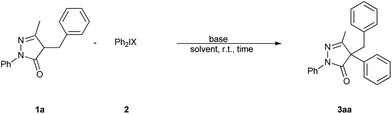
Base promoted direct C4-arylation of 4-substituted-pyrazolin-5-ones with diaryliodonium salts†
Song Maoa,
Xu Genga,
Yang Yanga,
Xiaofei Qiana,
Shengying Wua,
Jianwei Hanb and
Limin Wang*a
aKey Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: wanglimin@ecust.edu.cn; Fax: +86-21-64253881; Tel: +86-21-64253881
bShanghai-Hong Kong Joint Laboratory in Chemical Synthesis, Shanghai Institute of Organic Chemistry, The Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai 200032, P. R. China. E-mail: jianweihan@sioc.ac.cn; Fax: +86-21-54925383
First published on 17th April 2015
Abstract
A metal-free approach for the C4-arylation of 4-substituted-pyrazolin-5-ones with diaryliodonium salts was developed. The reaction proceeded smoothly at room temperature in the presence of DMAP (4-dimethylaminopyridine). As a result, a wide range of desired multi-substituted pyrazolin-5-one derivatives were obtained in good to excellent yields (20–98%).
Hypervalent iodine chemistry is currently a field of intense development. Thanks to a variety of possible derivatives, diaryliodonium salts have been used widely in organic synthesis because of their low toxicity, stability, high reactivity, and availability.1 Recently, metal-free arylation has become a rapidly growing area of extensive research for avoiding the drawbacks of organometallic chemistry, such as high economic cost, toxicity, and major problems regarding purification.2 Amongst these methods, the arylation of nucleophiles by using diaryliodonium salts without transition-metal catalysts was achieved via an oxidative activation strategy.1,3–12 The successful protocols included the α-arylation of enolates and azlactones,3 C-arylation of electron-rich aromatic compound,4 N-heteroarenes,5 naphthalene,6 and quinones;7 O-arylation of phenol, alcohols, carboxylic acids and oximes;8 N-arylation of anilines,9a aqueous ammonia9b and hydroxylamines;9c S-arylation of arylsulfonic acid salts;10 and even the dearomatizating arylation of phenols11 and indole derivatives.12 Despite the significant progress that has been made in this area, new and efficient methods for metal-free arylation of various nucleophiles with diaryliodonium salts are still in great demand.
On the other hand, the pyrazolone-based skeleton is found in a variety of biologically important structural components and pharmaceuticals.13 Compounds containing this moiety frequently exhibit various applications as pharmaceutical agents,14a–e synthetic scaffolds in combinatorial and medicinal chemistry,14f,g chelating agents in coordination chemistry,14h,i and analytical reagents in dye industry.14j,k For example, edaravone is used in inhibiting protease-resistant prion protein accumulation.15 Consequently, inspired by the broad utility of pyrazolones, it is not surprising that great efforts have been directed toward developing synthetic approaches for the construction of the pyrazolone cores.16 Very recently, the development of asymmetric methodologies to access pyrazolone derivatives with a quaternary stereogenic center at C4 position has attracted considerable attention.17 To our knowledge, the direct arylation to the C4 position of the pyrazolone ring remains unexplored. Herein, we present our results on the first efficient C4-arylation of 4-substituted-pyrazolin-5-ones by using diaryliodonium salts under metal-free conditions at room temperature.
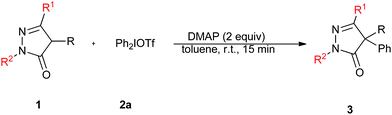
At the outset of the study, 4-benzyl-pyrazol-5-one (1a) and diphenyliodonium triflate (2a) were chosen as model substrates to establish the best reaction conditions (Table 1). A low conversion of 1a was obtained when either sodium carbonate or sodium hydroxide were used as the base (Table 1, entries 1 and 2). To our delight, with the treatment of 1a with 2 equiv. 2a in the presence of K3PO4 in toluene at room temperature for 6 h gave 93% yield of desired product (3aa, Table 1, entry 3). And other stronger bases were also efficient (Table 1, entries 4–6). Subsequently, a range of organic bases including NEt3, DBU, DMAP were screened, and DMAP was found to be the most suitable base, which gave the product with excellent yield after 15 min (94%, Table 1, entries 7–9). Next, the screening of solvents revealed that the conversion to 3aa was more efficient in toluene than in DCE, 1,4-dioxane, CH3CN, or DMF (Table 1, entries 10–13). Decreasing the amount of the base had a negative impact on yield (Table 1, entries 14 and 15). Furthermore, under the optimized conditions with the established model reaction of 4-benzyl-pyrazol-5-one and diphenyliodonium triflate, the influence of the counteranions of the diphenyliodonium salts was studied. Among all the tested reagents, diphenyliodonium triflate and p-toluenesulfonate furnished the desired product in excellent yields (Table 1, entries 9 and 17), whereas diphenyliodonium tetrafluoroborate gave a slightly lower yield and diphenyliodonium bromide only gave a trace amount of the desired product (Table 1, entries 16 and 18).
| Entry | X | Base | Solvent | Time (h) | Yieldb [%] |
|---|---|---|---|---|---|
| a Reaction conditions: 4-benzyl-pyrazol-5-one (0.2 mmol, 1 equiv.), diaryliodonium salts (0.4 mmol, 2 equiv.), base (0.4 mmol, 2 equiv.), solvent (3 mL).b Yield of isolated product.c DMAP (1.2 equiv.).d DMAP (0.3 equiv.). | |||||
| 1 | OTf | Na2CO3 | Toluene | 6 | Trace |
| 2 | OTf | NaOH | Toluene | 6 | 49 |
| 3 | OTf | K3PO4 | Toluene | 6 | 93 |
| 4 | OTf | Cs2CO3 | Toluene | 6 | 89 |
| 5 | OTf | tBuOK | Toluene | 6 | 81 |
| 6 | OTf | NaH | Toluene | 6 | 81 |
| 7 | OTf | NEt3 | Toluene | 0.25 | 93 |
| 8 | OTf | DBU | Toluene | 0.25 | 88 |
| 9 | OTf | DMAP | Toluene | 0.25 | 94 |
| 10 | OTf | DMAP | DCE | 1 | 81 |
| 11 | OTf | DMAP | 1,4-Dioxane | 1 | 68 |
| 12 | OTf | DMAP | CH3CN | 1 | 78 |
| 13 | OTf | DMAP | DMF | 1 | 78 |
| 14 | OTf | DMAPc | Toluene | 1 | 84 |
| 15 | OTf | DMAPd | Toluene | 12 | 29 |
| 16 | Br | DMAP | Toluene | 0.25 | Trace |
| 17 | OTs | DMAP | Toluene | 0.25 | 93 |
| 18 | BF4 | DMAP | Toluene | 0.25 | 85 |
With the optimized conditions in hand, the reaction scope of the C4-arylation of 4-benzyl-pyrazol-5-one by using a variety of symmetrical and unsymmetrical diaryliodonium salts was explored. As shown in Table 2, a number of substituted symmetrical diaryliodonium triflates such as di-(4-fluorophenyl)-iodonium triflate, di-(4-chlorophenyl)-iodonium triflate, di-(4-bromophenyl)-iodonium triflate, di-(4-tertbutylphenyl)-iodonium triflate, di-(4-methylphenyl)-iodonium triflate, di-(4-methoxyphenyl)-iodonium triflate and di-(3-methylphenyl)-iodonium triflate reacted efficiently with 4-benzyl-pyrazol-5-one (1a) to afford the corresponding arylation products with good to excellent yields (Table 2, entries 2–8). However, steric factors severely affected the reactivity, di-(2-methylphenyl)-iodonium triflate only gave desired product in 20% yield (Table 2, entry 9). Furthermore, almost no reaction took place with bis-(2,4,6-trimethyldiphenyl)iodonium triflate (2j) as the arylating reagent (Table 2, entry 10). Additionally, the use of unsymmetrical diaryliodonium salts was sequently investigated. 2k, 2l, and 2m preferentially transferred the aryl group with smaller steric hindrance to the 4-benzyl-pyrazol-5-one moiety in good yields (Table 2, entries 11–13), whereas 2n gave the desired product in moderate yield (Table 2, entry 14). In connection with the influence of the electronic properties on the reactivity, 4-methoxy-4′-nitrodiphenyliodonium salt (2o) was allowed to react with 1a under the standard conditions, selective transfer of p-nitrophenyl group to the desired product in excellent yield was observed (Table 2, entry 15), which is in accordance with the results of our previous report on metal-free arylation of hydroxylamines and oximes.9c
| Entry | 2 | Ar1 | Ar2 | Time (min) | 2 | Yieldb [%] |
|---|---|---|---|---|---|---|
| a Reaction conditions: pyrazol-5-ones (0.2 mmol, 1 equiv.), diphenyliodonium salts (0.4 mmol, 2 equiv.), DMAP (0.4 mmol, 2 equiv.), toluene (3 mL).b Yield of isolated product.c OTs salt 2o used. | ||||||
| 1 | 2a | Ph | Ph | 15 | 3aa | 94 |
| 2 | 2b | 4-FC6H4 | 4-FC6H4 | 15 | 3ab | 93 |
| 3 | 2c | 4-ClC6H4 | 4-ClC6H4 | 60 | 3ac | 85 |
| 4 | 2d | 4-BrC6H4 | 4-BrC6H4 | 15 | 3ad | 95 |
| 5 | 2e | 4-tBuC6H4 | 4-tBuC6H4 | 15 | 3ae | 93 |
| 6 | 2f | 4-MeC6H4 | 4-MeC6H4 | 120 | 3af | 95 |
| 7 | 2g | 4-MeOC6H4 | 4-MeOC6H4 | 60 | 3ag | 82 |
| 8 | 2h | 3-MeC6H4 | 3-MeC6H4 | 60 | 3ah | 92 |
| 9 | 2i | 2-MeC6H4 | 2-MeC6H4 | 120 | 3ai | 20 |
| 10 | 2j | Mesityl | Mesityl | 120 | 3aj | Trace |
| 11 | 2k | Mesityl | Ph | 30 | 3aa | 81 |
| 12 | 2l | Mesityl | 4-MeC6H4 | 60 | 3af | 86 |
| 13 | 2m | Mesityl | 4-CO2Et | 15 | 3ak | 79 |
| 14 | 2n | Mesityl | 4-NO2C6H4 | 120 | 3al | 44 |
| 15 | 2oc | 4-MeOC6H4 | 4-NO2C6H4 | 60 | 3al | 92 |
To further probe the scope of this reaction, a wide variety of pyrazolone derivatives bearing a benzyl group at the C4 position were investigated. Pyrazol-5-ones with fluoro-, chloro-, and bromo-groups on the aromatic ring of the R group reacted smoothly with 2a and formed the corresponding products in excellent yields (Table 3, entries 2–4). 4-Substituted pyrazol-5-ones containing electron-withdrawing substituents (4-CF3, 4-NO2) also gave the products in excellent yields (Table 3, entries 5 and 6). And the electron-donating methyl-group afforded the desired product in good yield (Table 3, entry 7). Especially, for the substrate containing an H at R in the pyrazolin-5-one ring system, diarylated product (3ha) was obtained in 72% yield (Table 3, entry 8). It is worth pointing out that the C4-arylation of 4-substituted pyrazol-5-ones successfully occurred when the R substituent was a linear group (Table 3, entries 9–11). The corresponding products 3ia–3ka were generated in good yields. In addition, substrate 1l with Phenyl group at R1 in the pyrazolin-5-one ring system reacted well, and the desired product was obtained in 83% yield (Table 3, entry 12), Furthermore, substrates, bearing a p-tolyl group or 4-(trifluoromethyl)phenyl group, also reacted smoothly with 2a to furnish the corresponding products in good yields (Table 3, entries 13 and 14).
| Entry | 1 | R | R1 | R2 | 3 | Yieldb [%] |
|---|---|---|---|---|---|---|
| a Reaction conditions: pyrazol-5-ones (0.2 mmol, 1 equiv.), diphenyliodonium salts (0.4 mmol, 2 equiv.), DMAP (0.4 mmol, 2 equiv.), toluene (3 mL).b Yield of isolated product. | ||||||
| 1 | 1a | Benzyl | Me | Ph | 3aa | 94 |
| 2 | 1b | 4-F-benzyl | Me | Ph | 3ba | 98 |
| 3 | 1c | 4-Cl-benzyl | Me | Ph | 3ca | 91 |
| 4 | 1d | 4-Br-benzyl | Me | Ph | 3da | 95 |
| 5 | 1e | 4-CF3-benzyl | Me | Ph | 3ea | 94 |
| 6 | 1f | 4-NO2-benzyl | Me | Ph | 3fa | 92 |
| 7 | 1g | 4-Me-benzyl | Me | Ph | 3ga | 88 |
| 8 | 1h | H | Me | Ph | 3ha | 72 |
| 9 | 1i | Me | Me | Ph | 3ia | 91 |
| 10 | 1j | Et | Me | Ph | 3ja | 90 |
| 11 | 1k | Allyl | Me | Ph | 3ka | 82 |
| 12 | 1l | Benzyl | Ph | Ph | 3la | 90 |
| 13 | 1m | Benzyl | Me | 4-MeC6H4 | 3ma | 83 |
| 14 | 1n | Benzyl | Me | 4-CF3 C6H4 | 3na | 95 |
Conclusions
In summary, we have disclosed the first C4-arylation of 4-substituted-pyrazolin-5-ones with diaryliodonium salts mediated by DMAP. The procedure is capable of tolerating a relatively wide range of substrates, and excellent results (up to 98% yield) can be obtained. Furthermore, this experimentally simple process, starting from readily available starting materials, facilitates the access to multiply substituted pyrazolin-5-ones derivatives, which are potential biologically active molecules.Experimental section
A mixture of 4-substituted-pyrazolin-5-ones 1 (0.2 mmol, 1 equiv.), diaryliodonium salt 2 (0.4 mmol, 2 equiv.), DMAP (0.4 mmol, 2 equiv.), and toluene (3 mL) was taken in a 10 mL reaction tube at room temperature for 15–120 min under vigorous stirring. After completion of the reaction, as indicated by TLC, the reaction mixture was concentrated under reduced pressure, and the crude compound was purified by chromatography on a silica gel column (ethyl acetate/petroleum ether (1/10)) to afford the desired product 3.Acknowledgements
The work was supported by the National Nature Science Foundation of China (NSFC, 21272069, 21202186) and the Fundamental Research Funds for the Central Universities, Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.Notes and references
- (a) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (c) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS PubMed; (d) L. F. Silva and B. Olofsson, Nat. Prod. Rep., 2011, 28, 1722 RSC; (e) M. S. Yusubov and V. V. Zhdankin, Curr. Org. Synth., 2012, 9, 247 CrossRef CAS.
- (a) S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673 CrossRef CAS PubMed; (b) W. Liu, H. Cao, H. Zhang, H. Zhang, K. H. Chung, C. He, H. Wang, F. Y. Kwong and A. Lei, J. Am. Chem. Soc., 2010, 132, 16737 CrossRef CAS PubMed; (c) E. Shirakawa, K. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed; (d) C. L. Sun, H. Li, D. G. Yu, M. Yu, X. Zhou, X. Y. Lu, K. Huang, S. F. Zheng, B. J. Li and Z. J. Shi, Nat. Chem., 2010, 2, 1044 CrossRef CAS PubMed.
- (a) P. Q. Norrby, T. B. Petersen, M. Bielawski and B. Olofsson, Chem.–Eur. J., 2010, 16, 8251 CrossRef CAS PubMed; (b) Z. Chai, B. Wang, J. N. Chen and G. S. Yang, Adv. Synth. Catal., 2014, 356, 2714 CrossRef CAS PubMed.
- (a) Y. Kita, K. Morimoto, M. Ito, C. Ogawa, A. Goto and T. Dohi, J. Am. Chem. Soc., 2009, 131, 1668 CrossRef CAS PubMed; (b) T. Dohi, M. Ito, N. Yanaoka, K. Morimoto, H. Fujioka and Y. Kita, Angew. Chem. Int. Ed., 2010, 49, 3334 (Angew. Chem., 2010, 122, 3406) CrossRef CAS PubMed; (c) N. Yamaoka, K. Sumida, I. Itani, H. Kubo, Y. Ohnishi, S. Sekiguchi, T. Dohi and Y. Kita, Chem.–Eur. J., 2013, 19, 15004 CrossRef CAS PubMed.
- (a) L. Ackermann, M. Dell'Acqua, S. Fenner, R. Vicente and R. Sandmann, Org. Lett., 2011, 13, 2358 CrossRef CAS PubMed; (b) J. Wen, R. Y. Zhang, S. Y. Chen, J. Zhang and X. Q. Yu, J. Org. Chem., 2012, 77, 766 CrossRef CAS PubMed.
- S. Castro, J. J. Fernández, R. Vicente, F. J. Fañanás and F. Rodríguez, Chem. Commun., 2012, 48, 9089 RSC.
- D. W. Wang, B. Y. Ge, L. Li, J. Shan and Y. Q. Ding, J. Org. Chem., 2014, 79, 8607 CrossRef CAS PubMed.
- (a) N. Jalalian, E. E. Ishikawa, L. F. Siva Jr and B. Olofsson, Org. Lett., 2011, 13, 1552 CrossRef CAS PubMed; (b) T. B. Petersen, R. Khan and B. Olofsson, Org. Lett., 2011, 13, 3462 CrossRef CAS PubMed; (c) N. Jalalian, T. B. Petersen and B. Olofsson, Chem.–Eur. J., 2012, 18, 14140 CrossRef CAS PubMed; (d) R. Ghosh, E. Lindstedt, N. Jalalian and B. Olofsson, ChemistryOpen, 2014, 3, 54 CrossRef CAS PubMed; (e) R. Ghosh and B. Olofsson, Org. Lett., 2014, 16, 1830 CrossRef CAS PubMed; (f) L. Chan, A. McNally, Q. Y. Toh, A. Mendoza and M. J. Gaunt, Chem. Sci., 2015, 6, 1277 RSC; (g) B. Q. Xiong, X. F. Feng, L. Z. Zhu, T. Q. Chen, Y. B. Zhou, C. T. Au and S. F. Yin, ACS Catal., 2015, 5, 537 CrossRef CAS; (h) H. Gao, Q. Xu, C. Keene and L. Kürti, Chem.–Eur. J., 2014, 20, 8883 CAS; (i) R. Ghosh, E. Stridfeldt and B. Olofsson, Chem.–Eur. J., 2014, 20, 8888 CrossRef CAS PubMed.
- (a) M. A. Carroll and R. A. Wood, Tetrahedron, 2007, 63, 11349 CrossRef CAS PubMed; (b) J. Li and L. Liu, RSC Adv., 2012, 2, 10485 RSC; (c) Y. Yang, X. S. Wu, J. W. Han, S. Mao, X. F. Qian and L. M. Wang, Eur. J. Org. Chem., 2014, 6854 CrossRef CAS PubMed.
- N. Umierski and G. Manolikakes, Org. Lett., 2013, 15, 188 CrossRef CAS PubMed.
- A. Ozanne-Beaudenon and S. Quideau, Angew. Chem., Int. Ed., 2005, 44, 7065 (Angew. Chem., 2005, 117, 7227) CrossRef CAS PubMed.
- K. P. Landge, K. S. Jang, S. Y. Lee and D. Y. Chi, J. Org. Chem., 2012, 77, 5705 CrossRef CAS PubMed.
- (a) R. H. Wiley and P. Wiley, Pyrazolones, Pyrazolidones, and Derivatives, ed. A. Weissberger, Interscience, New York, 1964, ch. VIII, vol. 20 of the series The Chemistry of Heterocyclic Compounds Search PubMed; (b) Agents and Actions Supplements. 100 Years of Pyrazolone Drugs. An Update, ed. K. Brune, BirkhaeuserVerlag, Basel, Switzerland, 1986, vol. 19, p. 355 Search PubMed; (c) G. Varvounis, Pyrazol-3-ones. Part IV: Synthesis and Applications, Adv. Heterocyclic Chem., ed. A. R. Katritzky, Academic Press, 2009, vol. 98, p. 143 Search PubMed.
- (a) N. Yokoyama, B. Ritter and A. D. Neubert, J. Med. Chem., 1982, 25, 337 CrossRef CAS; (b) R. I. Fryer, P. Zhang, R. Rios, Z. Q. Gu, A. S. Basile and P. Skolnick, J. Med. Chem., 1993, 36, 1669 CrossRef CAS; (c) L. Savini, P. Massarelli, C. Nencini, C. Pellerano, G. Biggio, A. Maciocco, G. Tuligi, A. Carrieri, N. Cinone and A. Carotti, Bioorg. Med. Chem., 1998, 6, 389 CrossRef CAS; (d) M. G. Ferlin, G. Chiarelotto, S. D. Acqua, E. Maciocco, M. P. Mascia, M. G. Pisu and G. Biggio, Bioorg. Med. Chem., 2005, 13, 3531 CrossRef CAS PubMed; (e) A. Kimata, H. Nakagawa, R. Ohyama, T. Fukuuchi, S. Ohta, T. Suzuki and N. Miyata, J. Med. Chem., 2007, 50, 5053 CrossRef CAS PubMed; (f) O. A. Attanasi, P. Filippone, B. Guidi, T. Hippe, F. Mantellini and L. F. Tietze, Tetrahedron Lett., 1999, 40, 9277 CrossRef CAS; (g) F. Lehmann, M. Holm and S. Laufer, J. Comb. Chem., 2008, 10, 364 CrossRef CAS PubMed; (h) F. Caruso, M. Rossi, J. Tanski, R. Sartori, R. Sariego, S. Moya, S. Diez, E. Navarrete, A. Cingolani, F. Marchetti and C. Pettinari, J. Med. Chem., 2000, 43, 3665 CrossRef CAS PubMed; (i) F. Caruso, C. Pettinari, F. Marchetti, P. Natanti, C. Phillips, J. Tanski and M. Rossi, Inorg. Chem., 2007, 46, 7553 CrossRef CAS PubMed; (j) Y. Li, S. Zhang, J. Yang, S. Jiang and Q. Li, Dyes Pigm., 2008, 76, 508 CrossRef CAS PubMed; (k) A. A. Metwally, M. E. Khalifa and F. A. Amer, Dyes Pigm., 2008, 76, 379 CrossRef PubMed.
- (a) T. Watanabe, S. Yuki, M. Egawa and H. Nishi, J. Pharmacol. Exp. Ther., 1994, 268, 1597 CAS; (b) H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and K. I. Saito, J. Pharmacol. Exp. Ther., 1997, 281, 921 CAS; (c) A. Graul and J. Castaner, Drugs Future, 1996, 21, 1014 CrossRef CAS.
- (a) J. B. Field, E. C. Dolendo, A. Mireles and B. H. Ershoff, Cancer Res., 1966, 26, 1371 CAS; (b) B. Foth, Cancer Res., 1972, 32, 804 Search PubMed; (c) C. K. Ghosh and K. K. Mukhopadhyay, Synthesis, 1978, 779 CrossRef CAS; (d) B. Chantegrel and S. Gelin, Synthesis, 1985, 548 CrossRef CAS PubMed; (e) V. Colotta, L. Cecchi, F. MeLani, G. Palazzino and G. Filacchioni, Tetrahedron Lett., 1987, 28, 5165 CrossRef CAS; (f) V. Colotta, L. Cecchi, F. Melani, G. Palazzino, G. Filacchioni, C. Martini, G. Giannaccini and A. Lucacchini, J. Pharm. Sci., 1989, 78, 239 CrossRef CAS PubMed; (g) R. K. Boeckman Jr, J. E. Reed and P. Ge, Org. Lett., 2001, 3, 3651 CrossRef CAS PubMed; (h) M. Abass and B. B. Mostafa, Bioorg. Med. Chem., 2005, 13, 6133 CrossRef CAS PubMed; (i) M. S. Chande, P. A. Barve and V. Suryanarayan, J. Heterocycl. Chem., 2007, 44, 49 CrossRef CAS PubMed; (j) G. Mariappan, B. P. Saha, N. R. Bhuyan, P. R. Bharti and D. Kumar, J. Adv. Pharm. Technol. Res., 2010, 1, 260 CAS; (k) R. Ma, J. Zhu, J. Liu, L. Chen, X. Shen, H. Jiang and J. Li, Molecules, 2010, 15, 3593 CrossRef CAS PubMed.
- (a) Y. H. Liao, W. B. Chen, Z. J. Wu, X. L. Du, L. F. Cun, X. M. Zhang and W. C. Yuan, Adv. Synth. Catal., 2010, 352, 827 CrossRef CAS PubMed; (b) S. Gogoi and C. G. Zhao, Tetrahedron Lett., 2009, 50, 2252 CrossRef CAS PubMed; (c) S. Gogoi, C. G. Zhao and D. Ding, Org. Lett., 2009, 11, 2249 CrossRef CAS PubMed; (d) A. N. Alba, A. Zea, G. Valero, T. Calbet, M. Font-Bardía, A. Mazzanti, A. Moyano and R. Rios, Eur. J. Org. Chem., 2011, 1318 CrossRef CAS PubMed; (e) A. Zea, A. N. R. Alba, A. Mazzanti, A. Moyano and R. Rios, Org. Biomol. Chem., 2011, 9, 6519 RSC; (f) A. Mazzanti, T. Calbet, M. Font-Bardía, A. Moyano and R. Rios, Org. Biomol. Chem., 2012, 10, 1645 RSC; (g) M. Šimek, M. Remeš, J. Veselý and R. Rios, Asian J. Org. Chem., 2013, 2, 64 CrossRef PubMed; (h) Z. G. Yang, Z. Wang, S. Bai, X. H. Liu, L. L. Lin and X. M. Feng, Org. Lett., 2011, 13, 596 CrossRef CAS PubMed; (i) Z. Wang, Z. G. Yang, D. H. Chen, X. H. Liu, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2011, 123, 5030 (Angew. Chem., 2011, 123, 5030) CrossRef PubMed; (j) Z. Wang, Z. L. Chen, S. Bai, W. Li, X. H. Liu, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2012, 51, 2776 (Angew. Chem., 2012, 124, 2830) CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03819g |
| This journal is © The Royal Society of Chemistry 2015 |