DOI:
10.1039/D4BM01097C
(Paper)
Biomater. Sci., 2025,
13, 275-286
A glucose responsive multifunctional hydrogel with antibacterial properties and real-time monitoring for diabetic wound treatment†
Received
19th August 2024
, Accepted 2nd November 2024
First published on 8th November 2024
Abstract
The healing of complex diabetic wounds with a hyperglycemic microenvironment and bacterial infection is considered an important clinical issue. In this study, glucose oxidase (GOx) and gold nanoclusters (AuNCs) were encapsulated in quaternary carboxymethyl chitosan (QCMCS)/sodium alginate oxide (OSA) hydrogels and were immersed in tannic acid (TA) solution to achieve antioxidant, antibacterial, pro-angiogenesis, pro-collagen deposition and real-time monitoring functions. In vitro studies showed that TA-QCMCS/OSA@GOx@AuNC hydrogels had inhibition rates of 98.99% and 99.99% against S. aureus and E. coli, respectively, and the survival rate of mouse fibroblasts (L929) was over 95%. In vivo studies showed that TA-QCMCS/OSA@GOx@AuNC hydrogels were 97.28% effective in healing diabetic wounds. In addition, image signals from TA-QCMCS/OSA@GOx@AuNC hydrogels can be collected in real time to accurately obtain glucose concentration values of diabetic wounds and reflect the healing status of diabetic wounds in a timely manner. The results showed that TA-QCMCS/OSA@GOx@AuNC hydrogels provide a novel idea for real-time monitoring of diabetic wound treatment.
1. Introduction
Long-term hyperglycemia can cause disorder and chronic damage to many tissues and organs.1,2 More than 15% of diabetic patients are expected to develop chronic wounds within their lifetime.3 Thus, diabetic chronic wounds have become a challenge and economic burden for health systems around the world. However, diabetic wounds are difficult to heal owing to distinct physiological microenvironments, such as elevated blood glucose levels,4 a high reactive oxygen species microenvironment,5 and recurrent bacterial infection.6 The wound glucose concentration can reflect the blood glucose level and serve as a prognostic indicator for diabetes; thus, its control and modulation are significant to guide the clinical treatment.7,8 Compared with conventional wound dressings, such as gauze, soft linen, natural or synthetic bandages, and cotton, hydrogels are considered as one of the most desirable wound treatment materials due to their distinguished ability to maintain a moist environment on injured sites, absorbing exudates, protecting the wound from microbial invasion and relieving the pain of patients.9,10 Therefore, a multifunctional hydrogel wound dressing with antibacterial, antioxidant and glucose monitoring activities to improve its therapeutic potential for diabetic wound healing becomes very desirable.
As a natural polysaccharide, chitosan has been widely used in wound treatment owing to its superior biocompatible, antibacterial and hemostatic properties.11,12 Quaternized carboxymethyl chitosan (QCMCS), as one of the functional derivatives of chitosan, has better water solubility and enhanced antibacterial activity.13 Therefore, it can provide a promising alternative for the development of antibacterial hydrogels. Sodium alginate (SA) is also a valuable polysaccharide consisting of 1, 4-linked β-D-mannuronic acid and α-L-guluronic acid and can be modified with sodium periodate to obtain oxidized sodium alginate (OSA) containing aldehyde groups,14 which can be further cross-linked with the free amino groups of QCMCS via the dynamic imine bonds to form dynamic Schiff-base hydrogels. However, the bond energy between hydrogels based on a single dynamic Schiff base is weak and exhibits poor mechanical strength. Tannic acid (TA) is a kind of organic acid composed of a benzene ring and a carboxyl group and has strong solubility and antioxidant properties.15,16 Most importantly, the presence of hydroxyl groups in TA's pyrogallol and pyrogallol ester groups leads to the formation of dense hydrogen bonds with various substrates of biological tissue materials.17,18 Therefore, TA modification of the QCMCS/OSA hydrogel can form a dense double network structure with QCMCS/OSA, while removing oxidative stress and improving the elasticity of the hydrogel dressing. However, the use of TA modified QCMCS/OSA multifunctional hydrogels in diabetic wound healing has not been reported.
Variation in wound glucose concentration can reflect the blood glucose level and serve as a prognostic indicator for diabetes.7,19 Early identification of the wound glucose concentration is therapeutically significant to guide the clinical treatment.20 Recently, gold nanoclusters (AuNCs) have attracted much attention owing to their great fluorescence characteristics and catalytic properties. Bovine serum albumin (BSA) stabilized AuNCs, together with GOx, could constitute a fluorescent bioprobe for glucose detection. At the same time, AuNCs are able to increase the electron transfer for the enhanced catalysis of enzymes.21 However, this bioprobe is unstable to be applied in vivo directly. Therefore, it is meaningful to take hydrogels as the stable carrier of the bioprobe to form a fluorescent hydrogel for biosensing.
Inspired by the above points, a tannic acid (TA) modified QCMCS/OSA hydrogel with AuNCs and GOx as the fluorescent bioprobes (TA-QCMCS/OSA@GOx@AuNCs) was developed for diabetic wound repair and glucose real-time monitoring. Firstly, dynamic Schiff base QCMCS/OSA hydrogels containing BSA stabilized AuNCs and GOx as fluorescent bioprobes for glucose detection were prepared successfully because the aldehyde group in OSA reacts with the amino group on QCMCS to form Schiff base bonds. Secondly, tannic acid (TA) was introduced into the QCMCS/OSA@AuNC hydrogels to prepare the TA-QCMCS/OSA@GOx@AuNC hydrogels, in which the hydroxyl group of TA can react with the amino groups in QCMCS/OSA@AuNC hydrogels to form hydrogen bonds. Furthermore, the physical and chemical properties and biocompatibility of the TA-QCMCS/OSA@GOx@AuNC hydrogels were systematically investigated. Finally, the effects of the TA-QCMCS/OSA@GOx@AuNC hydrogels on wound healing and real-time monitoring of a full-layer skin defect model in diabetic mice were evaluated (Scheme 1).
 |
| | Scheme 1 Schematic diagram illustrating the preparation process of TA-QCMCS/OSA@GOx@AuNC hydrogels and their subsequent application in diabetic wound healing and real-time monitoring. | |
2. Materials and methods
2.1. Materials
Carboxymethyl chitosan (CMCS, degree of carboxylation ≥ 90%), sodium alginate (SA, viscosity: 200 ± 20 mPa s), tannic acid (TA), glucose oxidase (GOx) and bovine serum protein (BSA) were provided by Maclean Biochemical Technology Co. Ltd (Shanghai, China). Chloroauric acid was provided by Merck Technology Co. Ltd, (Shanghai, China).
2.2. Fabrication of TA-QCMCS/OSA@GOx@AuNC hydrogels
The syntheses of QCMCS, OSA and AuNCs are based on our previous work.21,22 1 g of QCMCS, 0.6 g of OSA, 0.1 g of GOx and 200 μL of AuNCs were added into 2 mL of PBS solution, stirred for 30 minutes, and allowed to gel at room temperature. The above hydrogel was then immersed in a 2.5% TA solution and removed after 10 minutes to obtain the TA-QCMCS/OSA@GOx@AuNC hydrogels.
2.3. Characterization of TA-QCMCS/OSA@GOx@AuNC hydrogels
Transmission electron microscopy (TEM, JEM-2100F) was used to observe AuNCs. Fourier transform infrared spectroscopy (FTIR, Nexus) was used to analyze the functional groups of the hydrogels. The micromorphology of the hydrogels was observed under a scanning electron microscope (SEM, JSM-IT200).
2.4. Swelling rate of hydrogels
The initial weight of the hydrogels was recorded as W1, and the hydrogels were soaked in 50 mL PBS at room temperature. After equilibrium swelling was achieved, the hydrogel was removed and the excess PBS was removed with filter paper. The weight of the hydrogels after swelling was recorded as W2. All experiments were repeated three times. The swelling rate was calculated as follows:23| | 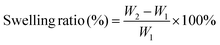 | (1) |
2.5. Antioxidant properties of hydrogels
The hydrogel was added to 8 mL of 1,1-diphenyl-2-pyridine (DPPH)-ethanol solution (0.04 mmol L−1). After incubation at room temperature for 30 min, 300 μL supernatant was collected and the optical density (OD) value of 517 nm was determined. The OD value of the hydrogel group was denoted as A1, the OD value of pure DPPH groups was denoted as A2, and the OD value of DPPH mixed solution with hydrogels was denoted as A3. The antioxidant property of the hydrogels obtained was denoted as D. All experiments were repeated three times. The DPPH free radical clearance rate was calculated as follows:24| |  | (2) |
2.6. Porosity of hydrogels
To calculate porosity, the samples were immersed in ethanol until reaching saturation. The sample weights were measured both prior to and following immersion in ethanol. The porosity was calculated as follows:25| |  | (3) |
where W1 and W2 represent the sample weights before and after immersion in ethanol, respectively, while V1 denotes the sample volume prior to immersion, and ρ is the density of ethanol.
2.7. Mechanical properties of hydrogels
Cylindrical hydrogel samples (20 mm in diameter and 15 mm in height) were subjected to compression testing using a 50 N sensor at a compression rate of 10 mm min−1. The compressive strength of the hydrogels was determined as the stress at 80% strain. The tests were conducted at 37 °C and 40% relative humidity using a CMT4503-5KN microcomputer-controlled electronic universal testing machine (Jinan, China).
2.8. Degradation properties of hydrogels
The hydrogels were initially prepared and weighed, with their initial mass recorded as W0. They were then immersed in a centrifuge tube containing 20 mL of phosphate-buffered saline (PBS) at pH 7.4 and placed in a temperature-controlled air bath at 37 °C with a rotation speed of 100 rpm. At predetermined intervals, the hydrogels were carefully removed, removing surface water, and their mass was measured and recorded as Wt. The degradation rate of the hydrogels was calculated using the following formula:| |  | (4) |
2.9. Antibacterial properties of hydrogels
S. aureus and E. coli were used to study the antibacterial properties of the hydrogels. The hydrogels were sterilized at 37 °C for 24 h, and the sterilized hydrogels were cultured in sterile water for 24 h to obtain the different hydrogel extracts.23 The hydrogel extracts were immersed in a 24-well plate Petri dish containing 1 × 105 CFU mL−1 bacterial suspension and incubated for 24 h. After 10-fold dilution, the resulting bacterial suspension was inoculated on TSA and incubated for 24 h. After this, photographs were taken and the number of colonies was calculated. The bacterial viability of the hydrogels was calculated as follows:
2.10. Blood compatibility of hydrogels
To estimate the hemolytic activity of the hydrogels, rat erythrocytes were used. A total of 2.0 mL of diluted erythrocytes was added to tubes, followed by the introduction of hydrogel samples (100 mg). Deionized water (100 mg) and phosphate-buffered saline (PBS) were employed as the positive and negative controls, respectively. The hydrogels were incubated at 37 °C for 3 h. Afterward, the supernatant was collected by centrifugation at 2500 rpm for 10 min, and the absorbance at 545 nm was measured.26| |  | (5) |
where OD1, OD2, and OD3 represent the optical density (OD) values for the hydrogels, PBS (negative controls), and deionized water (positive controls), respectively.
2.11. Cell activity of hydrogels
L929 cells were used to evaluate the biocompatibility of the hydrogels. The hydrogel extract was co-cultured with 1 × 104 cells in a 48 well and a CO2 incubator for 1, 3 and 5 days. The cell proliferation effects of different hydrogels were tested using the CCK8 kit, and cell viability was studied by staining live and dead cells as previously described.
2.12.
In vivo wound healing of hydrogels
The diabetic mouse model was set up as we have done before.21 Subsequently, all mice were divided into 5 groups, with the Tegaderm™ group of mice being used as control groups, and the remaining groups were treated with hydrogels. The hydrogels were changed every 2 days. The wound tissue on the 6th day of treatment was collected to evaluate the antibacterial effect of the material. The initial area of the wound was W1, and the area of the wound at different time periods was W2. The wound healing rate (WHR) follows the following formula:23| |  | (6) |
To assess wound healing at the histological level, the mice were euthanized on day 12 and wound tissue was collected using 10% paraformaldehyde and embedded in paraffin with ethanol gradient dehydration. 5 mm thick tissue sections were collected for H&E, Masson and CD31 immunofluorescence staining, and collagen deposition and CD31 expression levels were quantitatively analyzed.
2.13. Ethical statement
All animal procedures followed the National Research Council's Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Wuhan University of Technology.
2.14. Statistical analysis
Experiments were repeated three times for each sample. Statistical analysis was performed with Statistical Product and Service Solutions (SPSS) software, and results were expressed as the mean ± standard deviation. Differences were considered statistically significant if p < 0.05.
3. Results and discussion
3.1. Synthesis and characterization of TA-QCMCS/OSA@GOx@AuNC hydrogels
TEM was used to observe the microstructure of AuNCs, as shown in Fig. 1(a), the prepared AuNCs showed quasi-spherical particles, and the lattice spacing of 0.201 nm corresponds to the (200) plane of Au.27 In order to analyze the interactions of the various components in the hydrogel, as shown in Fig. 1(b), a broad hydroxyl group vibrational peak in the range 3000–4000 cm−1 of OSA was observed by FTIR. In addition, there is a weak peak at 1735 cm−1, which is the absorption vibrational peak of the aldehyde group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating that SA has been successfully oxidized by NaIO4.28 The maps of TA-QCMCS/OSA@GOx@AuNCs contain all the characteristic peaks of OSA and QCMCS/OSA. In addition, the spatial hindrance is increased due to the entanglement of cross-linked molecular chains. The absorption peak of OSA at 1420 cm−1 is redshifted to 1461 cm−1, the vibration peak at 1730 cm−1 is redshifted to the absorption peak of C
O, indicating that SA has been successfully oxidized by NaIO4.28 The maps of TA-QCMCS/OSA@GOx@AuNCs contain all the characteristic peaks of OSA and QCMCS/OSA. In addition, the spatial hindrance is increased due to the entanglement of cross-linked molecular chains. The absorption peak of OSA at 1420 cm−1 is redshifted to 1461 cm−1, the vibration peak at 1730 cm−1 is redshifted to the absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in TA, and the vibration peak at 1616 cm−1 is the absorption peak of C–O in TA. In SEM images, due to the cross-linking network formed by QCMCS and OSA, it was observed that QCMCS/OSA hydrogels showed interconnected pore structures, with a porosity of 73.13 ± 2.13% (Fig. 2(a)). After adding GOx, the porosity of the hydrogel was 65.02 ± 1.53%, and the porosity decreased somewhat. After the introduction of AuNCs, the porosity of hydrogels decreased further to 64.25 ± 1.02%, which may be due to the electrostatic interaction between GOx, AuNCs and hydrogels, which made the network structure of hydrogels more compact. After the addition of TA, due to the formation of hydrogen bonds between the polyphenol groups of TA and QCMCS and OSA hydrogels, the double network structure of the hydrogels resulted in more dense pores, and the porosity was further reduced to 55.85 ± 2.02%.
O in TA, and the vibration peak at 1616 cm−1 is the absorption peak of C–O in TA. In SEM images, due to the cross-linking network formed by QCMCS and OSA, it was observed that QCMCS/OSA hydrogels showed interconnected pore structures, with a porosity of 73.13 ± 2.13% (Fig. 2(a)). After adding GOx, the porosity of the hydrogel was 65.02 ± 1.53%, and the porosity decreased somewhat. After the introduction of AuNCs, the porosity of hydrogels decreased further to 64.25 ± 1.02%, which may be due to the electrostatic interaction between GOx, AuNCs and hydrogels, which made the network structure of hydrogels more compact. After the addition of TA, due to the formation of hydrogen bonds between the polyphenol groups of TA and QCMCS and OSA hydrogels, the double network structure of the hydrogels resulted in more dense pores, and the porosity was further reduced to 55.85 ± 2.02%.
 |
| | Fig. 1 (a) Characterization of AuNCs. (b) FTIR spectra of OSA, AuNCs, QCMCS/OSA, QCMCS/OSA@GOx@AuNCs and TA-QCMCS/OSA@GOx@AuNCs. (c) SEM images of different hydrogels. | |
 |
| | Fig. 2 (a) Porosity of the different hydrogels; (b) swelling ratio of the different hydrogels; (c) the DPPH scavenging percentage for different hydrogels; and (d) compression stress of the different hydrogels (80% strain), *p < 0.05. | |
3.2. Swelling properties of TA-QCMCS/OSA@GOx@AuNC hydrogels
Good swelling is sufficient to absorb the excess exudate from the wound in time to help it heal.29 As shown in Fig. 2(b), the swelling rate of QCMCS/OSA obtained in this study is 278.52 ± 12.52%. After adding GOx and AuNCs, the swelling rate of the hydrogel is reduced to 221.25 ± 15.82% and 215.85 ± 10.23%. The swelling rate of the TA-QCMCS/OSA@GOx@AuNCs hydrogel is 198.21 ± 9.85%. It can be clearly found that the swelling rate of the hydrogels is continuously decreasing, which may be attributed to the continuous reduction of the porosity.
3.3. Antioxidant properties of TA-QCMCS/OSA@GOx@AuNC hydrogels
Oxidative stress at diabetic wound sites is one of the leading causes of various diabetic wound complications and can cause cellular damage in the human body.30 In this study, DPPH free radical scavenging activity was used to assess the antioxidant properties of hydrogels. As shown in Fig. 2(c), the free radical clearance rate of QCMCS/OSA was 30.24 ± 1.08%, which was due to the antioxidant activities of QCMCS and OSA. In contrast, with the addition of GOx and AuNCs, the free radical scavenging rates of hydrogels were 35.27 ± 0.57% and 38.03 ± 0.98%, which did not improve the antioxidant properties of the hydrogels. After the introduction of TA, the free radical clearance rate of the hydrogels reached 77.44 ± 0.52%, which is because the polyphenol group of TA endows the hydrogel with good antioxidant properties, which can trap ROS and play a role in reducing oxidative stress in diabetic wounds.31
3.4. Mechanical properties of TA-QCMCS/OSA@GOx@AuNC hydrogels
Hydrogels have good mechanical properties and can adapt to irregular wound shapes.32 As shown in Fig. 2(d), all the hydrogels in this study show a similar trend. The maximum compressive stress of TA-QCMCS/OSA@GOx@AuNCs is higher than that of the other hydrogel groups, which is due to the tangent between TA and the double cross-linked network structure formed by QCMCS and OSA in the hydrogel. In addition, the electrostatic interactions between TA, QCMCS, OSA and GOx and AuNCs also play an important role in improving the mechanical properties of hydrogels.
3.5. Wound glucose monitoring properties of TA-QCMCS/OSA@GOx@AuNC hydrogels
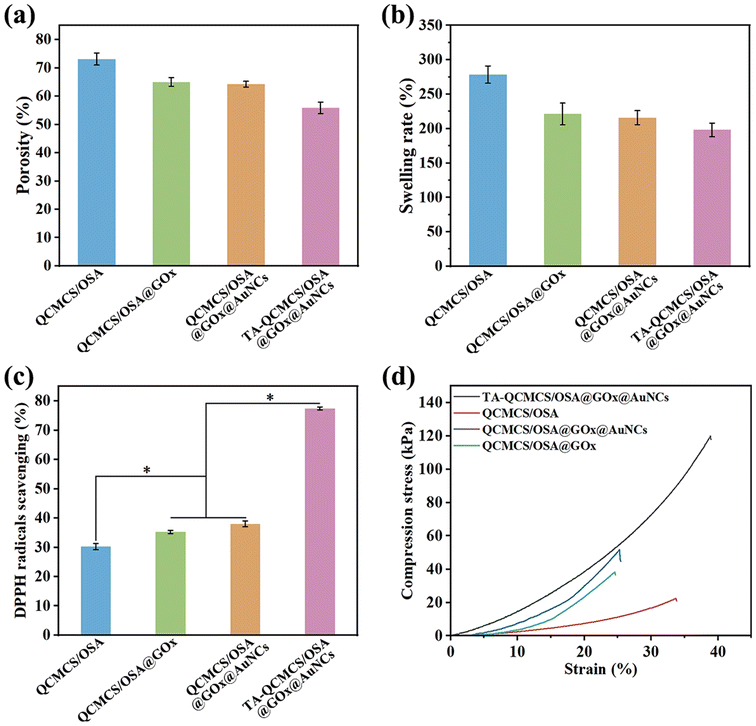
The main reason why diabetic wounds are difficult to heal is that high blood sugar at the wound site leads to bacterial growth and excessive oxidative stress, so glucose concentration plays a key role in the healing process of diabetic wounds.33 Based on the peroxidase-like nature of AuNCs, a glucose-dependent monitoring system with regular fluorescence intensity variations was developed. As shown in Fig. 3(a), with the increase of glucose concentration, the fluorescence intensity of AuNCs gradually decreases, which is because the GOx trapped in the hydrogel will decompose glucose into gluconic acid and H2O2, and H2O2 will lead to the quenching of AuNCs.34 As the glucose concentration increases, the H2O2 content gradually increases. The fluorescence intensity of AuNCs decreases continuously. In addition, we further tested the fluorescence changes of the hydrogel under different glucose concentrations, as shown in Fig. 3(b). Under the excitation of 365 nm UV light, the fluorescence intensity of the hydrogel gradually decreased with the increase of glucose concentration, which was consistent with the results in Fig. 3(a). In addition, by normalizing the fluorescence intensity, the fit between fluorescence intensity and different ordinary glucose concentrations reached 93% (Fig. 3(c)). It is reported that a wide variety of colors can be obtained by changing the three color channels of red (R), green (G) and blue (B) and superimposing them on each other.35 In this study, smartphones were used to perform color changes of the hydrogels at different stages and convert them to RGB values via the corresponding linear equations to accurately collect the glucose concentration at the wound site and adjust the treatment plan. As shown in Fig. 3(d), under excitation of 365 nm UV light, with the increase of glucose concentration, the arithmetic average value of RGB gradually decreases. Therefore, the signal of different glucose concentrations can be quantified by the arithmetic mean of the RGB, which can be calculated as follows:| |  | (7) |
 |
| | Fig. 3 (a) Fluorescence changes of AuNCs in different concentrations of glucose solution; (b) the fluorescence emission spectra of the TA-QCMCS/OSA@GOx@AuNC hydrogels at different concentrations of glucose solution; (c) the linear relationship between the fluorescence intensity and concentrations of glucose solution in the context of TA-QCMCS/OSA@GOx@AuNC hydrogels under 365 nm radiation; and (d) fitting curves of concentrations of glucose solution and R, G and B of TA-QCMCS/OSA@GOx@AuNC hydrogels under UV light. | |
3.6. Degradation properties of hydrogels
The degradability of wound dressings was a fundamental requirement for biomaterials. In this study, the in vitro degradation behavior of TA-QCMCS/OSA@GOx@AuNC hydrogels was assessed using a gravimetric method.36 Simultaneously, the in vivo degradation performance was evaluated by implanting the hydrogels into rats. Fig. S2(a)† illustrates the degradation curve of the TA-QCMCS/OSA@GOx@AuNC hydrogels in PBS. Initially, the hydrogels exhibited swelling, reaching maximum expansion after 1 day. Thereafter, the hydrogel's weight progressively decreased as degradation advanced. By day 7, the residual dry weight of the hydrogels was reduced to 21.2%, with the degradation rate potentially influenced by the degree of crosslinking. Furthermore, histological analysis of major organs in both the hydrogels and control groups was performed 19 days post subcutaneous implantation. As depicted in Fig. S2(b),† no significant inflammatory response was observed in the major organs following in vivo hydrogel degradation. These results demonstrate that the TA-QCMCS/OSA@GOx@AuNC hydrogels possess favorable degradability and biocompatibility.
3.7. Antibacterial properties of hydrogels
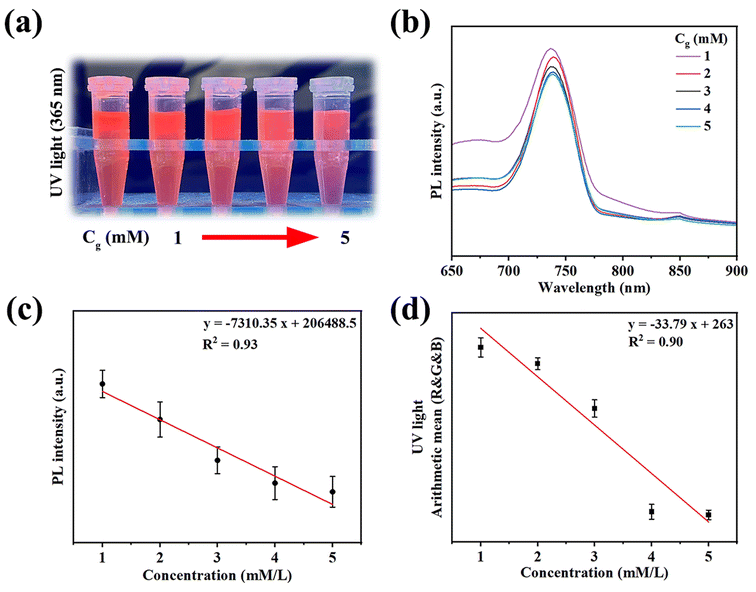
The antibacterial properties of the hydrogels against S. aureus and E. coli were quantitatively analyzed using the coated plate method after co-culture of the hydrogel extract with bacteria for 24 h. As shown in Fig. 4(a), after careful observation on the surfaces of the QCMCS/OSA group and the QCMCS/OSA@GOx group, it was found that there were fewer bacteria in the QCMCS/OSA@GOx group than in the QCMCS/OSA group. The antimicrobial rates against S. aureus reached 86.8 ± 1.2% and 94.4 ± 1.2% (Fig. 4(b)), and the antibacterial rates against E. coli reached 79.5 ± 1.5% and 88.8 ± 1.8% (Fig. 4(c)), confirming the good antibacterial properties of QCMCS and GOx. After the addition of AuNCs, the bacteria on the surface of the plates did not decrease, indicating that AuNCs do not have antimicrobial properties. After the addition of TA, the number of bacteria on the surface of the plates was significantly reduced, indicating that the addition of TA did not compromise the good antimicrobial properties of QCMCS/OSA@GOx@AuNCs. In addition, TA can kill bacteria by binding to surface proteins, polysaccharides, and lipids, changing the structure of the bacteria.37
 |
| | Fig. 4 (a) Image of living bacteria on agar plates after treatment with different hydrogels. (b and c) S. aureus and E. coli colony statistics on agar plates, *p < 0.05. | |
3.8. Cytocompatibility and hemostatic properties of hydrogels
The blood compatibility of hydrogels was studied using red blood cells. As shown in Fig. 5(a), the hemolysis rate of all hydrogel groups was lower than 5%, indicating the good blood compatibility of hydrogels. In addition, the hydrogel extract was co-cultured with L929 cells for 1, 3, and 5 days in two parts. In one part, the cell viability was measured using CCK8, as shown in Fig. 5(b), compared to the control groups, and the cell viability of the QCMCS/OSA group was similar. After the addition of GOx, the cell viability was reduced because GOx has a certain bactericidal effect. In the TA-QCMCS/OSA@GOx@AuNC group, the cell viability of the hydrogels remained above 90%, indicating good biocompatibility of the hydrogels. In the other part, cells were stained dead or alive using Calcein-AM/PI, as shown in Fig. 5(c). Cells in all groups showed a proliferation trend without severe cytotoxicity, consistent with the CCK8 results. Fortunately, no dead cells were found in any of the groups, further demonstrating the good biocompatibility of the hydrogels.
 |
| | Fig. 5 (a) Blood compatibility of different hydrogels; (b) cytotoxicity of hydrogels; and (c) Calcein-AM/PI staining images of L929 cells after 1 d, 3 d and 5 d. *p < 0.05. | |
3.9.
In vivo wound healing and monitoring capabilities of hydrogels
In vivo, diabetic complete skin wounds were constructed to further assess the wound healing effects of the TA-QCMCS/OSA@GOx@AuNCs hydrogel. The optical image of the wound at the specified time after hydrogel treatment is shown in Fig. 6(a and b). The healing effect of the QCMCS/OSA group was better than that of the Tegaderm™ group, possibly due to the inherent antibacterial activity of the hydrogel to eliminate infection. Compared with the QCMCS/OSA group, the healing efficacy of both the QCMCS/OSA@GOx and QCMCS/OSA@GOx@AuNCs groups was improved, suggesting that the antibacterial action of GOx had a favorable effect on the healing of diabetic wounds, as observed in the bacterial experiment (Fig. 4(a)). The TA-QCMCS/OSA@GOx@AuNC group showed the best healing results. On day 12, wound skin in the TA-QCMCS/OSA@GOx@AuNC group with 97.08 ± 2.4% WHR was close to normal appearance. In comparison, the WHR of the QCMCS/OSA group was 35.13 ± 2.1%, the WHR of the QCMCS/OSA@GOx group was 77.39 ± 2.7%, and the WHR of the QCMCS/OSA@GOx@AuNCs group was 74.66 ± 1.1%. Significant wounds were observed in all three unhealed tissues.
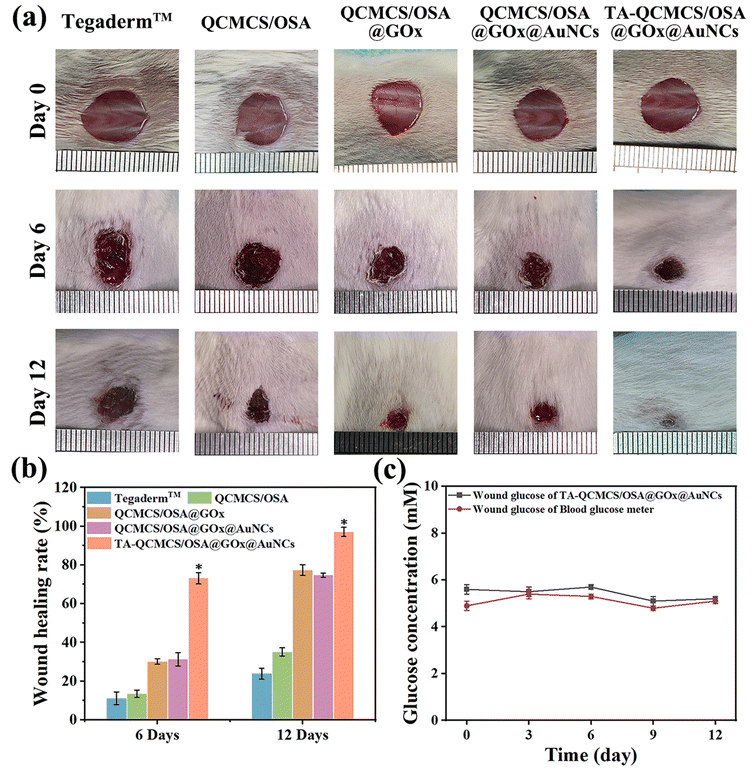 |
| | Fig. 6 (a) Optical images of the wounds on days 0, 6 and 12. (b) Wound healing rate. (c) Diabetic wound monitoring effect, *p < 0.05. | |
In addition, the effect of TA-QCMCS/OSA@GOx@AuNC hydrogels on blood glucose monitoring in vivo was studied. As shown in Fig. 6(c), within 12 days, the glucose concentration in wound tissue of diabetic mice treated with the TA-QCMCS/OSA@GOx@AuNCs hydrogel was stable at ∼5.4 × 10−3 mM. Fortunately, the glucose concentration in the wound tissue of diabetic mice was stable at ∼5.1 × 10−3 mM, which was almost the same. This shows that TA-QCMCS/OSA@GOx@AuNC hydrogels can accurately diagnose glucose concentrations.
The in vivo antibacterial efficacy of the hydrogels was further investigated by assessing the bacterial load on diabetic wound surfaces on day 6. As shown in Fig. S1,† the Tegaderm™ groups exhibited a substantial bacterial presence on the diabetic wound surface. In contrast, the bacterial load was notably reduced in the QCMCS/OSA, QCMCS/OSA@GOx, and QCMCS/OSA@GOx@AuNC groups, attributed to the potent antibacterial properties of QCMCS and GOx. Notably, the addition of TA led to a significant further reduction in bacterial load. These findings align with the results of in vitro antibacterial assays, confirming that the TA-QCMCS/OSA@GOx@AuNC hydrogels demonstrate robust antibacterial activity in vivo.
Fig. 7(a) shows the results of HE staining, Masson's tri-color staining, and CD31 immunofluorescence staining. The epithelial tissue of the QCMCS/OSA group grew as neatly as Tegaderm™. However, affected by infection, the Tegaderm™ group had fewer hair follicles than the QCMCS/OSA group. More importantly, the QCMCS/OSA@GOx group formed more new hair follicles than the Tegaderm™ group due to GOx further eliminating the infection. Crucially, more hair follicle tissue and thicker epithelial tissue were found in the TA-QCMCS/OSA@GOx@AuNC group compared to the QCMCS/OSA@GOx group. Masson's tri-color staining showed that the Tegaderm™ group had less collagen deposition than the rest of the group, suggesting that diabetic wound infections and excessive oxidative stress may have a negative effect on collagen deposition. After clearance of infection and oxidative stress, the highest collagen deposition occurred in the TA-QCMCS/OSA@GOx@AuNC group, distributed in the epidermis and dermis. Quantitative analysis results showed (Fig. 7(b)) that collagen deposition in the TA-QCMCS/OSA@GOx@AuNC group was significantly higher than that in other groups. Results from CD31 immunofluorescence staining showed that there were essentially no new blood vessels in the Tegaderm™ group, possibly due to excessive oxidative stress that damaged cells and thus impeded blood vessel formation. After excessive ROS clearance, the number of neovascularizations increased significantly in the TA-QCMCS/OSA@GOx@AuNC group (Fig. 7(c)). Results from HE staining, Masson's tricolor staining and CD31 immunofluorescence staining further confirm that the TA-QCMCS/OSA@GOx@AuNC group has the best healing effect for diabetic wounds among the five hydrogels.
 |
| | Fig. 7 (a) HE staining, Masson's trichrome staining and CD31 immunofluorescence staining. Quantitative analysis of (b) collagen deposition and (c) CD31. *p < 0.05. | |
4. Conclusions
In summary, a novel glucose responsive TA-QCMCS/OSA@GOX@AuNC hydrogel has been successfully developed. The experimental results showed that the hydrogel has good mechanical properties, excellent antibacterial properties and suitable biocompatibility. These properties enable the TA-QCMCS/OSA@GOX@AuNC hydrogel to provide a good repair environment and accelerate the rapid healing of diabetic wounds. Moreover, by intelligently collecting hydrogel images of the wound site and transforming them into digital models, the glucose concentration values of the wound can be obtained quickly and accurately, thus reflecting the healing status of diabetic wounds and enabling real-time monitoring of diabetic wounds. Therefore, the TA-QCMCS/OSA@GOX@AuNC hydrogel has great potential for the treatment and management of diabetic wounds.
Data availability
Data will be made available on request.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (52073220), the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (2021JJLH0071), the Knowledge Innovation Program of Wuhan-Basic Research (2022020801010175) and the Hubei Provincial Natural Science Foundation of China (2022CFB398).
References
- H. Yang, Y. M. Luo, X. L. Ren, M. Wu, X. L. He, B. W. Peng, K. J. Deng, D. Yan, H. Tang and H. Lin, Inf. Fusion, 2021, 75, 140–149 CrossRef.
- Z. R. Huang, Q. Z. Huang, K. W. Chen, Z. F. Huang, Y. Liu, R. B. Jia and B. Liu, Front. Nutr., 2022, 9, 1013466 CrossRef.
- G. Theocharidis, B. E. Thomas, D. Sarkar, H. L. Mumme, W. J. Pilcher, B. Dwivedi, T. Sandoval-Schaefer, R. F. Sîrbulescu, A. Kafanas and I. Mezghani, Nat. Commun., 2022, 13, 181 Search PubMed.
- M. Zhao, J. Wang, J. X. Zhang, J. M. Huang, L. Luo, Y. S. Yang, K. Shen, T. Jiao, Y. H. Jia and W. L. Lian, Mater. Today Bio, 2022, 16, 100334 CrossRef PubMed.
- M. R. Webster, M. E. Fane, G. M. Alicea, S. Basu, A. V. Kossenkov, G. E. Marino, S. M. Douglass, A. Kaur, B. L. Ecker, K. Gnanapradeepan, A. Ndoye, C. Kugel, A. Valiga, J. Palmer, Q. Liu, X. W. Xu, J. Morris, X. F. Yin, H. Wu, W. Xu, C. Zheng, G. C. Karakousis, R. K. Amaravadi, T. C. Mitchell, F. V. Almeida, M. Xiao, V. W. Rebecca, Y. J. Wang, L. M. Schuchter, M. Herlyn, M. E. Murphy and A. T. Weeraratna, Mol. Cell, 2020, 77(3), 633–644 CrossRef.
- Z. C. Yuan, L. X. Zhang, S. C. Jiang, M. Shafiq, Y. J. Cai, Y. J. Chen, J. H. Song, X. Yu, H. Ijima and Y. Xu, Smart Mater. Med., 2023, 4, 407–426 CrossRef.
- Y. Zhang, T. T. Li, R. M. Ma, Z. X. Yin, J. N. Wang, M. Z. He, D. D. Xu, X. Gao, Q. Wang and V. B. Kraus, Sci. Total Environ., 2020, 717, 137191 CrossRef.
- P. Mostafalu, M. Akbari, K. A. Alberti, Q. Xu, A. Khademhosseini and S. R. Sonkusale, Microsyst. Nanoeng., 2016, 2, 1–10 Search PubMed.
- Y. Wang, M. R. Yang, Z. F. Yang, J. Jiao, Z. Zhao and Y. C. Liu, Int. J. Biol. Macromol., 2024, 275, 133517 CrossRef.
- Z. W. Xie, C. B. Paras, H. Weng, P. Punnakitikashem, L. C. Su, K. Vu, L. P. Tang, J. Yang and K. T. Nguyen, Acta Biomater., 2013, 9, 9351–9359 CrossRef.
- E. S. Hosseini, L. Manjakkal, D. Shakthivel and R. Dahiya, ACS Appl. Mater. Interfaces, 2020, 12, 9008–9016 CrossRef PubMed.
- H. M. Geng, P. F. Zhang, L. Liu, Y. Shangguan, X. Cheng, H. R. Liu, Y. P. Zhao, J. C. Hao, W. W. Li and J. W. Cui, Mater. Today Chem., 2022, 25, 100968 CrossRef.
- X. Zhao, B. L. Guo, H. Wu, Y. P. Liang and P. X. Ma, Nat. Commun., 2018, 9, 2784 CrossRef PubMed.
- P. Jin, M. J. Xia, M. Hasany, P. Feng, J. Bai, J. Gao, W. Zhang, M. Mehrali and R. X. Wang, Interdiscip. Mater., 2023, 2, 771–788 Search PubMed.
- L. Hua, H. Qian, T. Lei, W. B. Liu, X. He, Y. H. Hu and P. F. Lei, Front. Bioeng. Biotechnol., 2021, 9, 796602 CrossRef.
- H. M. Geng, Z. W. Li, Z. Li, Y. Q. Zhang, Z. L. Gao, L. Sun, X. G. Li, J. W. Cui, S. L. Ni and J. C. Hao, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2220300120 CrossRef.
- Q. Cheng, H. M. Geng, C. Y. Zhang, X. H. Zhang, Y. Tian and J. W. Cui, ACS Appl. Mater. Interfaces, 2024, 16, 48607–48618 CrossRef PubMed.
- H. M. Geng, Q. Dai, H. F. Sun, L. P. Zhuang, A. X. Song, F. Caruso, J. C. Hao and J. W. Cui, ACS Appl. Bio Mater., 2020, 3, 1258–1266 CrossRef PubMed.
- W. J. Gu, Y. X. Liu, H. Y. Liu, G. Q. Yang, Q. H. Guo, J. Du, N. Jin, L. Zang, Z. H. Lv and J. M. Ba, Diabetol. Metab. Syndr., 2017, 9, 1–7 Search PubMed.
- Y. C. Ma, T. Qiang, M. J. Gao, J. G. Liang and Y. F. Jiang, Biosensors, 2021, 11, 484 CrossRef PubMed.
- Y. H. Shen, Z. L. Wang, Y. C. Wang, Z. Y. Meng and Z. Zhao, Carbohydr. Polym., 2021, 274, 118642 CrossRef PubMed.
- Z. F. Yang, C. Wang, Z. Y. Zhang, F. Z. Yu, Y. Wang, J. Q. Ding, Z. Zhao and Y. C. Liu, Int. J. Biol. Macromol., 2024, 264, 130741 CrossRef.
- Y. Wang, M. R. Yang and Z. Zhao, Carbohydr. Polym., 2023, 310, 120723 CrossRef.
- Y. P. Liang, X. Zhao, T. L. Hu, Y. Han and B. L. Guo, J. Colloid Interface Sci., 2019, 556, 514–528 CrossRef.
- M. T. Khorasani, A. Joorabloo, A. Moghaddam, H. Shamsi and Z. MansooriMoghadam, Int. J. Biol. Macromol., 2018, 114, 1203–1215 CrossRef.
- Y. Chen, C. Wang, Z. Y. Zhang, F. Z. Yu, Y. Wang, J. Q. Ding, Z. Zhao and Y. C. Liu, Int. J. Biol. Macromol., 2024, 268, 131637 CrossRef.
- X. H. Hou, C. L. Ma, H. P. Ji, S. S. Yi, L. Y. Zhang, Z. T. Zhang, Y. Wang, L. Yuan, D. L. Chen and Y. Zhou, Sens. Actuators, B, 2023, 393, 134241 CrossRef.
- Z. Li, J. Guo, F. C. Guan, J. H. Yin, Q. Yang, S. Zhang, J. Tian, Y. H. Zhang, M. F. Ding and W. M. Wang, Colloids Surf., A, 2023, 656, 130317 CrossRef.
- Y. Gong, P. Wang, R. Cao, J. Wu, H. R. Ji, M. S. Wang, C. Hu, P. Huang and X. S. Wang, ACS Nano, 2023, 17, 22355–22370 CrossRef.
- J. Yang, Z. Y. Chu, Y. C. Jiang, W. Zheng, J. W. Sun, L. L. Xu, Y. Ma, W. N. Wang, M. Shao and H. S. Qian, Adv. Healthcare Mater., 2023, 12, 2300725 CrossRef.
- H. K. Cao, D. Xiang, X. Zhou, P. P. Yue, Y. K. Zou, Z. B. Zhong, Y. S. Ma, L. Z. Wang, S. Q. Wu and Q. F. Ye, Carbohydr. Polym., 2023, 307, 120609 CrossRef.
- Y. Yuan, S. H. Shen and D. D. Fan, Biomaterials, 2021, 276, 120838 CrossRef.
- S. C. S. Hu and C. C. E. Lan, J. Dermatol., 2016, 84, 121–127 Search PubMed.
- Z. L. Wang, Y. H. Shen, X. Sun, Z. H. Li, X. Y. Wang and Z. Zhao, Microchem. J., 2020, 157, 105036 CrossRef.
- K. K. Zheng, Y. Tong, S. H. Zhang, R. Y. He, L. Xiao, Z. Iqbal, Y. H. Zhang, J. Gao, L. Zhang and L. B. Jiang, Adv. Funct. Mater., 2021, 31, 2102599 CrossRef.
- N. Wen, S. S. Li, H. Z. Jiang, J. C. Yang, W. B. Yang, Y. H. Song, J. L. Long, J. W. Zhao, Z. H. Lin and X. B. Yu, Adv. Funct. Mater., 2024, 2411959 CrossRef.
- W. L. Zhang, S. Roy, P. Ezati, D. P. Yang and J. W. Rhim, Trends Food Sci. Technol., 2023, 136, 11–23 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *ab
*ab

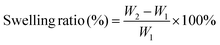





![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating that SA has been successfully oxidized by NaIO4.28 The maps of TA-QCMCS/OSA@GOx@AuNCs contain all the characteristic peaks of OSA and QCMCS/OSA. In addition, the spatial hindrance is increased due to the entanglement of cross-linked molecular chains. The absorption peak of OSA at 1420 cm−1 is redshifted to 1461 cm−1, the vibration peak at 1730 cm−1 is redshifted to the absorption peak of C
O, indicating that SA has been successfully oxidized by NaIO4.28 The maps of TA-QCMCS/OSA@GOx@AuNCs contain all the characteristic peaks of OSA and QCMCS/OSA. In addition, the spatial hindrance is increased due to the entanglement of cross-linked molecular chains. The absorption peak of OSA at 1420 cm−1 is redshifted to 1461 cm−1, the vibration peak at 1730 cm−1 is redshifted to the absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in TA, and the vibration peak at 1616 cm−1 is the absorption peak of C–O in TA. In SEM images, due to the cross-linking network formed by QCMCS and OSA, it was observed that QCMCS/OSA hydrogels showed interconnected pore structures, with a porosity of 73.13 ± 2.13% (Fig. 2(a)). After adding GOx, the porosity of the hydrogel was 65.02 ± 1.53%, and the porosity decreased somewhat. After the introduction of AuNCs, the porosity of hydrogels decreased further to 64.25 ± 1.02%, which may be due to the electrostatic interaction between GOx, AuNCs and hydrogels, which made the network structure of hydrogels more compact. After the addition of TA, due to the formation of hydrogen bonds between the polyphenol groups of TA and QCMCS and OSA hydrogels, the double network structure of the hydrogels resulted in more dense pores, and the porosity was further reduced to 55.85 ± 2.02%.
O in TA, and the vibration peak at 1616 cm−1 is the absorption peak of C–O in TA. In SEM images, due to the cross-linking network formed by QCMCS and OSA, it was observed that QCMCS/OSA hydrogels showed interconnected pore structures, with a porosity of 73.13 ± 2.13% (Fig. 2(a)). After adding GOx, the porosity of the hydrogel was 65.02 ± 1.53%, and the porosity decreased somewhat. After the introduction of AuNCs, the porosity of hydrogels decreased further to 64.25 ± 1.02%, which may be due to the electrostatic interaction between GOx, AuNCs and hydrogels, which made the network structure of hydrogels more compact. After the addition of TA, due to the formation of hydrogen bonds between the polyphenol groups of TA and QCMCS and OSA hydrogels, the double network structure of the hydrogels resulted in more dense pores, and the porosity was further reduced to 55.85 ± 2.02%.









