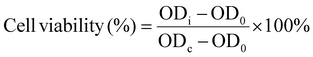Adhesive silk fibroin/magnesium composite films and their application for removable wound dressing†
Qiaolin
Chen‡
,
Kang
Wu‡
,
Jinrong
Yao
 ,
Zhengzhong
Shao
,
Zhengzhong
Shao
 and
Xin
Chen
and
Xin
Chen
 *
*
Department of Macromolecular Science, Shanghai Stomatological Hospital & School of Stomatology, State Key Laboratory of Molecular Engineering of Polymers, Laboratory of Advanced Materials, Fudan University, Shanghai, 200433, People's Republic of China. E-mail: chenx@fudan.edu.cn
First published on 12th November 2024
Abstract
Silk fibroin is a naturally abundant biomaterial renowned for its excellent biocompatibility and biodegradability, making it a promising candidate for biomedical applications like wound dressings. However, traditional silk fibroin materials often lack sufficient mechanical strength, adhesion, and the ability to modulate inflammation and oxidative stress—factors crucial for effective wound healing. To address these limitations, regenerated silk fibroin/magnesium ion [RSF/Mg(II)] composite films were developed by incorporating Mg(II) ions into RSF solutions. These films were characterized using Raman spectroscopy, mechanical testing, and biocompatibility assessments, and their wound-healing efficacy was evaluated in a mouse skin defect model. The RSF/Mg(II) composite films exhibited superior adhesion, higher transparency, and enhanced mechanical flexibility compared to pristine RSF films. They also demonstrated anti-inflammatory and antioxidative properties, effectively reducing cell apoptosis and reactive oxygen species levels in vitro. In vivo, the RSF/Mg Mg(II) composite films significantly accelerated wound healing in mice, improving epidermal thickness, collagen deposition, and promoting blood vessel formation. This study highlights the potential of RSF/Mg(II) composite films as advanced wound dressings with improved biocompatibility and biological activity, offering valuable insights for the development of Mg(II) ion-based biomaterials in wound healing and tissue regeneration applications.
Introduction
Silk fibroin, a naturally abundant biomacromolecule, exhibits exceptional biocompatibility, controllable biodegradability, low immunogenicity, high water absorbability, and excellent oxygen permeability. These properties make it an ideal candidate for the development of biomaterials in various medical applications.1 Over the years, researchers have developed regenerated silk fibroin (RSF)-based materials in diverse forms such as microsphere,2,3 fibers,4,5 films,6,7 hydrogels,8,9 and scaffolds,10,11 successfully applying them in drug delivery, tissue engineering, and wound healing.12–14 Additionally, due to its ease of processing and tunable mechanical and optical properties, RSF-based materials have garnered significant interest in the development of flexible sensors.15 Electronic and ionic conductors are often introduced into RSF matrices to achieve desired functionalities. Electronic conductors include carbon-based materials, metals, metal nanomaterials, and conductive polymers.16 For example, Zhu et al. incorporated reduced graphene oxide into a RSF matrix, forming covalent bonds to create a wearable smart sensor platform capable of dual humidity and temperature sensing.17 Our group developed a dual sensor by integrating a microstructured silver nanowire conductive layer onto RSF/calcium chloride hydrogels, enabling distinct sensing mechanisms for temperature and pressure.18 Zhang et al. also prepared conductive RSF/poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) composite electrodes for electrical stimulation of cell cultures by modifying RSF films with a water–ethanol dispersion.19In addition to electronic conductors, ionic conductors such as metal ions are widely used, with calcium ions being a common choice.20 The formic acid/calcium chloride system not only dissolves silk fibres but also introduces calcium ions, providing the resulting RSF materials with unique adhesive properties suitable for use in bio-based adhesives, particularly for sensor bioelectrode adhesion to skin.21,22 Ling et al. further discovered that this system possesses flame-retardant properties as well as temperature and humidity responsiveness, which has led to the design of automatic fire alarm system.23 In our previous research, we developed ionic skins with high sensitivity and cold resistance by adding calcium chloride to RSF solutions and casting them into films, resulting in effective temperature sensing capabilities.6 However, studies have shown that high concentrations of calcium ions can lead to adverse biological effects, such as cytotoxicity, inflammatory responses, and tissue calcification,24,25 raising concerns about the bio-safety of RSF/calcium ion composites in applications involving direct contact with biological tissues.
Magnesium, like calcium, is naturally present in silkworm glands and silk cocoons, albeit at slightly lower levels.26 Mg(II) ions play vital roles in biological processes, including cellular signalling, apoptosis regulation, and promotion of cell adhesion.27–29 Incorporating Mg(II) ions into RSF materials has the potential to enhance their biological functions, thereby improving their performance in biomedical applications. For instance, Lu et al. demonstrated that RSF nanocomplexes with high negative charge densities could interact with Mg(II) ions, inducing gelation and forming composites with angiogenic and osteogenic properties, promoting tissue regeneration.30,31 Despite this, studies investigating the effects of Mg(II) ions on the properties of silk fibroin materials are limited, particularly in regard to the systematic examination of the impact of varying Mg(II) ion concentrations on silk fibroin films.
As the largest organ in the human body, the skin serves as a primary defence against dehydration and external damage. When damaged, the skin loses its protective functions, leading to moisture and protein loss, metabolic dysfunction, immune imbalances, and other complication.32 Standard treatments for skin damage involve the application of medical dressings to promote wound healing. Ideal dressings should demonstrate excellent biocompatibility, appropriate adhesion, and the ability to conform to irregular wound surfaces for optimal protection.33 The practice of wound treatment dates back thousands of years, with early civilizations using plant leaves and later cotton and hemp fabrics to cover wounds. Traditional dressings, such as gauze and bandages, often adhere to wounds, and when removed, can damage newly formed tissue, slowing the healing proces.34 In 1962, George Winter introduced the concept of “moist wound healing”, demonstrating that maintaining a moist environment promotes faster healing.35 This led to the development of moisture-retentive dressings, such as hydrocolloids and hydrogels, which gained attention for their wound healing capabilities.36–38 However, like traditional dressings, moisture-retentive dressings often adhere to wound beds, requiring mechanical debridement during replacement. This disrupts newly formed epithelial tissue, causing rebleeding and significant pain, which can delay healing and negatively affect patient well-being.39–41 Thus, the development of non-invasive, easy-to-replace wound dressings is of significant clinical importance.
Given the strong potential of RSF-based materials for wound dressing13 and the bioactive properties of Mg(II) ions, this study aims to prepare RSF/Mg(II) composite films with excellent adhesive properties by incorporating Mg(II) ions into RSF solutions. We then systematically investigate the effects of varying Mg(II) ion concentrations on RSF conformation and composite film properties, exploring the underlying mechanisms of Mg(II) ion-regulated RSF film structure and performance. Finally, we evaluate the biocompatibility, removability, and wound healing promotion of the RSF/Mg(II) composite film when applied to wound dressings.
Experimental
Materials
Bombyx mori silkworm cocoons were obtained from local farmers in Nantong City, Jiangsu Province, China. Sodium bicarbonate (NaHCO3) and magnesium chloride hexahydrate (MgCl2·6H2O) were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Lithium bromide (LiBr) was acquired from Kuling Fine Chemical Co., Ltd, Shanghai, China. All reagents were used as received without any purification. Deionized water was used in all experiments (resistivity >18.2 MΩ cm).Preparation of RSF aqueous solution
Silkworm cocoons were boiled twice for 30 min each in a 0.5 wt% NaHCO3 solution for degumming. The degummed silk was washed thoroughly, dried, and stored. The dried silk fibres were dissolved in 9.3 mol L−1 LiBr solution at 60 °C for 1 h, then filtered through eight layers of gauze to remove impurities. The solution was transferred to dialysis tubes (MWCO: 12–14 kDa) and dialyzed against deionized water for 3 days to remove LiBr. The final solution, containing approximately 5 wt% RSF, was centrifuged, filtered, and stored at 4 °C.Preparation of RSF/Mg(II) composite films
Appropriate volume of 20 wt% MgCl2 solution was gradually added to the 5 wt% RSF solution under continuous stirring to achieve RSF/MgCl2 mass ratios of 90/10, 80/20, 70/30, and 60/40. The resulting solutions were centrifuged to remove bubbles, poured into polystyrene moulds, and air-dried in a desiccator at 5% relative humidity to form films. These films were labelled RSF/Mg10, RSF/Mg20, RSF/Mg30, and RSF/Mg40, respectively. The films were stored in a desiccator containing a saturated NaBr solution (57% relative humidity) for further use unless otherwise specified.Characterization of RSF/Mg(II) composite films
where d is the film thickness, R is resistance, and A is the cross-sectional area. Each sample was tested five times, and the average value was reported.
where m1 and m2 are the weights of the centrifuge tube before and after testing, t is transmission time (days), and B is the tube opening area.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. 4 mL sample extract, normal saline and deionized water were added to the centrifuge tube (normal saline as negative control and deionized water as positive control). After incubation at 37 °C for 30 min, 80 μL of rat blood was added to each tube and incubated for 60 min. The samples were centrifuged at 3500 rpm for 5 min, and 200 μL supernatants were transferred to a 96-well plate for OD measurement detected by Epoch2 enzyme-linked immunoassay (BioTek, USA) at 545 nm. Hemolysis rate was calculated as:
1 volume ratio. 4 mL sample extract, normal saline and deionized water were added to the centrifuge tube (normal saline as negative control and deionized water as positive control). After incubation at 37 °C for 30 min, 80 μL of rat blood was added to each tube and incubated for 60 min. The samples were centrifuged at 3500 rpm for 5 min, and 200 μL supernatants were transferred to a 96-well plate for OD measurement detected by Epoch2 enzyme-linked immunoassay (BioTek, USA) at 545 nm. Hemolysis rate was calculated as:where ODi is the sample absorbance, ODnc is the negative control, and ODpc is the positive control.
In vitro cell experiments
where ODi is the sample absorbance, ODc is the control, and OD0 is the background absorbance.
The cytocompatibility was further assessed using the direct contact method. Specifically, the RSF/MgCl2 solutions with varying Mg(II) ion concentrations were coated onto 24-well plates to form films under conditions of 25 °C and 5% relative humidity. After sterilization, HUVEC and NIH/3T3 cells were seeded onto the films at a density of 8 × 104 cells per well. The cells were stained for live/dead analysis using the Calcein-AM/PI kit, and images were captured with an LSM980 laser confocal microscope (Zeiss, Germany). Live cells appeared green, while dead cells appeared red.
Additionally, cell morphology on the RSF/Mg(II) composite films was observed by staining the cytoskeleton and nucleus. After 12 h of culture, rhodamine-labeled phalloidin was used to stain the cytoskeleton by incubating in the dark for 1 h. After washing three times with PBS, DAPI was added to counterstain the nucleus for 5 min then washed three more times with PBS. Finally, cell morphology was observed under a laser confocal microscope.
Mouse full-thickness skin defect repair experiment
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Fudan University and approved by the Animal Ethics Committee of Fudan University (202409030S). Full-thickness wounds (approximately 15 mm in diameter) were created on the dorsal skin of ICR mice (male, 10 weeks old, approximately 30 g). The samples were cut into slices with diameter of 15 mm and thickness of 1 mm and applied to the wounds, and wound contraction was calculated as:where At and A0 represent the wound area at day t and day 0, respectively.
Histological analysis was performed 20 days post-treatment, with sections stained using H&E, Masson, and CD31. Then, their histological morphology was observed under a DM2500P optical microscope (Leica, Germany).
Statistical analysis
All data were shown as mean ± SD. Sample size (n) for each statistical analysis was 3. The Shapiro–Wilk test was performed to evaluate the normality of the analysed data. Statistical comparisons were conducted by one-way ANOVA followed by Tukey's post-test. A value of p < 0.05 was considered statistically significant (*p < 0.05 and **p < 0.01). All statistical analysis was performed using the GraphPad Prism 9.0 (GraphPad Software Inc., USA).Results and discussion
Preparation of RSF/Mg(II) composite films
The process of preparing RSF/Mg(II) composite films, along with their potential applications, is illustrated in Fig. 1. The standard laboratory procedure of degumming, dissolving silk, and dialysis was used to obtain a RSF solution from silkworm cocoons.42 In this solution, RSF primarily adopts a random coil conformation, and the addition of MgCl2 does not induce the formation of β-sheets. Consequently, films produced via solution casting exhibited high transparency and a uniform appearance. As shown in Fig. 2a, the RSF/Mg(II) composite film with an 80/20 RSF/MgCl2 mass ratio (RSF/Mg20) displayed over 80% transmittance in the visible range, whereas pristine RSF films showed less than 60% transmittance. This enhanced optical performance suggests that RSF/Mg(II) composite films have promising applications in areas such as wound dressings, where transparency allows for direct observation of wounds.43 | ||
| Fig. 1 Schematic diagram for the preparation of RSF/Mg(II) composite films. Inset is the photograph of the transparent RSF/Mg20 film (thickness: 300 μm). | ||
To assess the impact of Mg(II) ion incorporation on the structure of silk fibroin, Raman spectroscopy was performed on composite films containing varying concentrations of MgCl2. The amide I (1600–1700 cm−1) and amide III (1220–1330 cm−1) bands were analysed. Fig. 2b reveals that the characteristic peak of the amide I band in pristine RSF films appears at around 1656 cm−1, indicating a predominant random coil structure. With the addition of Mg(II) ions, this peak gradually shifts to around 1682 cm−1, suggesting a transition state similar to the effect observed with calcium ions.6,26 Additionally, a minor shift in the amide III band also supports the conclusion that Mg(II) ions influence the molecular conformation of silk fibroin chains, but no significant β-sheet formation was detected. Prior research has shown that Mg(II) ions have a limited impact on silk fibroin transitioning from random coil to β-sheets, even at higher concentrations.26
The incorporation of Mg(II) ions into the films enhances their ability to absorb water from the environment, owing to the coordination effects of Mg(II) ions.44 We equilibrated the RSF/Mg(II) composite films with different Mg(II) ion concentrations in a 57% relative humidity environment (saturated NaBr solution) for 5 days and measured their water content. The results indicated that water content increased with higher MgCl2 content (Fig. 2c). Since absorbed water functions as a plasticizer, it improves the films’ flexibility.45 We then characterized the mechanical properties of the equilibrated films. As shown in Fig. 2d, the mechanical strength of the films varied significantly with increasing Mg(II) ion content. The tensile modulus of the composite films decreased from approximately 680 MPa to around 380 kPa as the MgCl2 content increased from 10 wt% to 20 wt%, a change of three orders of magnitude, approaching the elastic modulus of human skin.46 Moreover, the elongation at break for RSF/Mg20 was substantially higher than for RSF/Mg10, reaching more than 600%, and RSF/Mg30 displayed even greater extensibility (Fig. 2e). However, the RSF/Mg40 films absorbed excessive amounts of water, losing their self-supporting capacity, which precluded tensile testing.
Although silk fibroin itself is non-conductive, the incorporation of Mg(II) ions imparts ionic conductivity to the composite films. As demonstrated in Fig. 2f, the ionic conductivity of the RSF/Mg(II) composite films increased significantly with Mg(II) ion content. The conductivity of the RSF/Mg10 film was only 0.3 mS m−1, but it increased to 46.3 mS m−1 for RSF/Mg20 film and reached 153.6 mS m−1 for RSF/Mg30 film, indicating excellent ionic conductivity.
The presence of numerous coordination and hydrogen bonds within the structure of the RSF/Mg(II) composite films, particularly after water absorption, grants these films good flexibility and ductility. As a result, non-covalent interactions can reform following damage, providing the films with self-healing properties. We demonstrated this by cutting the composite films with a blade and placing them in an environment with approximately 60% relative humidity. Microscopy revealed that the cut marks, initially visible on the films, gradually disappeared over time and were fully healed (Fig. 2g). Furthermore, tensile tests performed on the healed films 1 h after self-repair showed that their mechanical properties were comparable to those of the original films.
Adhesion properties of RSF/Mg(II) composite films
The RSF/Mg(II) composite films demonstrate remarkable adhesion properties. As shown in Fig. 3a, a 10 × 10 mm RSF/Mg20 film was capable of adhering to surfaces such as glass, copper, and wood, supporting 100 g weights on both sides without detachment. Additionally, when applied to a single finger joint or the entire surface of the hand, the film adhered well to the skin, remaining in place even with continuous hand movement (Fig. 3b).To further characterize the adhesion strength of the composite films with varying Mg(II) ion content, lap-shear and T-peel tests were conducted. These tests evaluated adhesion performance on various natural and synthetic polymeric materials, including wood and paper (polysaccharides), hog skin (protein), polystyrene (plastic), as well as inorganic substrate like glass and metal substrate like copper (Fig. 3c and d). The results showed that shear strength and peel strength of the RSF/Mg(II) composite films varied based on Mg(II) ion content, revealing strong adhesion to wood, paper, and especially hog skin. For instance, the peel strength of RSF/Mg20 film on hog skin reached 132.0 N m−1, surpassing that of commercially available medical tapes and dressings.47 In contrast, the RSF/Mg(II) composite films exhibited weaker adhesion to less porous, hydrophobic materials such as polystyrene, glass, and copper. These findings suggest that the RSF/Mg(II) composite films tend to adhere more effectively to rough, hydrophilic surfaces that swell upon exposure to moisture.
The superior adhesion properties of the RSF/Mg(II) composite films can be attributed to the role of Mg(II) ions, which cause the majority of silk fibroin molecular chains to assume random coil or helical conformations. The absorbed water, facilitated by the presence of Mg(II) ions, reduces the modulus of the films, allowing them to form better contact with substrates and penetrate into surface pores.48,49 Substrates that are hydrophilic and soften upon swelling offer an even larger contact area for the RSF/Mg(II) composite films, enhancing adhesion. Additionally, the RSF/Mg(II) composite films contain numerous coordination and hydrogen bonds, which help dissipate energy at the interface during peeling, further increasing adhesive strength.50 This synergy of high energy dissipation, good interfacial contact, and appropriate cohesion grants the RSF/Mg(II) composite films their excellent adhesion characteristics.
As illustrated in Fig. 3c and d, RSF/Mg20 film displayed the best overall adhesion performance across various materials. The RSF/Mg10 films, with lower water absorption, exhibited minimal adhesion. For RSF/Mg20, RSF/Mg30, and RSF/Mg40 films, the system lacked cross-linking, resulting in higher cohesion strength and better adhesion at lower Mg(II) ion content (RSF/Mg20 film). However, with increased Mg(II) ion content, water absorption increased, and by the time the water content exceeded 30 wt% in RSF/Mg40 films (Fig. 2c), the mechanical properties of the films deteriorated, leading to a sharp reduction in adhesion across different substrates. Thus, RSF/Mg20 films were chosen for further exploration due to their optimal balance of adhesion and mechanical properties.
In vitro biocompatibility of RSF/Mg(II) composite films
To evaluate the suitability of RSF/Mg(II) composite films as biomaterials, a series of in vitro biocompatibility tests were conducted. First, hemolysis rate tests were performed to assess the effect of the composite films on red blood cells. Hemolysis testing is a crucial method to determine whether a material causes red blood cell lysis, which could result in hemotoxicity or other adverse effects. The results indicated that both pristine RSF films and RSF/Mg(II) composite films had hemolysis rates of less than 5% (Fig. 4a), which meets the biocompatibility standards.51 These results suggest that the films do not induce hemolysis and are safe for contact with blood.Next, the cytotoxicity of RSF/Mg(II) composite films was examined by culturing extraction solutions of the films with HUVECs and NIH/3T3 fibroblast cells. Cell viability was assessed using the CCK-8 assay. As shown in Fig. 4b and c, cell viability in all experimental groups exceeded 90% after 1 and 3 days of culture, indicating that the RSF/Mg(II) composite films exhibited minimal cytotoxicity. To further evaluate the biocompatibility, we seeded cells directly onto the surface of the composite films and performed live/dead staining after 1 and 3 days of culture. Confocal microscopy images revealed that both HUVECs and NIH/3T3 cells grew normally on the surfaces of both pristine RSF and RSF/Mg(II) composite films, with almost no dead cells observed (Fig. 4d). These results further confirm the excellent biocompatibility of RSF/Mg(II) composite films.
Additionally, to observe early cell attachment and growth, we stained the cytoskeleton and nuclei of cells cultured on the material surfaces for 12 h using phalloidin and DAPI. Fluorescence microscopy images demonstrated that both HUVECs and NIH/3T3 cells adhered well to the surface of the RSF/Mg(II) composite films, suggesting that these films support cell adhesion and infiltration, which is essential during the early stages of wound healing.
Inhibition of cell apoptosis and oxidative stress by RSF/Mg(II) composite films
To assess the potential of RSF/Mg(II) composite films as wound dressings, we explored their ability to promote wound healing in vitro by evaluating their anti-inflammatory and antioxidative properties. NIH/3T3 cells were exposed to the pro-inflammatory factor IL-1β to simulate an inflammatory environment, and the effect of RSF/Mg(II) composite films on cell apoptosis was examined. As depicted in the fluorescence images in Fig. 5a, numerous TUNEL-positive cells were observed in the wells and on pristine RSF films, indicating a significant level of apoptosis in NIH/3T3 cells. In contrast, cells cultured on RSF/Mg20 films displayed very few TUNEL-positive signals, suggesting minimal cell apoptosis. This finding demonstrates the anti-inflammatory properties of the RSF/Mg20 films, which help reduce apoptosis in inflamed cells.We investigated the ability of RSF/Mg20 films to suppress oxidative stress by measuring intracellular reactive oxygen species (ROS) levels in NIH/3T3 cells treated with hydrogen peroxide (H2O2). As shown in Fig. 5b, fluorescence imaging revealed significantly elevated ROS levels in the H2O2 group and in cells cultured on pristine RSF films (pristine RSF + H2O2 group) compared to the normal control group. However, cells cultured on RSF/Mg20 films (RSF/Mg20 + H2O2 group) showed markedly lower ROS levels, indicating that the composite films effectively suppress ROS expression under oxidative stress conditions. Quantitative analysis of ROS levels (Fig. 5c) showed that ROS levels in the H2O2 and pristine RSF + H2O2 groups were 2.07-fold and 2.12-fold higher, respectively, than in the control group. By contrast, ROS levels in the RSF/Mg20 + H2O2 group were only 1.24 times higher than in the control group, with no statistically significant difference. These results suggest that RSF/Mg20 films can mitigate excessive ROS production in cells subjected to oxidative stress, thereby reducing cellular damage and enhancing the healing environment.
Promotion of wound healing in mice by RSF/Mg(II) composite films
The previous results demonstrate that the RSF/Mg20 film possesses numerous beneficial properties, such as high water content, optimal adhesion, excellent biocompatibility, antioxidant activity, and the ability to prevent cell apoptosis in inflammatory environments. These characteristics make it a promising candidate for wound dressing applications. To further investigate its potential, we characterized the Mg(II) ion release profile and water vapor transmission rate (WVTR) of RSF/Mg20 films. The Mg(II) ion in the RSF/Mg20 film were released almost entirely within 24 h in PBS steadily without burst release (Fig. 6a). Notably, in wound applications, where only limited tissue fluid comes into contact with the dressing, the release rate would likely be slower than in PBS immersion. The WVTR of the RSF/Mg20 film was 1060 g m−2 day−1, approximately 76% of the value for medical gauze, and significantly higher than both pristine RSF film (419 g m−2 day−1) and commercial dressing Tegaderm™ (679 g m−2 day−1) (Fig. 6b). WVTR plays a critical role in maintaining moisture balance, which promotes granulation tissue formation and facilitates wound healing.52Another notable feature of the RSF/Mg20 film is its easy removal using water, unlike traditional dressings that require mechanical peeling off, which can cause secondary damage. As shown in Fig. 6c, a rhodamine B-stained RSF/Mg20 film applied to a wound and then the gauze soaked in sterile water is covered on the surface of the dressing. After a period of time, it can be completely wiped clean without any residue. If rinsed with sterile water or saline, the dressing will dissolve more quickly. Using sterile water to rinse the dressing placed on the surface of the hog skin for 20 s, it can be easily and completely removed (Video S1, ESI†). This simple method of removing the dressing avoids mechanical disruption to the wound and reduces patient discomfort during dressing changes.
We further evaluated the wound healing efficacy of the RSF/Mg20 film in a full-thickness skin defect model using mice (Fig. 7a). Fig. 7b shows the progression of wound healing in untreated mice (control group), and mice treated with RSF-based dressings (pristine RSF film and RSF/Mg20 film). On day 5, the control group exhibited noticeable yellow exudate and slow wound contraction, while the pristine RSF group had less exudate and faster contraction. The RSF/Mg20 group, however, showed almost no exudate, and wound healing progressed significantly faster.
Wound contraction was quantified at different time points (Fig. 7c). On day 5, wound contraction rates were 35.3% for the control group, 40.2% for the pristine RSF group, and 48.2% for the RSF/Mg20 group, with the latter showing significantly higher contraction. By day 10, contraction rates were 71.4%, 75.2%, and 84.4%, respectively, with the RSF/Mg20 group maintaining the highest contraction. By day 20, wound contraction reached 81.5% in the control group, 82.42% in the pristine RSF group, and 91.3% in the RSF/Mg20 group, suggesting nearly complete closure in the RSF/Mg20 film-treated wounds.
Histological analysis performed 20 days post-treatment provided further insights into the healing quality. H&E staining (Fig. 7d and e) showed that connective tissue in the RSF/Mg20 group was more organized, and the epidermal layer was significantly thicker than in the control and pristine RSF groups. Collagen deposition, a critical factor in wound healing, was evaluated using Masson staining. Collagen levels were highest in the RSF/Mg20 group (75.9%), compared to the control (60.5%) and pristine RSF groups (66.4%) (Fig. 7d and f). Moreover, CD31 immunohistochemical staining revealed increased blood vessel formation in the RSF/Mg20 group, with larger blood vessel diameters and higher blood vessel area percentage (2.8%) and density (170.8 mm−2) compared to the control group (1.5% and 98.8 mm−2) and pristine RSF group (1.9% and 126.1 mm−2) (Fig. 7d and g).
In summary, the RSF/Mg20 film not only accelerates wound healing but also improves healing quality by promoting wound contraction, increasing epidermal thickness, enhancing collagen deposition, and facilitating blood vessel formation. These benefits are attributed to the bioactivity of the RSF/Mg20 film. Wound healing typically occurs in four stages: hemostasis, inflammation, proliferation, and remodeling.53 Chronic wounds often exhibit prolonged inflammation, characterized by high levels of inflammatory mediators, which induce apoptosis and prevent progression to the proliferation and remodelling stages.54 Excessive reactive oxygen species (ROS) generated by neutrophils and monocytes in the wound bed further exacerbate apoptosis.55 Our in vitro studies demonstrate that the RSF/Mg20 film inhibits fibroblast apoptosis in inflammatory environments, likely through the activation of the PPAR-γ receptor by Mg(II) ions, which suppresses the NF-κB inflammatory signalling pathway.56 Additionally, the film's ability to reduce oxidative stress further modulates the wound bed microenvironment during early wound healing. Blood vessel formation is essential for delivering oxygen, nutrients, and growth factors to the wound, impacting both the speed and quality of healing.57 Our in vivo results confirm that the RSF/Mg20 film promotes blood vessel formation, enhancing wound healing. Beyond wound care, the RSF/Mg20 film, as a novel Mg(II) ion delivery system, holds potential for treating other vascularized soft tissue injuries, such as muscle damage, and may offer promise for integrated muscle-skin repair in cases of severe trauma involving large-scale muscle loss and skin injury.
Conclusions
In this study, we used a facile casting method to prepare transparent RSF/Mg(II) composite films. Although the addition of Mg(II) ion has minimal impact on the RSF conformation, it significantly influences the physical properties of the films. With the increase in Mg(II) ion content raises water retention from 10% to 30%, and reduces the tensile modulus from 680 MPa to 30 kPa, which significantly improves the flexibility of RSF/Mg(II) composite films. Moreover, the addition of Mg(II) ions introduces ionic conductivity, self-healing ability, and adhesive property to the films.An possible application of such a RSF/Mg(II) composite film is for wound healing as this study addresses several limitations of traditional RSF materials in biomedical applications. In vitro studies demonstrated that RSF/Mg(II) composite films possess excellent biocompatibility, with minimal cytotoxicity, low hemolysis rates, and significant antioxidant activity. The composite films effectively inhibited apoptosis and oxidative stress in inflammatory environments, contributing to a more favourable healing microenvironment. In vivo studies using a mouse skin defect model further confirmed the efficacy of RSF/Mg20 films in promoting wound healing. These films accelerated wound closure, enhanced epidermal regeneration, improved collagen deposition, and stimulated blood vessel formation, highlighting their potential as advanced wound dressings. The ability to easily remove the RSF/Mg(II) composite films without causing further damage to the wound offers a significant advantage over traditional dressings. Overall, this work provides valuable insights into the development of Mg(II) ion-based biomaterials, establishing RSF/Mg(II) composite films as promising candidates for wound care and other tissue regeneration applications. Future research may explore the broader applications of these films in treating vascularized soft tissue injuries.
Data availability
All data generated or analysed during this study are included in this published article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U2032123 and 21935002). We would like to extend our gratitude to Prof. Shengjie Ling at ShanghaiTech University for his assistance during the preparation of the manuscript.References
- C. Vepari and D. L. Kaplan, Prog. Polym. Sci., 2007, 32, 991–1007 CrossRef PubMed.
- Q. L. Chen, M. Wu, J. R. Yao, Z. Z. Shao and X. Chen, J. Mater. Chem. B, 2023, 11, 4529–4538 RSC.
- Y. Wang, M. Yang, J. Wang, Y. Shuai, Z. Xu, Q. Wan, S. Zhong, C. Mao, W. Ping and M. Yang, ACS Appl. Mater. Interfaces, 2024, 16, 15798–15808 CrossRef PubMed.
- Z. H. Liang, Z. Z. Zhou, J. Li, S. L. Zhang, B. H. Dong, L. Zhao, C. C. Wu, H. Y. Yang, F. X. Chen and S. M. Wang, Chem. Eng. J., 2021, 415, 9 Search PubMed.
- H. P. Wang, Q. L. Dong, J. R. Yao, Z. Z. Shao, J. M. Ma and X. Chen, Biomacromolecules, 2020, 21, 1596–1603 CrossRef PubMed.
- J. Liu, Q. Chen, Q. Liu, B. Zhao, S. Ling, J. Yao, Z. Shao and X. Chen, Adv. Mater. Technol., 2020, 5, 2000430 CrossRef CAS.
- Y. Wang, W. L. Gao, S. Yang, Q. L. Chen, C. Ye, H. Wang, Q. Zhang, J. Ren, Z. J. Ning, X. Chen, Z. Z. Shao, J. Li, Y. F. Liu and S. J. Ling, Nano-Micro Lett., 2023, 15, 17 CrossRef PubMed.
- R. Y. Huang, J. H. Hua, M. Ru, M. Yu, L. Wang, Y. Huang, S. Q. Yan, Q. Zhang and W. L. Xu, ACS Nano, 2024, 18, 15312–15325 CrossRef CAS PubMed.
- L. Y. Sun, M. L. Xiao, L. Chen, L. Y. Ni, X. X. Chen, L. N. Zhang, J. R. Yao, Z. Z. Shao, B. J. Zhao, X. Chen and Y. H. Liu, Adv. Healthcare Mater., 2024, 13, 2401460 CrossRef CAS PubMed.
- Y. Y. Luo, M. L. Xiao, B. S. Almaqrami, H. Kang, Z. Z. Shao, X. Chen and Y. Zhang, Biomater. Res., 2023, 27, 17 CrossRef PubMed.
- Q. Y. Wang, X. Y. Ran, J. Wang, S. A. Wang, P. L. Zhang, E. J. Gao, B. S. Bai, J. F. Zhang, G. D. Zhou and D. Lei, Adv. Fiber Mater., 2023, 5, 1008–1024 CrossRef CAS.
- Q. Deng, P. Lin, H. Gu, X. Zhuang and F. Wang, Biomacromolecules, 2024, 25, 1527–1540 CrossRef CAS PubMed.
- M. Farokhi, F. Mottaghitalab, Y. Fatahi, A. Khademhosseini and D. L. Kaplan, Trends Biotechnol., 2018, 36, 907–922 CrossRef CAS PubMed.
- M. L. Xiao, J. R. Yao, Z. Z. Shao and X. Chen, ACS Biomater. Sci. Eng., 2024, 10, 2827–2840 CrossRef CAS.
- D. L. Wen, D. H. Sun, P. Huang, W. Huang, M. Su, Y. Wang, M. D. Han, B. Kim, J. Brugger, H. X. Zhang and X. S. Zhang, Microsyst. Nanoeng., 2021, 7, 35 CrossRef CAS PubMed.
- C. Lu, X. Wang and X. Y. Liu, ACS Biomater. Sci. Eng., 2024, 10, 2784–2804 CrossRef CAS.
- Y. Jiang, J. Ma, L. Shen, W. Zhang, K. Yang, B. Zhu, Y. Yang, H. Ma, X. Chen, S. Bai and N. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 47196–47207 CrossRef CAS PubMed.
- Q. Chen, H. Tang, J. Liu, R. Wang, J. Sun, J. Yao, Z. Shao and X. Chen, Chem. Eng. J., 2021, 422, 130091 CrossRef.
- A. Zhuang, X. Huang, S. Fan, X. Yao, B. Zhu and Y. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 123–137 CrossRef.
- Q. Wen, L. Zhang, Y. Chen, Y. Su, J. Yu, P. Chen and T. Zheng, Sustainability, 2023, 15, 16053 CrossRef.
- J. W. Seo, H. Kim, K. Kim, S. Q. Choi and H. J. Lee, Adv. Funct. Mater., 2018, 28, 1800802 CrossRef.
- X. Wang, D. Tan, X. Zhang, Y. Lei and L. Xue, Biomimetics, 2017, 2, 10 CrossRef PubMed.
- Q. Liu, S. Yang, J. Ren and S. Ling, ACS Mater. Lett., 2020, 2, 712–720 CrossRef.
- S. Maeno, Y. Niki, H. Matsumoto, H. Morioka, T. Yatabe, A. Funayama, Y. Toyama, T. Taguchi and J. Tanaka, Biomaterials, 2005, 26, 4847–4855 CrossRef PubMed.
- A. Verkhratsky, Physiol. Rev., 2005, 85, 201–279 CrossRef PubMed.
- L. Zhou, X. Chen, Z. Z. Shao, Y. F. Huang and D. P. Knight, J. Phys. Chem. B, 2005, 109, 16937–16945 CrossRef PubMed.
- H. Zreiqat, C. R. Howlett, A. Zannettino, P. Evans, G. Schulze-Tanzil, C. Knabe and M. Shakibaei, J. Biomed. Mater. Res., 2002, 62, 175–184 CrossRef.
- X. Xing, G. Cheng, C. Yin, X. Cheng, Y. Cheng, Y. Ni, X. Zhou, H. Deng and Z. Li, Arabian J. Chem., 2020, 13, 5526–5538 CrossRef.
- L. Wei, Z. Du, C. Zhang, Y. Zhou, F. Zhu, Y. Chen, H. Zhao, F. Zhang, P. Dang, Y. Wang, Y. Meng, B. C. Heng, H. Zhang, J. Song, W. Liu, Q. Cai and X. Deng, Adv. Mater. Interfaces, 2023, 10, 2300224 CrossRef.
- Z. Ding, W. Cheng, L. Liu, G. Xu, Q. Lu and D. L. Kaplan, Adv. Healthc. Mater., 2023, 12, e2300887 CrossRef PubMed.
- W. Cheng, H. Yang, L. Xiao, G. Yang, Q. Lu and D. L. Kaplan, ACS Appl. Mater. Interfaces, 2024, 16, 9880–9889 CrossRef.
- J. Gould, Nature, 2018, 563, S84–S85 CrossRef.
- R. Dong and B. Guo, Nano Today, 2021, 41, 101290 CrossRef.
- V. J. Jones, Int. Wound J., 2006, 3, 79–86 CrossRef PubMed.
- G. D. Winter, Nature, 1962, 193, 293–294 CrossRef.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef PubMed.
- M. Naseri-Nosar and Z. M. Ziora, Carbohydr. Polym., 2018, 189, 379–398 CrossRef.
- K. Varaprasad, T. Jayaramudu, V. Kanikireddy, C. Toro and E. R. Sadiku, Carbohydr. Polym., 2020, 236, 116025 CrossRef PubMed.
- N. E. Atchison, P. F. Osgood, D. B. Carr and S. K. Szyfelbein, Pain, 1991, 47, 41–45 CrossRef.
- M. D. Konieczynska, J. C. Villa-Camacho, C. Ghobril, M. Perez-Viloria, K. M. Tevis, W. A. Blessing, A. Nazarian, E. K. Rodriguez and M. W. Grinstaff, Angew. Chem., Int. Ed., 2016, 55, 9984–9987 CrossRef.
- H. Luo, C. Cao, J. Zhong, J. Chen and Y. Cen, Wound Repair Regener., 2019, 27, 90–101 CrossRef.
- Q. Chen, M. Wu, J. Yao, Z. Shao and X. Chen, J. Mater. Chem. B, 2023, 11, 4529–4538 RSC.
- M. Kuddushi, A. A. Shah, C. Ayranci and X. H. Zhang, J. Mater. Chem. B, 2023, 11, 6201–6224 RSC.
- A. K. Katz, J. P. Glusker, S. A. Beebe and C. W. Bock, J. Am. Chem. Soc., 1996, 118, 5752–5763 CrossRef CAS.
- G. Chen, N. Matsuhisa, Z. Liu, D. Qi, P. Cai, Y. Jiang, C. Wan, Y. Cui, W. R. Leow, Z. Liu, S. Gong, K. Q. Zhang, Y. Cheng and X. Chen, Adv. Mater., 2018, 30, e1800129 CrossRef PubMed.
- R. Fu, L. Tu, Y. Zhou, L. Fan, F. Zhang, Z. Wang, J. Xing, D. Chen, C. Deng, G. Tan, P. Yu, L. Zhou and C. Ning, Chem. Mater., 2019, 31, 9850–9860 CrossRef.
- B. Zhao, Q. Chen, G. Da, J. Yao, Z. Shao and X. Chen, J. Mater. Chem. C, 2021, 9, 8955–8965 RSC.
- E. P. Chang, J. Adhes., 1997, 60, 233–248 CrossRef.
- C. Derail, A. Allal, G. Marin and P. Tordjeman, J. Adhes., 1997, 61, 123–157 CrossRef.
- S. Kim, A. M. Peterson and N. Holten-Andersen, Chem. Mater., 2018, 30, 3648–3655 CrossRef.
- G. H. Xi, W. Liu, M. Chen, Q. Li, X. Hao, M. S. Wang, X. Yang, Y. K. Feng, H. C. He, C. C. Shi and W. Z. Li, ACS Appl. Mater. Interfaces, 2019, 11, 46558–46571 CrossRef PubMed.
- P. Wu, A. C. Fisher, P. P. Foo, D. Queen and J. D. S. Gaylor, Biomaterials, 1995, 16, 171–175 CrossRef PubMed.
- Z. L. Tu, M. Chen, M. Wang, Z. X. Shao, X. Q. Jiang, K. Y. Wang, Z. Yao, S. W. Yang, X. X. Zhang, W. Y. Gao, C. Lin, B. Lei and C. Mao, Adv. Funct. Mater., 2021, 31, 18 Search PubMed.
- M. C. Ji, J. Y. Li, Y. Wang, F. Y. Li, J. Man, J. F. Li, C. W. Zhang, S. X. Peng and S. Q. Wang, Carbohydr. Polym., 2022, 297, 21 Search PubMed.
- H. Chang, P. F. Tian, L. Z. Hao, C. W. Hu, B. Liu, F. Z. Meng, X. Yi, X. H. Pan, X. H. Hu, H. Wang, X. Y. Zhai, X. Cui, J. P. Y. Cheung, X. Y. Liu, H. B. Pan, S. Q. Bian and X. L. Zhao, Chem. Eng. J., 2024, 481, 18 Search PubMed.
- W. X. Zhang, S. B. Zhao, Q. F. Guan, P. Li and Y. B. Fan, ACS Appl. Mater. Interfaces, 2024, 16, 8238–8249 CrossRef CAS.
- K. Wu, Q. Yang, L. Zhang, P. C. Xu, X. X. Wu, H. L. Yang, H. Zhou, X. Lin and L. Yang, J. Mater. Sci. Technol., 2023, 133, 123–134 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4bm01411a |
| ‡ These authos contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |