Themed collection New reactivity in organic chemistry

New reactivity in organic chemistry: a themed collection
Andrei Yudin and Corinna Schindler introduce the Chemical Science themed collection on the topic of new reactivity in organic chemistry.

Chem. Sci., 2020,11, 12385-12385
https://doi.org/10.1039/D0SC90262D
Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals
Hydrogen atom transfer from metal hydrides to alkenes appears to underlie widely used catalytic methods – the mechanistic implications are fascinating.

Chem. Sci., 2020,11, 12401-12422
https://doi.org/10.1039/D0SC04112B
Synthetic half-reactions
This perspective on reactivity introduces Synthetic Half-Reactions (SHRs) as a way to analyze chemical transformations.

Chem. Sci., 2020,11, 12423-12427
https://doi.org/10.1039/D0SC03876H
Electro-organic synthesis – a 21st century technique
This perspective provides insight into recent electro-organic methods and general trends in this field, and opens up prospects for future viewpoints.
Chem. Sci., 2020,11, 12386-12400
https://doi.org/10.1039/D0SC01848A
Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation
Cubane has attracted attention due to its unique 3D structure. Herein, we report the programmable synthesis of multiply arylated cubanes. The developed reaction allows the late-stage and regioselective installation of aryl groups.

Chem. Sci., 2020,11, 7672-7675
https://doi.org/10.1039/D0SC01909G
N-Heterocyclic carbene based catalytic platform for Hauser–Kraus annulations
The venerable Hauser–Kraus annulation is an effective and convergent method for generating oxygenated polycyclic aromatic compounds. A catalytic Hauser–Kraus annulation has been developed based on N-heterocyclic carbene catalysis.
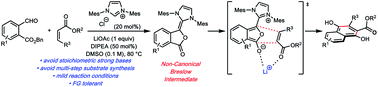
Chem. Sci., 2020,11, 7239-7243
https://doi.org/10.1039/D0SC03116J
Testing the limits of radical-anionic CH-amination: a 10-million-fold decrease in basicity opens a new path to hydroxyisoindolines via a mixed C–N/C–O-forming cascade
An intramolecular C(sp3)–H amidation proceeds in the presence of t-BuOK, molecular oxygen, and DMF.

Chem. Sci., 2020,11, 6539-6555
https://doi.org/10.1039/C9SC06511C
Diastereoselective olefin amidoacylation via photoredox PCET/nickel-dual catalysis: reaction scope and mechanistic insights
The selective 1,2-aminoacylation of olefins provides opportunities for the rapid construction of nitrogen-containing molecules.

Chem. Sci., 2020,11, 4131-4137
https://doi.org/10.1039/D0SC01459A
Effect of the enamine pyramidalization direction on the reactivity of secondary amine organocatalysts
Endo-pyramidalisation at nitrogen bestows enamines derived from α-substituted amines with higher reactivity compared to exo-pyramidalisation.
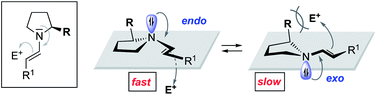
Chem. Sci., 2020,11, 1943-1947
https://doi.org/10.1039/C9SC05410C
Brønsted acid catalysis of photosensitized cycloadditions
Brønsted acids can catalyze triplet energy transfer reactions, and DFT computations suggest the unexpected importance of reorganization energy for catalysis.

Chem. Sci., 2020,11, 856-861
https://doi.org/10.1039/C9SC04822G
Sulfamides direct radical-mediated chlorination of aliphatic C–H bonds
Amine-anchored sulfamides direct radical-mediated chlorination of aliphatic C–H bonds. The site of C–H abstraction can be modulated by varying the sulfamide nitrogen substituents, a feature that has not been demonstrated with other substrate classes.

Chem. Sci., 2020,11, 217-223
https://doi.org/10.1039/C9SC03428E
Facile triflic acid-catalyzed α-1,2-cis-thio glycosylations: scope and application to the synthesis of S-linked oligosaccharides, glycolipids, sublancin glycopeptides, and TN/TF antigens
Studies of S-linked glycoconjugates have attracted growing interest because of their enhanced chemical stability and enzymatic resistance over O-glycoside counterparts.

Chem. Sci., 2019,10, 10475-10480
https://doi.org/10.1039/C9SC04079J
Saturated oxygen and nitrogen heterocycles via oxidative coupling of alkyltrifluoroborates with alkenols, alkenoic acids and protected alkenylamines
The oxidative coupling of alkyltrifluoroborates with heteroatom-tethered vinylarenes leads to a broad range of saturated oxygen and nitrogen heterocycles.

Chem. Sci., 2019,10, 9265-9269
https://doi.org/10.1039/C9SC02835H
Oxidant speciation and anionic ligand effects in the gold-catalyzed oxidative coupling of arenes and alkynes
The mechanism of the gold-catalyzed oxidative cross-coupling of arenes and alkynes has been studied in detail combining stoichiometric experiments with putative reaction intermediates and DFT calculations.

Chem. Sci., 2019,10, 8411-8420
https://doi.org/10.1039/C9SC02372K
Bio-inspired synthesis of xishacorenes A, B, and C, and a new congener from fuscol
In this manuscript, we describe the conversion of fuscol, a natural product isolated in 1978, to xishacorenes A, B, and C, isolated in 2017, as well as a previously unreported congener, which we have named xishacorene D.

Chem. Sci., 2019,10, 7788-7791
https://doi.org/10.1039/C9SC02572C
A regioselectivity switch in Pd-catalyzed hydroallylation of alkynes
Through rational evaluation of ligands and promoters, the reactivity of a key Pd(II) species towards transmetalation or β-H elimination is manipulated.

Chem. Sci., 2019,10, 6311-6315
https://doi.org/10.1039/C9SC01527B
Chiral phosphoric acid-catalyzed enantioselective construction of structurally diverse benzothiazolopyrimidines
Chiral phosphoric acid catalyzed the formal [4+2]-cycloaddition of 2-benzothiazolimines with enecarbamates to provide benzothiazolopyrimidines with up to 99% yield and >99% ee.
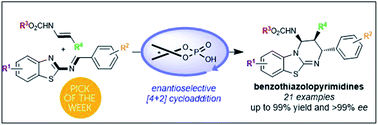
Chem. Sci., 2019,10, 3765-3769
https://doi.org/10.1039/C8SC05581E
Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride
Herein, we report the radiosynthesis of 18F-difluoromethylarenes via the assembly of three components, a boron reagent, ethyl bromofluoroacetate, and cyclotron-produced non-carrier added [18F]fluoride.
![Graphical abstract: Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride](/zh/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8SC05096A&imageInfo.ImageIdentifier.Year=2019)
Chem. Sci., 2019,10, 3237-3241
https://doi.org/10.1039/C8SC05096A
A modular approach to prepare enantioenriched cyclobutanes: synthesis of (+)-rumphellaone A
A modular synthesis of enantioenriched polyfunctionalized cyclobutanes was developed and applied to the synthesis of (+)-rumphellaone A.

Chem. Sci., 2019,10, 2315-2319
https://doi.org/10.1039/C8SC05444D
δ C–H (hetero)arylation via Cu-catalyzed radical relay
A radical relay strategy has been developed to enable selective δ C–H arylation. The approach employs a chiral copper catalyst, which serves the dual roles of generating an N-centered radical to promote intramolecular H-atom transfer, and then intercepting a distal C-centered radical for C–C bond formation with (hetero)aryl boronic acids.

Chem. Sci., 2019,10, 1207-1211
https://doi.org/10.1039/C8SC04366C
Direct introduction of nitrogen and oxygen functionality with spatial control using copper catalysis
Synthetic chemists have spent considerable effort optimizing the synthesis of nitrogen and oxygen containing compounds through a number of methods; however, direct introduction of N- and O-functionality remains challenging.
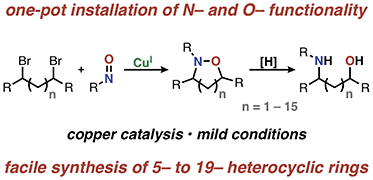
Chem. Sci., 2018,9, 8748-8752
https://doi.org/10.1039/C8SC03288B
Metal-free alkene oxy- and amino-perfluoroalkylations via carbocation formation by using perfluoro acid anhydrides: unique reactivity between styrenes and perfluoro diacyl peroxides
A practical metal-free perfluoroalkylation using acid anhydrides with unique reaction mode via carbocation has been developed.

Chem. Sci., 2018,9, 7115-7121
https://doi.org/10.1039/C8SC02547A
Catalyst-dependent selectivity in sulfonium ylide cycloisomerization reactions
An unusual example of π-acid catalyst-dependent selectivity in the cycloisomerization of alkene-tethered sulfonium ylides affords cyclopropanes or dihydrofurans with excellent control of chemoselectivity.
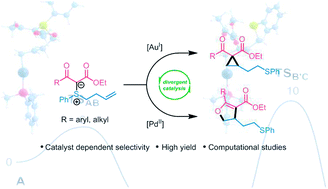
Chem. Sci., 2018,9, 7091-7095
https://doi.org/10.1039/C8SC02815J
Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks
By controlling the stability of dynamic covalent crosslinks with adjacent photoswitches, the stiffness of an adaptable hydrogel is tuned reversibly.

Chem. Sci., 2018,9, 5987-5993
https://doi.org/10.1039/C8SC02093K
Total synthesis of the reported structure of ceanothine D via a novel macrocyclization strategy
A novel regio- and stereocontrolled macrocyclization strategy was developed for the first synthesis of ceanothine D.

Chem. Sci., 2018,9, 2432-2436
https://doi.org/10.1039/C8SC00234G
Total chemical synthesis of glycocin F and analogues: S-glycosylation confers improved antimicrobial activity
Replacing the O-linked saccharide in the bacteriocin glycocin F with an S-linked version results in a peptidomimetic that increases the bacteriostatic effect.

Chem. Sci., 2018,9, 1686-1691
https://doi.org/10.1039/C7SC04383J
Ni-catalysed regioselective 1,2-diarylation of unactivated olefins by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles
Ni-catalysed diarylation of vinylpyridylsilanes with arylzinc reagents and aryl halides is reported by stabilizing Heck intermediates as pyridylsilyl-coordinated transient metallacycles.

Chem. Sci., 2018,9, 904-909
https://doi.org/10.1039/C7SC04351A
Mechanistic insights on the Pd-catalyzed addition of C–X bonds across alkynes – a combined experimental and computational study
A mechanistic study of the Pd-catalyzed intramolecular addition of carbamoyl chlorides and aryl halides across alkynes is presented.

Chem. Sci., 2017,8, 2914-2922
https://doi.org/10.1039/C6SC05001H
Palladium-catalyzed enantioselective Heck alkenylation of trisubstituted allylic alkenols: a redox-relay strategy to construct vicinal stereocenters
An enantioselective, redox-relay Heck alkenylation of trisubstituted allylic alkenol substrates has been developed.

Chem. Sci., 2017,8, 2277-2282
https://doi.org/10.1039/C6SC04585E
About this collection
Associate Editor Professor Andrei K. Yudin and Guest Editor Professor Corinna Schindler introduce this themed collection on the topic of new reactivity in organic chemistry. This collection is made up of a selection of previously published Edge content that has been carefully selected by Andrei and Corinna, to showcase what they feel represents new reactivity in organic chemistry around the topics of metal catalysis, difunctionalisation, strained ring systems, C-H functionalisation, oxidation, photochemistry, fluorination, organocatalysis, and biomolecule and natural product synthesis. This collection also features some specially curated review content on the topics of electro-organic synthesis, metal hydride hydrogen atom transfer, and synthetic half-reactions.