The gas phase ozonolysis and secondary OH production of cashmeran, a musk compound from fragrant volatile chemical products†
Ayomide A.
Akande
 and
Nadine
Borduas-Dedekind
and
Nadine
Borduas-Dedekind
 *
*
Department of Chemistry, University of British Columbia, Vancouver, Canada. E-mail: borduas@chem.ubc.ca; Tel: +1 604-822-4435
First published on 23rd October 2024
Abstract
Fragrant personal care products are a subset of volatile chemical products (VCPs), an emerging source of outdoor pollutants capable of impacting air quality. Fragrant molecules, such as musks, are used in perfumes and have been found in aquatic organisms, water bodies, indoor air, and urban environments. Considering the distribution of musk-smelling compounds, there is a need to constrain their atmospheric fate indoors and outdoors. Here, we used a Vocus proton-transfer-reaction time-of-flight mass spectrometer to quantify the atmospheric oxidative fate of cashmeran, a bicyclic musk compound, detected in a commercial perfume alongside galaxolide, astratone and rosamusk. Cashmeran concentrations rose up to 0.35 ppbv representing a mass yield of 0.33 ± 0.04% of the perfume. We determined the second order rate constant of the cyclo-addition of O3 with cashmeran to be (2.78 ± 0.31) × 10−19 cm3 molec−1 s−1 at 293 ± 1 K in N2. This rate constant corresponds to an 85 day lifetime against 20 ppbv of O3. Then, we repeated the ozonolysis experiments in air with 20% O2 and measured significant secondary OH concentrations up to 5.1 × 105 molec cm−3. Consequently, the lifetime of cashmeran in our experiment was shortened to 5 h. Thus, the oxidation of fragrant molecules, like cashmeran, could alter the oxidative capacity of indoor air via the production of secondary OH radicals. Furthermore, our results show that cashmeran is long-lived and could serve as a VCP tracer in urban air.
Environmental significanceMusk-smelling compounds, like cashmeran, are emitted from fragrant volatile chemical products. Using a Vocus mass spectrometer, we quantified the lifetime of cashmeran against 20 ppbv of O3 to be 85 days. However, in the presence of O2, cashmeran reacts with O3 to produce secondary OH radicals up to 5.1 × 105 molec cm−3, potentially influencing the oxidative capacity of indoor air. |
1 Introduction
Volatile chemical products (VCPs), including personal care products, cleaning agents, paints, and pesticides, have been identified as an emerging source of anthropogenic volatile organic compounds (VOCs) in urban air.1–3 These VOCs are precursors to ground-level O3 and secondary organic aerosols (SOA), impacting air quality.4–6 Additionally, VOCs can cause or aggravate respiratory diseases, headaches, and irritation of the eyes and nose.7,8 To understand and predict the sources of VCPs to the atmosphere, specific VOCs have been identified as tracers for different VCP categories.2 For example, D5-siloxane and limonene have been identified as reliable tracers for personal care products and fragrant VCPs, respectively.2,9–11Synthetic musk compounds are a group of molecules synthesized specifically for their distinctive musky fragrance, commonly used in personal care products marketed for men.12,13 These compounds are classified into four categories according to their structures: nitro-musks, polycyclic musks, macrocyclic musks, and alicyclic musks (see Fig. 1 for examples).14 Due to their high molecular weights ranging from 206 to 297 g mol−1 (ref. 15) and their vapour pressures less than 71 Pa at 293 K, musk compounds can be classified as semi-volatile organic compounds.16 There is growing interest in studying musk compounds because of their ubiquity in personal care products and their persistence in the environment.17,18 For instance, ∼4000 tons of polycyclic musks were used worldwide in 2000,19 and they are emerging environmental contaminants.20 Indeed, synthetic musk compounds have been detected in aquatic systems,21–23 wastewater treatment plants,15,24 aquatic organisms,13,22 human blood,25,26 human adipose tissue,27 human breast milk,28,29 outdoor air,15 precipitation,30 indoor air,14,31 and indoor dust32,33 (Table 1).
 | ||
| Fig. 1 Chemical structures of select synthetic musk compounds. Musk compounds detected in the commercial perfume used in this study are shown in black (top row), while other musk compounds reported in Table 1 are shown in grey (bottom row). | ||
| Molecule | Media | Description | N | Location | Concentration range (ref.) |
|---|---|---|---|---|---|
| a N is the number of samples and n.d refers to ’not detected’. | |||||
| Cashmeran | Air | Indoor air | 20 | Ontario, Canada | 0.028–160 ng m−3 (ref. 15) |
| Air from wastewater treatment plant | 32 | Ontario, Canada | n.d – 7.3 ng m−3 (ref. 15) | ||
| Air from cosmetic plant | 5 | Taiwan | 420–1000 ng m−3 (ref. 34) | ||
| Air from women's sport centre | 10 | Izmir, Turkey | 16.90 ± 6.7 ng m−3, mean (ref. 31) | ||
| Air from a primary school classroom | 10 | Izmir, Turkey | 84.5 ± 35.2 ng m−3, mean (ref. 31) | ||
| Aquatic | Surface waters | 10 | Ontario, Canada | n.d – 0.71 ng L−1 (ref. 15) | |
| Wastewater treatment plants effluent | 3 | Ontario, Canada | 76–9600 ng L−1 (ref. 15) | ||
| Surface waters from rivers | 34 | Portugal | n.d – 104.4 ng L−1 (ref. 35) | ||
| Carp fish species | 13 | Lake Chaohu, China | 0.24–7.69 ng g−1 (dry weight) (ref. 13) | ||
| Seafood | 10 | Tarragona, Spain | n.d – 11.2 ng g−1 (dry weight) (ref. 36) | ||
| Sediment samples | 12 | Kadicha river, Lebanon | n.d – 94.22 ng g−1 (dry weight) (ref. 37) | ||
| Human | Human blood | 108 | Vienna, Austria | n.d – 89 ng L−1 | |
| Galaxolide | Air | Air from cosmetic plant | 5 | Taiwan | 1000–1600 ng m−3 (ref. 34) |
| Indoor air | 20 | Ontario, Canada | 0.3–18 ng m−3 (ref. 15) | ||
| Air from women's sport centre | 10 | Izmir, Turkey | 144.0 ± 60.5 ng m−3, mean (ref. 31) | ||
| Air from a primary school classroom | 10 | Izmir, Turkey | 267.3 ± 56.0 ng m−3, mean(ref. 31) | ||
| Outdoor air, summer | 19 | Ontario, Canada | n.d – 2.1 ng m−3 (ref. 15) | ||
| Air from wastewater | 10 | Northern Germany | 5.2–407.2 ng m−3 (ref. 24) | ||
| Particulate matter | PM2.5 from women's sport centre | 10 | Izmir, Turkey | 0.94 ± 0.52 ng m−3, mean (ref. 31) | |
| PM2.5 from a primary school classroom | 10 | Izmir, Turkey | 2.50 ± 0.94 ng m−3, mean (ref. 31) | ||
| Indoor dust | 55 | China | 0.40–577 ng g−1 (ref. 32) | ||
| House dust | 10 | Kumamoto, Japan | 84–1600 ng g−1 (ref. 33) | ||
| Aquatic | Seafood | 10 | Tarragona, Spain | 17.1–367.3 ng g−1 (dry weight) (ref. 36) | |
| Carp fish species | 13 | Lake Chaohu, China | 3.63–28.9 ng g−1 (dry weight) (ref. 13) | ||
| Sediment samples | 12 | Kadicha river, Lebanon | n.d – 81.13 ng g−1 (dry weight) (ref. 37) | ||
| Surface waters from rivers | 34 | Portugal | n.d – 379.2 ng L−1 (ref. 35) | ||
| Surface waters | 10 | Ontario, Canada | n.d – 243 ng L−1 (ref. 15) | ||
| Precipitation | Snow samples | 42 | Beijing, China | 2.2–205.9 ng L−1 (ref. 30) | |
| Human | Human blood | 108 | Vienna, Austria | n.d – 6900 ng L−1 | |
| Human adipose | 49 | New York, USA | 12–798 ng g−1 (lipid weight) (ref. 38) | ||
| Human milk | 54 | Basel, Switzerland | 55.02 ± 57.87 ng g−1 (lipid weight), mean (ref. 29) | ||
| Tonalide | Air | Air from cosmetic plant | 5 | Taiwan | 610–960 ng m−3 (ref. 34) |
| Indoor air | 20 | Ontario, Canada | 0.09–17 ng m−3 (ref. 15) | ||
| Air from women's sport centre | 10 | Izmir, Turkey | 39.5 ± 14.7 ng m−3, mean (ref. 31) | ||
| Air from a primary school classroom | 10 | Izmir, Turkey | 59.6 ± 13.5 ng m−3, mean31 | ||
| Air from wastewater | 10 | Northern Germany | 0.3–65.1 ng m−3 (ref. 24) | ||
| Particulate matter | PM2.5 from women's sport centre | 10 | Izmir, Turkey | 1.34 ± 0.71 ng m−3, mean (ref. 31) | |
| PM2.5 from a primary school classroom | 10 | Izmir, Turkey | 1.19 ± 0.37 ng m−3, mean (ref. 31) | ||
| Indoor dust | 55 | China | n.d – 120 ng g−1 (ref. 32) | ||
| House dust | 10 | Kumamoto, Japan | n.d – 220 ng g−1 (ref. 33) | ||
| Aquatic | Seafood | 10 | Tarragona, Spain | n.d – 16.0 ng g−1 (dry weight) (ref. 36) | |
| Carp fish species | 13 | Lake Chaohu, China | 1.88–22.9 ng g−1 (dry weight) (ref. 13) | ||
| Sediment samples | 12 | Kadicha river, Lebanon | n.d – 64.58 ng g−1 (dry weight) (ref. 37) | ||
| Surface waters from rivers | 34 | Portugal | n.d – 60.6 ng L−1 (ref. 35) | ||
| Surface waters | 10 | Ontario, Canada | n.d – 41 ng L−1 (ref. 15) | ||
| Precipitation | Snow samples | 42 | Beijing, China | 6.5–754.1 ng L−1 (ref. 30) | |
| Human | Human blood | 108 | Vienna, Austria | n.d – 290 ng L−1 | |
| Human adipose | 49 | New York, USA | 8–134 ng g−1 (lipid weight) (ref. 38) | ||
| Human milk | 54 | Basel, Switzerland | 13.97 ± 9.96 ng g−1 (lipid weight), mean (ref. 29) | ||
| Astratone | Air | Air from wastewater | 32 | Ontario, Canada | n.d – 0.084 ng m−3 (ref. 15) |
| Particulate | House dust | 10 | Kumamoto, Japan | n.d – 220 ng g−1 (ref. 33) | |
| Celestolide | Air | Air from cosmetic plant | 5 | Taiwan | 230–310 ng m−3 (ref. 34) |
| Air from wastewater | 10 | Northern Germany | 0.01–1.7 ng m−3 (ref. 24) | ||
| Musk Xylene | Air | Air from a primary school classroom | 10 | Izmir, Turkey | 9.89 ± 2.76 ng m−3, mean (ref. 31) |
| Particulate matter | Indoor dust | 55 | China | n.d – 55.5 ng g−1 (ref. 32) | |
| Precipitation | Snow samples | 42 | Beijing, China | 4.4–16.1 ng L−1 (ref. 30) | |
| Human | Human blood | 152 | Heidelberg, Germany | 10–1183 ng L−1 (ref. 26) | |
| Human milk | 54 | Basel, Switzerland | 2.93 ± 6.17 ng g−1 (lipid weight) (ref. 29) | ||
| Musk Ketone | Particulate matter | Indoor dust | 55 | China | n.d – 203 ng g−1 (ref. 32) |
| House dust | 10 | Kumamoto, Japan | n.d – 390 ng g−1 (ref. 33) | ||
| Aquatic | Surface waters from rivers | 34 | Portugal | n.d – 78.2 ng L−1 (ref. 35) | |
| Precipitation | Snow samples | 42 | Beijing, China | 6.7–43.2 ng L−1 (ref. 30) | |
| Human | Human blood | 152 | Heidelberg, Germany | 10–518 ng L−1 (ref. 26) | |
| Human milk | 54 | Basel, Switzerland | 1.60 ± 2.77 ng g−1 (lipid weight) (ref. 29) | ||
Cashmeran is a commonly used bicyclic musk and accumulates in aquatic organisms due to its lipophilic nature. A study on fifteen aquatic species from Lake Chaohu, China, found cashmeran to be present in 54% of the samples analyzed from the 15 aquatic species and to accumulate in the fishes' gills and livers preferentially.13 Another study in Tarragona, Spain observed cashmeran in 3 out of 10 seafood species (Hake, Sole, and Cod) with concentrations of about 10 ng g−1.36 Furthermore, cashmeran was detected in 67%, 100%, 100%, and 88% of the 37 surface water samples taken from the Portuguese rivers: Ave, Leca, Antuã, and Cértima, respectively with concentrations up to 104.4 ng L−1.35 Additionally, cashmeran was the most abundant among five polycyclic musk compounds, galaxolide, cashmeran, celestolide, tonalide, and amberlan, detected in sediments collected along the Kadicha river basin in Lebanon, with concentrations up to 94.22 ng g−1 (Table 1).37 Moreover, cashmeran poses a potential risk to the aquatic environment due to bioaccumulation.39
Cashmeran has a vapour pressure of 1 Pa at 25 °C,40 and so cashmeran has also been detected in indoor air with offline techniques. Cashmeran was detected in air sampled from a primary school classroom and in window film samples collected from 10 offices in Turkey with average concentrations of 84.5 ± 35.2 ng m−3 and 18.3 ± 8.92 ng m−2 respectively.14,31 It was also detected in all samples collected from 10 homes and 10 offices in Ontario, Canada with concentrations up to 160 ng m−3.15 Furthermore, nitromusks have been identified as endocrine disruptors, but exposure to cashmeran is unlikely to cause any estrogen, androgen, or thyroid modalities.26,41 The detection of cashmeran in different environments indicates its emission from a range of fragrant products, including perfumes.
Here, we provide the first gas-phase real-time measurements of synthetic musk compounds from a commercial perfume using the Vocus proton-transfer-reaction time-of-flight mass spectrometer. We investigated the atmospheric ozonolysis of cashmeran which we identified as a major component of the perfume. We measured the lifetime of cashmeran under N2 and in air to investigate the mechanism of OH radical and SOA production. Overall, we propose that cashmeran, and by extension other synthetic musk compounds, are long-lived atmospheric compounds and can be used as VCP tracers for fragrant products.
2 Methods
2.1 Chemicals
A leading brand of a musk-smelling commercial perfume marketed for men was purchased from Amazon, Canada, and used here. Cashmeran (96%) was obtained from Toronto Research Chemicals. 1,3,5-Trimethylbenzene (98%), cyclohexane (99.9%) and acetonitrile (HPLC Plus, ≥99.9%) were purchased from Sigma-Aldrich and used without further purification. A solution of 350 mM of cashmeran was prepared by dissolving the solid in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mixture of ultrapure Milli-Q water (resistivity of >18.2 MΩ cm, PURELAB Option Q-7 system) and acetonitrile. An organic solvent was necessary to dissolve cashmeran and acetonitrile was chosen for its low m/z ratio and lower sensitivity on the Vocus.
30 mixture of ultrapure Milli-Q water (resistivity of >18.2 MΩ cm, PURELAB Option Q-7 system) and acetonitrile. An organic solvent was necessary to dissolve cashmeran and acetonitrile was chosen for its low m/z ratio and lower sensitivity on the Vocus.
2.2 Experimental setup
Experiments were carried out in a dark 8 m3 Teflon smog chamber (Ingeniven LLC) covered by black-out curtains (see Fig. S1 and S2†).42 1/4′′ OD fluorinated ethylene propylene (FEP)43,44 (Cole-Parmer LLC) tubing was used to connect the instruments to the middle of the chamber via stainless steel gaugeable 1/4 in. fittings (Swagelok). The instruments pulled a total of 9 L min−1 from the chamber during each experiment.Either N2 or particle-free air was used in this study. The particle-free air was supplied from an oil-less air compressor and passed through a particle filter, a water separator, and a dryer unit. For additional particle filtration, two particle filter cartridges (Parker Balston) were placed in line. To generate N2, the particle-free air was fed into a nitro pack generator (Parker Balston, Model 76-97) which removes O2, CO2, hydrocarbons, and particles from 0.01 microns.
To clean between experiments, 400–900 ppbv of excess O3 was left in the bag for an additional 12 h in batch mode to drive complete oxidation of any remaining VOCs. The chamber was then emptied at a flow of 20 L min−1, and because of a pulley system, the chamber was able to be completely deflated. Next, the chamber was refilled with N2 or particle-free air, and continuously purged for 24 h. After this cleaning procedure, the Vocus and a scanning mobility particle sizer (SMPS) were used to confirm that background signals of cashmeran and particles had been reached before the start of an experiment. To deep clean the smog chamber and to remove any accumulated organic matter on the walls, we pressure washed (Echo, MS-21H) the chamber with Milli-Q water and dried the chamber by purging with compressed air at 20 L min−1 for 72 h.
2.3 Instrumentation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and uses hydronium ions (H3O+), generated via a plasma discharge method, as its reagent ion.45,46 The Vocus was operated under optimized conditions with a drift pressure of 2.1 mbar, an ion source voltage of 427 V, an ion source current of 2 mA, an E/N ratio of 131 Td, a reagent ion flow rate of 20 sccm, and a molecular reactor temperature of 60 °C. The Vocus pulled at 6 L min−1 from the chamber, with 100 sccm subsampled into the instrument's focusing ion molecular reactor (FIMR). Mass spectra were recorded from 7 to 406 m/z with a single ion signal of 2.70 mV × ns and 1 Hz time resolution. A polytetrafluoroethylene (PTFE) filter (Savillex) was placed in front of the Vocus inlet to ensure that only gas-phase molecules were being sampled during the kinetics experiments. Data obtained were processed using either Tofware (Aerodyne/Tofwerk) v3.2.5 within Igor (Wavemetrics) Pro v8 or Tofware v3.3.0 within Igor Pro v9.
000 and uses hydronium ions (H3O+), generated via a plasma discharge method, as its reagent ion.45,46 The Vocus was operated under optimized conditions with a drift pressure of 2.1 mbar, an ion source voltage of 427 V, an ion source current of 2 mA, an E/N ratio of 131 Td, a reagent ion flow rate of 20 sccm, and a molecular reactor temperature of 60 °C. The Vocus pulled at 6 L min−1 from the chamber, with 100 sccm subsampled into the instrument's focusing ion molecular reactor (FIMR). Mass spectra were recorded from 7 to 406 m/z with a single ion signal of 2.70 mV × ns and 1 Hz time resolution. A polytetrafluoroethylene (PTFE) filter (Savillex) was placed in front of the Vocus inlet to ensure that only gas-phase molecules were being sampled during the kinetics experiments. Data obtained were processed using either Tofware (Aerodyne/Tofwerk) v3.2.5 within Igor (Wavemetrics) Pro v8 or Tofware v3.3.0 within Igor Pro v9.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (70
acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) solution with a double-beam UV/vis spectrophotometer (Carry-5000, Agilent). The measurements were baseline corrected using a solution of water:acetonitrile (70
30) solution with a double-beam UV/vis spectrophotometer (Carry-5000, Agilent). The measurements were baseline corrected using a solution of water:acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30).
30).
2.4 Analysis of musk-smelling compounds in perfume
The obtained sensitivity of 4115 cps ppb−1 for cashmeran (Fig. S4†) was then applied to galoxolide, rosamusk and astratone (Fig. 1). We acknowledge that applying the obtained sensitivity of cashmeran to the other musk compounds is an approximation considering their different functional groups, potential fragmentation, molecular weight, and the transmission efficiency posed by PTR-MS.47,48
2.5 Ozonolysis of cashmeran
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (70
acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) was introduced into the chamber with a syringe via either the U-shaped glass tube or a 3-neck round bottom flask, and heated gently with a heat gun with a flow of N2 (see Fig. S2†). When the signal of cashmeran steadied after 1 hour, excess O3 between 400 and 1000 ppbv was introduced into the chamber, and the reaction was allowed to proceed in batch mode (Fig. S8†). O3 was typically added over a period of 1 to 1.5 h before the O3 generator was stopped, ensuring a large excess of O3 compared to the analyte for pseudo-first order kinetics. The large O3 excess was necessary (1) to operate under pseudo-first order reaction kinetics, and (2) to accelerate the reaction rate to reasonable experimental times (less than 18 h).
30) was introduced into the chamber with a syringe via either the U-shaped glass tube or a 3-neck round bottom flask, and heated gently with a heat gun with a flow of N2 (see Fig. S2†). When the signal of cashmeran steadied after 1 hour, excess O3 between 400 and 1000 ppbv was introduced into the chamber, and the reaction was allowed to proceed in batch mode (Fig. S8†). O3 was typically added over a period of 1 to 1.5 h before the O3 generator was stopped, ensuring a large excess of O3 compared to the analyte for pseudo-first order kinetics. The large O3 excess was necessary (1) to operate under pseudo-first order reaction kinetics, and (2) to accelerate the reaction rate to reasonable experimental times (less than 18 h).
The change in concentration of cashmeran due to reactions with O3 and OH radicals is given by eqn (1). The integrated rate law is given by eqn (2).
 | (1) |
 | (2) |
The rate constant, kO3, of cashmeran with O3 was determined from experiments strictly under N2, to prevent OH radical production and to simplify eqn (2). The experiments were conducted in triplicate at temperatures of 292 K and 293 K (Fig. 3).
2.5.2.1 Predicted rate constant of cashmeran with OH radicals from a structure–activity relationship model. The integrated rate law, eqn (2), was used to estimate the production of secondary OH radicals during the experiments in air. Cashmeran's rate constant with OH radicals, kOH, was theoretically predicted using the Atmospheric Oxidation Program for Microsoft Windows (AOPWIN) from the United States Environmental Protection Agency Estimations Program Interface suite (EPIWEB 4.1).50 AOPWIN employs structural–activity relationships to estimate gas-phase rate constants between organic molecules and common oxidants such as O3 and OH radicals50 (see S9†). The ozonolysis experiments in the presence of O2, were carried out between 290 and 295 K. Since the rate constant is temperature-dependent according to the Arrhenius equation, there is an additional uncertainty in our reported concentrations, but one that would fall within the standard deviation of our reported rate constants.
2.5.2.2 Estimated OH radical production using TMB as a probe. In addition, 0.4 pptv of 1,3,5-trimethylbenzene (TMB) was introduced to an experiment conducted in particle-free air to estimate the concentration of secondary OH radicals produced.51,52 TMB reacts slowly with O3 (k(1,3,5-trimethylbenzene + O3) = 2.20 × 10−21 cm3 molec−1 s−1)53,54), but rapidly with OH radicals (k(1,3,5-trimethylbenzene + OH radicals) = 5.53 × 10−11 cm3 molec−1 s−1).52 Therefore, its decrease in concentration can be attributed solely to reactions with OH radicals.55 Finally, attempts at measuring the OH radical rate constant with cashmeran using TMB and the relative rate method were complicated by photolysis chemistry (Fig. S16†) and particle production, and so we opted to estimate OH radicals via AOPWIN.
3 Results and discussion
3.1 Cashmeran detected in a commercial perfume
First, we analyzed a commercial musk-smelling perfume with the Vocus to identify the emitted VOCs. We identified four structurally different musk compounds: cashmeran at m/z 207.1743, galaxolide at m/z 259.2056, astratone at m/z 271.1904, and rosamusk at m/z 199.1693 (Fig. 1 and 2). Cashmeran was the most prominent musk compound detected by the Vocus with a calibrated signal of up to 0.35 ppbv (Fig. 2). Since the Vocus only provides molecular formula information, we needed to confirm that the signal at m/z 207.1743 and the chemical formula C14H23O+ corresponded to cashmeran. To do so, we injected the commercial perfume into a GC-MS and matched the retention time on the GC to an internal standard of cashmeran (Fig. S3†). Furthermore, the fragmentation patterns of cashmeran in the perfume and the cashmeran standard were identical (Fig. S3†). We therefore confirmed the presence of cashmeran in the fragrance. | ||
| Fig. 3 The kinetics traces of cashmeran as a function of time are colour-coded by the O3 concentration. The data was pre-averaged by 1 minute intervals. The rate constant was determined to be (2.78 ± 0.31) × 10−19 cm3 molec−1 s−1. No wall loss corrections were necessary for the 8000 L chamber (see Fig. S5†). The rate constant was calculated by dividing the slope by the concentration of O3. | ||
Next, cashmeran's sensitivity (Fig. S4†) was applied to galaxolide, astratone and rosamusk, resulting in concentrations of up to 0.07 ppb, 0.01 ppb, and 0.003 ppb, respectively. Moreover, we determined the mass yield of each musk compound in the perfume (in triplicate, Fig. S7†). We report the mass yield of cashmeran, galaxolide, astratone, and rosamusk to be (0.33 ± 0.04)%, (0.08 ± 0.03)%, (0.02 ± 0.01)%, and (0.003 ± 0.001)% respectively (Table S2†). Together, these musk compounds account for ∼0.43% of the perfume's mass, with cashmeran as the dominant compound by an order of magnitude.
For comparison, the observed concentration of cashmeran in the musk-smelling commercial perfume was 45 times higher than concentrations measured in air sampled from homes and offices, where levels reached up to 160 ng m−3 (Table 1).15 The higher concentrations reported in our study are consistent with our experiments capturing the emissions directly from the perfume. The fragrant component of personal care products is an important source of VOCs indoors, yet product labels do not include fragrant VOC emissions.56
3.2 Ozonolysis of cashmeran
Additionally, we compared the ozonolysis rate constant obtained here with the estimated output from AOPWIN/US EPA EPI Suite, a quantitative structure–activity relationship model.50 The model estimates the rate constant of cashmeran with O3 to be 1.14 × 10−17 cm3 molec−1 s−1 at 298 K, which is two orders of magnitude faster than our reported rate constant, (2.78 ± 0.31) × 10−19 cm3 molec−1 s−1 in N2. This overestimation is likely due to the electron-deficient moiety of the α,β-unsaturated ketone that is not accurately accounted for in the predicted calculation. The carbonyl group in cashmeran pulls electron density away from the R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–R through a mesomeric effect, causing the C–C double bond to be less nucleophilic than a C–C double bond in a saturated molecule. This 2-order of magnitude overestimation by EPI Suite could be corrected by accounting for substituents on the C
C–R through a mesomeric effect, causing the C–C double bond to be less nucleophilic than a C–C double bond in a saturated molecule. This 2-order of magnitude overestimation by EPI Suite could be corrected by accounting for substituents on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C moiety.
C moiety.
To assess the formation of OH radicals in the chamber, we added cyclohexane as an OH radical probe,49 despite the fact that cyclohexane is not detected by the Vocus. Instead, we monitored with the Vocus the production of C6H11O+, most likely cyclohexanone, a known oxidation product of cyclohexane and OH radicals.65 Cyclohexanone increased with signals up to 1.2 × 104 counts s−1 in all experiments under air (Fig. 4). Next, we carried out a control experiment without cashmeran to verify that OH radical production originated from the ozonolysis of cashmeran. Indeed, no cyclexanone was observed without cashmeran nor in the absence of O2 (Fig. 4). Thus, cashmeran and likely other large alkenes used in fragrances, can produce OH radicals and so the next step was to quantify the amount of OH radicals produced.
3.2.3.1 Estimating OH radical production from estimating kOH. Considering the relatively long lifetime of cashmeran with O3 and its ability to produce OH radicals as a secondary product, we sought to quantify the relative contribution of each oxidant to the fate of cashmeran. We determined the OH radicals concentration from the integrated rate law of cashmeran reacting with both O3 and OH radicals (eqn (2)). To make this calculation, we used the theoretical rate constant output from the US EPA EPI Suite estimation model, kOH, = 1.08 × 10−10 cm3 molec−1 s−1 and our measured O3 rate constant, kO3, = 2.78 × 10−19 cm3 molec−1 s−1. Using eqn (2), we calculated the steady-state concentration of OH radicals in our triplicate experiments in the presence of O2 (Table 2). The average concentration of OH radicals produced was (3.29 ± 1.60) × 105 molec cm−3 (Fig. 5 and Table 2). This value is comparable with indoor steady-state indoor OH concentration such as 1.7 × 105 molec cm−3 predicted in indoor air,66 and measured up to 1.8 × 106 molec cm−3 in a classroom.67 While ozonolysis reaction can occur solely via the addition of O3 to cashmeran's C–C double bond, reactions with OH can proceed via two pathways: the addition of OH to the C–C double bond and the abstraction of a hydrogen atom.68–70 Thus, the reaction of cashmeran with OH radicals is less affected by the electron-deficient moiety of the α,β-unsaturated ketone compared to its reaction with O3. This rationale supports our use of the US EPA EPI Suite estimated rate constant of cashmeran's reaction with OH radicals despite the model's overestimation of the compound's rate constant with O3.
| Experimental runs | O3 concentration (molec cm−3) | Estimated OH radicals concentration (molec cm−3) |
|---|---|---|
| 1 | 9.75 × 1012 | 4.25 × 105 |
| 2 | 1.06 × 1013 | 5.05 × 105 |
| 3 | 1.14 × 1013 | 1.87 × 105 |
| 4 | 1.10 × 1013 | 1.99 × 105 |
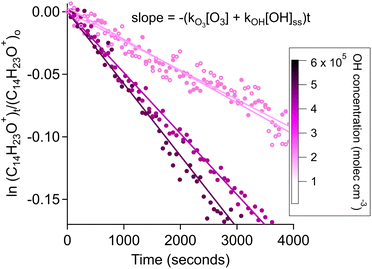 | ||
| Fig. 5 The decay slope of cashmeran is plotted as a function of time during ozonolysis in the presence of O2. The markers and linear fit are colour-coded with the estimated concentration of OH radicals produced within each reaction (Table 2). Four experiments are plotted and runs 3 & 4 are distinguished by open and filled markers. All data was pre-averaged by 1 minute interval. | ||
3.2.3.2 Estimating OH radical production using TMB as a probe. To further constrain this calculated OH radical concentration, we measured the concentration of OH radicals by monitoring the decay of a reference compound, 1,3,5-trimethylbenzene which has a well constrained rate constant with OH radicals of 5.53 × 10−11 cm3 molec−1 s−1 (Fig. S11 and S12†).52 We estimated the steady-state concentration of OH radicals produced to be 1.58 × 105 molec cm−3.52 TMB was chosen as the reference compound since its reaction with O3 to form secondary OH will not compete with the ozonolysis of cashmeran; indeed TMB's rate constant with O3 is 2 orders of magnitude slower at 2.20× 10−21 cm3 molec−1 s−1.53,54 Nevertheless, we recognize that TMB could react with secondary OH from cashmeran ozonolysis, generating more OH radicals and increasing the overall OH concentration. Therefore, the reported OH concentrations are an upper bound.
3.2.3.3 Overall secondary OH radical production. The agreement between the calculated value using kOH from EPI suite, (3.29 ± 1.60) × 105 molec cm−3, and the TMB experimental method, 1.58 × 105 molec cm−3, suggests that the expected secondary OH radical production is indeed on the order of 105 molec cm−3 during ozonolysis of cashmeran.
We calculated the OH molar yield (similar to eqn (S1)†) from the ozonolysis of cashmeran to be 0.004%, assuming the lowest estimate of one OH radical produced per cashmeran molecule. Although this yield is small, it increases the decay rate of cashmeran in air by an order of magnitude compared to in N2, indicating that OH radicals govern the atmospheric fate of cashmeran (Fig. S8†).
3.3 Oxidation products
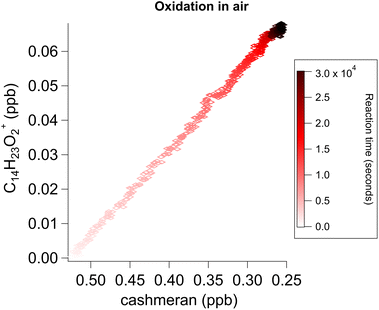 | ||
| Fig. 6 The evolution of C14H22O2 over time during the ozonolysis of cashmeran in air follows a linear correlation indicative of a first-generation product. The same plot was obtained under N2 (Fig. S14†). | ||
During ozonolysis of alkenes, the electrophilic O3 molecule attacks the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and undergoes a cyclo-addition forming the primary ozonide.71,72 The ozonide is an transient molecule with excess internal energy and quickly dissociates by breaking the O–O–O bond. This dissociation leads to the formation of the criegee intermediate, which can rearrange as well as act as an oxidant.73 We propose that the C14H23O2+ product is likely an epoxide via a dioxolane intermediate (see Fig. S13† for proposed structure).74
C bond and undergoes a cyclo-addition forming the primary ozonide.71,72 The ozonide is an transient molecule with excess internal energy and quickly dissociates by breaking the O–O–O bond. This dissociation leads to the formation of the criegee intermediate, which can rearrange as well as act as an oxidant.73 We propose that the C14H23O2+ product is likely an epoxide via a dioxolane intermediate (see Fig. S13† for proposed structure).74
We observed the trace of C14H23O2+ in the absence of cashmeran to determine whether it was being formed as an artifact (see Fig. S15†). While there was a slight increase in its signal, it was quickly removed at approximately 50 ppbv of O3. Thus, we conclude that this artifact does not significantly affect the quantification of the oxidation product detected at the same m/z. However, we acknowledge that the oxidation product (C14H23O2+) may fragment into cashmeran in the Vocus. If this fragmentation occurs, it implies that our reported rate is slightly lower than the true ozonolysis rate constant, indicating that we are providing a lower bound for the rate constant.
To illustrate the dependence of SOA production on cashmeran concentrations, we compared two experiments conducted under air at the same temperature (292 K) and with excess O3 (438 ppb), but with different cashmeran concentrations: 0.26 ppbv and 0.19 ppbv (Fig. 7). At 0.26 ppbv of cashmeran, we observed the onset of aerosol formation with a rapid increase in size and number concentrations. We calculated a number concentration of 3.24 × 106 #/cm3 with total aerosol mass concentration of 3.82 × 102 μg m−3. In contrast, no significant aerosol formation was observed at a lower cashmeran concentration of 0.19 ppb. The total particle number and mass concentrations measured were 1.40 × 105 #/cm3 and 19.3 μg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−3, respectively, at this lower concentration. The formation of SOA indoors from fragrant products has been observed in previous studies and was attributed to the presence of terpenes like limonene.75–78 However, our findings indicate that large compounds, like cashmeran, in these products could also affect the aerosol mass indoors.
m−3, respectively, at this lower concentration. The formation of SOA indoors from fragrant products has been observed in previous studies and was attributed to the presence of terpenes like limonene.75–78 However, our findings indicate that large compounds, like cashmeran, in these products could also affect the aerosol mass indoors.
Additionally, to minimize the formation of secondary organic aerosols (SOA), we determined the threshold mixing ratio for cashmeran at 292 K to be 0.19 ppbv. Above this concentration, we anticipate aerosol formation, as demonstrated in Fig. 7. Importantly, this concentration is lower than what we detected during the perfume analysis in Fig. 2. Thus, we demonstrate that the frequency and quantity of fragrant products used can impact indoor aerosol levels.
4 Atmospheric implication
We constrained the atmospheric fate of cashmeran, a dominant musk-smelling ingredient in perfumes, by investigating its fate against atmospheric oxidation. First, we determined the rate constant for the rate-limiting addition of O3 to the C–C double bond in cashmeran to be (2.78 ± 0.31) × 10−19 cm3 molec−1 s−1 at ambient temperature in N2. Cashmeran's lifetime in the presence of 20 ppbv of O3, typical of indoor air,61 would be 85 days. Moreover, the ozonolysis of cashmeran produced up to 5.1 × 105 molec cm−3 of OH radicals, a concentration comparable to typical steady-state levels indoors.66 These OH radicals, in turn, oxidize cashmeran at much faster rates, leading to a lifetime of 5 h under the maximum OH concentrations of 5.1 × 105 molec cm−3 measured.Furthermore, cashmeran will partition to surfaces, whether they be aerosols or indoor surfaces due to its low volatility, slow reactivity, and potential to form secondary organic aerosols. As a semi-volatile compound, we anticipate that cashmeran will sorb to the clothing and to diverse surfaces commonly found indoors, including carpets, upholstery, dust particles, and walls, after being emitted from a perfume.79–82 Also, we expect the rate of sorption to the fabric on which it was applied to vary depending on the type of fabric and polarity of the molecule.74,83 For example, as a polar molecule, cashmeran is likely to adsorb more readily to natural fibers like cotton and linen than to synthetic materials like polyester.84 Similar to other semivolatiles, cashmeran may then undergo heterogeneous surface chemistry with indoor oxidants like O3 and OH radicals or re-volatilize into the gas phase.74,85 Photodegradation may also play a role in determining the fate of this molecule, given its absorption at 320 nm (see Fig. S16†). Overall, taking typical indoor O3 and OH radical concentrations, we would expect 99% of cashmeran's fate to be governed by OH radicals.
Importantly, we identify the ability of cashmeran, and likely of other alkene VCPs to generate secondary OH radicals during ozonolysis. The ozonolysis of cashmeran is slow due to the electron-poor double bond in the α,β-ketone, which results in a lower OH yield of 0.004% compared to electron-rich compounds like 1,3-butadiene (8%), cyclohexene (68%), propene (0.33%), α-pinene (76%), 2-butanol (70%), and 2,3-dimethyl-2-butene (100%). Yet, fragrant VCPs may still impact the oxidative capacity of indoor environments.58,86,87 This yield of OH radicals from cashmeran ozonolysis decreases the lifetime of cashmeran by an order of magnitude (Fig. 8).
Finally, since cashmeran is relatively long-lived, it can spread throughout our environment (Table 1). Cashmeran is also a synthetic compound and is therefore indicative of anthropogenic activity. In addition, cashmeran is specifically emitted from fragrant personal care products. Based on this collective evidence, we propose that cashmeran, and likely similar musk compounds, are potential VCP tracers in urban air. Since cashmeran uniquely originates from perfumes, it can be added to the growing list of human-emitted chemical tracers like D5-siloxane in air quality studies.2,88
Data availability
Data presented in the figures of this study are available in the ESI† as a spreadsheet.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the Canada Foundation for Innovation, BC Knowledge Development Fund, Alfred P. Sloan Foundation (#2020-13956), and the University of British Columbia for infrastructure and funding support. We thank Rickey Lee for his MATLAB code for the SMPS data. We also thank Benjamin Herring for help with the GC-MS analysis.References
- G. I. Gkatzelis, M. M. Coggon, B. C. McDonald, J. Peischl, J. B. Gilman and K. C. Aikin, et al., Observations Confirm that Volatile Chemical Products Are a Major Source of Petrochemical Emissions in U.S. Cities, Environ. Sci. Technol., 2021, 55(8), 4332–4343, DOI:10.1021/acs.est.0c05471.
- G. I. Gkatzelis, M. M. Coggon, B. C. McDonald, J. Peischl, K. C. Aikin and J. B. Gilman, et al., Identifying Volatile Chemical Product Tracer Compounds in U.S. Cities, Environ. Sci. Technol., 2021, 55(1), 188–199, DOI:10.1021/acs.est.0c05467.
- M. M. Coggon, G. I. Gkatzelis, B. C. McDonald, J. B. Gilman, R. H. Schwantes and N. Abuhassan, et al., Volatile chemical product emissions enhance ozone and modulate urban chemistry, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(32), e2026653118, DOI:10.1073/pnas.2026653118.
- K. M. Seltzer, E. Pennington, V. Rao, B. N. Murphy, M. Strum and K. K. Isaacs, et al., Reactive organic carbon emissions from volatile chemical products, Atmos. Chem. Phys., 2021, 21(6), 5079–5100 CrossRef CAS PubMed , available from: https://acp.copernicus.org/articles/21/5079/2021/..
- K. M. Seltzer, B. N. Murphy, E. A. Pennington, C. Allen, K. Talgo and H. O. T. Pye, Volatile Chemical Product Enhancements to Criteria Pollutants in the United States, Environ. Sci. Technol., 2022, 56(11), 6905–6913, DOI:10.1021/acs.est.1c04298.
- M. Jerrett, R. T. Burnett, C. A. Pope, K. Ito, G. Thurston and D. Krewski, et al., Long-Term Ozone Exposure and Mortality, N. Engl. J. Med., 2009, 360(11), 1085–1095, DOI:10.1056/NEJMoa0803894.
- Canada H, Volatile Organic Compounds, 2017, last modified: 2021-01-15, available from: https://www.canada.ca/en/health-canada/services/air-quality/indoor-air-contaminants/volatile-organic-compounds.html Search PubMed.
- K. Rumchev, H. Brown and J. Spickett, Volatile Organic Compounds: Do they present a risk to our health?, Rev. Environ. Health, 2007, 22, 39–55 CAS.
- A. M. Yeoman, M. Shaw, N. Carslaw, T. Murrells, N. Passant and A. C. Lewis, Simplified speciation and atmospheric volatile organic compound emission rates from non-aerosol personal care products, Indoor Air, 2020, 30(3), 459–472 CrossRef CAS.
- X. Tang, P. K. Misztal, W. W. Nazaroff and A. H. Goldstein, Siloxanes Are the Most Abundant Volatile Organic Compound Emitted from Engineering Students in a Classroom, Environ. Sci. Technol. Lett., 2015, 2(11), 303–307, DOI:10.1021/acs.estlett.5b00256.
- A. Steinemann, Volatile emissions from common consumer products, Air Qual., Atmos. Health, 2015, 8(3), 273–281, DOI:10.1007/s11869-015-0327-6.
- A. M. Peck and K. C. Hornbuckle, Synthetic musk fragrances in urban and rural air of Iowa and the Great Lakes, Atmos. Environ., 2006, 40(32), 6101–6111 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1352231006005188..
- Y. Lyu, S. Ren, F. Zhong, X. Han, Y. He and Z. Tang, Occurrence and trophic transfer of synthetic musks in the freshwater food web of a large subtropical lake, Ecotoxicol. Environ. Saf., 2021, 213, 112074 CrossRef PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0147651321001858..
- E. Balci, M. Genisoglu, S. C. Sofuoglu and A. Sofuoglu, Indoor air partitioning of Synthetic Musk Compounds: Gas, particulate matter, house dust, and window film, Sci. Total Environ., 2020, 729, 138798 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0048969720323159..
- F. Wong, M. Robson, L. Melymuk, C. Shunthirasingham, N. Alexandrou and M. Shoeib, et al., Urban sources of synthetic musk compounds to the environment, Environ. Sci.: Processes Impacts, 2019, 21(1), 74–88 RSC , available from: https://pubs.rsc.org/en/content/articlelanding/2019/em/c8em00341f..
- C. J. Weschler and W. W. Nazaroff, Semivolatile organic compounds in indoor environments, Atmos. Environ., 2008, 42(40), 9018–9040 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1352231008008480..
- J. Liu, W. Zhang, Q. Zhou, Q. Zhou, Y. Zhang and L. Zhu, Polycyclic musks in the environment: A review of their concentrations and distribution, ecological effects and behavior, current concerns and future prospects, Crit. Rev. Environ. Sci. Technol., 2021, 51(4), 323–377, DOI:10.1080/10643389.2020.1724748.
- V. Arruda, M. Simões and I. B. Gomes, Synthetic Musk Fragrances in Water Systems and Their Impact on Microbial Communities, Water, 2022, 14(5), 692 CrossRef CAS , available from: https://www.proquest.com/docview/2637830447/abstract/83BE844CF6054F79PQ/1..
- D. Salvito, Synthetic Musk Compounds and Effects on Human Health?, Environ. Health Perspect., 2005, 113(12), A802 CrossRef , available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1314954/..
- X. Li, Z. Chu, J. Yang, M. Li, M. Du, X. Zhao, et al., Chapter Seven – Synthetic Musks: A Class of Commercial Fragrance Additives in Personal Care Products (PCPs) Causing Concern as Emerging Contaminants, Vol. 81 of Emerging Pollutants and Their Effects on Marine Ecosystems, ed. Chen B., Zhang B. H., Zhu Z. J. and Lee K., Academic Press, 2018. available from: https://www.sciencedirect.com/science/article/pii/S0065288118300257 Search PubMed.
- C. A. McDonough, P. A. Helm, D. Muir, G. Puggioni and R. Lohmann, Polycyclic Musks in the Air and Water of the Lower Great Lakes: Spatial Distribution and Volatilization from Surface Waters, Environ. Sci. Technol., 2016, 50(21), 11575–11583, DOI:10.1021/acs.est.6b03657.
- C. Lange, B. Kuch and J. W. Metzger, Occurrence and fate of synthetic musk fragrances in a small German river, J. Hazard. Mater., 2015, 282, 34–40 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0304389414004841..
- L. Melymuk, M. Robson, S. A. Csiszar, P. A. Helm, G. Kaltenecker and S. Backus, et al., From the City to the Lake: Loadings of PCBs, PBDEs, PAHs and PCMs from Toronto to Lake Ontario, Environ. Sci. Technol., 2014, 48(7), 3732–3741, DOI:10.1021/es403209z.
- I. Weinberg, A. Dreyer and R. Ebinghaus, Waste water treatment plants as sources of polyfluorinated compounds, polybrominated diphenyl ethers and musk fragrances to ambient air, Environ. Pollut., 2011, 159(1), 125–132 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0269749110004288..
- H. P. Hutter, P. Wallner, H. Moshammer, W. Hartl, R. Sattelberger and G. Lorbeer, et al., Blood concentrations of polycyclic musks in healthy young adults, Chemosphere, 2005, 59(4), 487–492 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0045653505002158..
- S. Eisenhardt, B. Runnebaum, K. Bauer and I. Gerhard, Nitromusk Compounds in Women with Gynecological and Endocrine Dysfunction, Environ. Res., 2001, 87(3), 123–130 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0013935101943026..
- K. Kannan, J. L. Reiner, S. H. Yun, E. E. Perrotta, L. Tao and B. Johnson-Restrepo, et al., Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States, Chemosphere, 2005, 61(5), 693–700 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0045653505004431..
- H. Wang, J. Zhang, F. Gao, Y. Yang, H. Duan and Y. Wu, et al., Simultaneous analysis of synthetic musks and triclosan in human breast milk by gas chromatography tandem mass spectrometry, J. Chromatogr. B, 2011, 879(21), 1861–1869 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1570023211003084..
- M. Schlumpf, K. Kypke, M. Wittassek, J. Angerer, H. Mascher and D. Mascher, et al., Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics, Chemosphere, 2010, 81(10), 1171–1183 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S004565351001132X..
- Z. Hu, Y. Shi, H. Niu and Y. Cai, Synthetic musk fragrances and heavy metals in snow samples of Beijing urban area, China, Atmos. Res., 2012, 104–105, 302–305 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0169809511002870..
- A. Sofuoglu, N. Kiymet, P. Kavcar and S. C. Sofuoglu, Polycyclic and nitro musks in indoor air: a primary school classroom and a women's sport center, Indoor Air, 2010, 20(6), 515–522, DOI:10.1111/j.1600-0668.2010.00674.x.
- Y. Lu, T. Yuan, S. H. Yun, W. Wang and K. Kannan, Occurrence of Synthetic Musks in Indoor Dust from China and Implications for Human Exposure, Arch. Environ. Contam. Toxicol., 2011, 60(1), 182–189, DOI:10.1007/s00244-010-9595-1.
- H. Nakata, M. Hinosaka and H. Yanagimoto, Macrocyclic-, polycyclic-, and nitro musks in cosmetics, household commodities and indoor dusts collected from Japan: Implications for their human exposure, Ecotoxicol. Environ. Saf., 2015, 111, 248–255 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0147651314004588..
- I. T. I. Wang, S. F. Cheng and S. W. Tsai, Determinations of airborne synthetic musks by polyurethane foam coupled with triple quadrupole gas chromatography tandem mass spectrometer, J. Chromatogr. A, 2014, 1330, 61–68 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0021967314000533..
- V. Homem, M. Llompart, M. Vila, A. R. L. Ribeiro, C. Garcia-Jares and N. Ratola, et al., Gone with the flow - Assessment of personal care products in Portuguese rivers, Chemosphere, 2022, 293, 133552 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0045653522000418..
- L. Trabalón, G. Cano-Sancho, E. Pocurull, M. Nadal, J. L. Domingo and F. Borrull, Exposure of the population of Catalonia (Spain) to musk fragrances through seafood consumption: Risk assessment, Environ. Res., 2015, 143, 116–122 CrossRef , available from: https://www.sciencedirect.com/science/article/pii/S0013935115001243..
- F. Merhabi, E. Gomez, H. Amine, D. Rosain, J. Halwani and H. Fenet, Occurrence, distribution, and ecological risk assessment of emerging and legacy contaminants in the Kadicha river in Lebanon, Environ. Sci. Pollut. Res., 2021, 28(44), 62499–62518, DOI:10.1007/s11356-021-15049-0.
- K. Kannan, J. L. Reiner, S. Yun, E. Perrotta, L. Tao and B. Johnson-Restrepo, et al., Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States, Chemosphere, 2005, 61(5), 693–700 CrossRef CAS PubMed , available from: https://www.semanticscholar.org/paper/3b5eff30206e8c09b256f791bf339e2c70442834..
- A. M. Api, D. Belsito, S. Biserta, D. Botelho, M. Bruze and G. A. Burton, et al., RIFM fragrance ingredient safety assessment, 6,7-dihydro-1,1,2,3,3-pentamethyl-4(5H)-indanone, CAS Registry Number 33704-61-9, Food Chem. Toxicol., 2021, 149, 111929 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S027869152030819X..
- Austrialian Industrial Chemicals Introdution Scheme, Cashmeran (4H-Inden-4-One, 1,2,3,5,6,7-Hexahydro-1,1,2,3,3-Pentamethyl-), Evaluation Statement – 14, September, 2021 Search PubMed.
- S. Tayal, M. A. Schults, P. Sterchele, E. Hulzebos and G. S. Ladics, Cashmeran as a potential endocrine disrupting chemical: What does the weight-of-the-evidence indicate?, Food Chem. Toxicol., 2024, 184, 114351 CrossRef CAS PubMed , available from: https://www.sciencedirect.com/science/article/pii/S0278691523007536..
- R. J. M. Lee, A. A. Akande, P. A. Heine, P. Padihar, D. Tonkin, W. Rusinoff, C. Manke, D. Lovrity, T. Mittertreiner, M. A. Rezaei and N. Borduas-Dedekind, The development of the UBC ATMOX chamber: An 8 m3 LED-powered wavelength-dependent modular environmental chamber for indoor and outdoor atmospheric chemistry, to be submitted Search PubMed.
- B. L. Deming, D. Pagonis, X. Liu, D. A. Day, R. Talukdar and J. E. Krechmer, et al., Measurements of delays of gas-phase compounds in a wide variety of tubing materials due to gas–wall interactions, Atmos. Meas. Tech., 2019, 12(6), 3453–3461 CrossRef CAS , available from: https://amt.copernicus.org/articles/12/3453/2019/..
- D. Pagonis, J. E. Krechmer, J. de Gouw, J. L. Jimenez and P. J. Ziemann, Effects of gas–wall partitioning in Teflon tubing and instrumentation on time-resolved measurements of gas-phase organic compounds, Atmos. Meas. Tech., 2017, 10(12), 4687–4696 CrossRef CAS , available from: https://amt.copernicus.org/articles/10/4687/2017/..
- J. Krechmer, F. Lopez-Hilfiker, A. Koss, M. Hutterli, C. Stoermer and B. Deming, et al., Evaluation of a New Reagent-Ion Source and Focusing Ion–Molecule Reactor for Use in Proton-Transfer-Reaction Mass Spectrometry, Anal. Chem., 2018, 90(20), 12011–12018, DOI:10.1021/acs.analchem.8b02641.
- H. Li, T. G. Almeida, Y. Luo, J. Zhao, B. B. Palm and C. D. Daub, et al., Fragmentation inside proton-transfer-reaction-based mass spectrometers limits the detection of ROOR and ROOH peroxides, Atmos. Meas. Tech., 2022, 15(6), 1811–1827 CrossRef CAS , available from: https://amt.copernicus.org/articles/15/1811/2022/..
- T. Reinecke, M. Leiminger, A. Jordan, A. Wisthaler and M. Müller, Ultrahigh Sensitivity PTR-MS Instrument with a Well-Defined Ion Chemistry, Anal. Chem., 2023, 95(32), 11879–11884, DOI:10.1021/acs.analchem.3c02669.
- K. Sekimoto, S. M. Li, B. Yuan, A. Koss, M. Coggon and C. Warneke, et al., Calculation of the sensitivity of proton-transfer-reaction mass spectrometry (PTR-MS) for organic trace gases using molecular properties, Int. J. Mass Spectrom., 2017, 421, 71–94 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1387380616302494..
- M. D. Keywood, J. H. Kroll, V. Varutbangkul, R. Bahreini, R. C. Flagan and J. H. Seinfeld, Secondary Organic Aerosol Formation from Cyclohexene Ozonolysis: Effect of OH Scavenger and the Role of Radical Chemistry, Environ. Sci. Technol., 2004, 38(12), 3343–3350, DOI:10.1021/es049725j.
- EPA U and US EPA, Estimation Programs Interface Suite™ for Microsoft® Windows, V 11, United States Environmental Protection Agency, Washington, DC, USA, 2024 Search PubMed.
- R. Atkinson, Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions, Chem. Rev., 1986, 86(1), 69–201, DOI:10.1021/cr00071a004.
- B. Bohn and C. Zetzsch, Kinetics and mechanism of the reaction of OH with the trimethylbenzenes – experimental evidence for the formation of adduct isomers, Phys. Chem. Chem. Phys., 2012, 14(40), 13933–13948 RSC , available from: https://pubs.rsc.org/en/content/articlelanding/2012/cp/c2cp42434g..
- C. T. Pate, R. Atkinson and J. N. Pitts Jr, The gas phase reaction of O3 with a series of aromatic hydrocarbons, J. Environ. Sci. Health, Part A: Environ. Sci. Eng., 1976, 11(1), 1–10, DOI:10.1080/10934527609385750.
- F. Kramp and S. E. Paulson, On the Uncertainties in the Rate Coefficients for OH Reactions with Hydrocarbons, and the Rate Coefficients of the 1,3,5-Trimethylbenzene and m-Xylene Reactions with OH Radicals in the Gas Phase, J. Phys. Chem. A, 1998, 102(16), 2685–2690, DOI:10.1021/jp973289o.
- S. E. Paulson, J. D. Fenske, A. D. Sen and T. W. Callahan, A Novel Small-Ratio Relative-Rate Technique for Measuring OH Formation Yields from the Reactions of O3 with Alkenes in the Gas Phase, and Its Application to the Reactions of Ethene and Propene, J. Phys. Chem. A, 1999, 103(13), 2050–2059, DOI:10.1021/jp984140v.
- A. C. Steinemann, Fragranced consumer products and undisclosed ingredients, Environ. Impact Assess. Rev., 2009, 29(1), 32–38 CrossRef , available from: https://www.sciencedirect.com/science/article/pii/S0195925508000899..
- S. E. Paulson and J. J. Orlando, The reactions of ozone with alkenes: An important source of HOx in the boundary layer, Geophys. Res. Lett., 1996, 23(25), 3727–3730, DOI:10.1029/96GL03477.
- R. Atkinson and S. M. Aschmann, Hydroxyl radical production from the gas-phase reactions of ozone with a series of alkenes under atmospheric conditions, Environ. Sci. Technol., 1993, 27(7), 1357–1363, DOI:10.1021/es00044a010.
- D. K. Farmer, M. E. Vance, J. P. D. Abbatt, A. Abeleira, M. R. Alves and C. Arata, et al., Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry, Environ. Sci.: Processes Impacts, 2019, 21(8), 1280–1300 RSC , available from: http://xlink.rsc.org/?DOI=C9EM00228F..
- D. K. Farmer, M. E. Vance, D. Poppendieck, J. Abbatt, M. R. Alves and K. C. Dannemiller, et al., The chemical assessment of surfaces and air (CASA) study: using chemical and physical perturbations in a test house to investigate indoor processes, Environ. Sci.: Processes Impacts, 2024 10.1039/d4em00209a , available from: https://pubs.rsc.org/en/content/articlelanding/2024/em/d4em00209a..
- Canada H, Residential Indoor Air Quality Guidelines [education and Awareness], 2015, https://www.canada.ca/en/health-canada/services/air-quality/residential-indoor-air-quality-guidelines.html Search PubMed.
- Z. Zhao, W. Zhang, T. Alexander, X. Zhang, D. B. C. Martin and H. Zhang, Isolating α -Pinene Ozonolysis Pathways Reveals New Insights into Peroxy Radical Chemistry and Secondary Organic Aerosol Formation, Environ. Sci. Technol., 2021, 55(10), 6700–6709, DOI:10.1021/acs.est.1c02107.
- M. Siese, K. H. Becker, K. J. Brockmann, H. Geiger, A. Hofzumahaus and F. Holland, et al., Direct Measurement of OH Radicals from Ozonolysis of Selected Alkenes: A EUPHORE Simulation Chamber Study, Environ. Sci. Technol., 2001, 35(23), 4660–4667, DOI:10.1021/es010150p.
- K. S. Docherty and P. J. Ziemann, Effects of Stabilized Criegee Intermediate and OH Radical Scavengers on Aerosol Formation from Reactions of β -Pinene with O3, Aerosol Sci. Technol., 2003, 37(11), 877–891, DOI:10.1080/02786820300930.
- S. M. Aschmann, A. A. Chew, J. Arey and R. Atkinson, Products of the Gas-Phase Reaction of OH Radicals with Cyclohexane: Reactions of the Cyclohexoxy Radical, J. Phys. Chem. A, 1997, 101(43), 8042–8048, DOI:10.1021/jp971869f.
- C. J. Weschler and H. C. Shields, Production of the Hydroxyl Radical in Indoor Air, Environ. Sci. Technol., 1996, 30(11), 3250–3258, DOI:10.1021/es960032f.
- E. Gomez Alvarez, D. Amedro, C. Afif, S. Gligorovski, C. Schoemaecker and C. Fittschen, et al., Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(33), 13294–13299, DOI:10.1073/pnas.1308310110.
- M. R. McGillen, C. J. Percival, D. E. Shallcross and J. N. Harvey, Is hydrogen abstraction an important pathway in the reaction of alkenes with the OH radical?, Phys. Chem. Chem. Phys., 2007, 9(31), 4349–4356 RSC , available from: https://pubs.rsc.org/en/content/articlelanding/2007/cp/b703035e..
- Q. D. Wang, M. M. Sun and J. H. Liang, Reaction Mechanisms and Kinetics of the Hydrogen Abstraction Reactions of C4–C6 Alkenes with Hydroxyl Radical: A Theoretical Exploration, Int. J. Mol. Sci., 2019, 20(6), 1275 CrossRef PubMed , available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6471405/..
- Z. Tian, Y. Liu, J. Li and Y. Yan, Theoretical study of H-abstraction and addition reactions of cyclohexene with H, OH and HO2, Fuel, 2023, 332, 125891 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0016236122027156..
- D. Johnson and G. Marston, The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere, Chem. Soc. Rev., 2008, 37(4), 699–716 RSC , available from: https://pubs.rsc.org/en/content/articlelanding/2008/cs/b704260b..
- S. A. Epstein and N. M. Donahue, The Kinetics of Tetramethylethene Ozonolysis: Decomposition of the Primary Ozonide and Subsequent Product Formation in the Condensed Phase, J. Phys. Chem. A, 2008, 112(51), 13535–13541, DOI:10.1021/jp807682y.
- R. Chhantyal-Pun, M. A. H. Khan, C. A. Taatjes, C. J. Percival, A. J. Orr-Ewing and D. E. Shallcross, Criegee intermediates: production, detection and reactivity, Int. Rev. Phys. Chem., 2020, 39(3), 385–424, DOI:10.1080/0144235X.2020.1792104.
- A. D. L. Wylie and J. P. D. Abbatt, Heterogeneous Ozonolysis of Tetrahydrocannabinol: Implications for Thirdhand Cannabis Smoke, Environ. Sci. Technol., 2020, 54(22), 14215–14223, DOI:10.1021/acs.est.0c03728.
- S. Rossignol, C. Rio, A. Ustache, S. Fable, J. Nicolle and A. Même, et al., The use of a housecleaning product in an indoor environment leading to oxygenated polar compounds and SOA formation: Gas and particulate phase chemical characterization, Atmos. Environ., 2013, 75, 196–205 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1352231013002215..
- G. Sarwar, D. A. Olson, R. L. Corsi and C. J. Weschler, Indoor Fine Particles: The Role of Terpene Emissions from Consumer Products, J. Air Waste Manage. Assoc., 2004, 54(3), 367–377, DOI:10.1080/10473289.2004.10470910.
- C. M. F. Rosales, J. Jiang, A. Lahib, B. P. Bottorff, E. K. Reidy and V. Kumar, et al., Chemistry and human exposure implications of secondary organic aerosol production from indoor terpene ozonolysis, Sci. Adv., 2022, 8(8), eabj9156, DOI:10.1126/sciadv.abj9156.
- T. Wu, T. Müller, N. Wang, J. Byron, S. Langer and J. Williams, et al., Indoor Emission, Oxidation, and New Particle Formation of Personal Care Product Related Volatile Organic Compounds, Environ. Sci. Technol. Lett., 2024, 11(10), 1053–1061, DOI:10.1021/acs.estlett.4c00353.
- J. P. D. Abbatt and C. Wang, The atmospheric chemistry of indoor environments, Environ. Sci.: Processes Impacts, 2020, 22(1), 25–48 RSC , available from: https://pubs.rsc.org/en/content/articlelanding/2020/em/c9em00386j..
- R. B. Jørgensen, O. Bjørseth and B. Malvik, Chamber testing of adsorption of volatile organic compounds (VOCs) on material surfaces, Indoor Air, 1999, 9(1), 2–9 CrossRef PubMed.
- N. Borduas, J. G. Murphy, C. Wang, G. da Silva and J. P. D. Abbatt, Gas Phase Oxidation of Nicotine by OH Radicals: Kinetics, Mechanisms, and Formation of HNCO, Environ. Sci. Technol. Lett., 2016, 3(9), 327–331, DOI:10.1021/acs.estlett.6b00231.
- B. A. Tichenor, Z. Guo, J. E. Dunn, L. E. Sparks and M. A. Mason, The Interaction of Vapour Phase Organic Compounds with Indoor Sinks, Indoor Air, 1991, 1(1), 23–35, DOI:10.1111/j.1600-0668.1991.03-11.x.
- J. Yu, F. Wania and J. P. D. Abbatt, A New Approach to Characterizing the Partitioning of Volatile Organic Compounds to Cotton Fabric, Environ. Sci. Technol., 2022, 56(6), 3365–3374, DOI:10.1021/acs.est.1c08239.
- A. Saini, J. O. Okeme, J. Mark Parnis, R. H. McQueen and M. L. Diamond, From air to clothing: characterizing the accumulation of semi-volatile organic compounds to fabrics in indoor environments, Indoor Air, 2017, 27(3), 631–641, DOI:10.1111/ina.12328.
- K. Yeh, L. Li, F. Wania and J. P. D. Abbatt, Thirdhand smoke from tobacco, e-cigarettes, cannabis, methamphetamine and cocaine: Partitioning, reactive fate, and human exposure in indoor environments, Environ. Int., 2022, 160, 107063 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S0160412021006887..
- G. E. Orzechowska and S. E. Paulson, Production of OH radicals from the reactions of C4–C6 internal alkenes and styrenes with ozone in the gas phase, Atmos. Environ., 2002, 36(3), 571–581 CrossRef CAS , available from: https://www.sciencedirect.com/science/article/pii/S1352231001004459..
- A. A. Chew and R. Atkinson, OH radical formation yields from the gas-phase reactions of O3 with alkenes and monoterpenes, J. Geophys. Res.: Atmos., 1996, 101(D22), 28649–28653, DOI:10.1029/96JD02722.
- L. H. Rivellini, S. Jorga, Y. Wang, A. K. Y. Lee, J. G. Murphy and A. W. Chan, et al., Sources of Wintertime Atmospheric Organic Pollutants in a Large Canadian City: Insights from Particle and Gas Phase Measurements, ACS ES&T Air, 2024, 1(7), 690–703, DOI:10.1021/acsestair.4c00039.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00452c |
| This journal is © The Royal Society of Chemistry 2025 |




