Open Access Article
Open Access ArticleRatiometric fluorescence imaging of Golgi H2O2 reveals a correlation between Golgi oxidative stress and hypertension†
Hui
Wang‡
,
Zixu
He‡
,
Yuyun
Yang
,
Jiao
Zhang
,
Wei
Zhang
,
Wen
Zhang
,
Ping
Li
* and
Bo
Tang
 *
*
College of Chemistry, Chemical Engineering and Materials Science, Institute of Biomedical Sciences, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan 250014, PR China. E-mail: tangb@sdnu.edu.cn; lip@sdnu.edu.cn
First published on 7th October 2019
Abstract
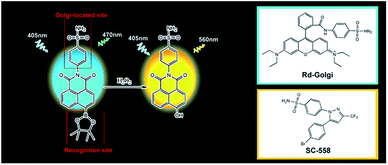
Golgi oxidative stress is significantly associated with the occurrence and progression of hypertension. Notably, the concentration of hydrogen peroxide (H2O2) is directly proportional to the degree of Golgi oxidative stress. Therefore, based on a novel Golgi-targeting phenylsulfonamide group, we developed a two-photon (TP) fluorescent probe, Np-Golgi, for in situ H2O2 ratiometric imaging in living systems. The phenylsulfonamide moiety effectively assists Np-Golgi in the precise location of Golgi apparatus. In addition, the raw material of phenylsulfonamide is easily available, and chemical modification is easily implemented. By application of Np-Golgi, we explored the generation of H2O2 during Golgi oxidative stress, and also successfully revealed increases on the levels of Golgi H2O2 in the kidneys of mice with hypertension. This work provides an ideal tool to monitor Golgi oxidative stress for the first time and novel drug targets for the future treatment of hypertension.
Introduction
Hypertension is a major risk factor for the development of cardiovascular disease (CVD).1 It is predicted that the prevalence of hypertension will increase by more than 50% during the next 30 years.2,3 Until now, despite receiving antihypertensive treatment, many people with the disorder still cannot adequately control their blood pressure. Thus, novel therapies are urgently needed to address resistant hypertension. A large amount of studies suggest that oxidative stress plays a central role in the pathogenesis of hypertension by perturbing the balance between reactive oxygen species (ROS) and antioxidant defenses.4–7 Excess ROS promote hypertension by inducing endothelial dysfunction.8,9 Therefore, complete understanding of the mechanisms of oxidative stress could contribute to the development of new therapies.Since the Golgi complex acts as a key trafficking and sorting station and a vital biosynthetic centre for glycoproteins and lipids,10 Golgi oxidative stress plays both physiological and pathophysiological roles in cells along with extensive ROS production.11–13 Therefore, quantitative detection of various ROS is essential to study the mechanism of Golgi oxidative stress, especially hydrogen peroxide (H2O2),14,15 an indicator of oxidative stress. However, the concentration and generation of H2O2 in the Golgi complex remain poorly understood to date, which is mainly due to a lack of tools for specific measurement of Golgi-located H2O2in situ. This ultimately causes difficulties in revealing the direct relevance between H2O2 levels and hypertension.
Two-photon (TP) fluorescence imaging is a noninvasive approach for in situ detection of various biomolecules.16–19 It exhibits increased tissue penetration depth, higher temporal resolution and less specimen photodamage than one-photon fluorescence imaging.20 To date, many TP fluorescent probes have been developed to visualize H2O2 in various organelles in cells,21–25 but in situ bioimaging of Golgi H2O2 is still scarce. Developing a TP fluorescent probe for tracing Golgi H2O2 in living systems could contribute to defining the relationship between hypertension and Golgi oxidative stress. However, the main obstacle for the development of a TP fluorescent probe for Golgi H2O2 detection is a lack of precise Golgi-targeting groups. To date, two kinds of Golgi-targeting groups have been reported, including Golgi-targeting polypeptides and cysteine.26,27 Golgi-targeting polypeptides exhibit significant localization ability but are hard to synthesize. Cysteine has limitations in the synthesis of fluorescent probes due to its low lipid solubility. Therefore, it is urgent to develop a novel Golgi-targeting group, which should meet the following requirements: (1) precise localization, (2) easily available raw material, and (3) facile chemical modification.
Previous reports have shown that the selectivity of SC-558, a Golgi protein cyclooxygenase-2 (COX-2) inhibitor, results from a phenylsulfonamide moiety that binds in specific pockets in complexes of COX-2.28,29 Based on this work, we supposed that phenylsulfonamide could be used as a Golgi-targeting group to deliver fluorescent probes into the Golgi apparatus. We herein report the first TP ratiometric fluorescent probe, NP-Golgi, (Scheme 1) with a phenylsulfonamide moiety as the Golgi-targeting group for H2O2 bioimaging in the Golgi apparatus. NP-Golgi comprises phenylsulfonamide-modified 1,8-naphthalimide as a fluorescent reporter and boric acid ester as a specific H2O2 recognition group.30,31 The boric acid ester converts to an electron-donating hydroxyl group upon reaction with H2O2, thus promoting the push–pull electron effect of the naphthalimide-conjugated system and subsequently resulting in strong fluorescence emission. In accordance with our prediction, Np-Golgi could effectively accumulate in the Golgi complex to sense H2O2 with high selectivity and sensitivity both in vivo and in vitro. The generation of Golgi H2O2 was also investigated for the first time with the assistance of Np-Golgi. With the aid of TP fluorescence microscopy, Np-Golgi was successfully applied to detect Golgi H2O2 fluctuations in mice with hypertension, suggesting Np-Golgi as a new instrument with which to explore the correlations between Golgi oxidative stress and diseases.
Results and discussion
Photophysical properties and selectivity of Np-Golgi
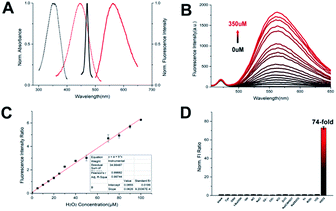
The details of the synthesis routes and characterization of Np-Golgi, and Rd-Golgi are shown in the ESI.†We first assessed the spectroscopic properties of Np-Golgi under physiological conditions. The absorption and fluorescence spectra of Np-Golgi were measured in the absence and presence of H2O2. As shown in Fig. 1A, along with a colour change, a red-shift of the absorption peak of Np-Golgi from 350 nm to 446 nm occurred after reaction with H2O2. Meanwhile, the fluorescence emission peak was red-shifted from 470 nm to 560 nm. This demonstrated that Np-Golgi exhibited obvious changes in spectroscopic properties upon the addition of H2O2. We subsequently confirmed the reaction mechanism between Np-Golgi and H2O2 with high-performance liquid chromatography (HPLC).32 The HPLC results showed that most Np-Golgi (HPLC retention time, TR = 50.8 min) was converted to Np-OH (TR = 25.7 min) after incubation with H2O2 (Fig. S1†). Meanwhile, the fluorescence quantum yield increased from 0.30 to 0.39, and the fluorescence of the mixture (Np-Golgi and H2O2) was stable for at least 60 min (Fig. S2†). Then, we calculated the TP absorption cross section of Np-Golgi, which was 72 GM upon treatment with H2O2 (Fig. S3†).33,34 All these results suggest that Np-Golgi potentially possesses the ability to detect H2O2 as a TP fluorescent probe.
We subsequently detected the fluorescence responses of Np-Golgi to various H2O2 levels. Upon addition of H2O2, the fluorescent intensity (FI) of Np-Golgi (2 μM) at 470 nm was constant, while the FI at 560 nm was significantly enhanced with higher concentrations of H2O2 (Fig. 1B). Notably, the F560/F470 ratio increased 130-fold in the presence of H2O2 (350 μM) compared with the absence of H2O2. There were two linear relationships between the F560/F470 ratio and the concentration of H2O2 (1–100 μM: R2 = 0.997; 100–350 μM: R2 = 0.982). The detection limit of Np-Golgi to H2O2 is 0.20 μM (Fig. 1C and S4†). These results demonstrate that Np-Golgi exhibits highly sensitive responses to H2O2 and can quantitatively detect H2O2 over a wide range.
To apply Np-Golgi to image H2O2 in cells and in vivo, we investigated the fluorescence responses of Np-Golgi to various biomolecules in cells. As shown in Fig. 1D, the FI ratio F560/F470 of Np-Golgi was prominently increased after incubation with H2O2, whereas F560/F470 was scarcely changed after incubation with other biomolecules. This result indicated that Np-Golgi exhibited remarkable selectivity for H2O2. We also monitored the influence of different pH values on F560/F470. The results showed that Np-Golgi exhibited obvious fluorescence responses to H2O2 at pH 6.0–8.0 (Fig. S5†). The data indicates that Np-Golgi could serve as a superior sensor for fluorescence imaging of H2O2 in living system.
Validation of Np-Golgi for imaging H2O2 in cells
To assess whether Np-Golgi could be utilized in biological systems, a standard MTT assay was performed in various cells to evaluate the cytotoxicity of Np-Golgi. The probe showed inconspicuous cytotoxicity at concentrations less than 20 μM (Fig. S6–S8†), which demonstrated that Np-Golgi could be used in living systems. We next applied Np-Golgi for endogenous H2O2 detection in human hepatoma cells (SMMC7721). Phorbol 12-myristate 13-acetate (PMA, 1 μg mL−1) was used to induce generation of H2O2 in cells. The fluorescence of Np-Golgi in the green channel (530–580 nm) increased significantly upon the addition of PMA, while the fluorescence in the blue channel (430–480 nm) was constant. The ratio of Fgreen/Fblue in PMA-treated cells showed 1.5-fold enhancement than in control cells. To confirm that the fluorescence enhancement was caused by increased H2O2 concentrations, we also evaluated the FI ratio Fgreen/Fblue of Np-Golgi in N-acetylcysteine35 (NAC: H2O2 eliminator) incubated cells. As shown in Fig. S9,† both NAC-stimulated cells and PMA + NAC-stimulated cells presented negligible fluorescence in the green channel with lower Fgreen/Fblue ratios than control cells. This phenomenon demonstrates that intracellular H2O2 has been effectively eliminated. These observations validate that Np-Golgi can be used for fluorescence imaging of H2O2 in cells with high selectivity and good sensitivity to quantify the concentrations of H2O2. Next, we explored the intracellular photostability of Np-Golgi (Fig. S10†). The fluorescence of Np-Golgi was consistent for 60 min after cells were rinsed thoroughly with PBS. The result implies that Np-Golgi can be applied for long-term imaging of H2O2in vivo.36Subcellular localization of Np-Glogi
To verify the Golgi complex-targeting ability of Np-Golgi, a colocalization experiment was performed to observe the subcellular distribution of the probe. As shown in Fig. 2, the green fluorescence of Np-Golgi overlapped well with the fluorescence of the commercial probe Golgi-Tracker Red (Pearson's colocalization coefficient: 0.94) in human cervical carcinoma cells (HeLa). In the meantime, the fluorescence of Np-Golgi showed poor overlap with that of Mito-Tracker Red (0.10), Lyso-Tracker Red (0.54) and ER-Tracker Red (0.44). To further confirm that Np-Golgi possessed universal Golgi-targeting capability, colocalization experiments were also performed in SMMC7721 and human hepatic cells (HL-7702). As expected, the Np-Golgi probe also showed excellent Golgi-targeting characteristics in these cells (Fig. S11 and S12†). Collectively, Np-Golgi exhibits superior Golgi complex-locating capability in various cell types.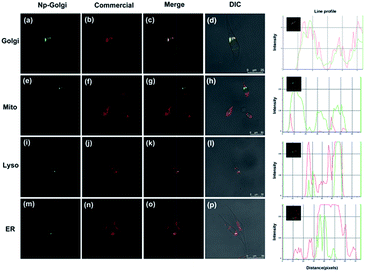 | ||
| Fig. 2 Co-localization cell imaging of Np-Golgi and commercial dyes including Golgi-Red (a–d), Mito-Red (e–h), Lyso-Red (i–l), ER-Red (m–p) in HeLa cells. | ||
Subsequently, to validate that the targetability of the Np-Golgi to the Golgi complex was dependent on the phenylsulfonamide moiety, we synthesized a new phenylsulfonamide-modified fluorescent probe Rd-Golgi (Fig. S13†). Then the Golgi-targeting ability of Rd-Golgi was investigated. Rd-Golgi and Golgi-Tracker Red presented remarkable fluorescence overlap in cells with the Pearson's colocalization coefficient 0.95 (Fig. S14†). This result suggests that Rd-Golgi possesses Golgi-targeting ability and phenylsulfonamide is the crucial part of the probe for effective targeting of the Golgi apparatus. These outcomes establish that phenylsulfonamide is a powerful and universal Golgi-targeting group. In addition, compared with the reported Golgi-targeting peptide and the chemical group cysteine, phenylsulfonamide exhibits the following benefits: (1) the raw material is easily available; (2) chemical modification is simple; and (3) long-term accumulation in the Golgi is possible.
The oxidative stress of Golgi apparatus
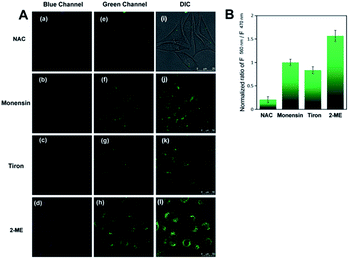
We next applied the H2O2-responsive, excellently Golgi-targeting TP fluorescent probe Np-Golgi to imaging of the fluctuations in intracellular H2O2 associated with Golgi oxidative stress. Monensin37,38 was used to induce Golgi oxidative stress by disturbing pH homeostasis to reduce the activity of Golgi proteins. Under NAC-pretreated conditions, monensin-stimulated cells exhibited brighter green fluorescence than NAC-incubated cells, and the F560/F470 ratio was enhanced by 4.9-fold (Fig. 3A). This result shows the level of Golgi H2O2 is obviously increased in the Golgi oxidative stress process.Many studies show that a large proportion of intracellular H2O2 is generated from superoxide anions (O2−) by catalysis reactions of various oxidases, such as superoxide dismutase (SOD). To seek evidence that Golgi H2O2 was produced from O2− in the Golgi oxidative stress, the fluorescence of Np-Golgi was detected after incubating cells with O2− inducer (2-methoxyestradiol,39 1.0 μg mL−1) or O2− scavenger (Tiron,40 10 μM). In order to avoid the interference from cytoplasmic H2O2 and induce Golgi oxidative stress of the cells, NAC was used to eliminate the intracellular H2O2 at first, and then monensin was added to cause higher level of H2O2. In this case, we investigated the relations between O2− and H2O2 by using 2-ME and Tiron. As illustrated in Fig. 3, stronger green fluorescence was observed in both 2-methoxyestradiol (2-ME) and monensin co-incubated cells, and the F560/F470 ratio was enhanced by 1.57-fold compared with monensin incubated cells. After the addition of Tiron to eliminate O2−, the FI of Np-Golgi in Tiron and monensin co-incubated cells decayed with a decreasing F560/F470 ratio (by 0.83-fold) compared with monensin incubated cells. The above results indicate that the concentration of Golgi H2O2 has positive correlation with O2− levels. Collectively, these data are in excellent agreement with our prediction that Golgi H2O2 is mainly generated from O2− in Golgi oxidative stress.
TP fluorescence imaging of HBP mice in situ
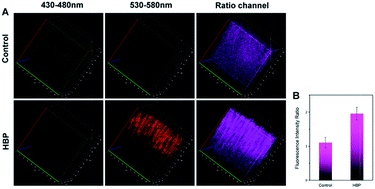
To prove that the probe could detect H2O2 levels in vivo, we applied Np-Golgi for TP fluorescence imaging of H2O2 in the kidneys of mice. The animal experiments were performed in compliance with the relevant laws and guidelines issued by the Ethical Committee of Shandong University and were in agreement with the guidelines of the Institutional Animal Care and Use Committee. The probe (10 μM, 100 μL) in the LPS-injected area of the abdominal cavity of the mice showed remarkable fluorescence, and the Fred/Fgreen ratio was increased by 1.9-fold in the probe-injected area compared with the normal saline-injected area (Fig. S15†). This suggests that Np-Golgi could quantitatively monitor fluctuations of H2O2in vivo through TP fluorescence imaging.Whether high blood pressure (HBP) is accompanied by Golgi oxidative stress was further investigated. Mice were injected with either 0.9% saline (control mice) or 50 μg ouabain per day (HBP mice) for 20 days, and the blood pressure of the mice was measured three times per day. Eventually, the blood pressure of HBP mice exceeded 160 mmHg (Fig. S16†), and the results of Masson's trichrome staining of the kidneys showed that there was more collagen deposition in HBP mice than in control mice (Fig. S17†), proving that the HBP mouse model was successfully constructed. After incubation of the kidneys of mice with Np-Golgi (10 μM, 100 μL) for 30 min, the Fred/Fgreen ratio of the probe was compared between HBP mice and control mice combined with two-photon imaging technology. As shown in Fig. 4, the Fred/Fgreen ratio in HBP mice was significantly increased (by 1.8-fold) compared with that in control mice, indicating increased H2O2 levels in the mice with HBP. These data demonstrate that pathological changes in kidney tissue in HBP mice cause severe Golgi oxidative stress and excess H2O2 accumulation in the process, which suggests that hypertension is closely related to Golgi oxidative stress.
Conclusion
To discover the role of Golgi oxidative stress in hypertension, we developed a novel TP ratiometric fluorescent sensor, Np-Golgi, to quantitatively detect Golgi H2O2 levels in situ. By application of Np-Golgi, we successfully detected increased levels of H2O2 in the kidneys of mice with hypertension. This work not only reveals the relationship between hypertension and Golgi oxidative stress, but also provides a robust tool for uncovering the connections between Golgi oxidative stress and severe diseases in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21535004, 91753111, 21405097, 21390411 and 21475079), Technological Special Project for “Significant New Drugs Development” (2017ZX09301030004), the Key Research and Development Program of Shandong Province (2018YFJH0502), Natural Science Foundation of Shandong Province (ZR2017ZC0225).Notes and references
- S. Mendis, Cardiovasc Diagn Ther., 2017, 7, S32–S38 CrossRef PubMed.
- P. M. Kearney, M. Whelton, K. Reynolds, P. Muntner, P. K. Whelton and J. He, Lancet, 2005, 365, 217–223 CrossRef.
- C. J. McAloon, L. M. Boylan, T. Hamborg, N. Stallard, F. Osman, P. B. Lim and S. A. Hayat, Int. J. Cardiol., 2016, 224, 256–264 CrossRef PubMed.
- T. Finkel and N. J. Holbrook, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- H. Y. Small, S. Migliarino, M. Czesnikiewicz-Guzik and T. J. Guzik, Free Radical Biol. Med., 2018, 125, 104–115 CrossRef CAS PubMed.
- M. Korsager Larsen and V. V. Matchkov, Medicina (Kaunas), 2016, 52, 19–27 CrossRef PubMed.
- W. Xu, Z. Zeng, J. H. Jiang, Y. T. Chang and L. Yuan, Angew. Chem., Int. Ed., 2016, 55, 13658–13699 CrossRef CAS PubMed.
- H. Matsuoka, S. Miyata, N. Okumura, T. Watanabe, K. Hashimoto, M. Nagahara, K. Kato, S. Sobue, K. Takeda, M. Ichihara, T. Iwamoto and A. Noda, Clin. Exp. Hypertens., 2019, 41, 307–311 CrossRef CAS PubMed.
- S. I. Dikalov and A. E. Dikalova, Antioxid Redox Signal, 2019 Search PubMed.
- F. Zappa, M. Failli and M. A. De Matteis, Curr. Opin. Cell Biol., 2018, 50, 102–116 CrossRef CAS PubMed.
- W. Zhong, Clin. Exp. Hypertens., 2011, 3, a005363 Search PubMed.
- P. Mayinger, Clin. Exp. Hypertens., 2011, 3, a005314 Search PubMed.
- S. W. Hicks and C. E. Machamer, Biochim. Biophys. Acta, 2005, 1744, 406–414 CrossRef CAS PubMed.
- J. B. Pi, W. Qu, J. M. Reece, Y. Kumagai and M. P. Waalkes, Exp. Cell Res., 2003, 290, 234–245 CrossRef CAS PubMed.
- Q. Li, M. M. Harraz, W. Zhou, L. N. Zhang, W. Ding, Y. Zhang, T. Eggleston, C. Yeaman, B. Banfi and J. F. Engelhardt, Mol. Cell. Biol., 2006, 26, 140–154 CrossRef CAS PubMed.
- G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518–3520 RSC.
- H. W. Liu, X. B. Zhang, J. Zhang, Q. Q. Wang, X. X. Hu, P. Wang and W. Tan, Anal. Chem., 2015, 87, 8896–8903 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
- W. Zhao, Y. Li, S. Yang, Y. Chen, J. Zheng, C. Liu, Z. Qing, J. Li and R. Yang, Anal. Chem., 2016, 88, 4833–4840 CrossRef CAS PubMed.
- E. J. Sanchez, L. Novotny, G. R. Holtom and X. S. Xie, J. Phys. Chem. A, 1997, 101, 7019–7023 CrossRef CAS.
- B. C. Dickinson, Y. Tang, Z. Chang and C. J. Chang, Chem. Biol., 2011, 18, 943–948 CrossRef CAS PubMed.
- C. Chung, D. Srikun, C. S. Lim, C. J. Chang and B. R. Cho, Chem. Commun., 2011, 47, 9618–9620 RSC.
- D. Kim, G. Kim, S. J. Nam, J. Yin and J. Yoon, Sci. Rep., 2015, 5, 8488 CrossRef CAS PubMed.
- J. Xu, Y. Zhang, H. Yu, X. Gao and S. Shao, Anal. Chem., 2016, 88, 1455–1461 CrossRef CAS PubMed.
- M. Ren, B. Deng, J. Y. Wang, X. Kong, Z. R. Liu, K. Zhou, L. He and W. Lin, Biosens. Bioelectron., 2016, 79, 237–243 CrossRef CAS PubMed.
- D. Srikun, A. E. Albers, C. I. Nam, A. T. Iavarone and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 4455–4465 CrossRef CAS PubMed.
- R. S. Li, P. F. Gao, H. Z. Zhang, L. L. Zheng, C. M. Li, J. Wang, Y. F. Li, F. Liu, N. Li and C. Z. Huang, Chem. Sci., 2017, 8, 6829–6835 RSC.
- R. G. Kurumbail, A. M. Stevens, J. K. Gierse, J. J. McDonald, R. A. Stegeman, J. Y. Pak, D. Gildehaus, J. M. Miyashiro, T. D. Penning, K. Seibert, P. C. Isakson and W. C. Stallings, Nature, 1996, 384, 644–648 CrossRef CAS PubMed.
- H. Zhang, J. Fan, J. Wang, S. Zhang, B. Dou and X. Peng, J. Am. Chem. Soc., 2013, 135, 11663–11669 CrossRef CAS PubMed.
- M. C. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392–15393 CrossRef CAS PubMed.
- A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed.
- J. Xu, Q. Yang, X. Qian, J. Samuelsson and J. C. Janson, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 847, 82–87 CrossRef CAS PubMed.
- Z. R. Dai, G. B. Ge, L. Feng, J. Ning, L. H. Hu, Q. Jin, D. D. Wang, X. Lv, T. Y. Dou, J. N. Cui and L. Yang, J. Am. Chem. Soc., 2015, 137, 14488–14495 CrossRef CAS PubMed.
- S. Xu, H. W. Liu, X. X. Hu, S. Y. Huan, J. Zhang, Y. C. Liu, L. Yuan, F. L. Qu, X. B. Zhang and W. Tan, Anal. Chem., 2017, 89, 7641–7648 CrossRef CAS PubMed.
- J. Xiao, J. Deng, L. Lv, Q. Kang, P. Ma, F. Yan, X. Song, B. Gao, Y. Zhang and J. Xu, Viruses, 2015, 7, 2816–2833 CrossRef CAS PubMed.
- H. Y. Li, X. H. Li, W. Shi, Y. H. Xu and H. M. Ma, Angew. Chem., Int. Ed., 2018, 57, 12830–12834 CrossRef CAS PubMed.
- C. E. Machamer, Front. Neurosci., 2015, 9, 421 Search PubMed.
- J. I. Sbodio, B. D. Paul, C. E. Machamer and S. H. Snyder, Cell Rep., 2013, 4, 890–897 CrossRef CAS PubMed.
- P. Huang, L. Feng, E. A. Oldham, M. J. Keating and W. Plunkett, Nature, 2000, 407, 390–395 CrossRef CAS PubMed.
- A. N. Ledenev, A. A. Konstantinov, E. Popova and E. K. Ruuge, Biochem. Int., 1986, 13, 391–396 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc04384e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |