. Ball milling with iron powder as co-grinding particles can achieve organic pollutant decomposition and promote iron powder to transfer electrons, aiding in debromination.71 It has been reported that iron powder/silica grinding for a long time can achieve 90% debromination.72 Chen et al.73 used zero-valent nano-iron as a dehalogenation agent, providing electrons to increase the C–Br bond length in pentabromodiphenyl ether, making it easier to break the bonds during ball milling. The content of bromine on the surface of particles was reduced by half after treatment. If iron powder co-ball milling reduces energy and solvent use compared to liquid-phase recovery, biodegradation simplifies the process and reduces pollution further. Gusse et al.74 first proposed the use of fungi to achieve the biodegradation of PF resin. Many organic pollutants, including DDT, PCBS, and trinitrotoluene, can be degraded by lignin enzymes produced by white-rot fungi.75 Their ability to break down lignin has led researchers to question whether they can be used to break down phenolic resins (Fig. 18c); one type, named Phanerochaete chrysosporium, has been shown to biodegrade PF resin after testing a variety of white-rot fungi. As shown in Fig. 18d, after the bacterial colonies on the PF resin surface were cleaned by ethanol, holes and pores with large differences in morphology from the surrounding areas could be observed through scanning electron microscopy images. After this study, Lawrance et al.76 used Trichoderma aureoviridae, a Trichoderma, to successfully break down the phenolic resin components in a phenolic leather tanning agent (Basyntan DI). Fig. 18e and f show that COD, BOD, and TOC of the tanning agent decreased with an increase in the number of biodegradation days. As shown in the figure, the degradation effect changes with a change in the Basyntan DI concentration, and the degradation efficiency at high concentration is the most obvious. At 1500 ppm, COD drops to 65% of the initial value after 8 d, BOD is 16.8% of the initial value, and TOC is reduced by nearly 80%.
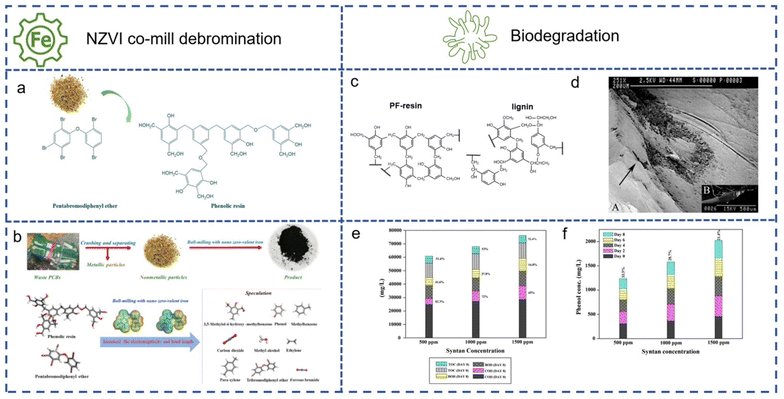 | ||
| Fig. 18 (a) The two main components of phenolic resin containing flame retardants. (b) The mechanism and product from NZVI co-ball milling of PF resin. Reproduced with permission from ref. 72; copyright: 2020 American Chemical Society. (c) The two-dimensional chemical structures of PF resin and lignin. (d) A scanning electron microscope image of the PF resin surface degraded by lignin enzyme. Reproduced with permission from ref. 73; copyright: 2006 American Chemical Society. (e) Changes in COD, BOD and TOC with a change in the number of biodegradation days and Basyntan DI concentration. (f) Changes in total phenol concentration after biodegradation. Reproduced with permission from ref. 75; copyright: 2018 Springer. | ||
In summary, the depolymerization of thermosetting resins into small monomer molecules requires high energy compared with conventional depolymerization methods. For example, depolymerizing PF resin in supercritical and subcritical fluids requires high temperatures and pressures, making large-scale applications challenging. In seeking to reduce the energy demands, the application of organic solvents will bring about the possibility of secondary pollution. The use of mechanical energy and electron transfer to help break chemical bonds is another direction that can be investigated. In addition, the application of bioengineering in resin degradation can also be explored; using fungi to degrade resins is a worthwhile approach.
4.2. Conversion into carbon materials
Technology for the depolymerization of phenolic resin is being promoted, but the energy required to destroy all chemical bonds between and within the molecules of thermosetting resins cannot be reduced. Research is focusing on transforming waste plastics into various functional carbon-based materials, such as carbon nanotubes, carbon fibers, and heteroatom-doped carbon.77 These materials are used in various industries and represent a nonrenewable resource retention method with good environmental and economic benefits. Lithium-ion batteries (LIBs) have been successful in the current energy field, but the uneven global distribution of lithium limits the scale of future grid development; as recycling technology for LIBs is not mature, sodium-ion batteries (SIBs) may be a substitute.78 The use of SIBs on a large scale requires an alternative to graphite anodes due to the larger volume of sodium ions compared to lithium ions. Hard carbon with larger layer spacing and many defects and vacancies is considered suitable as an anode in SIBs. Phenolic resin has been considered as a potential hard carbon precursor due to its low cost and rich carbon content. However, the carbonization of PF resin cannot directly obtain a precursor that can be used to manufacture hard carbon; gas molecules generated at high temperature will destroy the surface structure of the precursor, so PF resin needs to be pretreated (Fig. 19).79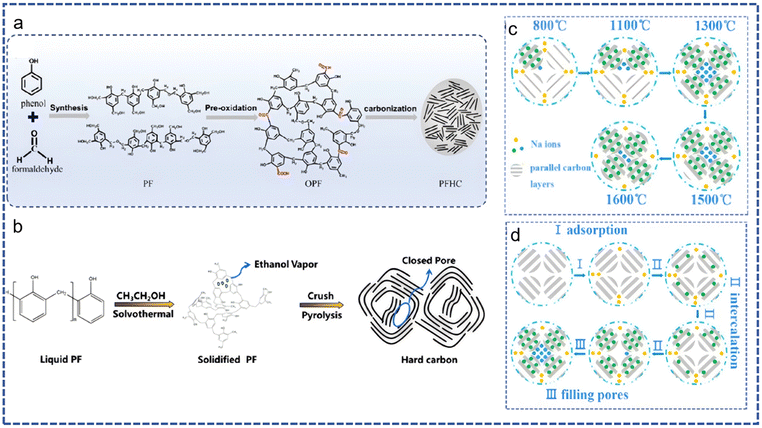 | ||
| Fig. 19 (a) PF resin synthesis and the HC production process; reproduced with permission from ref. 80; copyright: 2021 American Chemical Society. (b) The HC closed pore formation mechanism; (c) sodium-ion filling as temperature increases; and (d) the sodium-ion filling process; reproduced with permission from ref. 81; copyright: 2024 American Chemical Society. | ||
CO2 emissions contribute significantly to global warming. Under the directive of low-carbon policies, a range of methods are employed to reduce CO2, from curbing sources to absorbing it. Porous materials are essential for CO2 adsorption, especially those that are low cost and use a wide range of raw materials, while further requirements such as surface adjustability and chemical/thermal stability are constantly emerging. Phenolic resin, with high carbon content, is used as a carbon precursor for porous carbon. Yue et al.82 used urea to dope phenolic resin with nitrogen, then activated it with KOH to create nitrogen-doped porous carbon. This type of carbon has improved adsorption capacity due to the presence of basic nitrogen-containing groups.83Fig. 20a–c shows SEM images of phenolic resin (R), urea-modified carbonized resin (RU), and KOH-activated RU (RUK). Fig. 20d shows a TEM image of RUK. It can be seen that there is only a size gap between R and RU, and their surfaces are smooth without pores, while the surface of RUK has pores due to the etching action of KOH, and more micropores can be found after further magnification. The pore size distribution of porous carbon calculated by density functional theory (DFT) shows that the pore size of porous carbon is mainly about 1 nm at normal temperature (600 °C), and the pore size increases when the temperature rises to 700 °C. Subsequent analysis also shows that a larger pore size is not conducive to gas adsorption (Fig. 20f). The adsorption experiment results showed that when the mass ratio of KOH/resin was 3 and the activation temperature was 600 °C (RUK-600-3), the adsorption efficiency of the porous carbon could reach 7.13 mmol g−1 at 1 bar pressure and 0 °C. When the temperature rose to room temperature (25 °C), the adsorption efficiency was 4.61 mmol g−1. Liu et al.84 improved the production process of porous carbon by first mixing the resin with KOH solution and drying it, and then placing the dry product into a reactor for direct activation, thus skipping the carbonization step. Due to the lack of nitrogen doping, although the pore distribution is consistent with the product obtained by Yue et al.,82 its CO2 absorption efficiency is slightly lower. However, at room temperature, adsorption of 4.12 mmol g−1 can be achieved, and it has good cycling stability (Fig. 20g).
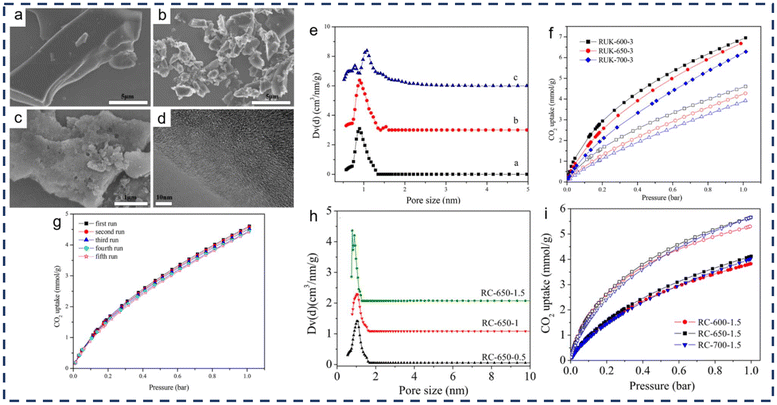 | ||
| Fig. 20 (a)–(c) SEM images of R, RU, and RUK. (d) A TEM image of RUK. (e) The pore size distributions of porous carbon calculated by DFT: (a) RUK-600-3, (b) RUK-600-4, and (c) RUK-700-4. (f) The CO2 adsorption efficiency of products at different pressures, with solid markers at 0 °C and hollow markers at 25 °C. Reproduced with permission from ref. 81; copyright: 2018, American Chemical Society. (g) The adsorption capacity curves of porous carbon (RC) prepared by direct activation over multiple cycles; (h) the pore size distributions of RC calculated by DFT; and (i) the adsorption capacity of RC for CO2 at different temperatures and pressures, with hollow markers at 0 °C and solid markers at 25 °C; reproduced with permission from ref. 83; copyright: 2019, American Chemical Society. | ||
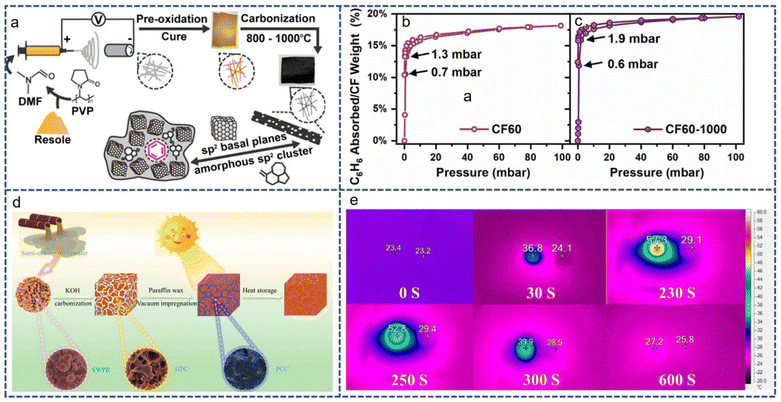
In addition to the adsorption of greenhouse gases such as CO2, activated carbon prepared from PF resin can be modified by other processes to adsorb organic pollutants. Due to the properties of the fiber giving the material a larger surface area, phenolic-based activated carbon fiber has a stronger adsorption capacity than granular activated carbon; however, initially its high price limited development. An initial improvement involved using phenolic-resin-coated glass fibers and then activating them to obtain the target fiber85 using electrospinning technology. It is also an option to obtain and carbonize phenolic resin fibers directly by electrospinning. Zhao et al.86 used electrospinning to produce phenolic resin fibers. As phenolic resin is divided into two categories, thermosetting and thermoplastic, the properties displayed in the remelting process will also be different. The addition of a curing agent leads to electrostatic textile thermoplastic resin having a greater risk of secondary pollution. The degree of activator input, pre-oxidation time and carbonization temperature have been included as experimental variables. In testing, the thermosetting phenolic resin was first made into fibers by electrospinning, then pre-oxidized for 72 h (kept at 120 °C for 12 h and heated to 180 °C for 60 h) and carbonized at 1000 °C. The final product (CF60-1000) shows high selective adsorption of benzene, which is speculated result from the strong π–π interactions on its surface. At the same time, due to a lack of functional groups on its surface, its absorption capacity for other organic volatiles, such as ethyl acetate, is weak. In addition to being beneficial for gas adsorption, the porous structure of activated carbon can also be used for energy storage. Wang et al.87 increased the thermal conductivity of the composite by adding paraffin wax (PW) to porous carbon made from phenolic resin. They used semi-coking wastewater to produce phenolic resin (SWPR). The porous carbon is also prepared by a KOH activation–carbonization process similar to previous ones, and the obtained hierarchical porous carbon (HPC) is soaked in PW in a vacuum oven to obtain phase-change composite materials (PCCs). Monitoring results showed that the thermal conductivity of PCC increased by 166% compared with pure PW, and infrared images in sunlight also showed high heat absorption/release efficiency (Fig. 21e).
 | ||
| Fig. 21 (a) The electrospinning–pre-oxidation–carbonization process for the production of organic-volatile-adsorbing fibers. (b) and (c) The effect of carbonization on the adsorption efficiency toward benzene. Reproduced with permission from ref. 85; copyright: 2024, American Chemical Society. (d) The preparation of phase-change composites using phenolic resin obtained from semi-coking wastewater. (e) A comparison of the heating and cooling rates of phase-change composites and paraffin wax under sunlight. Reproduced with permission from ref. 86; copyright: 2023, American Chemical Society. | ||
Compared with the depolymerization of resin, the energy required for activation and carbonization may be lower; in addition, the use of organic solvents is quite limited. The activated carbon obtained from the carbonization of PF resin can have many applications; the porous characteristics of activated carbon can be used for electrode manufacturing, gas adsorption, energy storage, and in other fields. Therefore, the conversion of phenolic resins into functional carbon materials is currently more economical and environmentally friendly than depolymerization.
5. Conclusions
Discussion of the sustainability of phenolic resin has yielded more optimistic results than may have been first assumed. Sustainability improvements can be made at all stages of the phenolic resin lifecycle, including but not limited to finding more economical and environmentally friendly raw materials to replace phenol and formaldehyde, such as lignin, which always has a positive effect on resin performance and synthesis cost. During the synthesis process, scavenger agents can also be added to make the phenolic resin product release as little formaldehyde as possible during usage. Improved synthetic processes can produce more durable phenolic resin products, also meeting the requirements of sustainability. Finally, from the perspective of recovery, the introduction of dynamic covalent bonds will make it easier to decompose waste phenolic resin and reduce the recovery cost. However, among the latest recovery technologies, there is still some resistance to the development of technology for depolymerizing phenolic resin into small-molecule monomers. Due to the existence of intermolecular cross-linking networks, breaking these molecular bonds without other auxiliary conditions requires high energy, which is not conducive to the large-scale application of these processes. Activated carbon is one of the most suitable conversion products when converting phenolic resins to carbon materials, because its porous properties can be used in many fields. Through the analysis and summary of these processes, it can be seen that even these polymer materials with the longest development history still have a very broad research space.Conflicts of interest
The authors declare no competing interests.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
This research was financially supported by Innovation Capability Improvement Project of Science and Technology smes of Shandong Province (grant no. 2024TSGC0935).References
- K. Hirano and M. Asami, Phenolic resins-100 years of progress and their future, React. Funct. Polym., 2013, 73, 256–269 CrossRef CAS.
- B. Zhu, X. Y. Jiang, S. J. Li and M. Y. Zhu, An Overview of Recycling Phenolic Resin, Polymers, 2024, 16, 1255 CrossRef CAS.
- Z. L. Yu, Y. C. Gao, B. Qin, Z. Y. Ma and S. H. Yu, Revitalizing Traditional Phenolic Resin toward a Versatile Platform for Advanced Materials, Acc. Mater. Res., 2024, 5, 146–159 CrossRef CAS.
- J. Liu, S. Z. Qiao, H. Liu, J. Chen, A. Orpe, D. Zhao and G. Q. Lu, Extension of The Stber Method to the Preparation of Monodisperse Resorcinol–Formaldehyde Resin Polymer and Carbon Spheres**, Angew. Chem., Int. Ed., 2011, 50, 5947–5951 CrossRef CAS PubMed.
- A. H. Lu, B. Spliethoff and F. Schüth, Aqueous Synthesis of Ordered Mesoporous Carbon via Self-Assembly Catalyzed by Amino Acid, Chem. Mater., 2008, 20(16), 5314–5319 CrossRef CAS.
- C. Liang and S. Dai, Synthesis of Mesoporous Carbon Materials via Enhanced Hydrogen-Bonding Interaction, J. Am. Chem. Soc., 2006, 128(16), 5316–5317 CrossRef CAS.
- A. Kobayashi and G.-i. Konishi, Rapid Synthesis of Phenolic Resins by Microwave-Assisted Self-Condensation of Hydroxybenzyl Alcohol Derivatives, Polym. J., 2008, 40(7), 590–591 CrossRef CAS.
- Q. M. Xu, Z. X. Zhang, Y. H. Li, F. M. Zeng, P. Y. Deng, J. W. Zhu and Z. M. Su, Synthesis of thermosetting phenolic resin and its montmorillonite/silica nanocomposites under alkali-free conditions, J. Adhes. Sci. Technol., 2023, 37(16), 2317–2327 CrossRef CAS.
- W. Xiong, X. Li, X. Chen, C. Zhang and J. Luo, A strategy to improve tribological performances of solvent-based phenol−formaldehyde resin by using oil-containing nanocapsules, Carbon, 2023, 213, 118172 CrossRef CAS.
- B. Wang, Q. Fu, Y. Liu, Z. Lu, Y. Lu and S. Cao, Multi-walled carbon nanotubes/carbon fiber fabric multiscale hybrid materials for improving the mechanical and tribological properties of phenolic resin composites, J. Appl. Polym. Sci., 2022, 139, 48 Search PubMed.
- P. Cai, T. Wang and Q. Wang, Effect of several solid lubricants on the mechanical and tribological properties of phenolic resin-based composites, Polym. Compos., 2015, 36(12), 2203–2211 CrossRef CAS.
- H. J. Song and Z. Z. Zhang, Study on the tribological and hydrophobic behaviors of phenolic coatings reinforced with PFW, PTFE and FEP, Surf. Coat. Technol., 2006, 201, 1037–1044 CrossRef CAS.
- M. Yang, Z. Zhang, X. Zhu, X. Men and G. Ren, In situ reduction and functionalization of graphene oxide to improve the tribological behavior of a phenol formaldehyde composite coating, Friction, 2015, 3, 72–81 CrossRef CAS.
- L. F. Wu, Z. Z. Zhang, M. M. Yang, J. Y. Yuan, P. L. Li, F. Guo and X. H. Men, One-step synthesis of g-C3N4 nanosheets to improve tribological properties of phenolic coating, Tribol. Int., 2019, 132, 221–227 CrossRef CAS.
- L. F. Wu, Z. Z. Zhang, M. M. Yang, J. Y. Yuan, X. H. Men and F. Guo, A comparative study on wear and friction characteristics of phenolic composite coatings filled with different morphologies ZnO, Polym. Adv. Technol., 2019, 30(5), 1173–1181 CrossRef CAS.
- L. Liu, B. Pan, J. Sun, X. Han, Y. A. Lei, Y. Niu, J. Luo and H. Wang, Effect of organic-Mo on the wear behavior of phenolic resin composites, J. Macromol. Sci., Part B: Phys., 2020, 59(5), 284–294 CrossRef CAS.
- V. Ramdugwar, H. Fernandes and P. Gadekar, Study of scavengers for free formaldehyde reduction in phenolic resins used in polychloroprene based contact adhesives, Int. J. Adhes. Adhes., 2022, 115, 103122 CrossRef CAS.
- S. Boran, M. Usta and E. Gümüskaya, Decreasing formaldehyde emission from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin, Int. J. Adhes. Adhes., 2011, 31(7), 674–678 CrossRef CAS.
- D. J. Merline, S. Vukusic and A. A. Abdala, Melamine formaldehyde: curing studies and reaction mechanism, Polym. J., 2012, 45(4), 413–419 CrossRef.
- Y. Li, C. Ji, Y. Lu, L. Wu, S. Sun, R. Qu, C. Sun, Y. Zhang and Z. Xue, In situ synthesis of carbon/g-C3N4 composites for visible light catalysis by facile one-step pyrolysis of partially formaldehyde-modified dicyandiamide, Mater. Chem. Phys., 2018, 214, 28–33 CrossRef CAS.
- N. A. Costa, J. Pereira, J. Ferra, P. Cruz, J. Martins, F. D. Magalhes, A. Mendes and A. Mendes, Scavengers for achieving zero formaldehyde emission of wood-based panels, Wood Sci. Technol., 2013, 47(6), 1261–1272 CrossRef CAS.
- D. Dziurka, J. Lecka and R. Mirski, The effect of grafting particles with acetoacetyl groups on the properties of particleboards, J. Appl. Polym. Sci., 2003, 6, 2 Search PubMed.
- P. P. Selakjani, A. Dorieh, A. Pizzi, M. H. Shahavi, A. Hasankhah, S. Shekarsaraee, M. Ashouri, S. G. Movahed and M. N. Abatari, Reducing free formaldehyde emission, improvement of thickness swelling and increasing storage stability of novel medium density fiberboard by urea-formaldehyde adhesive modified by phenol derivatives, Int. J. Adhes. Adhes., 2021, 111, 102962 CrossRef CAS.
- Y. Li, C. Ji, Y. Lu, L. Wu, S. Sun, R. Qu, C. Sun, Y. Zhang and Z. Xue, In situ synthesis of carbon/g-C3N4 composites for visible light catalysis by facile one-step pyrolysis of partially formaldehyde-modified dicyandiamide, Mater. Chem. Phys., 2018, 214, 28–33 CrossRef CAS.
- H. Dong, X. M. Hu, J. D. Liu, Y. T. Liang, W. Wang, H. Tang, F. S. Zhu, Z. L. He and P. Wang, Study of preparation and properties of environmentally friendly phenolic resin for mining, J. Appl. Polym. Sci., 2023, 140, 23 Search PubMed.
- W. Li, Q. Lin, M. Yan and Y. Zou, Reducing the contents of free phenol and formaldehyde in phenolic foam, J. Appl. Polym. Sci., 2003, 90(9), 2333–2336 CrossRef CAS.
- K. Yang, X. Gong, L. Bai, Y. Zhang and N. Zhou, Glucose-lignin-based phenolic resin: an environmentally friendly low-formaldehyde wood adhesive, Pigm. Resin Technol, 2023, 56, 742–750 Search PubMed.
- D. Fan, J. Li, J. Chang, J. Gou and J. Jiang, Chemical Structure and Curing Behavior of Phenol–Urea–Formaldehyde Cocondensed Resins of High Urea Content, J. Adhes. Sci. Technol., 2009, 23(13–14), 1787–1797 CrossRef CAS.
- N. Chaussoy, D. Brandt, J. F. Gerard and J. F. Versatile, Method To Reduce the Free Formaldehyde Content in Phenolic Resins for High-Temperature Applications, ACS Appl. Polym. Mater., 2022, 4(6), 4454–4463 CrossRef CAS.
- A. Demir, Determination of the effect of valonia tannin when used as a filler on the formaldehyde emission and adhesion properties of plywood with artificial neural network analysis, Int. J. Adhes. Adhes., 2023, 123, 103346 CrossRef CAS.
- S. Kim and H. J. Kim, Evaluation of formaldehyde emission of pine and wattle tannin-based adhesives by gas chromatography, Holz Roh – Werkst., 2004, 62(2), 101–106 CrossRef CAS.
- N. L. M. Hafiz, P. M. D. Tahir, L. S. Hua, Z. Z. Abidin, F. A. Sabaruddin and N. M. Yunus, et al., Curing and thermal properties of co-polymerized tannin phenol-formaldehyde resin for bonding wood veneers, J. Mater. Res. Technol., 2020, 9, 6994–7001 CrossRef.
- O. Gonultas and M. Balaban, Ucar Chemical composition of some commercial tannins produced in Turkey. Proc 55th Int Conv Soc Wood Sci Technol. 2012, 1–9.
- D. Dukarska, J. Kawalerczyk and J. Kmieciak, Modified pine needles as a formaldehyde scavenger for urea-formaldehyde resin in plywood production, Eur. J. Wood Wood Prod., 2023, 82(1), 147–158 CrossRef.
- A. A. Benhamou, M. H. Salim, A. Boussetta, M. Mennani, K. Essifi, Z. Kassab, M. El Achaby and A. Moubarik, Graphene oxide a promising formaldehyde scavenger of phenolic resin-bonded plywood: From elaboration to evaluation, J. Appl. Polym. Sci., 2024, 141(11), e55093 CrossRef.
- X. Gong, Y. Meng, J. Lu, Y. Tao, Y. Cheng and H. Wang, A Review on Lignin-Based Phenolic Resin Adhesive, Macromol. Chem. Phys., 2022, 223, 210043 CrossRef.
- J. Li, W. Wang, S. Zhang, Q. Gao, W. Zhang and J. Li, Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins, RSC Adv., 2016, 6, 67435–67443 RSC.
- Y. Song, Z. Wang, N. Yan, R. Zhang and J. Li, Demethylation of Wheat Straw Alkali Lignin for Application in Phenol Formaldehyde Adhesives, Polymers, 2016, 8, 209 CrossRef PubMed.
- W. Yang, L. Jiao, X. Wang, W. Wu, H. Lian and H. Dai, Formaldehyde-free self-polymerization of lignin-derived monomers for synthesis of renewable phenolic resin, Int. J. Biol. Macromol., 2021, 166, 1312–1319 CrossRef CAS.
- W. Yang, X. Du, W. Liu, Z. Wang, H. Dai and Y. Deng, Direct valorization of lignocellulosic biomass into value-added chemicals by polyoxometalate catalyzed oxidation under mild conditions, Ind. Eng. Chem. Res., 2019, 58, 22996–23004 CrossRef CAS.
- S. Wang, S. Ma, C. Xu, Y. Liu, J. Dai, Z. Wang, X. Liu, J. Chen, X. Shen and J. Wei, Vanillin-derived high-performance flame retardant epoxy resins: facile synthesis and properties, Macromolecules, 2017, 50, 1892–1901 CrossRef CAS.
- R. F. Nystrom and W. G. Brown, Reduction of organic compounds by lithium aluminum hydride. I. Aldehydes, ketones, esters, acid chlorides and acid anhydrides, J. Am. Chem. Soc., 1947, 69, 1197–1199 CrossRef CAS.
- N. Chaussoy, D. Brandt, J. F. Gerard and J. F. Versatile, Method To Reduce the Free Formaldehyde Content in Phenolic Resins for High-Temperature Applications, ACS Appl. Polym. Mater., 2022, 4(6), 4454–4463 CrossRef CAS.
- N. Chaussoy, D. Brandt and J. F. Gerard, Formaldehyde-Free and Phenol-Free Non-toxic Phenolic Resins with High Thermostability, ACS Appl. Polym. Mater., 2023, 5, 5630–5640 CrossRef CAS.
- D. Balgude and A. S. Sabnis, CNSL: an environment friendly alternative for the modern coating industry, J. Coat. Technol. Res., 2014, 11(2), 169–183 CrossRef CAS.
- M. Telascrêa, A. L. Leao, M. Z. Ferreira, H. F. F. Pupo, B. M. Cherian and S. Narine, Use of a Cashew Nut Shell Liquid Resin as a Potential Replacement for Phenolic Resins in the Preparation of Panels – A Review, Mol. Cryst. Liq. Cryst., 2014, 604, 222–232 CrossRef.
- K. T. da Silva, B. S. Oliveira, L. R. R. da Silva, A. L. A. Mattos, S. E. Mazzetto and D. Lomonaco, Bio-based novolac resins from cashew nut processing waste: Alternative resource for the development of high-value sustainable products, J. Appl. Polym. Sci., 2023, 140(13), e53661 CrossRef CAS.
- T. M. Loganathan, M. T. H. Sultan, Q. Ahsan, A. U. M. Shah, M. Jawaid, A. R. Abu Talib and A. A. Basri, Physico–mechanical and Flammability Properties of Cyrtostachys renda Fibers Reinforced Phenolic Resin Bio-composites, J. Polym. Environ., 2021, 29(11), 3703–3720 CrossRef CAS.
- M. Wang, Z. Yuan, S. Cheng, M. Leitch and C. C. Xu, Synthesis of Novolac-Type Phenolic Resins Using Glucoseas the Substitute for Formaldehyde, J. Appl. Polym. Sci., 2010,(118), 1191–1197 CrossRef CAS.
- L. Granado, R. Tavernier, S. Henry, R. O. Auke, G. Foyer, G. David and S. Caillol, Toward Sustainable Phenolic Thermosets with High Thermal Performances, ACS Sustainable Chem. Eng., 2019, 7(7), 7209–7217 CrossRef CAS.
- S. Corezzi, D. Fioretto, G. Santucci and J. M. Kenny, Modeling Diffusion-Control in the Cure Kinetics of Epoxy-Amine Thermoset Resins: An Approach Based on Configurational Entropy, Polymer, 2010, 51(24), 5833–5845 CrossRef CAS.
- S. Wang, S. Q. Ma, Q. Li, X. W. Xu, B. B. Wang, K. F. Huang, Y. L. Liu and J. Zhu, Facile preparation of polyimine vitrimers with enhanced creep resistance and thermal and mechanical properties via metal coordination, Macromolecules, 2020, 53(8), 2919–2931 CrossRef CAS.
- A. Walter and S. Schlögl, The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications, Polymers, 2020, 12, 1660 CrossRef PubMed.
- J. Chen, K. Zhang, K. Zhang, B. Jiang and Y. Huang, Facile preparation of reprocessable and degradable phenolic resin based on dynamic acetal motifs, Polym. Degrad. Stab., 2022, 196, 109818 CrossRef CAS.
- Q. Li, S. Q. Ma, S. Wang, W. C. Yuan, X. W. Xu, B. B. Wang, K. F. Huang and J. Zhu, Facile catalyst-free synthesis, exchanging, and hydrolysis of an acetal motif for dynamic covalent networks, J. Mater. Chem. A, 2019, 7, 18039–18049 RSC.
- X. Zhang, Y. Zhao, S. Wanga and X. Jing, Cross-linked polymers based on B–O bonds: synthesis, structure and properties, Mater. Chem. Front., 2021, 5, 5534–5548 RSC.
- O. R. Cromwell, J. Chung and Z. Guan, Malleable and self-healing covalent polymer networks through tunable dynamic boronic ester bonds, J. Am. Chem. Soc., 2015, 137, 7492–6495 CrossRef PubMed.
- M. Rottger, T. Domenech, R. van der Weegen, A. B. R. Nicolay and L. Leibler, High-performance vitrimers from commodity thermoplastics through diox-aborolane metathesis, Science, 2017, 356(6333), 62–65 CrossRef PubMed.
- S. Wang, X. Xing, X. Zhang, X. Wang and X. Jing, Room-temperature fully recyclable carbon fibre reinforced phenolic composites through dynamic covalent boronic ester bonds, J. Mater. Chem. A, 2018, 6, 10868–10878 RSC.
- L. Li, X. Chen, K. Jin and J. M. Torkelson, Vitrimers designed both to strongly suppress creep and to recover original cross-link density after reprocessing: Quantitative theory and experiments, Macromolecules, 2018, 51, 5537–5546 CrossRef CAS.
- J. J. Cash, T. Kubo, D. J. Dobbins and B. S. Sumerlin, Maximizing the symbiosis of static and dynamic bonds in self-healing boronic ester networks, Polym. Chem., 2018, 9, 2011–2020 RSC.
- C. Kim, H. Ejima and N. Yoshie, Polymers with autonomous self-healing ability and remarkable reprocessability under ambient humidity conditions, J. Mater. Chem. A, 2018, 6, 19643–19652 RSC.
- Z. W. Wicks, New developments in the field of blocked isocyanates, Prog. Org. Coat., 1981, 9, 3–28 CrossRef CAS.
- L. Jiang, Z. M. Liu, Y. Lei, Y. Yuan, B. Wu and J. X. Lei, Sustainable thermosetting polyurea vitrimers based on a catalyst-free process with reprocessability, permanent shape reconfiguration and self-healing performance, ACS Appl. Polym. Mater., 2019, 1, 3261–3268 CrossRef CAS.
- X. Liu, Y. Li, X. Xing, G. Zhang and X. Jing, Fully recyclable and high performance phenolic resin based on dynamic urethane bonds and its application in self-repairable composites, Polymer, 2021, 229, 124022 CrossRef CAS.
- J. R. Williams, A. A. Clifford and S. H. R. Al-Saidi, Supercritical Fluids and Their Applications in Biotechnology and Related Areas, Mol. Biotechnol., 2002, 22, 263–286 CrossRef CAS.
- H. Tagaya, Y. Suzuki, T. Asou, J. Kadokawa and K. Chiba, Reaction of Model Compounds of Phenol Resin and Molding Materials of Phenol Resin in Supercritical Water for Chemical Recycling of Polymer Waste, Chem. Lett., 1998, 27, 937–938 CrossRef.
- K. Li, L. G. Zhang and Z. M. Xu, Decomposition behavior and mechanism of epoxy resin from waste integrated circuits under supercritical water condition, J. Hazard. Mater., 2019, 374, 356–364 CrossRef CAS PubMed.
- Y. Chen, J. K. Yang, S. Liang, J. P. Hu, H. J. Hou, B. C. Liu, K. K. Xiao, W. B. Yu and H. L. Deng, New insights into the debromination mechanism of non-metallic fractions of waste printed circuit boards via alkaline-enhanced subcritical water route, Resour., Conserv. Recycl., 2021, 165, 105227 CrossRef CAS.
- J. Zhu, X. Chen, Z. Yao, Y. Yin, K. Lin, H. Liu, J. Huang, J. Ruan and R. Qiu, Directional concentration of bromine from nonmetallic particles of crushed waste printed circuit boards by vacuum-gasification-condensation, J. Cleaner Prod., 2019, 231, 462–467 CrossRef CAS.
- K. Zhang, J. Huang, H. Wang, K. Liu, G. Yu, S. Deng and B. Wang, Mechanochemical degradation of hexabromocyclododecane and approaches for the remediation of its contaminated soil, Chemosphere, 2014, 116, 40–45 CrossRef CAS.
- G. Cagnetta, J. Huang, M. Lu, B. Wang, Y. Wang, S. Deng and G. Yu, Defect engineered oxides for enhanced mechanochemical destruction of halogenated organic pollutants, Chemosphere, 2017, 184, 879–883 CrossRef CAS PubMed.
- X. Chen, J. Zhu, J. J. Ruan, Y. T. Tang and R. L. Qiu, Debromination and Decomposition Mechanisms of Phenolic Resin Molecules in Ball Milling with Nano-Zerovalent Iron, ACS Sustainable Chem. Eng., 2020, 8, 172–178 CrossRef CAS.
- A. C. Gusse, P. D. Miller and T. J. Volk, White-Rot Fungi Demonstrate First Biodegradation of Phenolic Resin, Environ. Sci. Technol., 2006, 4196–4199 CrossRef CAS.
- D. P. Barr and S. D. Aust, MECHANISMS WHITE ROT FUNGI USE TO DEGRADE POLLUTANTS, Environ. Sci. Technol., 1994, 28, 78A–87A CrossRef CAS PubMed.
- I. Lawrance, V. Sivaranjani, A. Michael Selvakumar, Y. Khambhaty and P. Saravanan, Biodegradation of phenolic resin used in leather processing by laccase producing Trichoderma aureoviridae, Int. J. Environ. Sci. Technol., 2019, 6857–6862 CrossRef CAS.
- L. Zhu, W. Zheng, H. Xie and K. Zhang, Two types of nitrogen and sulfur co-doped carbons derived from soybean sprouts enabling high performance lithium-sulfur batteries, J. Energy Storage, 2023, 68, 107790 CrossRef.
- J. Y. Hwang, S. T. Myung and Y. K. Sun, Sodium-Ion Batteries: Present and Future, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- H. Zhou, N. Sun, J. Yu, R. A. Soomro, S. Zhang and B. Xu, Microstructure Regulation of Resin-Based Hard Carbons via Esterification Cross-Linking for High-Performance Sodium-Ion Batteries, Inorg. Chem. Front., 2023, 10, 2404–2413 RSC.
- G. Zhang, L. Zhang, Q. Ren, L. Yan, F. Zhang, W. Lv and Z. Shi, Tailoring a Phenolic Resin Precursor by Facile Pre-Oxidation Tactics to Realize a High-Initial-Coulombic-Efficiency Hard Carbon Anode for Sodium-Ion Batteries, ACS Appl. Mater. Interfaces, 2021, 13, 31650–31659 CrossRef CAS PubMed.
- S. C. Dey, B. Worfolk, L. Lower, W. J. Sagues, M. R. Nimlos, S. S. Kelley and S. Park, Phenolic Resin Derived Hard Carbon Anode for Sodium-Ion Batteries: A Review, ACS Energy Lett., 2024, 9, 2590–2614 CrossRef CAS.
- L. M. Yue, L. L. Rao, L. L. Wang, Y. Sun, Z. Z. Wu, H. DaCsota and X. Hu, Enhanced CO2 Adsorption on Nitrogen-Doped Porous Carbons Derived from Commercial Phenolic Resin, Energy Fuels, 2018, 32, 2081–2088 CrossRef CAS.
- G. Sethia and A. Sayari, Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture, Carbon, 2015, 93, 68–80 CrossRef CAS.
- S. Liu, Q. Li, L. Wang, R. Ma, J. Zou, L. Huang and X. Hu, Facile Single-Step Synthesis of Porous Carbons as Efficient CO2 Adsorbents, Energy Fuels, 2019, 11544 CrossRef CAS.
- C. L. Mangun, Z. R. Yue and J. Economy, Adsorption of Organic Contaminants from water Using Tailored ACSs, Chem. Mater., 2001, 13, 2356–2360 CrossRef CAS.
- Z. Y. Zhao, W. Q. Wu, W. P. Li, X. X. Wang, G. G. Li, J. Chen, Z. S. Zhang and Z. P. Hao, Thermosetting Phenolic Resin-Derived Activated Carbon Fibers for Volatile Organic Compound Adsorption: Electrospinning, Preoxidation, and Carbonization, ACS ES&T Eng., 2024, 4, 1635–1643 Search PubMed.
- X. J. Wang, L. Yan, H. Q. Liu, K. Y. Sun, Y. F. Wang, J. Li, Q. Q. Liu and Q. Shi, Porous Carbon-Based Phase Change Material Host Matrix from Semicoking Wastewater, ACS Appl. Eng. Mater., 2023, 1, 2533–2542 CrossRef CAS.
- Z. Gao, X. Lang, S. Chen and C. Zhao, Mini-Review on the Synthesis of Lignin-Based Phenolic Resin, Energy fuel, 2021, 35, 18385–18395 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |




