2-in-1: catalyst and reaction medium
Rebecca A.
Haley
,
James
Mack
* and
Hairong
Guan
 *
*
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, Ohio 45221-0172, USA. E-mail: james.mack@uc.edu; hairong.guan@uc.edu
First published on 29th November 2016
Abstract
Mechanochemistry, more specifically high-speed ball milling, has garnered significant attention in several areas of chemistry, particularly for the synthesis of inorganic materials, cocrystals, organic compounds, discrete metal complexes and metal organic frameworks. This methodology is creating exciting research opportunities because, unlike traditional synthesis, a reaction carried out in a high-speed ball mill does not necessarily need a solvent, thus representing an environmentally friendly solution to the issue of solvent waste. This Chemistry Frontiers article delves into a unique area of ball milling that capitalizes on the solventless nature of the synthesis by using reaction vials, balls, foils, and pellets as both reaction medium and the catalyst. Several examples are highlighted, from nanoparticle synthesis and nanocatalysis to using transition metals in their metallic forms as catalysts. This article is aimed to show both the advantages and challenges present in the field, and to spark interest in further development of this research area.
The procedure to grind cinnabar to elemental mercury, a mechanochemical reaction, was used as early as in the fourth century BC, making mechanochemistry a longstanding method of chemical synthesis.1 As defined by IUPAC, a mechanochemical reaction is a “chemical reaction that is induced by the direct adsorption of mechanical energy.”2 In present day, this mechanical energy typically comes from grinding reactants in ball mills, but some of the earliest mechanochemical reactions (recorded as early as 1820) used a mortar and pestle.3 That reaction, performed by Faraday, involved the reduction of AgCl to Ag with Zn. By extending the reaction to other reducing agents, including Cu, Sn, and Fe, Faraday laid the groundwork for mechanochemistry to be largely developed in the realm of inorganic materials.4 In fact, mechanical alloying, or the combination of different elements to produce a homogeneous alloy, was the first type of application that brings mechanochemistry into the modern era of chemistry.5 With growing industrial interest in nanoparticles, mechanochemical research has evolved from traditional inorganic synthesis to a more versatile methodology that includes the synthesis of nanoparticles.6 For example, many chemical reactions that produce nanoparticles require high temperatures to retain the homogeneous phase of reactants or products. In mechanochemical milling, however, the mechanical energy allows for the synthesis of several different kinds of nanoparticles, all with comparable or superior features to those synthesized by other methods.7 For example, Moores and co-workers have illustrated these advantages through rapid and scalable mechanochemical synthesis of gold nanoparticles (AuNPs).8 In that study, instead of using often wasteful external reducing agents, the authors were able to use steel balls to reduce Au(III) to Au(0). In addition to showing that ultra-small nanoparticles with controllable size could be synthesized under mechanochemical conditions, it also highlights an example of incorporating milling media into the chemical reaction. Considering that nanocatalysis is a burgeoning field in catalysis, exploring the mechanochemical formation of and catalysis with nanoparticles would be an attractive, streamlined method of performing metal-catalyzed organic transformations.9 Hapiot and coworkers have recently demonstrated the possibility of combining nanoparticle synthesis with nanocatalysis. They achieved this by using gold nanoparticles that catalyzed sodium borohydride reduction of nitrobenzene using cyclodextrins as additives (Scheme 1).10
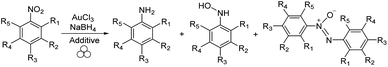 | ||
| Scheme 1 One-pot Au-nanoparticle synthesis and subsequent Au-nanoparticle catalyzed reduction of nitrobenzene derivatives in a high-speed ball mill (as suggested by Hanusa,11 we use the three circle sign here to denote ball milling). | ||
Given the strong historical foundation of mechanochemistry in metals, it is no wonder that ongoing work in this field has segued into organic synthesis through the use of transition-metal catalysis. In addition to the simplicity for synthesis, mechanochemistry has gained significant traction in the organic chemistry community due to the avoidance of bulk solvents used for the reaction. One of the 12 principles of green chemistry is to use safer solvents and auxiliaries and make them “unnecessary wherever possible,” thus making mechanochemistry an attractive strategy for designing green processes.12 Modern organic mechanosynthesis has grown rapidly, and examples include classic reactions such as Suzuki, Heck–Mizoroki and Sonogashira coupling, and olefin metathesis. An excellent comprehensive review of these reactions has recently been published by Hernández and Friščić.13 This Chemistry Frontiers article delves into an emerging mechanochemical method of metal-catalyzed reactions where the catalyst itself is the reaction medium. This type of catalysis takes advantage of the solventless system by using metal powders, balls, pellets, foils, or reaction vials to catalyze various organic transformations. This methodology essentially creates a bridge between nanocatalysis and mechanosynthesis, and it is simple and inexpensive, requiring no catalyst synthesis other than the processing of the elemental metal into the desired shape (ball, foil, vial, etc.).
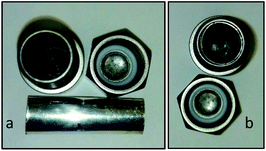
In 2000, Loeker and Leitner reported the use of a steel reactor to promote the oxidation of olefins in supercritical CO2.14 The exploration of using the reaction medium as a catalyst under solvent-free mechanochemical conditions started in the Mack group with the investigation of solventless Sonogashira reaction.15 After optimizing the reaction conditions, a study ensued of how a “copper-free” Sonogashira reaction would proceed. It was found that without having any form of copper, reaction yields were low. However, when a copper ball was added to the reaction mixture in lieu of an inert ball bearing (stainless steel, tungsten carbide, or Teflon), the reaction yields improved, but not enough to be synthetically useful for coupling a variety of aryl halides. In order to solve this problem, a custom-made screw-capped copper vial was fashioned, and the Sonogashira reaction was performed using the copper vial and a copper ball. To envision what these reaction media look like, an example of the copper vial and ball is shown in Fig. 1. When performing these reactions, the cap from the vial is removed, the ball is added to the vial along with any chemical reagents needed, and a Teflon O-ring is placed in the cap to prevent leakage. The assembly is then secured in a high-speed ball mill, where under vigorous shaking, the chemicals are allowed to react as long as needed. The results showed that with the combination of copper media, yields greatly increased and were comparable to the reactions that used copper iodide as a co-catalyst (Table 1).
| X | R | Percent yielda with CuI | Percent yielda without copper | Percent yielda with Cu ball | Percent yielda with Cu ball and Cu vial |
|---|---|---|---|---|---|
| a Reported as isolated yields. b 1,2-Disubstituted. | |||||
| I | H | 95 | 39 | 87 | 88 |
| I | Me | 84 | 17 | 46 | 83 |
| I | Br | 88 | 43 | 31 | 89 |
| I | Cl | 87 | 37 | 52 | 86 |
| I | OMe | 84 | 58 | 40 | 42 |
| Br | CHO | 87 | 44 | 85 | 84 |
| Br | CHOb | 89 | 46 | 88 | 90 |
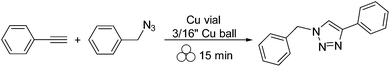
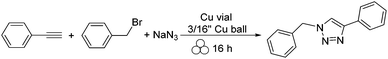
To continue this thought of using copper vials to perform copper mediated reactions, a copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction was studied.16 The success of the reaction was evident as the copper vial and copper ball could catalyze the coupling of a terminal alkyne with an organic azide to give the desired triazole in as little as fifteen minutes (Scheme 2). Furthermore, it was shown that the organic azide could be generated in situ using sodium azide and an alkyl bromide (Scheme 3). Considering no alarming exothermicity was observed during the reaction, this methodology might be safer than the traditional solution based synthesis, which is usually carried out in a glass vessel.
Added benefits of using copper as the reaction medium include high recyclability of copper sources and low copper contamination to the products. It was recorded that the copper vial and copper balls were used more than 100 times over the course of this study, thus making this methodology not only efficient but also economically attractive. Additionally, according to ICP-MS analysis of the products, no significant leaching of copper into the products was observed. In a separate study, Friščić et al. examined a variety of copper sources as catalysts for the mechanosynthesis of sulfonyl thioureas.17 In addition to typical copper halides, copper powder was shown to catalyze the coupling of p-toluenesulfonamide with n-butyl isocyanate, providing the pharmaceutically relevant compound tolbutamide in 88% yield.17 Brass, an alloy of copper and zinc, was also used in the optimization and yielded the coupling product in 87% yield.
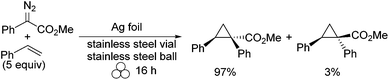
Copper and brass are fairly robust in their solid metallic state, and therefore vials and balls made from these materials do not break or bend easily under the mechanochemical conditions. In contrast, metals such as palladium and silver are very soft and too costly to be made into vials and balls. Yet, these metals are known as excellent catalysts for many reactions. The soft nature of the metals should not be a deterrent, but rather be taken advantage of especially when it is in the foil form. Silver has been shown to bend readily into a cylindrical shape that fits securely in a stainless steel vial. The reaction illustrating the use of silver foil as a catalyst is the intermolecular [2 + 1] cyclopropanation of alkenes with diazoacetates.18 This reaction often proceeds through precious metal-based catalysts, which can be cost prohibitive. In lieu of trying to reduce cost by immobilizing these catalysts on a solid support for recyclability, the ball-milling methodology uses a recyclable silver foil that can be applied economically. The reaction showed high diastereoselectivity with an average E/Z ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Scheme 4). Recyclability for the silver foil was also shown to be high, as it was used five consecutive times with no significant decrease in yield or selectivity. An example of the set-up is illustrated in Fig. 2.
3 (Scheme 4). Recyclability for the silver foil was also shown to be high, as it was used five consecutive times with no significant decrease in yield or selectivity. An example of the set-up is illustrated in Fig. 2.
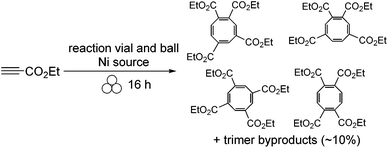
The most recent endeavour in using vials, balls, pellets, or foil as both a catalyst and reaction medium involves the nickel metal. Here, the goal is to not only have a recyclable catalyst, but also perform a reaction that is normally catalyzed in solution by expensive and air-sensitive metal complexes such as Ni(COD)2 and Ni(PPh3)4.19 To test if nickel metal acts as a viable replacement to the homogeneous nickel catalysts, cycloaddition of terminal alkynes was studied (Scheme 5).20 Interestingly, substituted cyclooctatetraenes were isolated as the major products, in strong contrast to solution chemistry in which the major cycloaddition products are substituted benzenes. This work examined all forms of nickel (vial, ball, foil, and pellet) and ultimately found that filling a stainless steel vial with nickel pellets was the most effective form of catalyst and reaction medium. The pellets (as shown in Fig. 3) give a frictional energy while at the same time revealing a fresh nickel surface to perform the reaction.
In summary, we have highlighted recent advances in a unique area of mechanochemistry, where the reaction medium (such as vial, ball, foil, and pellet) in its metallic form is also capable of catalyzing a reaction. While the examples described here show great promise for the future of catalysis in mechanochemistry, there are challenges. For instance, while using a copper vial does not seem to create much loose copper after the reaction, other metals potentially can generate particles to be filtered off as waste. Nickel has presented this drawback, with the mass of the nickel vial weighing slightly less after performing each reaction, indicating that some of the metal was lost during the workup. For optimal recyclability, all materials used for this methodology should not lose any mass. Additionally, studying reactions under ball milling is generally challenging because of the lack of analytical tools to monitor the reaction in real-time. Most mechanistic information has been derived from control experiments and product distribution after the reaction has been stopped. As an example, when using a stainless steel vial, the results will be compared to those from a control reaction using a Teflon vial to determine whether or not the reaction was catalyzed by iron or trace amounts of chromium present in a stainless steel vial. Despite the obstacles, Friščić and coworkers have recently developed a method of using powder X-ray diffraction to monitor the mechanochemical syntheses of metal–organic frameworks in real-time.21
Looking forward, we believe that the opportunities in mechanochemical synthesis are endless as the physical methods used to study reactions under high-speed ball milling conditions continue to evolve. For the specific type of mechanochemistry mentioned in this paper, a novel and very useful advancement would be to find a method to examine the metal surface as the catalysis is occurring. This would enable researchers to gain more information about the reaction mechanisms, thus giving further insight into how to design and improve mechanochemical catalytic reactions. Overall, this methodology is in alignment with the green chemistry movement while at the same time providing novel products and simple procedures. Thus there is no doubt that the momentum to bridge catalysis and mechanochemistry will be strong.
Acknowledgements
We are grateful for financial support from the National Science Foundation (CHE-1465110 for J. M. and CHE-1464734 for H. G.). Additionally, we would like to thank P. E. O. (Philanthropic Educational Organization) International and Chapter R of West Virginia for their continued support for women in science and higher education.Notes and references
- K. D. M. Harris, Nat. Chem., 2013, 5, 12–14 CrossRef CAS PubMed.
- A. D. McNaught and A. Wilkinson, IUPAC Compendium of Chemical Terminology, Blackwell Scientific Publications, Oxford, 2nd edn, 1997 Search PubMed.
- L. Takacs, J. Therm. Anal. Calorim., 2007, 90, 81–84 CrossRef CAS.
- W. Jones and M. D. Eddleston, Faraday Discuss., 2014, 170, 9–34 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- G. Kaupp, CrystEngComm, 2009, 11, 388–403 RSC.
- T. Tsuzuki and P. G. McCormick, J. Mater. Sci., 2004, 39, 5143–5146 CrossRef CAS.
- M. J. Rak, N. K. Saadé, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- N. Yan, C. Xiao and Y. Kou, Coord. Chem. Rev., 2010, 254, 1179–1218 CrossRef CAS.
- S. Menuel, B. Léger, A. Addad, E. Monflier and F. Hapiot, Green Chem., 2016, 18, 5500–5509 RSC.
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 2000 Search PubMed.
- J. G. Hernández and T. Friščić, Tetrahedron Lett., 2015, 56, 4253–4265 CrossRef.
- F. Loeker and W. Leitner, Chem. – Eur. J., 2000, 6, 2011–2015 CrossRef CAS.
- D. A. Fulmer, W. C. Shearouse, S. T. Medonza and J. Mack, Green Chem., 2009, 11, 1821–1825 RSC.
- T. L. Cook, J. A. Walker Jr. and J. Mack, Green Chem., 2013, 15, 617–619 RSC.
- D. Tan, V. Štrukil, C. Mottillo and T. Friščić, Chem. Commun., 2014, 50, 5248–5250 RSC.
- L. Chen, M. O. Bovee, B. E. Lemma, K. S. M. Keithley, S. L. Pilson, M. G. Coleman and J. Mack, Angew. Chem., Int. Ed., 2015, 54, 11084–11087 CrossRef CAS PubMed.
- S. K. Rodrigo, I. V. Powell, M. G. Coleman, J. A. Krause and H. Guan, Org. Biomol. Chem., 2013, 11, 7653–7657 CAS.
- R. A. Haley, A. R. Zellner, J. A. Krause, H. Guan and J. Mack, ACS Sustainable Chem. Eng., 2016, 4, 2464–2469 CrossRef CAS.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed.
| This journal is © the Partner Organisations 2017 |