Toxicity of halloysite clay nanotubes in vivo: a Caenorhabditis elegans study†
Gölnur I.
Fakhrullina
a,
Farida S.
Akhatova
a,
Yuri M.
Lvov
b and
Rawil F.
Fakhrullin
*a
aBionanotechnology Lab, Department of Microbiology, Institute of Fundamental Medicine and Biology, Kazan Federal University, Kreml uramı 18, Kazan, Republic of Tatarstan 420008, Russian Federation. E-mail: kazanbio@gmail.com
bInstitute for Micromanufacturing, Louisiana Tech University, 911 Hergot Ave., Ruston, LA 71272, USA
First published on 3rd November 2014
Abstract
Here we investigated the toxicity of halloysite clay nanotubes in vivo employing a Caenorhabditis elegans nematode as a model organism. Using enhanced dark-field microscopy and physiological tests, we found that halloysite is localised exclusively in the alimentary system and does not induce severe toxic effects on nematodes.
Nano impactHalloysite is a nanomaterial which is already used in tens of thousands of tons in the ceramic and polymeric composite industries. These clay tubes are excavated from the mines as stone minerals and processed by milling to fine powder of tubes with a diameter of 50 nm and a length of 1.5 μm. This treatment converts environmentally safe minerals to potentially dangerous nano-dispersed materials. The halloysite nanotubes were found to be nontoxic for isolated cell cultures, but no in vivo studies were performed for whole organisms. We analysed the nanosafety of halloysite for soil nematodes (Caenorhabditis elegans), one of the first organisms which may encounter these nanotubes in the polluted soil. Halloysite nanotubes were found safe for C. elegans at a concentration up to 1 mg mL−1 which is about 1000 times higher than the possible soil contamination concentrations. |
Halloysite nanotubes (HNTs) are regarded as one of the most promising natural nanoscale materials. These clay nanomaterials have recently attracted attention due to their extraordinary chemical and physical properties, cheap production and availability in thousands of tons. Halloysite is a natural clay material, chemically identical to kaolin, with an outer diameter of 40–70 nm, an inner diameter of 10–20 nm and a length of 500–1500 nm.1 Halloysite tubes are rolled kaolin alumosilicate sheets, where the internal side is composed of Al2O3 while the external is SiO2, which allows for the selective chemical modification of these outer/inner surfaces.2 Halloysite is excavated from the mines as white rocks containing 95–99 wt.% of nanotubes with the remaining being kaolin, quartz and iron oxide admixtures. Halloysite is well mixable with many polymers: from synthetic polyethylene, polypropylene, epoxy resins and polyamides to natural polysaccharides and gelatin. Typically, doping polymers with 4–5 wt.% halloysite increases the composite strength and adhesivity by 40–50% and effectively improves its flame retardancy.1 The large surface area and oppositely charged inner and outer layers facilitate loading of negatively charged biomacromolecules into a positive tube's lumen (e.g., DNA encapsulation). As a result, halloysite nanotubes have been found to be applicable for fabrication of novel biomedical materials with controlled release, i.e. drug3–6 or enzyme7 carriers, gene delivery vehicles,8 antibacterial coatings,9 nanostructured coatings for improved adhesion of human cells,10,11 cell surface engineering,12 and scaffolds for tissue engineering.13 Equally important, pristine and functionalized halloysite nanotubes were utilised in numerous industrial applications as inorganic micelles to capture hydrocarbon and aromatic oils,14 corrosion inhibitors,15,16 organic film stabilizers,17 filtration membranes18 and catalyst supports,19,20 among many others.
Annually, approximately 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of halloysite clay minerals are excavated worldwide and processed to dispersed nanotubes.1 These nanotubes are added into ceramic and polymer composites; besides, potentially doping them into auto tire rubber which may increase drastically halloysite pollution is under consideration.21 Rapidly expanding the use of halloysite nanotubes in the porcelain and polymer composite industries22 suggests a high probability of undesired release of HNTs into the environment, bringing them into the direct contact with organisms in their natural habitats, which may potentially cause unwanted damage to cells, tissues and organs. Therefore, the elucidation of the toxicity of halloysite nanotubes towards living organisms is crucially important.
000 tons of halloysite clay minerals are excavated worldwide and processed to dispersed nanotubes.1 These nanotubes are added into ceramic and polymer composites; besides, potentially doping them into auto tire rubber which may increase drastically halloysite pollution is under consideration.21 Rapidly expanding the use of halloysite nanotubes in the porcelain and polymer composite industries22 suggests a high probability of undesired release of HNTs into the environment, bringing them into the direct contact with organisms in their natural habitats, which may potentially cause unwanted damage to cells, tissues and organs. Therefore, the elucidation of the toxicity of halloysite nanotubes towards living organisms is crucially important.
Several recent reports demonstrate the investigation of halloysite nanotoxicity in vitro, employing human cell cultures and microbial cells. The toxicity and cellular uptake of halloysite nanotubes were investigated using human breast cancer cells and human epithelial adenocarcinoma cells.23 The cells were cultivated in media supplemented with increasing halloysite concentrations; consequently the distribution of nanotubes in cytoplasm was mapped by confocal microscopy, while the viability of the cells was assessed using enzymatic activity tests. The results suggest that the viability of the halloysite-treated cells (up to 0.075 mg mL−1) was preserved (up to 70% of viable cells); however, at higher concentrations of HNTs (from 1 mg mL−1), cell death was induced in both types of cells. Relatively low toxicity of chitosan-based scaffolds for tissue engineering was demonstrated by monitoring the growth of fibroblasts on nanocomposites.13 No significant effects of fibroblast attachment and development on chitosan-doped scaffold were observed. In proteomic analysis, exposure-specific changes in expression observed among 4081 proteins have shown pro-inflammatory effects at halloysite exposures as low as 1 mg mL−1 and significant changes in protein expression at very high concentration of 100 mg mL−1. Based on these findings, halloysite clay nanotubes appear unlikely to have toxic effects at moderate levels of exposure. Bioinformatic analysis of differentially expressed protein profiles suggests that halloysite stimulates processes related to cell growth and proliferation, subtle responses to cell infection, irritation and injury, and enhanced antioxidant capability, all characteristic of an overall adaptive response to exposure.24 Moreover, halloysite-doped polymer dental scaffolds stimulated the growth of dental pulp fibroblast cells.25
The extent of toxicity of HNTs on microbial communities is not completely understood yet. According to Zhang et al., pristine HNTs exhibit little toxicity towards Escherichia coli bacteria;26 however, another report suggests that the pristine halloysite exhibits the highest toxicity towards E. coli.27 The toxic effects of pristine HNTs are likely to be caused by the direct contact of nanotubes with cell walls and reactive oxygen generation.27 On the other hand, HNTs were shown to be non-toxic towards yeast cells.12 These ambiguous results stimulate further investigation of halloysite toxicity. Importantly, in vivo investigations are required28 since the toxic effects of nanomaterials on an isolated cell culture may not be directly extrapolated onto the whole organisms.
Here we report for the first time the in vivo toxicity testing of halloysite nanotubes employing a free-living Caenorhabditis elegans nematode as a model organism. These nematodes have been extensively used in a number of biological studies, including toxicity assays. The C. elegans nematode is an extremely important tool in molecular biology because its fully sequenced genome is closely homologous to the human genome, its relatively short lifespan takes only three weeks and its tiny transparent ~1 mm long body is built up from about 960 cells.29,30 Previously, C. elegans nematodes were found to be versatile animal models for nanotoxicity assays to evaluate the toxicity of carbon nanotubes,31,32 gold,33 silica34 and metallofullerene nanoparticles,35 and graphite nanoplatelets.36 Aiming at the toxicity tests of halloysite nanotubes, we have chosen wild-type C. elegans microworms (Bristol N2) for the following reasons: 1) C. elegans naturally populate soils, and therefore, they are likely to encounter the product-released HNTs; 2) they are optically transparent and small-sized animals, which allows one to directly visualise HNTs distribution using enhanced dark-field microscopy inside the live worms without sophisticated sample preparation techniques; and 3) there is a well-established and simple methodology to estimate the toxic effects of nanomaterials based on certain physiological parameters of microworms.
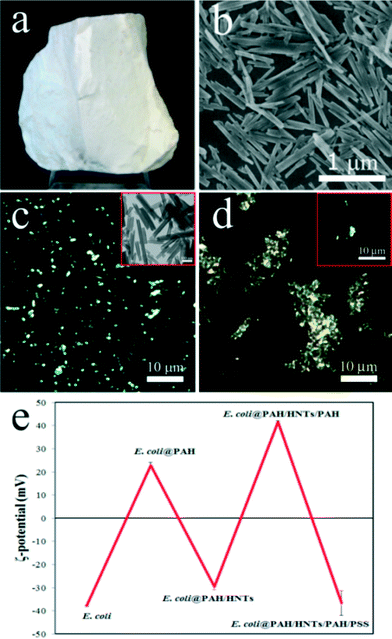
We investigated the toxicity of the pristine halloysite nanotube (Fig. 1a) obtained from Applied Minerals Dragon Mine, USA, which is also one of the products distributed by Sigma-Aldrich referred in many halloysite publications. The scanning electron microscopy image (Fig. 1b) confirms the typical sizes of the halloysite nanotubes (ca. 15 nm lumen, 50 nm outer diameter, and 1–1.5 μm length). Importantly, halloysite nanotubes suspended in aqueous solutions can be visualised in situ using enhanced dark-field (EDF) microscopy. As shown in Fig. 1c, HNTs are clearly seen in the EDF microscopy image as bright spots retaining the intrinsic rod-like geometry (a real-time footage showing the movement of an isolated nanotube is shown in Video 1 in the ESI†). In water, HNTs exhibit a negative zeta-potential of −32 ± 2 mV.
The primary pathway of the nanoparticle entry into the nematodes is the intestinal uptake,32 which occurs while the worms feed on E. coli bacteria and spontaneously ingest the nanoparticles. However, HNTs' linear dimensions are large enough to anticipate that the worms may try avoiding the areas enriched with the nanotubes. Accordingly, we applied a simple behavioural test to assess the taxis of C. elegans microworms towards the HNTs. Starved L1 nematodes were introduced into the agar-based nematode growth media (NGM) on Petri dishes where 50 μL of pure food (E. coli, 1010 cells mL−1) and bacteria directly mixed with HNTs (1 mg mL−1) were dropped onto the opposite sides of the dish, as demonstrated in Fig. S1 in the ESI.† After 8 hours, the dishes were screened under a stereomicroscope to count the number of worms feeding on pure and HNTs-mixed bacteria. Most of the worms (63%) were detected feeding on pure E. coli, while just the remaining 37% were spotted in the HNTs-doped spots. It is worth noting that the animals found on HNTs-containing bacteria drops were considerably smaller than the ones found on HNTs-free drops (Fig. S2 in the ESI†). This suggests that the worms actively avoid HNTs mixed with food, preferring the pure bacteria ration. We hypothesized that testing the toxic effects of HNTs using the traditional approach based on mixing bacterial food with nanoparticles37 can be influenced by the behaviour of the worms trying deliberately to choose the HNTs-free bacterial cells during feeding. To overcome this, we employed the recently proposed nanoparticle delivery method based on “nanobaits” – microbial cells coated with nanoparticles sandwiched between polyelectrolyte nanolayers.38 We deposited halloysite nanotubes on E. coli cells via the sequential layer-by-layer deposition of (poly)allylamine (PAH) and (poly)stryrene sulphonate (PSS) polymers. The final architecture of nanocoatings on bacteria was PAH/HNTs/PAH/PSS, which ensure the resulting negative charge of HNTs-coated cells similar to that of intact cells. The effective immobilisation of HNTs on E. coli cells was confirmed by EDF microscopy (Fig. 1d) and by monitoring of zeta-potential inversion after each deposition step (Fig. 1e). Next, the HNTs-coated E. coli “nanobaits” were supplied to the C. elegans nematodes as the sole food source. Synchronised adult hermaphrodite animals were starved overnight, then the HNTs-coated cells were added onto the Petri dishes and the worms were allowed to feed freely on them for 1 hour. Next, the animals were collected and fixed for microscopy monitoring of HNTs localisation.
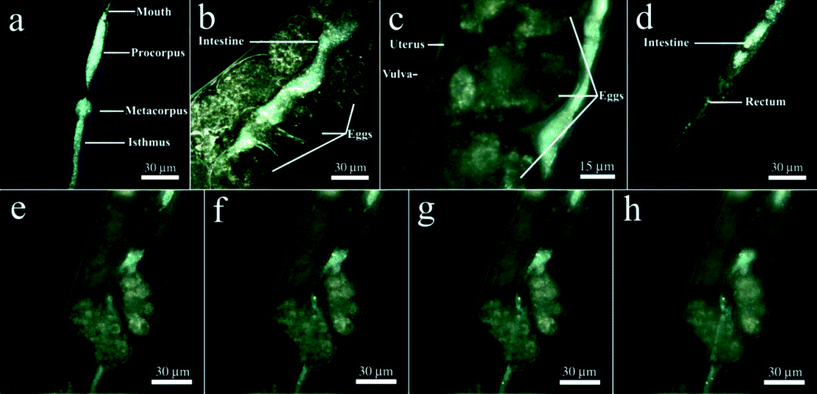
First, we labelled HNTs with rhodamine B prior to exposure to nematodes and then inspected the sample with a confocal microscope finding out that the confocal images do not allow us to visualise the HNTs inside the worms with the same precision as EDF microscopy (Fig. S3 in the ESI†). High contrast of dark-field images of HNTs ingested during the feeding of the worms on HNTs allowed for the effective visualisation of the nanotubes without use of fluorescent dyes,32 which may leak from the carrier particles or chemical fixation and thin sectioning, followed by electron microscopy imaging, which may result in washing off the nanotubes from the worms. In EDF microscopy images, halloysite nanotubes were found exclusively in the alimentary system of the worms (Fig. 2a–d). We detected the HNTs along the whole intestine, starting from the buccal cavity to the anus, with prominent aggregation in the interior bulb and terminal bulb (Fig. 2a). In the midgut and hindgut areas, HNTs were also clearly visible; however, less aggregation was observed (Fig. 2b, d). The distribution of HNTs in a whole nematode is shown in Video 2 (ESI†). Importantly, we did not detect nanotubes outside the intestines of the nematodes. Previous reports suggest that the silica nanoparticle entry into the C. elegans occurs not only through the mouth but also through the vulva, whence they travel further and are consequently internalised by the singe vulval cells.37 In the case of HNTs, we do not see any aggregation of the nanotubes near the vulva (Fig. 2c); moreover, no free HNTs were detected inside the uterus or in the embryos, suggesting that no uptake happens through the vulva. We attribute this to the relatively large sizes of HNTs (up to 1500 nm) if compared with the 50 nm silica used in the previous study.37 Importantly, no HNTs were detected in the vulva, ovaries or spermatheca in samples where the worms were incubated with free HNTs (data not shown), suggesting that HNTs not immobilised onto E. coli cells do not enter the vulva as well. The intestinal localisation of HNTs in nematodes can be seen more clearly in Fig. 2e–h. In this image, the focal plane was moved to demonstrate the intestine filled with randomly distributed HNTs, whereas no nanotubes were detected outside the intestines in the same focal planes. Interestingly, no HNTs were detected in the embryos, which corresponds well with the inhomogeneous distribution of oxidised single-walled carbon nanotubes in C. elegans.32
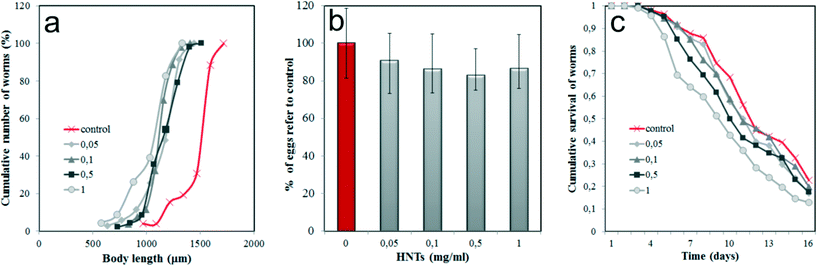
Next, we investigated the toxic effects of HNTs by monitoring several physiological parameters of HNTs-treated nematodes. First, we focused on the body size of the worms (Fig. 3a), which is one of the integral parameters of toxic effects. HNTs within the 0.05–1 mg mL−1 concentration range inhibited the normal body growth of the nematodes if compared with the untreated samples, indicating the development deficit. However, the ingestion of HNTs-coated E. coli cells does not reduce the body size as much as reported for amine-modified single-walled carbon nanotubes,39 where almost a twofold reduction of the body length in C. elegans was detected. This suggests that the uptake of HNTs with food does not induce the starvation of the animals; more likely a different mechanism is responsible for the size reduction.
As noted, the reproductive organs, such as the ovaries, uterus and spermatheca, were free of HNTs. To explore the possible effects of HNTs on the reproduction of the worms, the number of eggs per hermaphrodite in HNTs-treated worms was counted (Fig. 3b). As expected, we found that HNTs have no significant effect on fertility of the microworms; thus, no statistically significant reduction of egg number occurred.
Finally, we investigated the longevity of the HNTs-treated C. elegans. Synchronised adult nematodes were kept in 96-well plates (~10 worms per well), treated with fluorodeoxyuridine (to inhibit the reproduction), fed with HNTs-coated bacteria (100 μL−1) and monitored for viability by touching with a thin wire (the non-viable worms were counted if no tactile reaction was detected). Cumulative survival analysis demonstrated that no significant negative effect on the lifespan was induced in nematodes within all the concentrations of the HNTs studied (Fig. 3c). The detailed statistical analysis of data presented in Fig. 3c is shown in Table S1 (ESI†). Lower concentrations of HNTs (0.05, 0.1 mg mL−1) did not decrease the longevity of the worms, whereas higher concentrations (0.5, 1 mg mL−1) somewhat reduced the mean lifespan (up to ~15% if compared with untreated animals), although this reduction was not statistically significant (P > 0.05).
The results obtained suggest that HNTs have no profound toxic effects on C. elegans nematodes unlike other nanomaterials, such as single-walled39 or multi-walled31 carbon nanotubes, graphene oxide,40 TiO2 nanoparticles40 or platinum nanoparticles.41 We suppose that the low toxicity of HNTs in comparison with other nanomaterials is outlined by the relatively low (if any) uptake of nanotubes by intestinal cells and very limited transport to other tissues and organs. For instance, highly soluble single-walled carbon nanotubes (SWCNTs) severely reduced the body length in nematodes, whereas pristine SWCNTs were almost nontoxic.39 Here, HNTs are ingested by nematodes via feeding on HNTs-coated cells, but then they are not adsorbed by the intestinal cells due to their sizes and are later safely removed via excretion. This is confirmed by observing the nematodes 2 hours after feeding (Fig. S4 in the ESI†). Additionally, we performed another set of experiments where we coated C. elegans eggs with PAH\HNTs\PAH\PSS (Fig. 4 inset) and then incubated them normally and monitored HNTs in larvae and adult worms. In all cases, we did not detect any HNTs inside the animals. Moreover, only a slight reduction in body length was observed in nematodes hatched from HNTs-coated eggs (Fig. 4). In this case, the microworms can take up the nanotubes through the egg cuticle only, and after hatching the nematodes apparently try to avoid ingesting nanotubes during feeding on bacteria.
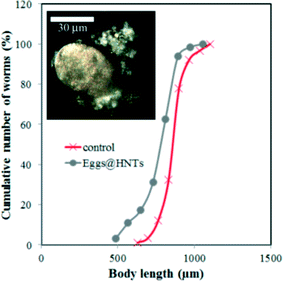 | ||
| Fig. 4 The cumulative curves of the body length of nematodes hatched from the HNTs-coated eggs (the inset shows a typical EDF microscopy image of the HNTs-coated egg). | ||
Finally, we tried to elucidate the mechanism of the slightly toxic effect of higher concentrations of HNTs on the body length of the nematodes. We suppose that this might be caused by the irritation inflicted by rod-shaped nanotubes contacting with the intestinal cuticle of the worms. Using the EDF microscopy, we observed the spontaneous intensive rotational movement of single isolated nanotubes and larger aggregates of nanotubes inside the intestines of the immobilised living adult nematodes along the whole length of the gut. Typical real-time footages demonstrating these movements in the grinder and intestines are given in the ESI† (Video 3 and Video 4, respectively). We suppose that the moving nanotubes can harm and irritate the intestines of microworms, thus affecting the ingestion and, as a consequence, the body length, whereas the number of eggs and the overall longevity are not reduced. Normally, micron-sized food particles (bacterial cells) as well as artificial microparticles reside in the intestine for around 60–110 seconds, being constantly excreted from the hindgut during defecation.42 The very fast digestion and excretion of bacteria provides the nematodes with nutrients; however, in our study, we found that HNTs persisted in intestines even after 2 hours upon ingestion (after feeding with HNTs-coated bacteria, the worms were transferred into HNTs-free dishes). The EDF microscopy images of the nematodes (data not shown) clearly demonstrate that the nanotubes are still seen inside the intestines, although in this case they are primarily located in the hindgut region, which suggests that HNTs migrate very slowly if compared with normal diet (E. coli) or 2 μm polystyrene beads.42 It is likely that the HNTs reversibly adsorb onto the intestinal microvilli and thus irritate the intestinal cells. It is worth noting that no bacterial layers were observed inside the intestine,44 suggesting that the irritating HNTs effect is temporary, and worms eventually restore normal digestion. However, the persisting aggregates and moving nanotubes are expected to induce acute effects on digestion, which is supported by the reduced body sizes of HNTs-treated nematodes.
Conclusions
Our study suggests that the HNTs within the concentrations investigated are not capable of severely damaging the organism of the nematodes, inflicting only mechanical stress on the alimentary system. We believe that the microworms intentionally avoid the nanotubes; therefore the only effective way of delivery is based on HNTs-modified cells. Regarding the potential applications of HNTs in C. elegans studies, pH-sensitive sensors, currently fabricated using silica nanoparticles,43 in future can be produced using HNTs, greatly reducing the unwanted toxic effects of silica.37 During manufacturing and halloysite product usage, these alumosilicate nanotubes will be eventually returned to the environment as fine nano-powder; therefore its toxicity assessment is important. Overall, low toxicity of halloysite to soil nematodes demonstrated in this work suggests that its quickly growing industrial application is likely to be environmentally safe.Acknowledgements
The work is performed according to the Russian Government Program of Competitive Growth of Kazan Federal University. This work was funded by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities. This publication is based on the work supported by grant FSAX-14-60155-0 from the U.S. Civilian Research & Development Foundation (CRDF Global). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of CRDF Global.References
- Y. Lvov and E. Abdullayev, Prog. Polym. Sci., 2013, 38, 1690–1719 CrossRef CAS PubMed.
- E. Abdullayev, A. Joshi, W. Wei, Y. Zhao and Y. Lvov, ACS Nano, 2012, 6, 7216–7226 CrossRef CAS PubMed.
- N. Veerabadran, R. Price and Y. Lvov, Nano, 2007, 2, 215–222 CrossRef.
- V. Vergaro, Y. Lvov and S. Leporatti, Macromol. Biosci., 2012, 12, 1265–1271 CrossRef CAS PubMed.
- Y. Lee, G. Jung, S. J. Cho, K. E. Geckeler and H. Fuchs, Nanoscale, 2013, 5, 8577–8585 RSC.
- K. M. Rao, S. Nagappan, D. J. Seo and C.-S. Ha, Appl. Clay Sci., 2014, 97–98, 33–42 CrossRef CAS PubMed.
- C. Chao, J. Liu, J. Wang, Y. Zhang, B. Zhang, Y. Zhang, X. Xiang and R. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 10559–10564 CAS.
- Y. Shi, Z. Tian, Y. Zhang, H. Shen and N. Jia, Nanoscale Res. Lett., 2011, 6, 608, DOI:10.1186/1556-276X-6-608.
- W. Wei, R. Minullina, E. Abdullayev, R. Fakhrullin, D. Mills and Y. Lvov, RSC Adv., 2014, 4, 488–494 RSC.
- A. D. Hughes and M. R. King, Langmuir, 2010, 26, 12155–12164 CrossRef CAS PubMed.
- M. J. Mitchell, C. S. Chen, V. Ponmud, A. D. Hughes and M. R. King, J. Controlled Release, 2012, 160, 609–617 CrossRef CAS PubMed.
- S. A. Konnova, I. R. Sharipova, T. A. Demina, Y. N. Osin, D. R. Yarullina, O. N. Ilinskaya, Y. M. Lvov and R. F. Fakhrullin, Chem. Commun., 2013, 49, 4208–4210 RSC.
- M. Liu, C. Wu, Y. Jiao, S. Xiong and C. Zhou, J. Mater. Chem. B, 2013, 1, 2078–2089 RSC.
- G. Cavallaro, G. Lazzara, S. Milioto, F. Parisi and V. Sanzillo, ACS Appl. Mater. Interfaces, 2014, 6, 606–612 CAS.
- E. Abdullayev, V. Abbasov, A. Tursunbayeva, V. Portnov, H. Ibrahimov, G. Mukhtarova and Y. Lvov, ACS Appl. Mater. Interfaces, 2013, 5, 4464–4471 CAS.
- E. Abdullayev, R. Price, D. Shchukin and Y. Lvov, ACS Appl. Mater. Interfaces, 2009, 1, 1437–1443 CAS.
- J. Qiao, J. Adams and D. Johannsmann, Langmuir, 2012, 28, 8674–8680 CrossRef CAS PubMed.
- J. Zhang, Y. Zhang, Y. Chen, L. Du, B. Zhang, H. Zhang, J. Liu and K. Wang, Ind. Eng. Chem. Res., 2012, 51, 3081–3090 CrossRef CAS.
- L. Wang, J. Chen, L. Ge, Z. Zhu and V. Rudolph, Energy Fuels, 2011, 25, 3408–3416 CrossRef CAS.
- L. Wang, J. Chen, L. Ge, V. Rudolph and Z. Zhu, J. Phys. Chem. C, 2013, 117, 4141–4151 CAS.
- Y. Fu, D. Zhao, W. Wang and Y. Lvov, IOP Conf. Ser.: Mater. Sci. Eng., 2014, 63, 132–136, DOI:10.1088/1757-899X/64/1/012047.
- M. Du, B. Guo and D. Jia, Polym. Int., 2010, 59, 574–595 CAS.
- V. Vergaro, E. Abdullayev, Y. M. Lvov, A. Zeitoun, R. Cingolani, R. Rinaldi and S. Leporatti, Biomacromolecules, 2010, 11, 820–826 CrossRef CAS PubMed.
- X. Lai, M. Agarwal, Y. Lvov, C. Pachpande, K. Varahramyan and F. Witzmann, J. Appl. Toxicol., 2013, 33, 1316–1329 CAS.
- M. C. Bottino, G. H. Yassen, J. A. Platt, N. Labban, L. J. Windsor, K. J. Spolnik and A. H. Bressiani, J. Tissue Eng. Regener. Med., 2013 DOI:10.1002/term.1712.
- Y. Zhang, Y. Chen, H. Zhang, B. Zhang and J. Liu, J. Inorg. Biochem., 2013, 118, 59–64 CrossRef CAS PubMed.
- H.-J. Choi, T. J. Stazak Jr. and C. D. Montemagno, J. Nanopart. Res., 2013, 15, 2008, DOI:10.1007/s11051-013-2008-4.
- J. S. Bozich, S. E. Lohse, M. D. Torelli, C. J. Murphy, R. J. Hamers and R. D. Klaper, Environ. Sci.: Nano, 2014, 1, 260–270 RSC.
- E. Culetto and D. B. Sattelle, Hum. Mol. Genet., 2000, 9, 869–877 CrossRef CAS PubMed.
- T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–399 CrossRef CAS PubMed.
- A. Nouara, Q. Wu, Y. Li, M. Tang, H. Wang, Y. Zhao and D. Wang, Nanoscale, 2013, 5, 6088–6096 RSC.
- C. M. Goodwin, G. G. Lewis, A. Fiorella, M. D. Ellison and R. Kohn, RSC Adv., 2014, 4, 5893–5900 RSC.
- S. W. Kim, J. I. Kwak and Y.-J. An, Environ. Sci. Technol., 2013, 47, 5393–5399 CrossRef CAS PubMed.
- A. Pluskota, E. Horzowski, O. Bossinger and A. von Mikecz, PLoS One, 2009, 4, e6622 Search PubMed.
- W. Zhang, B. Sun, L. Zhang, B. Zhao, G. Nie and Y. Zhao, Nanoscale, 2011, 3, 2636–2641 RSC.
- E. Zanni, G. De Bellis, M. P. Bracciale, A. Broggi, M. L. Santarelli, M. S. Sarto, C. Palleschi and D. Uccelletti, Nano Lett., 2012, 12, 2740–2744 CrossRef CAS PubMed.
- A. Scharf, A. Piechulek and A. von Mikecz, ACS Nano, 2013, 7, 10695–10703 CrossRef CAS PubMed.
- G. I. Däwlätşina, R. T. Minullina and R. F. Fakhrullin, Nanoscale, 2013, 5, 11761–11769 RSC.
- P.-H. Chen, K.-M. Hsiao and C.-C. Chou, Biomaterials, 2013, 34, 5661–5669 CrossRef CAS PubMed.
- Q. Wu, Y. Zhao, G. Zhao and D. Wang, Nanomedicine, 2014, 10, 1263–1271 CrossRef CAS PubMed.
- J. Kim, T. Shirasawa and Y. Miyamoto, Biomaterials, 2010, 31, 5849–5854 CrossRef CAS PubMed.
- S. Ghafouri and J. D. McGhee, Nematology, 2007, 9, 87–91 CrossRef PubMed.
- N. D. Mathew, M. D. Mathew and P. P. Surawski, Anal. Biochem., 2014, 450, 52–56 CrossRef CAS PubMed.
- Y. J. Cha, J. Lee and S. S. Choi, Chemosphere, 2012, 87, 49–54 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional figures, a table and real-time footage, as noted in the text. See DOI: 10.1039/c4en00135d |
| This journal is © The Royal Society of Chemistry 2015 |