The repressive effect of miR-520a on NF-κB/IL-6/STAT-3 signal involved in the glabridin-induced anti-angiogenesis in human breast cancer cells†
Juan Mu‡
,
Shilong Ning‡,
Xingxing Wang,
Lu Si,
Fei Jiang,
Yuan Li and
Zhong Li*
Department of Nutrition and Food Hygiene, The Key Laboratory of Modern Toxicology, Ministry of Education, School of Public Health, Nanjing Medical University, Nanjing, 211166, China. E-mail: lz-ny@njmu.edu.cn; Fax: +86-25-8652-7613; Tel: +86-25-8686-8451
First published on 30th March 2015
Abstract
The formation of blood vessels plays a vital role in the growth of tumors. Breast cancer is a type of vascular dependent tumor, and angiogenesis plays an important role in its invasion and metastasis. Thus, the continued search for new mechanisms that inhibit the formation of blood vessels is becoming a strategy for the treatment of breast cancer. Glabridin (GLA) is a novel anti-tumor agent that inhibits oxidization, inflammation, and proliferation in human cancer cells. However, the functions of GLA in the regulation of angiogenesis in breast cancer and its underlying molecular mechanisms remain largely uninvestigated. In our present study, GLA attenuated the angiogenic ability by the microRNA-520a (miR-520a)-mediated inhibition of the NF-κB/IL-6/STAT-3 signal pathway in MDA-MB-231 and Hs-578T cells. Briefly, in these cells, GLA up-regulated the expression of miR-520a in a time-dependent manner; miR-520a, which targeted the NF-κB/RelA-3′UTR, decreased the expression/function of NF-κB, leading to the inactivation of IL-6/STAT-3 signaling; the knockdown of miR-520a abolished the GLA-induced inhibitions of the NF-κB/IL-6/STAT-3 signal pathway, the VEGF secretion, and the angiogenesis. By understanding the novel mechanism in which GLA inhibits the angiogenic potential in human breast cancer cells, our study would help in the design of future strategies in terms of developing GLA as a potential chemopreventive agent, when used alone or in combination with other current anticancer drugs.
1 Introduction
Breast cancer is one of the most common cancers in women worldwide, and more than 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 women die from this disease every year.1 In solid tumors, cancer cells recruit new blood vessels for their growth, maintenance, and metastasis; tumors cannot grow once the original blood supply is exhausted.2 As a consequence, angiogenesis plays a crucial role in tumorigenesis and tumor development, including breast cancer.3,4 In the past few years, the two major mechanisms that regulate angiogenesis in human cancers have been identified, namely, the hypoxia-regulated signaling pathways and the nuclear factor-κB (NF-κB) signaling pathways; both synergize in the regulation of vascular endothelial growth factor (VEGF) expression.5 On the one hand, as a classical angiogenesis inducer, the hypoxia-inducible factor mediates the phosphatidylinositol-3-kinase/mTOR-induced tumor cell growth via the transcriptional up-regulation of the VEGF gene;6 on the other hand, NF-κB elevates the VEGF expression by IL-6/STAT-3 signaling.7 Importantly, the NF-κB-mediated autocrine IL-6 signaling has been identified as a key regulator in the malignant progression of breast cancer.8 Thus, the suppression of NF-κB activity, which attenuates the tumor-induced development of new blood vessels, is an important strategy for the treatment of breast cancer.
000 women die from this disease every year.1 In solid tumors, cancer cells recruit new blood vessels for their growth, maintenance, and metastasis; tumors cannot grow once the original blood supply is exhausted.2 As a consequence, angiogenesis plays a crucial role in tumorigenesis and tumor development, including breast cancer.3,4 In the past few years, the two major mechanisms that regulate angiogenesis in human cancers have been identified, namely, the hypoxia-regulated signaling pathways and the nuclear factor-κB (NF-κB) signaling pathways; both synergize in the regulation of vascular endothelial growth factor (VEGF) expression.5 On the one hand, as a classical angiogenesis inducer, the hypoxia-inducible factor mediates the phosphatidylinositol-3-kinase/mTOR-induced tumor cell growth via the transcriptional up-regulation of the VEGF gene;6 on the other hand, NF-κB elevates the VEGF expression by IL-6/STAT-3 signaling.7 Importantly, the NF-κB-mediated autocrine IL-6 signaling has been identified as a key regulator in the malignant progression of breast cancer.8 Thus, the suppression of NF-κB activity, which attenuates the tumor-induced development of new blood vessels, is an important strategy for the treatment of breast cancer.
The root of Glycyrrhiza glabra (licorice) has been used for many centuries in Asia and Europe as an antioxidant, antidote, demulcent, expectorant and a remedy for allergic inflammation; moreover, it is a flavoring and sweetening agent.9 Glabridin [GLA, (R)-4-(3,4-dihydro-8,8-dimethyl)-2H,8H-benzo[1,2-b:3,4-b′]dipyran-3yl)-1,3-benzenediol] is a polyphenolic flavonoid and is a main constituent in the hydrophobic fraction of licorice extract.10 In addition to estrogenic effects, GLA exhibits a wide range of biological activities including neuro-protective, cardiovascular-protective and anti-inflammatory.11 Recent studies indicate that GLA presents anti-tumor effects with the attenuation of proliferation, migration/invasion, angiogenesis, and cancer stem cells-like properties in a wide variety of human cancers, including breast cancer.12,13 However, the relationship between GLA and NF-κB/IL-6/STAT-3 signaling pathway in the angiogenesis of breast cancer and the molecular mechanisms involved between them remain unclear. Herein, to identify the molecular mechanisms underlying the effects of GLA on angiogenesis and to identify new targets for breast cancer therapy, MDA-MB-231 and Hs-578T cells were treated with GLA and early molecular changes were assessed with an emphasis on the NF-κB/IL-6/STAT-3 signal pathway.
2 Materials and methods
2.1 Cell culture and reagents
Human breast cancer cell lines, MDA-MB-231 and Hs-578T, were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Briefly, MDA-MB-231 cells were maintained at 37 °C in the absence of CO2 in L-15 medium (Life Technologies/Gibco, Grand Island, NY, USA), while the Hs-578T cells were maintained in 5% CO2 at 37 °C in Dulbecco's modified Eagle's medium (DMEM, Life Technologies/Gibco). Both the media were supplemented with 10% fetal bovine serum (FBS, Life Technologies/Gibco), 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin (Life Technologies/Gibco). Glabridin (GLA, 99.0% purity) was purchased from Sigma Chemical (St. Louis, MO, USA). All the other reagents used were of analytical grade or the highest grade available.2.2 Determination of angiogenic potential
The abilities of angiogenesis in breast cancer cells were analyzed by VEGF secretion and tube formation in vitro. Briefly, after the cells were treated as indicated, the conditioned media were collected, purified by centrifugation and stored at −80 °C. For analysing VEGF secretion, enzyme-linked immunosorbent assay (ELISA) was performed using the human VEGF Quantikine kit (R&D Systems, USA) according to the company protocol. Recombinant human VEGF (R&D Systems) was used for calibration. For the tube formation assay, human umbilical vein endothelial cells (HUVECs), maintained in 5% CO2 at 37 °C in RPMI-1640 (Roswell Park Memorial Institute-1640, Life Technologies/Gibco), were trypsinized and seeded at 5 × 104 cells per well in a 48-well plate on matrigel (BD), which had been polymerized for 30 min at 37 °C. Subsequently, the cells were incubated in the conditioned media as described above for 6 h. Capillary morphogenesis was evaluated using an inverted microscope (Olympus, Tokyo, Japan).2.3 Quantitative real-time polymerase chain reaction (qRT-PCR)
The primers used are listed in Table 1. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, USA) according to the manufacturer's recommendations. For the detection of mRNAs, total RNA (2 μg) was transcribed into cDNA using AMV reverse transcriptase (Promega, Madison, USA). For the detection of miRNAs, 2 μg of total RNA, miRNAs-specific stem-loop RT primers, and MMLV reverse transcriptase (Promega) were used in reverse transcription following the manufacturer's protocol. qRT-PCR was performed using an ABI 7300 real-time PCR detection system (Applied Biosystems by Life Technologies, Grand Island, NY, USA). Fold changes in the expression of each gene were calculated by a comparative threshold cycle (Ct) method using the formula 2−(ΔΔCt).| IL-6 | Forward | 5′-AGTAGTGAGGAACAAGCCAGA-3′ |
| Reverse | 5′-TACATTTGCCGAAGAGCC-3′ | |
| NF-κB/RelA | Forward | 5′-GAAGGAGATAGGGTGTTGGC-3′ |
| Reverse | 5′-GGGATGACGTAAAGGGATAG-3′ | |
| VEGFA | Forward | 5′-CCTTGCTGCTCTACCTCC-3′ |
| Reverse | 5′-AAATGCTTTCTCCGCTCT-3′ | |
| GAPDH | Forward | 5′-GACCTGACCTGCCGTCTA-3′ |
| Reverse | 5′-GGAGTGGGTGTCGCTGT-3′ |
2.4 Western blots
Cells were washed twice with ice-cold PBS and then scraped-off in 0.2 ml of lysate buffer (Beyotime) and incubated on ice for 30 min, followed by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. Protein concentrations were measured using the BCA protein assay (Beyotime). Subsequently, proteins were diluted to equal concentrations (20 μg), boiled for 10 min, and separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Then, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, USA), which were probed with a primary antibody [RelA, p-RelA (ser536), STAT-3, p-STAT-3 (Tyr 705), and IL-6 receptor alpha (IL-6Rα) Cell Signaling Technology, Beverly, MA, USA, dilutions, 1
000 rpm for 15 min. Protein concentrations were measured using the BCA protein assay (Beyotime). Subsequently, proteins were diluted to equal concentrations (20 μg), boiled for 10 min, and separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Then, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, USA), which were probed with a primary antibody [RelA, p-RelA (ser536), STAT-3, p-STAT-3 (Tyr 705), and IL-6 receptor alpha (IL-6Rα) Cell Signaling Technology, Beverly, MA, USA, dilutions, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500] at 4 °C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beyotime) for 1 h at room temperature. The immune complexes were detected by enhanced chemiluminescence (Cell Signaling Technology). Glyceraldehyde phosphate dehydrogenase (GAPDH, Sigma, dilutions, 1
500] at 4 °C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beyotime) for 1 h at room temperature. The immune complexes were detected by enhanced chemiluminescence (Cell Signaling Technology). Glyceraldehyde phosphate dehydrogenase (GAPDH, Sigma, dilutions, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) was used to normalize the protein loading. For densitometric analyses, the bands on the blots were measured using an Eagle Eye II imaging system.
1000) was used to normalize the protein loading. For densitometric analyses, the bands on the blots were measured using an Eagle Eye II imaging system.
2.5 MicroRNA transfection
Con-mimic, miR-520a-mimic, anti-con, and anti-miR-520a were obtained from RiBoBio Co (Guangzhou, China). The cells were transiently transfected using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. Briefly, cells were seeded in 6-well plates at a density of 1 × 105 per well. After 48 h, the cells were transfected with 50 nM anti-miR-520a or 20 nM miR-520a-mimic for 12 h. After transfection, the cells were cultured in a fresh DMEM medium supplemented with 10% FBS (Gibco) for another 24 h before using it for other experiments.2.6 RNA interference
Control-siRNA and RelA-siRNA were purchased from Cell Signaling Technology. Transfections were performed with the N-TERTM Nanoparticle siRNA Transfection System (Sigma). Briefly, 1 × 105 cells were seeded into each well of 6-well plates for 48 h, and then a nanoparticle formation solution containing 20 nM target siRNA was transfected into each well for another 12 h. Then, the cells were conventionally cultured as described above, and then harvested and subjected to further experiments.2.7 Cell viability
A total of 2 × 103 MDA-MB-231 cells were seeded in 96-well plates. After 24 h, they were treated with 10 μM GLA for 24, 48, or 72 h. Then, the cells were washed with 1× PBS and incubated with CCK-8 (Dojindo Molecular Technologies, Inc, Kumamoto, Japan) for another 4 h. The absorbance at 450 nm was measured using a multi-well plate reader (Model 680, Bio-Rad, USA). The relative ratio of cell vitality was measured.2.8 Statistical analysis
Derived values are presented as the mean ± SD. A Student's t test and a one-way analysis of variance followed by Dunnett's t test were used to assess significant differences between groups. P values <0.05 were considered statistically significant.3 Results
3.1 Glabridin reduces the angiogenic capacity in MDA-MB-231 cells
We first investigated the effect of glabridin (GLA) on cell viability. GLA did not appreciably affect the vitality of MDA-MB-231 breast cancer cells at the concentration of 10 μM (Fig. S1A†). Thus, we used the concentration of 10 μM GLA for further investigating the roles of GLA on angiogenesis in breast cancer cells. We determined the effects of GLA on VEGF secretion in MDA-MB-231 cells. As shown in Fig. 1A, GLA inhibited the secretion of VEGF in a time-dependent manner. Subsequently, we used a tube formation assay to further detect the functions of GLA for angiogenic abilities. MDA-MB-231 cells were pre-treated by 0 or 10 μM GLA for 72 h, and then the media were replaced with fresh media containing 1% serum. After 24 h, the conditioned media were collected, and were used to treat human umbilical vein endothelial cells (HUVECs). As shown in Fig. 1B and C, the formation of tubes decreased in HUVECs incubated with the conditioned media, which were collected from the GLA-treated group. These results suggest that GLA attenuates the angiogenic abilities of MDA-MB-231 cells, in which the inhibition of VEGF secretion is involved.3.2 GLA blocks the NF-κB/IL-6/STAT-3 signal in MDA-MB-231 cells
NF-κB activation plays a major role in metastasis, carcinogenesis and angiogenesis, and stimulated NF-κB is phosphorylated into nucleus, activating the transcription of NF-κB target genes, such as IL-6.14,15 IL-6, a secreted inflammatory cytokine, combines with the IL-6 receptor (IL-6R) on the target cells to initiate the activators of STAT-3, causing a constant transcription of VEGF.16Herein, to reveal the effects of NF-κB on the angiogenic abilities in breast cancer cells, RNA interference was conducted, and the efficacy of gene transfection can be seen in Fig. S1B.† As shown in Fig. 2A and B, the knockdown of NF-κB/RelA blocked the expression and secretion of IL-6 and VEGF in MDA-MB-231 cells. These results indicate that NF-κB may be a key manager that regulates the angiogenesis in breast cancer cells. Then, we further determined the effects of GLA on the NF-κB/IL-6/STAT-3 axis. MDA-MB-231 cells were treated with 10 μM GLA for 24, 48, and 72 h. In accordance with NF-κB knockdown, GLA decreased the expressions of p-NF-κB/RelA, p-STAT3, and IL-6R in a time-dependent manner (Fig. 2C and D). Furthermore, with the increase in the time of GLA exposure, there was decreased expression/secretion of IL-6 (Fig. 2E). Interestingly, in addition to the blocking of NF-κB/RelA phosphorylation, GLA also decreased the expression of total NF-κB/RelA mRNA and protein (Fig. 2C, D, and F). Collectively, these results indicate that the anti-angiogenesis induced by GLA involves the blockage of the NF-κB-mediated IL-6/STAT-3/VEGF pathway, and that the repressive effects of GLA on the endogenous NF-κB/RelA may not occur via the classical phosphorylation signaling, but via the miRNAs, which caused the degradation of mRNAs.
3.3 GLA up-regulates the expressions of miR-372/520s in MDA-MB-231 cells
A previous study suggests that miR-372/520 family negatively regulates the invasion of breast cancer cells by targeting NF-κB.8 Herein, as shown in Fig. 3 and S1E,† GLA cause a significant up-regulation of miR-520a in a time-dependent manner with no changes in the other members of miR-372/520 family in GLA-treated MDA-MB-231 cells. Thus, we targeted miR-520a for further investigation.3.4 MiR-520a is involved in the interference of NF-κB/IL-6/STAT-3 signal pathway in GLA-treated human breast cancer cells
The transfection of con-mimic or miR-520a-mimic into MDA-MB-231 and Hs-578T cells and the efficacy of gene transfection are shown in Fig. S1C.† qRT-PCR and western blots showed that the expression/activation of NF-κB/RelA was significantly suppressed (Fig. 4A–C). These results confirm the previous findings that miR-520a blocks NF-κB in breast cancer cells.Subsequently, we determined the effects of miR-520a on NF-κB in GLA-treated cells. After MDA-MB-231 or Hs-578T cells were transfected by anti-con or anti-miR-520a for 12 h, they were exposed to 0.0 or 10.0 μM of GLA for 72 h, and the efficacy of gene transfection is shown in Fig. S1D.† As shown in Fig. 4D–F, the knockdown of miR-520a eliminated the GLA-induced blockage of NF-κB/RelA (as determined by the expression and phosphorylation of NF-κB/RelA).
Furthermore, we detected the effects of miR-520a on the NF-κB-mediated angiogenesis signals (IL-6/STAT-3). MDA-MB-231 and Hs-578T cells were treated as described above, and the conditioned media were collected. As shown in Fig. 5, in these cells, GLA decreased the expression/secretion of IL-6; however, the knockdown of miR-520a abolished such phenomenon. Collectively, these results indicate that GLA blocks the NF-κB/IL-6/STAT-3 signal pathway by miR-520a in breast cancer cells.
3.5 Functions of miR-520a in GLA-induced angiogenesis in breast cancer cells
Based on the results listed above, we hypothesize that the GLA-induced anti-angiogenesis in breast cancer cells involves the attenuation of the NF-κB dependent IL-6/STAT-3 signal by miR-520a. To verify this hypothesis, we finally treated miR-520a knockdown MDA-MB-231 and Hs-578T cells with GLA to determine their angiogenic abilities. As shown in Fig. 6A–C, the knockdown of miR-520a blocked the GLA-induced inhibitions of VEGF expression/secretion and tube formation in these cells.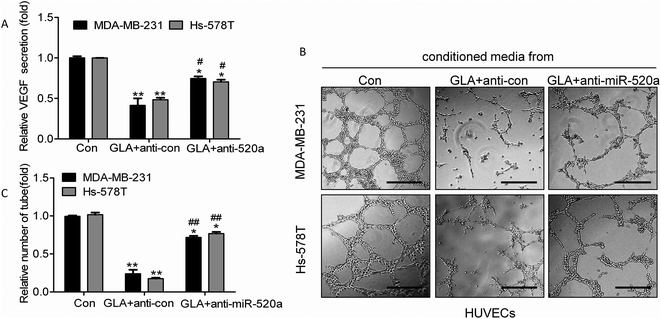 | ||
| Fig. 6 Functions of miR-520a in GLA-induced angiogenesis. Cells were treated as described in Fig. 5 and the conditioned media were collected. (A) ELISA was used to detect VEGF secretion (mean ± SD, n = 3). The previous media with anti-miRNAs and GLA were removed and the cells were washed by 1× PBS to replace the fresh media with 1% serum for 24 h; subsequently, the conditioned media were collected. (B) Tube formation assay. (C) Quantitative analysis of the tube numbers (mean ± SD, n = 5). *P < 0.05 and **P < 0.01 compared with medium control cells or cells transfected by con-mimic; #P < 0.05 and ##P < 0.01 compared with cells transfected by anti-con. | ||
4 Discussion
Naturally occurring plant products, especially from food and dietary supplements (DSs), are attracting increasing attention because of their potential use in the intervention against the malignant invasive progression of cancers.17 Glycyrrhiza has been widely used as supplementary treatments in both traditional and modern medicine.18 GLA, isolated from isoflavans, presents numerous chemical and biological properties such as antioxidant,19 anticancer,20 neuroprotective,21 anti-inflammatory,22 antiplasmodial,11 antifungal,23 anti-angiogenesis,12,13 skin-whitening,17 anti-obesity,24 and chemo-preventive effects.25 Previous studies have suggested that GLA induces HL-60 cell apoptosis by subsequently stimulating the activation of caspase-3, -8, and -9 through the phosphorylation of JNK1/2 and p38 MAPK pathway.17 Besides, GLA reduces the CSCs-like properties of hepatocellular carcinoma (HCC) cells to prevent post-surgical recurrence by miR-148a-mediated inhibition of TGF-β/SMAD2 signal pathway.25 Furthermore, it inhibits migration, invasion, and angiogenesis by inhibiting the FAK/Rho signaling pathway.12,13 Herein, we found that GLA attenuated the tube formation of endothelial cells by reducing the activity of the NF-κB/IL-6/STAT-3 signal pathway. To our knowledge, there are two important points about the formation of blood vessels: one is that they are directly produced by the proliferation and migration of vessel endothelial cell;26 the other is that they are indirectly stimulated by the tumor-derived angiogenic factor.27 In ref. 12, the authors revealed a type of direct anti-angiogenesis, and they found that glabridin decreased HUVECs migration to inhibit angiogenesis by blocking the FAK/Rho signaling pathway. Here, in our study, we showed that GLA could also decrease the tumor-derived angiogenic factors (IL-6 and VEGF) and indirectly inhibit angiogenesis. There was no conflict between our findings and previous studies. Thus, our results suggest that the attenuation of tumor-derived angiogenic factors is one of the underlying putative mechanisms for the inhibition of angiogenesis by GLA.For the transcriptional regulation of IL-6, two major factors in human cancers have been identified, namely, NF-κB and STAT-3.28 In fact, IL-6, NF-κB, and STAT-3 consist of a positive feed-back loop. On the one hand, NF-κB, and/or STAT-3, elevates the IL-6 expression at the transcriptional level.28 On the other hand, IL-6 activates NF-κB and STAT-3 via its specific pathways.29 For example, IL-6 induces classical IKKβ-IκBα-NF-κB/p65 signal activation via the suppression of miR-200c.30 Moreover, by binding to its receptor complex, which is comprised of IL-6Rα and glycoprotein 130, IL-6 activates the Janus kinases (JAK)/STAT-3 signaling.16 Furthermore, IL-6 also mediates the cross-talk between NF-κB and STAT-3 in human cancer cells.31 Thus, the relationships between IL-6, NF-κB, and STAT-3 are very complex. In this study, we also found that GLA decreased the expression of IL-6R. IL-6 and IL-6R aggregate into a complex, which activates the JAK/STAT3 signal. This process accelerates tumor survival, invasion, epithelial–mesenchymal transition and angiogenesis.32 Moreover, the attenuation of either IL-6 or IL-6R will be beneficial to suppress tumor progression.33,34 In our study, we found that not only the production of IL-6 was decreased by GLA, but also the IL-6R signaling was blocked. Furthermore, we found that NF-κB could decrease the production of IL-6. Whether GLA can down-regulate the expression of IL-6R via NF-κB remains to be further investigated. Besides, GLA decreased the expressions of total NF-κB/RelA, which indicates an exhaustive abolishment of NF-κB-IL-6-STAT-3 feed-back loop. Furthermore, we identified that the repressive effects of GLA on NF-κB were primarily attributed to the improvement of miR-520a expression.
MiRNAs are a class of endogenous non-coding small RNAs, which regulate gene expression by binding to 3′-UTR and the other regions of the protein-coding mRNA sequences of their target mRNAs, thus causing mRNA degradation or inhibiting its translation.35 Numerous reports document that some miRNAs are involved in many key cellular processes, including development, differentiation, metabolism, apoptosis, and proliferation.36 Furthermore, studies also indicate that individual miRNAs can regulate the multiple hallmarks of cancer, such as cell invasion, metastasis, chemotherapeutic resistance and angiogenesis, as tumor suppressor genes or oncogenes.37
miR-372/520 family gene is located on the genomic chromosome at 19q13.42. Some reporters consider miR-372 to be an oncogenic factor that regulates cell growth and apoptosis by down-regulating TNFAIP1 and positively regulating NF-κB expression in gastric cancer.38 However, in hepatocellular carcinoma, miR-372 can suppress the expression of ATAD2 by directly targeting their regulation, which inhibits tumor cell growth and decreases their invasive and migratory capacity.39 A previous study identified that miR-373 and miR-520c can promote tumor invasion and metastasis by suppressing CD44 in human breast cancer MCF-7 cell line.40 On the contrary, recent studies have reported that miR-373 and miR-520c can function as a tumor suppressor to inhibit tumor progression, metastasis and inflammation in estrogen receptor negative breast cancer by targeting the NF-κB and TGF-β signaling pathways;8 moreover, miR-520a suppresses the proliferation and invasion of esophageal squamous cell carcinoma by targeting ErbB4, which suggest its role as a tumor suppressor.41 Other studies found that miR-520d can induce hepatoma cells to form normal liver tissues via a stemness-mediated process.42 However, little evidence has elucidated the connection between miR-372/520 family and breast cancer. Above all, the regulation of 372/520s in breast cancer depends on the expression of ER. Herein, we found that GLA improved the miR-520a expression in ER negative human breast cancer cells. Furthermore, we found that by targeting NF-κB/RelA, miR-520a mediated the repressive effects of GLA on the NF-κB/IL-6/STAT-3 signal pathway, the VEGF secretion, and the angiogenesis.
To avoid the chemical effects of GLA on HUVECs and highlight the role of VEGF in angiogenesis, in our study, we decided that human breast cancer cells pre-treated by GLA will be cultured in fresh media with 1% serum for 24 h, and the conditioned media would be collected. Finally, in human breast cancer cells pre-treated by GLA, up-regulated miR-520a remained and decreased the expression of NF-κB/RelA and p-NF-κB/RelA, and lowered the secretion of VEGF and IL-6.
These results further confirmed that GLA can inhibit angiogenesis by blocking the NF-κB/IL-6/STAT-3 signal pathway and reducing the secretion of VEGF, which will be implemented through miR-520a. The results can be seen in Fig. S2A–D.†
5 Conclusion
In conclusion, our present study indicates that GLA inhibits the formation of blood vessels in human breast cancer MDA-MB-231 and Hs-578T cells by blocking the NF-κB/IL-6/STAT-3 signal pathway, which in turn reduces the secretion of the pro-angiogenesis factor, VEGF. Indeed, GLA improved the expression of miR-520a, which directly targeted RelA and blocked the activation of NF-κB. The knockdown of miR-520a abolished the GLA-induced intervention of NF-κB/IL-6/STAT-3 signal pathway, the VEGF secretion, and the angiogenesis. Our study offers a novel mechanism in which GLA inhibits the angiogenesis in breast cells, which might help to provide new approaches for the treatment of breast cancer.6 Conflict of interest
The authors declare that there is no conflict of interest.Acknowledgements
This work was supported by National Natural Science Foundation of China (81171987), a Research Fund for the Doctoral Program of Higher Education of China (20133234110007), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.References
- J. Mandelblatt, N. van Ravesteyn, C. Schechter, Y. Chang, A. T. Huang, A. M. Near, H. de Koning and A. Jemal, Cancer, 2013, 119, 2541–2548 CrossRef PubMed.
- E. Salvati, P. Zizza, A. Rizzo, S. Iachettini, C. Cingolani, C. D'Angelo, M. Porru, A. Randazzo, B. Pagano, E. Novellino, M. E. Pisanu, A. Stoppacciaro, F. Spinella, A. Bagnato, E. Gilson, C. Leonetti and A. Biroccio, Nucleic Acids Res., 2014, 42, 2945–2957 CrossRef CAS PubMed.
- T. Donnem, K. Lonvik, K. Eklo, T. Berg, S. W. Sorbye, K. Al-Shibli, S. Al-Saad, S. Andersen, H. Stenvold, R. M. Bremnes and L. T. Busund, Cancer, 2011, 117, 3193–3200 CrossRef CAS PubMed.
- J. Ma, Y. Xue, W. Cui, Y. Li, Q. Zhao, W. Ye, J. Zheng, Y. Cheng, Y. Ma, S. Li, T. Han, L. Miao, L. Yao, J. Zhang and W. Liu, Cancer, 2012, 118, 4105–4116 CrossRef CAS PubMed.
- I. Kukreja, P. Kapoor, R. Deshmukh and V. Kulkarni, J. Oral Maxillofac. Pathol., 2013, 17, 367–373 CrossRef PubMed.
- S. Y. Nam, Y. S. Ko, J. Jung, J. Yoon, Y. H. Kim, Y. J. Choi, J. W. Park, M. S. Chang, W. H. Kim and B. L. Lee, Br. J. Cancer, 2011, 104, 166–174 CrossRef CAS PubMed.
- A. A. Wani, S. M. Jafarnejad, J. Zhou and G. Li, Oncogene, 2011, 30, 2778–2788 CrossRef CAS PubMed.
- I. Keklikoglou, C. Koerner, C. Schmidt, J. D. Zhang, D. Heckmann, A. Shavinskaya, H. Allgayer, B. Guckel, T. Fehm, A. Schneeweiss, O. Sahin, S. Wiemann and U. Tschulena, Oncogene, 2012, 31, 4150–4163 CrossRef CAS PubMed.
- K. K. Chakravarthi and R. Avadhani, J. Nat. Sci., Biol. Med., 2013, 4, 420–425 CrossRef PubMed.
- V. Tantishaiyakul, K. Suknuntha, S. Saithong and C. Pakawatchai, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o3501 CAS.
- H. S. Cheema, O. Prakash, A. Pal, F. Khan, D. U. Bawankule and M. P. Darokar, Parasitol. Int., 2014, 63, 349–358 CrossRef CAS PubMed.
- Y. L. Hsu, L. Y. Wu, M. F. Hou, E. M. Tsai, J. N. Lee, H. L. Liang, Y. J. Jong, C. H. Hung and P. L. Kuo, Mol. Nutr. Food Res., 2011, 55, 318–327 CAS.
- Y. M. Tsai, C. J. Yang, Y. L. Hsu, L. Y. Wu, Y. C. Tsai, J. Y. Hung, C. T. Lien, M. S. Huang and P. L. Kuo, Integr. Cancer Ther., 2011, 10, 341–349 CrossRef CAS PubMed.
- C. Lin, L. Song, H. Gong, A. Liu, X. Lin, J. Wu, M. Li and J. Li, Cancer Res., 2013, 73, 3638–3648 CrossRef CAS PubMed.
- J. Yang, S. Kantrow, J. Sai, O. E. Hawkins, M. Boothby, G. D. Ayers, E. D. Young, E. G. Demicco, A. J. Lazar and D. Lev, Cancer Res., 2012, 72, 4682–4695 CrossRef CAS PubMed.
- J. L. Su, K. P. Lai, C. A. Chen, C. Y. Yang, P. S. Chen, C. C. Chang, C. H. Chou, C. L. Hu, M. L. Kuo, C. Y. Hsieh and L. H. Wei, Cancer Res., 2005, 65, 4827–4835 CrossRef CAS PubMed.
- H. L. Huang, M. J. Hsieh, M. H. Chien, H. Y. Chen, S. F. Yang and P. C. Hsiao, PLoS One, 2014, 9, e98943 Search PubMed.
- M. Adianti, C. Aoki, M. Komoto, L. Deng, I. Shoji, T. S. Wahyuni, M. I. Lusida, Soetjipto, H. Fuchino, N. Kawahara and H. Hotta, Microbiol. Immunol., 2014, 58, 180–187 CrossRef CAS PubMed.
- D. Atrahimovich, J. Vaya and S. Khatib, Bioorg. Med. Chem., 2013, 21, 3348–3355 CrossRef CAS PubMed.
- S. Tamir, M. Eizenberg, D. Somjen, N. Stern, R. Shelach, A. Kaye and J. Vaya, Cancer Res., 2000, 60, 5704–5709 CAS.
- X.-Q. Yu, C. C. Xue, Z.-W. Zhou, C.-G. Li, Y.-M. Du, J. Liang and S.-F. Zhou, Life Sci., 2008, 82, 68–78 CrossRef CAS PubMed.
- T. Yokota, H. Nishio, Y. Kubota and M. Mizoguchi, Pigm. Cell Res., 1998, 11, 355–361 CrossRef CAS PubMed.
- W. Liu, L. P. Li, J. D. Zhang, Q. Li, H. Shen, S. M. Chen, L. J. He, L. Yan, G. T. Xu, M. M. An and Y. Y. Jiang, PLoS One, 2014, 9, e103442 Search PubMed.
- J. Ahn, H. Lee, J. Jang, S. Kim and T. Ha, Food Chem. Toxicol., 2013, 51, 439–445 CrossRef CAS PubMed.
- F. Jiang, J. Mu, X. Wang, X. Ye, L. Si, S. Ning, Z. Li and Y. Li, PLoS One, 2014, 9, e96698 Search PubMed.
- E. Y. Yi, K. S. Han and Y. J. Kim, J. Cancer Prev., 2014, 19, 247–252 CrossRef PubMed.
- Y. P. Li, F. G. Tian, P. C. Shi, L. Y. Guo, H. M. Wu, R. Q. Chen and J. M. Xue, Asian Pac. J. Cancer Prev., 2014, 15, 10151–10156 CrossRef.
- R. Strippoli, F. Carvello, R. Scianaro, L. De Pasquale, M. Vivarelli, S. Petrini, L. Bracci-Laudiero and F. De Benedetti, Arthritis Rheum., 2012, 64, 1680–1688 CrossRef CAS PubMed.
- K. Yokota, K. Sato, T. Miyazaki, H. Kitaura, H. Kayama, F. Miyoshi, Y. Araki, Y. Akiyama, K. Takeda and T. Mimura, Arthritis Rheumatol., 2014, 66, 121–129 CrossRef CAS PubMed.
- M. Rokavec, W. Wu and J. L. Luo, Mol. Cell, 2012, 45, 777–789 CrossRef CAS PubMed.
- M. Xiang, N. J. Birkbak, V. Vafaizadeh, S. R. Walker, J. E. Yeh, S. Liu, Y. Kroll, M. Boldin, K. Taganov, B. Groner, A. L. Richardson and D. A. Frank, Sci. Signaling, 2014, 7, ra11 CrossRef PubMed.
- G. Zhang, C. M. Tsang, W. Deng, Y. L. Yip, V. W. Lui, S. C. Wong, A. L. Cheung, P. M. Hau, M. Zeng, M. L. Lung, H. Chen, K. W. Lo, K. Takada and S. W. Tsao, PLoS One, 2013, 8, e62284 CAS.
- D. Liu, C. Liu, X. Wang, S. Ingvarsson and H. Chen, Cancer Epidemiol., 2014, 38, 85–92 CrossRef PubMed.
- U. M. Litzenburger, C. A. Opitz, F. Sahm, K. J. Rauschenbach, S. Trump, M. Winter, M. Ott, K. Ochs, C. Lutz, X. Liu, N. Anastasov, I. Lehmann, T. Hofer, A. von Deimling, W. Wick and M. Platten, Oncotarget, 2014, 5, 1038–1051 Search PubMed.
- H. Yu, L. Jiang, C. Sun, L. Li Guo, M. Lin, J. Huang and L. Zhu, Gene, 2014, 534, 60–65 CrossRef CAS PubMed.
- Z. Wang, J. Wang, Y. Yang, B. Hao, R. Wang, Y. Li and Q. Wu, J. Exp. Clin. Cancer Res., 2013, 32, 76 CrossRef PubMed.
- T. Yu, J. Li, M. Yan, L. Liu, H. Lin, F. Zhao, L. Sun, Y. Zhang, Y. Cui, F. Zhang, J. Li, X. He and M. Yao, Oncogene, 2015, 34, 413–423 CrossRef CAS PubMed.
- C. Zhou, X. Li, X. Zhang, X. Liu, Z. Tan, C. Yang and J. Zhang, Int. J. Oncol., 2013, 42, 635–642 CAS.
- G. Wu, H. Liu, H. He, Y. Wang, X. Lu, Y. Yu, S. Xia, X. Meng and Y. Liu, BMC Cancer, 2014, 14, 107 CrossRef PubMed.
- Q. Huang, K. Gumireddy, M. Schrier, C. le Sage, R. Nagel, S. Nair, D. A. Egan, A. Li, G. Huang, A. J. Klein-Szanto, P. A. Gimotty, D. Katsaros, G. Coukos, L. Zhang, E. Pure and R. Agami, Nat. Cell Biol., 2008, 10, 202–210 CrossRef CAS PubMed.
- W. Ye, Q. Yao, M. Zhang, Q. Wen and J. Wang, Nanfang Yike Daxue Xuebao, 2014, 34, 164–168 Search PubMed.
- S. Tsuno, X. Wang, K. Shomori, J. Hasegawa and N. Miura, Sci. Rep., 2014, 4, 3852 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra17062h |
| ‡ Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |





