Catalytic properties and biomedical applications of cerium oxide nanoparticles†
Carl
Walkey
a,
Soumen
Das
b,
Sudipta
Seal
b,
Joseph
Erlichman
c,
Karin
Heckman
c,
Lina
Ghibelli
d,
Enrico
Traversa
e,
James F.
McGinnis
f and
William T.
Self
*g
aIntegrated Nanotechnology and Biomedical Sciences Laboratory, Terrence Donnelly Building, University of Toronto, 160 College St., Toronto, ON M5S 3G9, Canada
bAdvanced Materials Processing and Analysis Centre, Nanoscience Technology Center, University of Central Florida, Orlando, FL, USA
cDepartment of Biology, St. Lawrence University, Johnson Hall of Science, 23 Romoda Drive, Canton, NY 13617, USA
dDepartment of Biology, Università di Roma Tor Vergata, Via della Ricerca Scientifica, 00133 Roma, Italy
eKing Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia
fDean A. McGee Eye Institute, Department of Ophthalmology, 608 Stanton L. Young, Blvd., Oklahoma City, OK 73126, USA
gBurnett School of Biomedical Science, College of Medicine, University of Central Florida, Orlando, Florida 32816, USA. E-mail: william.self@ucf.edu
First published on 10th November 2014
Abstract
Cerium oxide nanoparticles (nanoceria) have shown promise as catalytic antioxidants in the test tube, cell culture models and animal models of disease. However given the reactivity that is well established at the surface of these nanoparticles, the biological utilization of nanoceria as a therapeutic still poses many challenges. Moreover the form that these particles take in a biological environment, such as the changes that can occur due to a protein corona, are not well established. This review aims to summarize the existing literature on biological use of nanoceria, and to raise questions about what further study is needed to apply this interesting catalytic material to biomedical applications. These questions include: 1) How does preparation, exposure dose, route and experimental model influence the reported effects of nanoceria in animal studies? 2) What are the considerations to develop nanoceria as a therapeutic agent in regards to these parameters? 3) What biological targets of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are relevant to this targeting, and how do these properties also influence the safety of these nanomaterials?
Soumen Das received his PhD from Indian Institute of Technology, Kharagpur in 2010. Currently he is working as a Research assistant professor in Advanced Materials Processing and Analysis Center and Nanoscience Technology Center, University of Central Florida, Orlando. His research is focused on the synthesis and conjugation of biomolecules to rare earth nanoparticles as targeted therapeutic agents and understanding the interaction mechanism of nanoparticles with cells. He is coauthor of 36 papers and 1 patent has been awarded. He has own GRC speaker award, UCF 2012 innovator award, Dr. D.S. Kothari Postdoctoral fellowship and CSIR fellowship. |
Sudipta Seal is a UCF Distinguished Professor and Pegasis Professor in Materials Science and Engineering, School of medicine and interim chair and director of the Advanced Materials Processing and Analysis Center and the Nanoscience Technology Center, University of Central Florida. He is a Fellow of ASM, AVS, IoN, AAAS, NAI, ECS, AIMBE and a recipient of Office of Naval Research Young Investigator Award (ONR YIP). His current research involves functional nanoparticles for energy, biomedical and sensor applications, and green manufacturing. He has coauthored more than 300 papers, numerous book chapters, three books on nanotechnology, and has been awarded 40 patents. |
Joe Erlichman received his Ph.D. from Dartmouth Medical School and additional post-doctoral training at Dartmouth Medical School and Boonschoft School of Medicine in Dayton, Ohio. He is a past recipient of the American Physiological Societies Van Harreveld Memorial Award in central nervous system studies and currently is professor at St. Lawrence University in the Department of Biology/Program in Neuroscience. In addition to his academic appointments, Dr. Erlichman co-founded Neuroredox, LLC and Cerion NRx, LLC and currently serves the Chief Scientific Officer for these organizations. His current research interests involve the development of novel, ceria-based therapeutics in the treatment of neurodegenerative diseases. |
Karin L. Heckman PhD, RD is an Assistant Professor of Biology at St. Lawrence University in Canton, NY. She earned her BS degree in Community/Medical Dietetics at Viterbo University in La Crosse, WI prior to becoming credentialed as a Registered Dietitian. She went on to complete her PhD in Biomedical Science: Immunology at the Mayo Clinic College of Medicine in Rochester, MN. |
Lina Ghibelli PhD in Biology (Universitá La Sapienza Roma); Research Associate, University of Chicago, Visitor at the European Molecular Biology Laboratory in Heidelberg; Visiting Scientist at the Karoloinska Institutet; Dipartimento di Biologia, Universitá di Roma Tor Vergata; Professor of General Pathology, Universitá di Urbino. From 1991 she has led a research group studying the process of cell death by apoptosis, oxidative stress, survival pathways, the biological roles of magnetic fields and the homeostatic role of melatonin. Recently, she has focused on the bio-compatibility of nanoparticles, including nanotoxicology and biological exploitation of carbon nanotubes, graphene and cerium oxide nanoparticles, with special attention to interaction with cells and the resulting signal transduction. Author of >200 scientific full papers in high impact peer-reviewed international journals. |
Enrico Traversa received his PhD in Chemical Engineering from the University of Rome La Sapienza. In 2013 he joined the King Abdullah University of Science and Technology (KAUST), Saudi Arabia, as Professor of Materials Science and Engineering, after being the Director of the Department of Fuel Cell Research at the International Center for Renewable Energy, Xi'an Jiaotong University, China. He joined the University of Rome Tor Vergata, Italy, in 1988, where he is a Professor of Materials Science and Technology, now on leave of absence. From January 2009 to March 2012, he was a Principal Investigator at the International Research Center for Materials Nanoarchitectonics (MANA) at the National Institute for Materials Science (NIMS), Tsukuba, Japan, leading a unit on Sustainability Materials. His research interests include nanostructured materials for environment, energy, and healthcare, with special attention to sustainable development, and he was elected as a Fellow of the Electrochemical Society in 2013. |
James F. McGinnis is a Professor in the departments of Cell Biology and Ophthalmology at Oklahoma University Center for Health Sciences, Director of the Dean McGee Eye Institute Cell Imaging Core, and Associate Director of the Oklahoma Center for Neuroscience. Over the last ten years, his lab has been using nanoceria to protect retinal neurons from Reactive Oxygen Species induced death in multiple animal models for recessive and dominant forms of inherited retinal degeneration (RP), Age-related Macular Degeneration (AMD), Diabetic Retinopathy (DR), Retinoblastoma (Rb) and Retinopathy of Prematurity (ROP). Current efforts are focused on the demonstration of the molecular mechanisms by which nanoceria function in vivo and the advancement of nanoceria as an FDA approved Investigational New Drug (IND) for safe use in humans. |
William T. Self obtained his Ph.D. in Microbiology from the University of Florida in 1998, working with Dr. K. T. Shanmugam and studying microbial physiology using Escherichia coli as a model. He moved to the NIH in Bethesda, Maryland and was a Research Fellow under the tutelage of Dr. Thressa Stadtman studying selenoprotein biochemistry. He moved to UCF in 2003 and is now an Associate Professor in the Burnett School of Biomedical Science. His lab is now more focused on the study of the catalytic behavior of cerium oxide nanomaterials and moving this material into clinical applications in medicine. |
Nano impactThis critical review of the biological applications of cerium oxide nanoparticles brings together several research groups that have been working towards biomedical use of cerium oxide. The reactivity, fate and distribution of these nanomaterials in humans is critical to assessing their safety in biomedicine, given the growing evidence that this nanomaterial has potent anti-inflammatory properties. |
I. Overview – introduction and overview
Cerium oxide nanoparticles, also known as nanoceria, have been utilized for decades for applications in glass polishing and chemical mechanical polishing applications.1,2 In addition, considerable interest has also arisen in the use of cerium oxide based fuel additives to reduce soot and increase efficiency of Diesel engines.3 In addition the study of cerium oxide in polishing and catalysis has been well developed for a number of years, and is reviewed in other works in the Environmental Science: Nano Nanoceria Research themed collection. However the biological application of this rare earth oxide began in earnest around 2006 with some groundbreaking studies that showed nanoceria exhibited antioxidant character in cell culture models.4–9These studies ignited an area of research that now has a larger group of scientists engaged in the study of nanoceria for biomedical applications. A number of these scientists are authoring this report to establish a foothold on where we are in terms of biomedical applications of nanoceria, and also to set forth the challenges faced to safely and effectively use this metal oxide for biomedicine.
This critical review summarizes the findings of studies that have shown nanoceria to act in a beneficial manner in cell culture and animal studies. In addition this review emphasizes the correlation between surface chemistry of nanoceria and its catalytic properties. The surface corona is also discussed and integrated into discussion and review of surface modifications. This review does not discuss the toxicology or toxicity of nanoceria, as this is the focus of another review in this special issue.10 The discussion of surface chemistry and material science in this review is also tied closely with another review in this special issue that asks broader questions about ceria and it uses in various applications.11 A third closely related review discusses more of the nanoparticle aspects of cerium oxide that also relates to the catalytic nature of nanoceria that is presented in this work.12 Since these works were developed in parallel, extensive cross-referencing is made difficult and thus the reader is encouraged to seek out information in all the reviews of this special issue on nanoceria.
II. The biological identity of nanoceria
The biological behavior of a nanoparticle, including its biodistribution, pharmacokinetics, toxicity, dissolution, and elimination, depends on its physical and chemical properties. In the past, it was assumed that the properties of a nanoparticle within a biological system are the same as the properties it had following synthesis. Recently, researchers have discovered that nanoparticles interact with a diverse collection of soluble biomolecules when they enter a biological environment. Biomolecule–nanoparticle interactions lead to the formation of an adsorbed biomolecular corona.13,14 The biomolecular corona changes the size, surface charge, and composition of the nanoparticle, giving it a biological identity that is distinct from its synthetic identity.15 It is the biological identity that is ‘seen’ by the components of a biological system.16 Biomolecule–nanoparticle interactions can also change the aggregation state, activity, and dissolution characteristics of a nanoparticle. As a result, biomolecule–nanoparticle interactions within a biological environment influence a nanoparticle's biological behavior. While biomolecule–nanoparticle interactions within biofluids have been studied for a wide array of different nanoparticle types, there is a relative scarcity of research specifically considering ceria nanoparticles (nanoceria). This section briefly describes general principles governing the formation of the biological identity and its influence on downstream biological interactions. Interested readers are referred to more comprehensive reviews for a detailed discussion of this topic.14,15,17 Where appropriate, the highlighted principles are applied to the specific case of nanoceria. In the future, it may be possible to deliberately engineer the biological identity of nanoceria to maximize efficacy and safety, enable new applications, and speed clinical translation. Although the biomolecular corona typically consists of proteins, lipids, sugars, and small molecules, the remainder of this section will focus on protein–nanoparticle interactions because they hold particular biological significance and are the most widely studied.Formation of the biological identity of nanoceria
Biofluids typically contain diverse collections of soluble proteins. Blood plasma, for example, contains over 3700 unique proteins, varying in abundance by over 10 orders of magnitude.18 When a nanoparticle enters a biofluid, proteins begin migrating towards its surface. The most abundant proteins will, on average, arrive first. Often, the most abundant proteins associate weakly with the nanoparticle surface, desorb rapidly and return to solution. Over time, proteins with lower abundance but higher affinity replace proteins with high abundance but low affinity.19,20 Although there is no fixed time period over which the exchange process takes place, the protein corona around many nanoparticle formulations reaches a quasi-equilibrium state within minutes.21,22 Because the nanoparticle surface tends to enrich low abundance proteins, the relative abundance of proteins in the adsorbed protein corona does not, in general, follow the relative abundance of the same proteins in the biofluid.23,24 This has led to the somewhat unexpected observation that the most abundant protein in blood plasma, serum albumin, is found at low abundance within the protein corona around most nanoparticle formulations.15,25 Although it has not yet been established experimentally, the protein corona around nanoceria will presumably enrich low abundance proteins.Proteins within the protein corona are conceptually divided into two classes depending on their adsorption affinity.13,24 Proteins within the ‘hard’ corona are strongly-bound and remain associated with the nanoparticle for minutes or hours. Proteins within the ‘soft’ corona, in contrast, form a dynamic equilibrium with the nanoparticle and rapidly desorb when the biofluid is removed. Casals et al. observed that, over a time period spanning days, the protein corona around nanoceria exposed to fetal bovine serum evolved from a dynamic reversible state to an irreversible state. The authors referred to this process as hardening.26,27 Hardening of the protein corona may be a result of changes in the conformation and orientation of adsorbed proteins.
Because of their prolonged residence time, proteins within the hard corona remain associated with the nanoparticle as it interacts with components of the biological system, including cells. The hard corona thus defines, in part or in whole, the bioactive interface of a nanoparticle in a biological environment, while the soft corona is thought to play a less important role.
Since the hard corona can be isolated from the surrounding biological environment, and given its importance in mediating the biological response, its composition is relatively well characterized. A priori, it is unclear which of the hundreds or thousands of distinct proteins within a biofluid will be incorporated in the hard protein corona around a given nanoparticle formulation. To characterize the composition of the hard protein corona, researchers are relying increasingly on modern quantitative mass spectrometry techniques adapted from the field of proteomics.22,28,29 These techniques are capable of simultaneously identifying and quantifying the relative abundance of proteins within a complex sample by rapidly sequencing peptide fragments generated by proteolysis. Given the prevalence of intravenous injection as a route of nanoparticle administration, the majority of researchers have characterized the composition of the protein corona following exposure to isolated blood plasma or serum. The plasma protein corona around a wide range of distinct nanoparticles including carbon nanotubes,30 metal oxide nanoparticles,31 polymer nanoparticles,23 liposomes,32 silica nanoparticles,24 and gold nanoparticles33 has been extensively characterized. These studies have shown that the plasma protein corona is complex, routinely consisting of dozens of distinct proteins spanning orders of magnitude in relative abundance.14,15 Adsorbed plasma proteins tend to be involved in complement activation, coagulation, opsonization, acute phase responses, and inflammation. However, there is no ‘universal’ plasma protein corona formed around all nanoparticles. Instead, the identities and relative abundances of the adsorbed proteins depend on the nanoparticle composition, size, shape, and surface modification. At the time of writing, no detailed proteomic studies characterizing the plasma protein corona around nanoceria have been published.
It has been widely reported that irrespective of the surface charge of a nanoparticle following synthesis, it acquires a negative surface charge after being incubated in blood plasma.21,33,34 When proteins adsorb to the surface of a nanoparticle, the charge of the nanoparticle reflects the charge of the adsorbed proteins, not the underlying synthesized nanoparticle surface. Because proteins in plasma tend to carry a net negative charge at physiological pH, their adsorption imparts a negative charge to the nanoparticle. Even nanoparticles with cationic surfaces after synthesis undergo a charge reversal upon incubation in blood plasma.35 This observation calls into question the long-standing belief that cationic nanoceria associate with cells by interacting electrostatically with anionic cell-surface glycoproteins.
Upon exposure to a biofluid, protein–nanoparticle interactions may induce nanoparticle aggregation or promote colloidal stability.36 The adsorption of large, bulky, and hydrophilic proteins, such as serum albumin, has been shown to stabilize nanoparticles against aggregation by sterically inhibiting direct nanoparticle contact, whereas the adsorption of smaller proteins that are more hydrophobic or those that carry a charge that is opposed to the that of the nanoparticles tends to promote aggregation.37,38 Protein–nanoparticle interactions can also act to pull apart nanoparticles that are aggregated upon entering a biofluid. Protein–nanoparticle interactions may have a significant influence on the aggregation state of nanoceria within biofluids given that most preparations are aggregated after synthesis.39 Biomolecule interactions within the environment may also alter nanoceria stability, altering its availability and ecotoxicity.40
Influence of the biological identity on the biological response to nanoceria
The mechanism of interaction between nanoparticles and cells, along with the resulting cellular response is typically studied using in vitro cell culture systems. Studies have shown that the presence of serum proteins in the cell culture media decreases the association of carbon nanotubes,41 titanium dioxide nanoparticles,39 silica nanoparticles,42 polystyrene nanoparticles,43 quantum dots,44 gold nanoparticles,44 and graphene oxide nanosheets45 with multiple cell types from distinct tissue sources. Lesniak et al. suggest that, in the absence of serum proteins, nanoparticles with high surface activity interact strongly with biomolecules within the cell membrane, which promotes cell association and uptake.46 When serum is present in the culture media, adsorbed serum proteins ‘passivate’ the nanoparticle surface, rendering it hydrophilic and creating a steric shield that inhibits direct interactions between the cell membrane and the underlying nanoparticle surface.The passivation of a nanoparticle's surface by adsorbed serum proteins can also inhibit physical damage to the cell membrane and cell-surface biomolecules that results in cytotoxicity.47 It has been suggested that the formation of an artificial biomolecular corona may be used as a means of ‘detoxifying’ nanoparticle formulations, including nanoceria, prior to use in medical applications or disposal and release in the environment.48 However, this approach must be taken with care, since degradation or loss of the protective coating could restore the toxicity of the formulations.49
While the formation of a protein corona may suppress non-specific interactions between the pristine nanoparticle surface and the cell surface, specific interactions between cell-surface receptors and adsorbed proteins are also possible. The adsorption of plasma protein opsonins, in particular immunoglobulins and some complement factors, makes nanoparticles more ‘visible’ to tissue-resident macrophages in the liver and spleen.50–52 Macrophages selectively express opsonin receptors including Fc and complement receptors that recognize surface-adsorbed opsonins, leading to efficient nanoparticle uptake.53 Opsonin adsorption and induced aggregation upon exposure to blood plasma are considered two of the most important causes of the rapid clearance of nanoparticles from the blood following intravenous administration.54
Designing nanoceria with controlled biological identities and biological responses
The physical and chemical properties of a nanoparticle determine the nature of protein–nanoparticle interactions within a biofluid and the composition and structure of the protein corona. This is because the propensity of a nanoparticle to engage in interactions with specific amino acid side chains will determine the relative protein affinities.55 Changes in the charge, hydrophobicity, and chemical structure of a nanoparticle's surface will thus influence the composition of the resulting protein corona. For example, acidic carboxy-functionalized polystyrene nanoparticles preferentially adsorb basic proteins (pI > 5.5) from plasma, whereas amine-functionalized polystyrene nanoparticles preferentially adsorb acidic proteins (pI < 5.5).56 It is likely that changes in the physical and chemical properties of nanoceria will influence the composition and structure of the resulting protein corona.Nanoparticle size influences lateral interactions between adjacent adsorbed proteins and the local geometry and structure of the protein binding sites on a nanoparticle. As such, the interaction of biomolecules with nanoparticles tends to be distinct from the interaction of biomolecules with ‘bulk’ materials with the same composition.28 However, there are no obvious correlations between protein molecular weight, isoelectric point, and the trend in relative adsorption between nanoparticle sizes. It is likely that the effect of nanoparticle size on the adsorbed abundance of individual proteins is a complex function of protein size, shape, amino acid composition, and 3D structure. Yet, it appears that the effect of nanoparticle size becomes significant only when it approaches the size of the protein, which typically occurs in the sub-30 nm range.29,57,58 Since nanoceria formulations are in the sub-10 nm range, changes in nanoceria size are expected to influence the composition of the protein corona.
The adsorbed surface stabilizers that are incorporated either synthetically or post-synthetically influence the surface chemistry of a nanoparticle. As such, a given nanoparticle core with a specific geometry, chemical composition, and crystallinity has a range of possible protein coronae, depending on the chemistry, structure, and surface arrangement of the associated surface stabilizers. Rational design of the surface stabilizing molecules is one method that can be used to control protein–nanoparticle interactions and thus the biological behavior of a nanoparticle. Because uncontrolled protein adsorption can often lead to detrimental effects, such as the rapid clearance of nanoparticles by tissue-resident macrophages of the liver and spleen, surface modification strategies have been developed with the goal of altogether eliminating protein adsorption. One of the most popular strategies is to graft the surface of a nanoparticle with long chains of the hydrophilic and conformationally-flexible polymer poly(ethylene glycol) (PEG).59 PEG grafting prevents protein adsorption by blocking protein-binding sites and creating a steric barrier that prevents protein diffusion to the nanoparticle surface.60 While PEG grafting is effective at reducing plasma protein adsorption and macrophage uptake, and hence increasing blood half-life, it also reduces interactions with target cells, which can lower target-site localization.29,61,62
Instead of designing the surface of a nanoparticle to eliminate protein adsorption, it may instead be designed to selectively adsorb specific plasma proteins that promote target site localization. Using nanoceria as a reactive oxygen species scavenger for the treatment of neurodegenerative diseases is one of its most promising therapeutic applications. The delivery of functional nanoceria to the brain is a prerequisite for such applications, but is also a significant technical challenge. If nanoceria are administered intravenously, they must not only evade capture by tissue-resident macrophages, but must also cross the layer of endothelial cells that comprises the blood–brain barrier (BBB). Studies have shown that coating polymer nanoparticles with polysorbate 80 can significantly enhance their transport across the BBB.63 Adsorbed polysorbate 80 promotes the selective adsorption of apolipoproteins B and E to the nanoparticle surface, which facilitate transcytosis by interacting with lipoprotein receptors expressed on the surface of BBB endothelial cells.64,65 Surface-modifying nanoceria to selectively adsorb apoliproteins B and E from blood plasma may also facilitate their transport across the BBB. However, it is not yet clear whether polysorbate 80 modification of nanoceria will promote the adsorption of apolipoproteins B and E.
The importance of protein–nanoparticle interactions in the fields of nanomedicine and nanotoxicity has begun to emerge, suggesting a critical role of the protein ‘corona’ in mediating the biological effects of nanoparticles. Development of the corona is a dynamic, two-step process that results in the evolution of a stable, ‘hard corona’ and a more labile ‘soft corona’. The cellular effects of the protein–nanoparticle interface are diverse and the have only recently been studied in simplified biological systems. The effects of protein binding include cell membrane receptor activation and altered routes of endocytosis and cellular trafficking. The nature of this protein mantle is dictated by the size, composition and surface properties of the nanoparticle. The astounding divergence in the abundance and nature of proteins comprising this layer has been described with only very modest changes in physico-chemical attributes.66 Given the complexity of these interactions, a formidable task lies ahead in understanding the nature of these interactions, predicting the biological outcome of nanoparticle–protein interactions and studying these effects in intact, living systems. This is particularly relevant for harnessing the potential of nanoceria in biological applications. The inherent ability of nanoceria to participate in redox-coupled reactions reflects their significant surface reactivity. The adsorption of proteins to nanoceria may reduce its catalytic potential but, more importantly, may alter the conformation of proteins bound to the particle, exposing novel biological epitopes that may drive downstream cellular effects not normally associated with the native protein. Gaining insight into the nature of these complex interactions is critical for the rational design of nanoceria in the future.
III. Cerium oxide nanoparticles act as biological mimics of enzymes involved in oxidative stress defense
Cerium oxide nanoparticles (nanoceria) act as superoxide dismutase mimetics
Superoxide (O2˙−), the product of a one electron reduction of the dioxygen diradical (O2), is a key reactive oxygen species in biological systems.67 It is generated as a by-product of a number of metabolic processes such as the respiratory chain in the mitochondria as well as enzymes such as xanthine oxidase.68 Although it can spontaneously decompose into hydrogen peroxide, it also can react with other radicals such as the nitric oxide radical (˙NO) to generate other oxidants including peroxynitrite (ONOO−). Because of these reactions, mammals and many microbes produce catalysts to limit the steady state concentration of superoxide in the cell, primarily superoxide dismutases and superoxide reductases.69 However during inflammation, the production of superoxide can overwhelm these defenses leading to increased reactive oxygen species (ROS) and reactive nitrogen species (RNS). Thus keeping superoxide levels in check is a key determinant in the limitation of inflammation.Nanoceria were first shown to be superoxide dismutase (SOD) mimetics by Korsvik et al.70 in 2007. In this report two preparations of nanoceria were studied – one with very few cerium atoms in the +3 oxidation state at the surface and a second material with higher levels of cerium atoms in the +3 oxidation state based on X-ray photoelectron spectroscopy (XPS) analysis. This was the first report of a catalytic activity for this material under biologically relevant conditions (aqueous buffered near neutrality). Indeed it also established an important parameter – that the synthesis of the nanoparticles and the surface chemical properties of this material can make a significant difference in terms of catalytic activity of the material. A follow up study focused on further analysis of this surface chemistry revealed that the nanoparticles with higher amounts of cerium atoms in the +3 oxidation state at the surface could be altered by excess hydrogen peroxide to revert to a surface chemistry with lower levels of this reduced state.71 In line with Korsvik et al., this change in surface chemistry also correlated with a reduction in the SOD mimetic activity.
The impact of PEGylation on the SOD mimetic activity by nanoceria
The first study to address the generation of a biocompatible surface of cerium oxide utilized polyethylene glycol polymers (PEGylation). The presence of PEG on the surface of the nanoceria did not prevent SOD mimetic activity, and the level of cerium in the +3 oxidation state again correlated well with the SOD mimetic activity.72 However the extent of PEG coverage may alter the reactivity of the surface depending on the synthesis procedures used to make the nanoceria. Another attempt to make nanoceria more stable was reported in 2012 using poly(lactic-co-glycolic acid) (PLGA).73 The PLGA coated nanoceria retained SOD mimetic activity for a longer period of time (90 days) after synthesis, a process known as ‘aging’. The precise changes and the mechanism of these changes that occur during aging are not well understood. In addition to surface changes for biomaterial applications, another report showed that phosphate ions could also shift the surface oxidation state of nanoceria and result in changes in SOD mimetic activity.74 This study presumed that the phosphate ions are binding in or near oxygen vacancies that are predicted to lead to reduced cerium atoms at the surface, although it should be noted that phosphate binding to other cerium oxide nanoparticles may or may not affect their catalytic activities depending on the presence of stabilizing groups. Recent work showed that phosphate ions are binding on ceria nanoparticles enriched in the +3 oxidation state while they do not adsorb when Ce is mostly in the +4 valence state.75Evidence for SOD mimetic activity in vivo
Estevez et al. demonstrated in an ischemic brain slice model that cerium oxide nanoparticles exhibited potent SOD mimetic activity.76 A more recent report by Ganesana et al. studied SOD mimetic activity of nanoceria in a brain slice model and demonstrated that administration of nanoceria did lead to reduction in the superoxide levels in this ex vivo system. In this study the authors were able to show that 5 micromolar levels of nanoceria is equivalent to 580 units of SOD activity – a remarkable level of catalytic activity in this model.77 This key finding indicates that under biologically relevant conditions nanoceria do exhibit SOD mimetic activity.77 Thus all these reports have validated the initial report that nanoceria can exhibit SOD mimetic activity by a variety of assay systems and also show that this activity is likely occurring in vivo. Studies that tie this activity to efficacy of nanoceria are not discussed here but are discussed elsewhere in this article and compendium.Although there are significant amounts of wet chemistry data that correlate the SOD mimetic activity with surface chemistry and material synthesis, the core reaction mechanism is still not well understood. A recent study that used a combination of molecular dynamics (MD) and density functional theory (DFT) alongside wet chemical measurements has shed some light on the reactivity of nanoceria with superoxide.78 In this study, the presence of water on the surface of nanoceria was investigated and the results suggest that cerium oxide that is synthesized in water contains reactive regions that are altered upon dehydration. To confirm these predictions, water based nanoceria were synthesized and shown to have SOD mimetic activity only prior to dehydration. Removal of water from this preparation significantly reduced the SOD mimetic activity of the nanoparticles. This study can lead to future work to better understand the mechanism of superoxide dismutase activity of nanoceria.
Nanoceria display catalase mimetic activity
Pirmohamed et al. have also described the catalase mimetic activity of nanoceria.79 Several classes of enzymes are involved in the modulation of hydrogen peroxide levels in mammals, including catalase, glutathione peroxidases and peroxiredoxins. The overall catalytically weak activity of nanoceria as compared to the highly efficient catalyst catalase initially suggested this activity might not be relevant in vivo.79 However studies have shown that peroxides are likely the most stable and abundant ROS in vivo, and thus a catalyst that can reduce peroxide levels could prove critical during inflammation or to prevent metal catalyzed oxidation reactions (Fenton reactions) from occurring. However the role that peroxides play in biology is growing, and the level of peroxides is modulated by a number of enzymes (glutathione peroxidases, peroxiredoxins, catalase), many of which require reducing potential in the form of thioredoxin and glutaredoxin.80Moreover the catalase mimetic activity of nanoceria is inversely related to the SOD mimetic activity in terms of the surface charge of cerium. In other words, when the concentration of cerium atoms at the surface of the nanoparticle in the +3 oxidation state are more abundant, the catalase mimetic activity is weak.79 It was in this initial study that we stumbled upon (by accident) the effect of phosphate ions on these activities. A follow up study showed that phosphate can interconvert nanoceria from SOD to catalase mimetics in vitro, correlating with cerium reduction state.74 This suggests overall that reduced cerium sites, i.e. oxygen vacancies, do not contribute to catalase mimetic activity and thus reduce the overall reactivity of the nanoparticle. This also indicates that, given that the level of phosphate in most biological systems is in the millimolar range, the reactivity of nanoceria with phosphate must be taken into account when correlating chemical reactions in a test tube to chemistry occurring in vivo. In contrast, a very recent report by Vicki Colvin's group has shown that nanoceria produced by an alternate method (not water based) are good catalase mimetics, and the authors of this study suggest that oxygen vacancies are critical to the reactivity with hydrogen peroxide.81 In addition the authors found that when coated with oleic acid, these nanoceria were quite stable over a long period of time and were more robust as catalysts in successive reactions. This adds to our understanding of CeNP stability and the properties resulting from synthesis, but will also lead to renewed efforts to understand the catalysis that is occurring at the surface as well. Another study has shown that aqueous nanoceria have both SOD mimetic and catalase mimetic in vivo.82 It should also be noted that some groups have questioned the presence of cerium in the 3+ oxidation state,83 thus given the disparity in the results of several studies there is likely an underlying mechanism that has yet to be elucidated.
Evidence for scavenging of nitric oxide radical
The first report that conclusively demonstrated the scavenging of reactive nitrogen species is that of Estevez et al.76 This study was done in an intact brain tissue that generated reactive oxygen and nitrogen species in vitro, and thus it is clear that nanoceria can directly affect (reduce) RNS in vivo. In parallel work in vitro, Dowding et al. investigated whether nanoceria could react with biologically relevant reactive nitrogen species. The radical gas nitric oxide (˙NO) can be followed by a number of techniques and can be generated by a series of chemicals known as NONOates. Dowding measured the level of nitric oxide generated from NONOates using two independent methods,84 and followed the reaction of nitric oxide with the ferrous form of hemoglobin. This study found that in the presence of nanoceria with a low level of cerium in the +3 oxidation state, the nanoceria could prevent the interaction with ferrous hemoglobin in a dose dependent manner.84 This indicated that nitric oxide was interacting with nanoceria. An alternate NONOate was also tested by an alternate method to follow nitric oxide, a copper fluorescein complex. Again the presence of nanoceria with lower levels of cerium in the +3 oxidation state reduced the reaction of NO with the copper complex, indicating a reactivity with nanoceria.84 In both cases positive controls such as glutathione or a radical scavenger (DEPMPO) were also shown to compete. These results suggest strongly that nanoceria can scavenge nitric oxide. Because the level of nitric oxide is difficult to follow in vitro, the kinetics of this reaction has not yet been established.Nanoceria accelerate the decay of peroxynitrite
Peroxynitrite (ONOO−) is a strong oxidizing agent that is formed primarily from the reaction of superoxide and nitric oxide radicals.85 Given the significant reduction in nitrotyrosine levels (about 70%) observed by Estevez,76 the possibility that nanoceria reacted with peroxynitrite was raised. This potent oxidant and nitrating species is stable under basic conditions, yet spontaneously decomposes at neutral or near neutral pH. This allowed for the reaction of peroxynitrite in a controlled (pH dependent) fashion with nanoceria.86 Dowding observed a significant increase in the decay of peroxynitrite when nanoceria were present in a buffered reaction near physiological pH levels.87 A comparable peroxynitrite scavenger, uric acid, was needed in millimolar levels to show the same accelerated decay (Fig. 1). Thus it is likely that nanoceria are potent reactants with peroxynitrite in vivo, yet this is difficult at best to study in vitro, given the cadre of end products that occur upon peroxynitrite decay.85 The boronate probe used to follow peroxynitrite levels have been shown to be preferentially detecting peroxynitrite.87 Nanoceria were also shown to reduce 3-nitrotyrosine levels in proteins when exposed to peroxynitrite in a test tube,87 mirroring the results obtained in vitro.76 This indicates by a variety of methods that nanoceria are reacting with peroxynitrite directly or one of its many radical end products during decomposition, such as the carbonate radical.85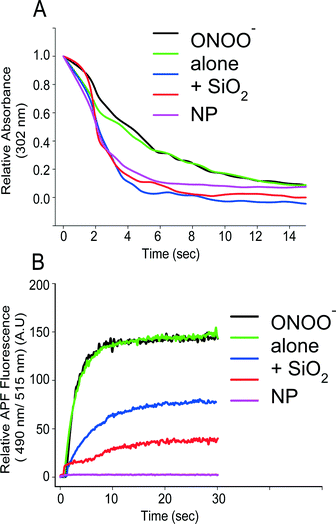 | ||
| Fig. 1 CeO2 NPs accelerate the decay of peroxynitrite in vitro. (A) Relative absorbance of peroxynitrite (25 μM) at 320 nm over time (seconds) either in the absence or presence of CeO2 nanoparticles (100 μM), SiO2 nanoparticles (100 μM), uric acid (UA) (1 mM), or glutathione (GSH) (0.5 mM). (B) Relative APF (10 μM) fluorescence at 490 nm excitation and 515 nm emission wavelength of either peroxynitrite (20 μm) alone, or in combination with CeO2 nanoparticles (100 μM), SiO2 nanoparticles (100 μM), uric acid (1 mM), or glutathione (0.5 mM) measured over a time period of 14 seconds. Data are representative of three or more experiments. Reproduced with permission from Drug Delivery Transl. Res.87 | ||
Given the wide distribution of the types of nanoceria, and the differences observed in catalytic activity, a summary of a wide range of studies in which the surface chemistry of nanoceria has been combined with the study of catalysis is listed in Table S1 (ESI†). This table enables a comparison of the types of nanoceria synthesis method, nanoceria catalysis and references the study from which the data are derived. Albeit not all inclusive, this can give the reader a guide to the kinds of correlations that have been observed for surface chemistry analysis and catalytic activities.
IV. Uptake and distribution of cerium oxide using in vitro cell culture models and in vivo models
Redox active cerium oxide nanoparticles (nanoceria) can scavenge reactive oxygen (ROS) as well as reactive nitrogen species (RNS). It is interesting to mention that the regenerative nature of nanoceria' surface make these nanoparticles different from other known antioxidant molecules. Different bio-medical applications of nanoceria that have been explored in the last few years include inhibition of neovascular macular degeneration,88–90 protection against laser induced eye damage,5 protection against radiation induced tissue damage,4,91,92 induction of pro-angiogenesis and wound healing,93–96 management of ischemic stroke, neurodegenerative diseases,9,97–100 anti-angiogenesis and inhibition of tumor stroma interaction.101–104 These studies, using both in vitro and in vivo models, have shown that nanoceria have the potential to be developed as a therapeutic agent for a wide range of pathologies associated with oxidative injury.Subcellular localization of nanoceria
Nanoparticles' cellular interaction, cellular uptake and subcellular localization are determined by physical properties of the nanoparticles which include nanoparticles' size/agglomeration status in media, surface charge, and residual surfactant/presence of functional group/coating on the surface of the nanoparticles. Negatively charged nanoceria particles were taken up effectively in a cell culture model (A549) in one of the first studies to address uptake.105 These nanoceria were bare, but the potential for difference in uptake in cells based on surface bound chemicals (ions, lipids, proteins, protein corona) likely can alter uptake efficiency and the mechanism(s) of uptake. Residual hexamethylenetetramine (HMT) on the surface of the nanoceria not only changes the catalytic property of the nanoparticles (increased phosphatase activity, and loss of SOD and catalase mimetic activity), but also led to increases in the cellular uptake (HUVEC cell culture). Higher surface charge nanoparticles nanoceria2 (30.2 mV) and HMT-nanoceria (34.6 mV) were found to be taken up with greater efficiency as compared to nanoceria1 (18 mV). Moreover, cellular uptake of HMT-nanoceria was two fold higher compared to nanoceria2, which suggests that the presence of a functional group/stabilizer can influences nanoparticle–cell interaction and therefore cellular uptake.86 In our previous study we have shown that for nanoceria1 and nanoceria2 after incubation in HUVEC cell culture medium both show ~(−)9 mV surface charge,93 therefore differential cellular uptake may be due to difference in physicochemical properties. In particular, presence of functional groups/molecule on the surface of the nanoparticles can have greater influence on nanoparticle–cell interaction then surface charge, which may explain different trend in the uptake observed in these two studies.86,105 Moreover, two different cell lines used in these studies cannot be ignored. Since these nanoceria were made in different ways this emphasizes influences of the variability of the nanoparticles physiochemical properties in biological systems.The cellular uptake mechanism of nanoceria has also been studied using carboxy-fluorescence conjugated nanoceria (F-nanoceria). Energy dependent, clathrin and caveolae-mediated endocytosis was reported for the cellular uptake of F-nanoceria. F-nanoceria were observed localized in lysosomes, the endoplasmic reticulum and mitochondria, in addition to the cytoplasm and nucleus.106 The presence of nanoceria in all major subcellular compartments ensures protection against a variety of oxidative stresses. However it should be noted that the behavior of bare nanoceria as compared to that conjugated with a dye is likely quite different.
Cerium oxide bio-distribution and route of administration
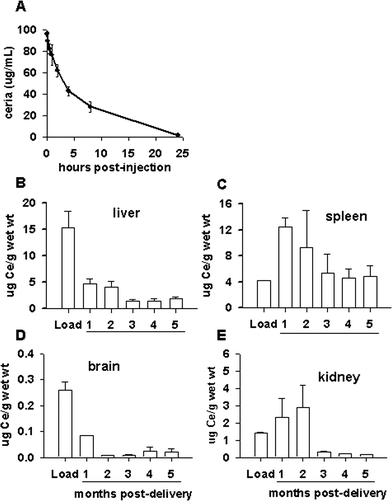
The route of administration of the nanoparticles is another important parameter to assess in delivering materials to particular target organs. achieve maximum benefits of nanoceria. In vivo real time imaging revealed that intravenous injection of F-nanoceria deposited in liver within 3 h of injection. Moreover, F-nanoceria were mainly found deposited in the liver, spleen and lungs and persisted 2 weeks after F-nanoceria injection.To examine bio-distribution of bare nanoceria, 0.5 mg kg−1 of body weight of nanoceria (once in a week) were administered in three different routes, perorally (PO), intravenously (IV), or intraperitoneally (IP) for two and five weeks. IV administration resulted in the greatest tissue deposition (liver, kidney, heart, brain, spleen and lung) of nanoceria, followed by the IP and PO routes, results that were quantified using inductively coupled plasma mass spectroscopy. For IV and IP routes, nanoceria mainly deposited in the spleen and liver whereas very low amounts were found in the lungs and kidneys. It is important to mention that this specific formulation of nanoceria was not found in heart or brain. Nanoceria administered in PO route showed minimal organ deposition. Tissue accumulation was highest following IV administration. Multiple weekly systemic injections of nanoceria over a period of 5 weeks did not show any overt pathology in different organs.107
On the other hand citric acid coated nanoceria showed different tissue distribution and in addition to spleen and liver citric acid nanoceria were also found in brain when injected though IV route. Citric acid coated nanoceria are reported to have increased blood circulation time. Citric acid nanoceria were even found in blood circulation after 30 days of administration, and as expected, longer blood circulation times were observed for smaller nanoparticles (5 nm) as compared to larger nanoceria (55 nm).108 Therefore, bio-distribution and pharmacokinetics data of the nanoparticles will help to select nanoceria formulation for specific application.
V. Mechanistic studies on the cell effects of nanoceria
The bio-effects of nanoceria are generally attributed to the Ce3+/Ce4+ redox switch.109 The oxygen vacancies may also theoretically exert a biological activity of their own, i.e., independently of the redox cycle. Modifications of the materials through nanoceria doping with Sm, which consists in the substitution of Sm that has a fix 3+ valence in the Ce lattice sites, ends up in eliminating the redox switch activity and in decreasing the Ce3+ concentration, thereby keeping the number of oxygen vacancies almost constant. The comparison of the effects exerted by nanoceria and Sm-doped nanoceria allows discriminating the eventual roles played by oxygen vacancies or the Ce3+/Ce4+ redox couple. With this approach, it was possible to demonstrate that the antioxidant and antiapoptotic activity of nanoceria in a system of human monocytes are due to the redox activity of nanoceria, since they were abolished by Sm-doping.110 It is uncertain whether oxygen vacancies possess biological activity, and so far none has been described.Nanoceria was shown to increase cell survival in conditions of stress by inhibiting apoptosis.109 Apoptosis is often a consequence of oxidative stress, therefore it is conceivable that anti-oxidant agents such as nanoceria, decreasing the insult, increase cell survival. This was demonstrated in a study on U937 cells, where oxidative vs. non-oxidative apoptosis can be recognized by a different cell morphology.111 Nanoceria was shown to inhibit only oxidative apoptosis, whereas the non-oxidative pathway remains unaffected.110 In the search for the mechanism at the basis of this effect, it was observed that the depletion of glutathione (the main intracellular antioxidant), a phenomenon that is a hallmark of stress-induced apoptosis,112 is prevented by nanoceria,110 suggesting that nanoceria may act on glutathione metabolism, and specifically on its transporters, which are protein complexes present on the cell membrane that allow the export of glutathione outside cells, and are redox-regulated proteins.113 Recently the antioxidant and cell protective action of nanoceria was confirmed in a cell inflammatory model, where it is shown that nanoceria protect from alterations in oxidative metabolism induced by the cytotoxic agent tumor necrosis factor (TNFα).114
Nanoceria were shown to affect endogenous cell defenses, modulating the activity of redox-sensitive nuclear transcription factors such as nuclear factor-kappa B (NF-κB)91,115,116 and activator protein 1 (AP1),91 which are devoted to the promotion of cell survival pathways. Moreover, nanoceria increase the expression of superoxide dismutase, thereby ameliorating the ROS scavenging effect in response to ionizing radiation.91,115 Nanoceria possess anti-inflammatory ability by inhibiting NF-κB activation, thereby reducing the expression of the downstream pro-inflammatory proteins,116–118such as inducible nitric oxide synthase (iNOS) and the pro-inflammatory cytokines produced by stimulated macrophages.117,119
Several studies show that nanoceria selectively protect from damage normal but not cancer cells; in fact, in cancer cells nanoceria is even pro-apoptotic.91,115 These findings are of paramount importance, because to date no agents that are selectively toxic for cancer cells have been found.
It was hypothesized that the selective toxicity of nanoceria against cancer cells is due to the inhibition of nanoceria catalase-like activity occurring in acidic (pH 4.3) environments:120 the hypothesis is based on the assumption that pH of cancer microenvironment is acidic due to the Warburg effect.91 Moreover, the pH-dependent inhibition of catalase might play a role in the selective toxicity of nanoceria on tumor cells considering the very acidic pH (~4) present within the lysosomal cell compartment.121 Interestingly, it is shown that the cytosolic localization of nanoceria is different in normal vs. cancer cells, being cytosolic vs. lysosomal, respectively. Internalization of the same preparation of nanoceria vary in tumor vs. normal cells, being localized in lysosomes vs. cytosol, respectively.121 Future research will undoubtedly focus on the reason why tumor vs. normal cells impart a different localization to nanoceria.122
Cerium oxide nanoparticles protect cells from ionizing radiations.115 However, in some instances irradiated nanoceria behave as pro-oxidant agents. Indeed, it was shown that specific intensities of X-rays, peaking at 50–60 keV, induce the production of reactive oxygen species.123 At the same time, the Ce3+/Ce4+ redox couple on nanoceria surface scavenges the reactive oxygen species produced by irradiation, and therefore the resulting effects depend on the balance between these two opposite mechanisms.91
Oxidative stress has important implications in cardiovascular diseases as their reason and/or consequence.124 Cardiac progenitor cells are promising as source for autologous tissue engineering for cardiac repair.125 ROS are paramount for reducing stem cell lifetime, inducing senescence,126 and impairing self-renewal.127 Nanoceria may counteract these effects.128 Thus, the internalized nanoparticles worked as a potent, long-lasting ROS scavenger able to protect cells from the oxidative damage.
In the near future, much effort will be devoted to: i) elucidate the cellular mechanisms behind the action of cerium oxide as a function of the cell type; ii) understand the interactions between this promising material and cells; iii) understand the peculiar differential effects nanoceria exert on tumor vs. normal cells, with the goal of proposing novel approaches to cancer therapy; iv) to implement the studies on the effects of nanoceria in the improvement of tissue engineering.
VI. Traversing physiological barriers: differences in biological test beds
Most studies using nanoceria over the past decade have examined biological effects using reduced, in vitro preparations that do not fully recapitulate in vivo interactions.4,6–9,91,117 Most often, in vitro approaches have made use of immortalized cell lines that differ in their genetic and phenotypic profile compared to endogenous, native cells. The principle advantage of in vitro studies is the ability to make detailed, single cell measurements of primary cell cultures or tissues slices that retain intact architecture and more accurately reflect cellular function of native tissues. However, these preparations may not be ideal as there are fewer barriers between the bulk solution and cellular uptake of the particles compared to in vivo models that have many more anatomical barriers that must be overcome for nanoceria to reach a biological target. For example, particles entering the lung or gastrointestinal tract destined for the blood stream would need to traverse at least ten separate biological barriers before reaching their target,129 and the number of barriers could exceed fifteen if the target cell were a neuron (depending on the route of delivery). At each barrier, the nanoparticle would encounter cell membranes that may vary in their composition as a function of their polarity (i.e. apical and basolateral membranes) and the tortuosity of paracellular fluid movement through tissue or uptake by mobile cell types (immune cells). As the nanoceria transitions across barriers, the fluid compartments it encounters will also vary in their ionic strength, pH and protein content, all of which can profoundly influence both the physical and chemical attributes of the particle and hence its biological activity.Importance of nanoparticle stabilizers
To date, studies exploring the therapeutic potential of nanoceria using intact animals are limited,89,91,100,130 and most investigators have utilized nanoceria ranging in size from 3 to 55 nm, often with very negative zeta potentials (< −50 mV; with the exception of the eye) administered as spherical, nanodispersions of bare ceria particles or nominally stabilized with citrate or polyethylene glycol.100,108 In rodent models, bare particles have very short plasma half-lives and are prone to aggregation and accumulation in reticulendothelial organs.89,108,131 Indeed, non-stabilized nanoparticles frequently bind complement or other proteins that lead to rapid removal of the nanomaterial by circulating and tissue resident macrophages.29,132 Commercially available, unstabilized or citrate-stabilized cerium oxide nanoparticles deposit in the highest levels in the spleen and liver in rodents.89,108,131,133,134 Delivery of non-stabilized, 3–5 nm particles (cumulative dose 2.5 mg kg−1) over a 5 week period resulted in ceria deposition of >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ug g−1 in the liver and spleen one week after the final injection.107 In contrast, single injections of 5 nm (85 mg kg−1) or 30 nm (70 mg kg−1) citrate-stabilized nanoceria resulted in 500–1000 ug g−1 nanoceria in the liver and spleen.108 Thus, non-stabilized nanoceria material is often retained in the reticuloendothelial system to a much greater extent than citrate-stabilized particles, regardless of particle size. Not surprisingly, the plasma half-life of these formulations of nanoceria are also short: ~7 minutes for non-stabilized nanoceria and 1 hour for citrate stabilized particles, suggesting that more durable coatings increase circulation times and reduce reticuloendothelial system uptake.133,135 However, many of the stabilizers (i.e. citrate, acetate) that are often added to prevent aggregation and optimize surface charge are readily washed off when the particles are exposed to physiological salt solutions. PEGylation appears to desorb from the particle less quickly, thereby increasing plasma circulation times, reducing scavenging by tissue-resident or circulating monocytes as well as reducing deposition in the liver and spleen.72 Although the characteristics imparted by PEG are a distinct advantage in improving biocompatibility and increasing target tissue accumulation associated with increased circulation times, these benefits are offset by the possibility of diminished catalytic activity of ceria with high density PEG coating in which the surface chemistry dominates chemical reactivity. In addition, depending on the molecular weight of the PEG used in synthesis, significant increases in particle size can occur. Intravenous delivery of 3 nm, PEGylated nanoparticles significantly reduced infarct size following middle cerebral artery occlusion, and the particles resisted agglomeration in high salt solutions for days, illustrative of a highly durable coating that retained catalytic activity for weeks in solution.100 Curiously, these tissue sparing effects were present only at two similar doses (0.5 and 0.7 mg kg−1), but not at higher or lower dosing (range = 0.1–1.5 mg kg−1 intravenous injections).
000 ug g−1 in the liver and spleen one week after the final injection.107 In contrast, single injections of 5 nm (85 mg kg−1) or 30 nm (70 mg kg−1) citrate-stabilized nanoceria resulted in 500–1000 ug g−1 nanoceria in the liver and spleen.108 Thus, non-stabilized nanoceria material is often retained in the reticuloendothelial system to a much greater extent than citrate-stabilized particles, regardless of particle size. Not surprisingly, the plasma half-life of these formulations of nanoceria are also short: ~7 minutes for non-stabilized nanoceria and 1 hour for citrate stabilized particles, suggesting that more durable coatings increase circulation times and reduce reticuloendothelial system uptake.133,135 However, many of the stabilizers (i.e. citrate, acetate) that are often added to prevent aggregation and optimize surface charge are readily washed off when the particles are exposed to physiological salt solutions. PEGylation appears to desorb from the particle less quickly, thereby increasing plasma circulation times, reducing scavenging by tissue-resident or circulating monocytes as well as reducing deposition in the liver and spleen.72 Although the characteristics imparted by PEG are a distinct advantage in improving biocompatibility and increasing target tissue accumulation associated with increased circulation times, these benefits are offset by the possibility of diminished catalytic activity of ceria with high density PEG coating in which the surface chemistry dominates chemical reactivity. In addition, depending on the molecular weight of the PEG used in synthesis, significant increases in particle size can occur. Intravenous delivery of 3 nm, PEGylated nanoparticles significantly reduced infarct size following middle cerebral artery occlusion, and the particles resisted agglomeration in high salt solutions for days, illustrative of a highly durable coating that retained catalytic activity for weeks in solution.100 Curiously, these tissue sparing effects were present only at two similar doses (0.5 and 0.7 mg kg−1), but not at higher or lower dosing (range = 0.1–1.5 mg kg−1 intravenous injections).
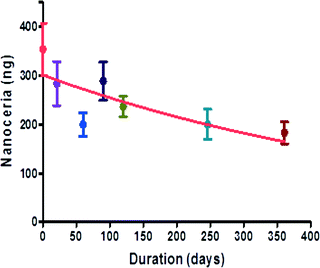
A unique stabilizer package was recently developed that includes both citrate and the metal chelator EDTA on a 2.9 nm ceria nanoparticle; these nanoceria distribute to the brain when injected intravenously into mice.82 This combination of citric acid and EDTA results in highly monodispersed particles that resist agglomeration in high salt solutions and cannot be pelleted out using ultracentrifugation (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 degrees Celsius). A hallmark of the importance of this acid/chelator combination is our finding that removal of the citrate from this stabilizer package by either aggressive washing and centrifugation or synthesizing Ce–EDTA particles without citrate eliminates both the ROS neutralizing activity of the particles and their deposition in the brain (unpublished results). Pharmacokinetic studies of these particles administered intravenously in the rat revealed that the half-life of citrate/EDTA nanoceria was 3.7 hours (Fig. 2A), considerably longer than ceria stabilized with citrate alone and far longer than ‘bare’ particles. Again, this supports the use of a more durable stabilizer that seems to avoid active removal by cells of the immune system. Consistent with this notion of reduced clearance by reticuloendothelial organs, tissue levels of nanoceria in the liver and spleen were extremely low in healthy animals82 compared to previous studies using 5 nm, citrate stabilized nanoceria at similar time points.108 Renal clearance of negatively charged particles is thought to occur quickly with particles less than 6 nm in size, but nanomaterials larger than 6 nm are likely to end up in the liver and the spleen.136 The stabilizer package of citric acid and EDTA appears to be unique. Extensive screening of a variety of other biocompatible acids and metal chelator combinations have yielded lower performing particles (unpublished results) tested in a hippocampal brain slice model of oxidative injury76 than the combination of citrate and EDTA. Although the chemistry associated with this combination of stabilizers is not yet clear, it is possible that the citric acid may serve as an electron transfer agent between the particle surface and the reactive oxygen species, whereas the EDTA may serve to hold the ceria/citrate complex together. Given that the zeta potential of the citrate/EDTA particle (−23.5 mV) exceeds that of citrate (−20.8 mV) or EDTA alone (−15.7 mV), there may be a synergy with the combination of compounds. Clearly, parsing apart the chemistry will be necessary to understand the chemical interactions of this complex to optimize its biological action.
000 × g, 4 degrees Celsius). A hallmark of the importance of this acid/chelator combination is our finding that removal of the citrate from this stabilizer package by either aggressive washing and centrifugation or synthesizing Ce–EDTA particles without citrate eliminates both the ROS neutralizing activity of the particles and their deposition in the brain (unpublished results). Pharmacokinetic studies of these particles administered intravenously in the rat revealed that the half-life of citrate/EDTA nanoceria was 3.7 hours (Fig. 2A), considerably longer than ceria stabilized with citrate alone and far longer than ‘bare’ particles. Again, this supports the use of a more durable stabilizer that seems to avoid active removal by cells of the immune system. Consistent with this notion of reduced clearance by reticuloendothelial organs, tissue levels of nanoceria in the liver and spleen were extremely low in healthy animals82 compared to previous studies using 5 nm, citrate stabilized nanoceria at similar time points.108 Renal clearance of negatively charged particles is thought to occur quickly with particles less than 6 nm in size, but nanomaterials larger than 6 nm are likely to end up in the liver and the spleen.136 The stabilizer package of citric acid and EDTA appears to be unique. Extensive screening of a variety of other biocompatible acids and metal chelator combinations have yielded lower performing particles (unpublished results) tested in a hippocampal brain slice model of oxidative injury76 than the combination of citrate and EDTA. Although the chemistry associated with this combination of stabilizers is not yet clear, it is possible that the citric acid may serve as an electron transfer agent between the particle surface and the reactive oxygen species, whereas the EDTA may serve to hold the ceria/citrate complex together. Given that the zeta potential of the citrate/EDTA particle (−23.5 mV) exceeds that of citrate (−20.8 mV) or EDTA alone (−15.7 mV), there may be a synergy with the combination of compounds. Clearly, parsing apart the chemistry will be necessary to understand the chemical interactions of this complex to optimize its biological action.
Clearance of nanoceria
The clearance of nanoparticles also reflects how the body views the material as a biological entity. High levels of ‘bare’ or citrate stabilized nanoparticles are retained in the liver in rodents up to 90 days post-administration, and, importantly, little decrement was observed in tissue levels over time.133 The high concentration and long duration of residence may likely contribute to the observed toxicity in the liver and the spleen associated with these formulations of ceria.133,137 In contrast to these findings, a single 20 mg kg−1 intravenous dose of citrate/EDTA stabilized nanoceria showed progressive clearance from the brain, liver and spleen over a six month period with large decreases being observed after 3 months in the tissues examined (Fig. 2b–e) and ref. 82. The mechanism(s) of ceria clearance in intact animals have not been well studied and are not currently known. Although decreases in ceria content were noted, low levels of ceria in the liver, spleen and brain persist for months,82 though by 12 months post-injection, ceria levels are near detection limits for most organs (unpublished data). Histopathology of livers harvested from mice administered 30 mg kg−1 of citrate–EDTA stabilized nanoceria per week for 5 weeks were unremarkable compared to saline injected controls,82 however the biological effects of lengthy residence times is not currently known. Although quantitative estimates of cerium elimination are rare, it appears that the primary route of elimination for cerium, regardless of route of administration, is through the feces, with smaller (<10%) amounts eliminated in the urine.138,139 It has been suggested that the fecal excretion of systemically absorbed cerium is due to elimination in the bile,138 since hepatic clearance was due primarily to biliary function.Moving forward, additional studies will be needed to identify the clearance routes and evaluate the biological consequences of persistent low levels of ceria in tissues for extended periods of time. Taken together, these data show that the choice of stabilizers and their durability are critical in evaluating the biological impact of metal oxides like nanoceria and their potential for drug development.
Nanoceria's impact on immune system homeostasis
As with any other foreign molecule, the introduction of nanoceria to an organism has the potential to modulate the function of the intact immune system. Typically, the size of individual ceria nanoparticles would allow them to escape detection by the immune system, but any tendency of nanoceria to accumulate into larger aggregates increases the likelihood of uptake by phagocytic immune cells and the potential to initiate an immune response.140 Further, while nanoceria itself may not be immunogenic, the particles could act as haptens by adsorbing to endogenous proteins of the necessary size and complexity to function as carriers.141 The resultant response is similar to the development of an allergy to drugs like penicillin that become immunogenic in some patients when interacting with host proteins. Thus, selecting the appropriate stabilizer for nanoceria synthesis is also important for minimization of self-aggregation and protein adsorption in the context of the immune system.Cells of the innate immune system, particularly phagocytic macrophage and neutrophil cells, are those most likely to trigger immediate inflammatory responses to nanoceria exposure. In the skin, resident Langerhans cells (a type of dendritic cell) and rapidly recruited neutrophils and macrophages can clear the area of nanomaterials, but may be triggered by nanoceria aggregates or nanoceria complexing to other proteins to release pro-inflammatory cytokines. Human primary neutrophils exposed to sodium polyacrylate stabilized nanoceria generated increased levels of matrix-metalloproteinase 9 and myeloperoxidase and upregulated markers of degranulation.142 While these characteristics would be desirable during a response to pathogen exposure, unnecessary release of such tissue destructive enzymes could be damaging to healthy tissues. However, the authors noted aggregation of these nanoparticles,142 which increases the likelihood that scavenger receptors and complement receptors might be engaged to initiate this behavior. Stabilizing the nanoceria with a coating less likely to trigger aggregation may in fact alleviate this effect. Indeed, the choice of stabilizer and the resulting chemical characteristics did impact uptake by non-immune cell lines in several studies.121,143 Increased cellular uptake of heparin-functionalized nanoceria compared to non-stabilized nanoceria by a human monocyte cell line is some evidence that stabilizer choice does in fact affect immune cell responses.144 However, regardless of stabilizer coating, the nanoceria was able to reduce ROS levels in this activated monocyte cell line.144,145 Decreased ROS production was also observed in two murine macrophage cell lines that were either unstimulated146 or stimulated.117 Uptake of nanoceria and ROS level reduction was observed in a size-dependent manner in activated monocytes,145 further suggesting that larger individual nanoceria particles (and theoretically nanoceria aggregates) have the potential to be more immunomodulatory than smaller nanoceria that can evade aggregation with itself or other proteins. A consequence of such reduced ROS levels could be impaired innate immune cell destruction of pathogens. Patients with chronic granulomatous disease (CGD) whose macrophages and neutrophils lack the ROS-producing enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase suffer from chronic fungal and bacterial infections due to an inability of these cells to generate sufficient ROS to kill pathogens.147 Though it is unclear whether nanoceria could reduce ROS to the same levels as in the cells of CGD patients, any degree of ROS reduction may interfere with the ability of innate cells to clear pathogens, thus rendering patients vulnerable to infection.
Though such in vitro observations provide some insight into the potential response of immune cells to nanoceria exposure, studies published thus far provide an incomplete picture of how the immune system could be affected by such exposure. First, many studies (with the exception of ref. 142 and 148) utilize cell lines which, even when they are human lines as opposed to murine cell lines, cannot serve as a perfect proxy for primary human cells. Second, though not a major source of ROS and hence not as involved in oxidative stress, dendritic cells (DCs) must be considered, given their predominant role as the link between the innate and adaptive immune systems. Any immunomodulatory effects on DCs can directly impact the incidence of T cell activation which, if improperly timed, could promote autoimmunity. Third, while in vitro cultures allow for focus on specific cell types and specific cell functions, immune cells do not function in isolation in the body. For example, would nanoceria-induced decreases in ROS production in neutrophils and macrophages shift the burden of pathogen clearance and destruction to the complement system or to the slower-acting adaptive immune system? Since immune cells function as a system, it is quite difficult to fully grasp the extent of nanoceria's effects without studying a whole organism.
Application of nanoceria to oxidative stress-mediated disease
Autoimmune diseases that involve oxidative stress provide a unique opportunity to examine the simultaneous effects of nanoceria on inappropriately activated immune cells and on levels of ROS that also contribute to disease pathology. The autoimmune disease multiple sclerosis (MS) is complex in that is characterized by different disease progression patterns in part due to the involvement of distinct immune cell populations or combinations of cell populations and their respective cellular secretions.149 Disease pathogenesis results in the loss of the myelin sheath coating neurons in the central nervous system; neurons lacking this insulating structure cannot efficiently convey nerve impulses that allow communication between neurons and control of muscles.150 As a result, MS patients suffer loss of motor function and paralysis.150Rodent models called experimental autoimmune encephalomyelitis (EAE) replicate the symptoms of MS and are used to investigate both disease pathogenesis as well as potential therapeutic reagents.151 The murine model of chronic progressive MS is induced in C57BL/6 mice by targeting immune cell activation against the myelin oligodendrocyte glycoprotein (MOG), eliciting both helper T (TH) cell responses and the involvement of infiltrating macrophages.151 TH cells orchestrate the autoimmune destruction of myelin via cytokine production.150 In particular, TH cell-derived cytokines activate macrophages, initiating and amplifying production of pro-inflammatory cytokines and ROS by the macrophages themselves. These ROS generate oxidative stress in the CNS that damages myelin and other macromolecules.150 Application of nanoceria to this disease model would thus enable analysis of two questions about nanoceria. First, can nanoceria retain its functional activity in the intact animal (despite needing to cross numerous biological membrane barriers and forming a protein corona) upon reaching the brain and encountering ROS? Second, how does nanoceria affect immune cells in vivo as part of an intact system? If nanoceria was viewed by the body as “foreign” and activated the immune system, one would expect nanoceria to amplify the deleterious effects of immune cells and potentially exacerbate disease symptoms.
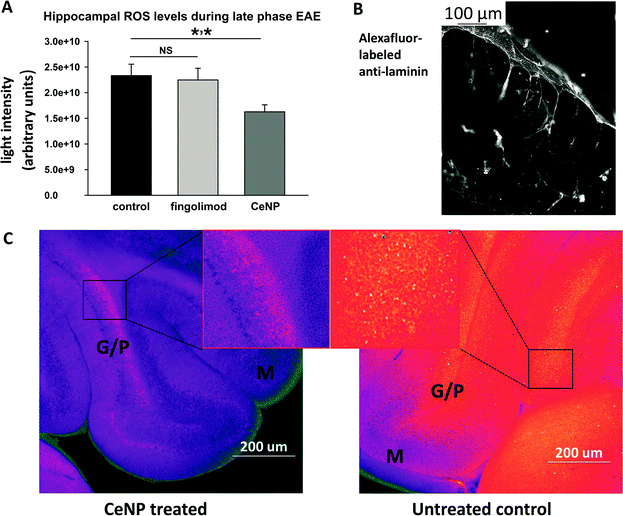
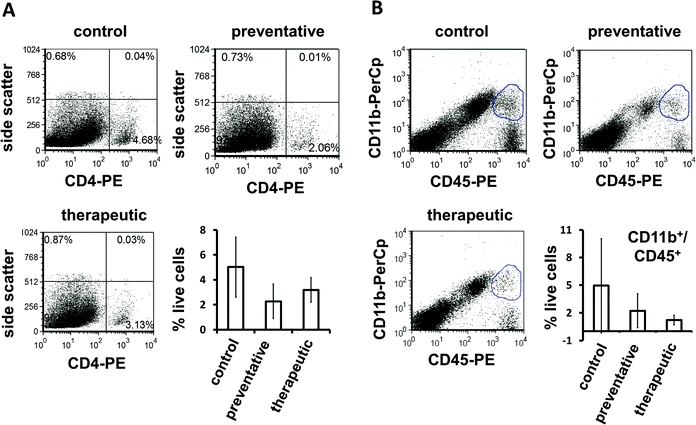
When treated with intravenous doses of sodium-citrate stabilized nanoceria, MOG EAE mice displayed fewer motor deficits and less severe clinical symptoms than control animals.82 The dose of nanoceria delivered correlated with the reduction in disease severity observed as well as with the amount of material detected in the brains of these animals. Brain sections harvested from nanoceria-treated EAE animals late in the disease course also exhibited lower levels of ROS than those harvested from control and fingolimod treated EAE animals, indicating that the antioxidant function of the nanoceria was preserved after entering the CNS compartment (Fig. 3). The alleviation of disease symptoms suggests that nanoceria at least did not worsen the pro-inflammatory function of TH and macrophage cells, which is also supported by the observation of similar numbers of these cells in the brains of nanoceria vs. control animals (Fig. 4). A more complete analysis of the cytokine secretion and phenotype of these cells is certainly required before a lack of immunomodulatory activity can be concluded. However, several important points can be concluded from this study. First, the stabilized nanoceria was detected in the brain of healthy and EAE animals, in contrast to others who did not detect nanoceria in the brain.107 The sodium citrate stabilization appears to have conferred a favorable distribution pattern to the nanoceria, yielding sufficient levels of particles in the brain to have therapeutic effect while maintaining inherent ROS-neutralizing properties. Second, use of the animal model allowed for analysis of effects on immune cells altogether in the intact system as opposed to in vitro studies of isolated cell types; that the stabilized nanoceria did not appear to aggravate or promote immune cell function is as exciting as the potential therapeutic efficacy observed in this disease model. Thus, when comparing this work to others, it appears that the nuances of particle formulation and stabilization drive the unique distribution, clearance, and functional capacity of nanoceria.
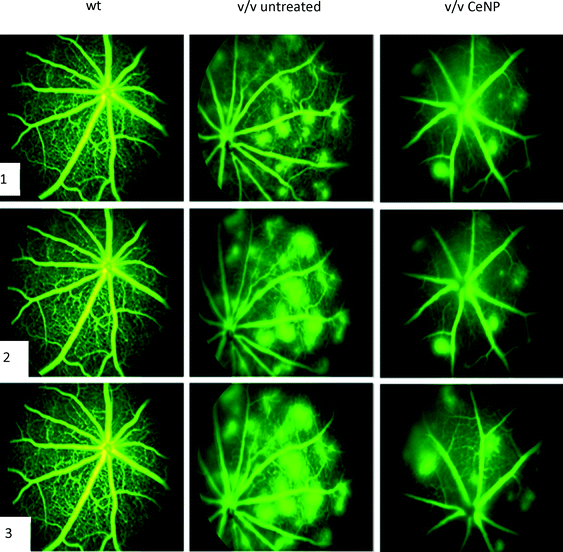
Nanoceria and eye diseases – in vivo
Recent research has focused on the identification and characterization of agents that provide protection to the retina (primarily photoreceptors) against diseases and injury. For the past ten years we have focused primarily on the use of cerium oxide nanoparticles (nanoceria) to prolong vision in animal models of human diseases. The eye is a globe consisting of a tough exterior coat, the sclera, which is continuous with the clear cornea in the front of the eye. The anterior chamber is a fluid filled space beneath the cornea and above the iris (colored part) of the eye. The lens helps to form the bottom part of the anterior chamber and is suspended in the a gel-liquid matrix (vitreous) which fills the interior of the globe and keeps the neural part, the retina, against a thick (Bruch's) membrane which is in contact with a layer of protective/nourishing cells called Retina Pigment Epithelial (RPE) in the back of the eye. The choroid (blood supply) is beneath the RPE and attached to the interior of the sclera. Light passes successively through the cornea, anterior chamber, lens, vitreous and is detected in the back of the eye by the photoreceptors of the retina.Like the testis and brain, the eye is an immune privileged organ which means that it is not under surveillance by the immune system and the presence of a foreign antigen does not normally produce an inflammatory response. Many neurodegenerative eye diseases have been shown to progress through a common metabolic connection, irrespective of the primary defect causing the disease. This common node is the chronic or acute accumulation of toxic Reactive Oxygen Species (ROS) in excess of the cellular defenses. At some undefined threshold of ROS damage, the cell initiates apoptosis or necrosis and dies. It is logical to think that by preventing the ROS damage, one might prevent the death of the cells or at least prolong their lives and function, which in the case of the retina is vision.
Distribution and retention of nanoceria in the eye
The eye has unique advantages for the study of therapeutic agents because the volume of the eye is small and therefore smaller amounts of a therapeutic agent can be effective. Nanoceria are catalytic antioxidants which mimic the enzymatic activities of superoxide dismutase (SOD) and catalase.119 Using inductively coupled plasma mass spectrometry (ICP-MS), we investigated152 the temporal and spatial distributions of nanoceria in healthy rat retinas after a single intravitreal injection. The data demonstrated (Fig. 5) that nanoceria were rapidly and preferentially taken up by the retina and, most surprising to us, the majority of nanoceria were retained in the retina with a half-life of over one year (414 days). No acute or long-term negative effects of nanoceria on retinal function (electroretinography – ERG) or cytoarchitecture (histology) were detected at any time point, even after prolonged exposure. These properties indicate that small doses of nanoceria have the potential to provide powerful, long lasting protection against the destructive effects of retinal diseases.Nanoceria destroy ROS and prevent light-induced retinal degeneration
We initially tested CeNP in primary cell cultures of retinal neurons and found them to be effective in preventing H2O2-induced cell death.5 This led us to test their effectiveness in our “blindness on demand” albino rat model for Age-related Macular Degeneration (AMD) in which the extent of retinal degeneration can be controlled by the intensity of light and the duration of exposure. Because the retina is completely contained within the eye, we thought that direct injection of CeNP into the vitreous would enable us to limit the amount of CeNP and provide the highest concentration of CeNP to the retina without requiring that the whole animal be dosed. The retina is about 250 um thick with the photoreceptor cell layer (125 um thick) being the furthest distance (~125 um) from the vitreous. This means that to be effective, the nanoceria must pass through and/or around multiple cell types and must enter photoreceptors to be effective against intracellular ROS which only travel Angstrom distances before reacting with other molecules.Our experimental paradigm was to inject nanoceria or vehicle into the vitreous of the eye on day 0; three days later expose the rat to bright light for 6 hours; and 7 days later determine retinal function by electroretinography (ERG) and end the experiment. Light damage to the retina proceeds through ROS production and our data suggested that nanoceria decreased light induced ROS in the retina. To evaluate the effectiveness of the CeNP, we examined the histological morphology of the retina and measured its thickness which is directly proportional to the number of rod photoreceptor cells in the retina. Our data indicated that the morphology and rod content of the retina was protected against light damage by CeNP. More significantly, using electroretinography (ERG), the data showed (Fig. 6) a concentration dependent protection of retinal function with less than 1/2 nanogram providing significant protection and 3.5 nanograms providing almost complete protection. This study5 led us to examine other animal models of human eye diseases.
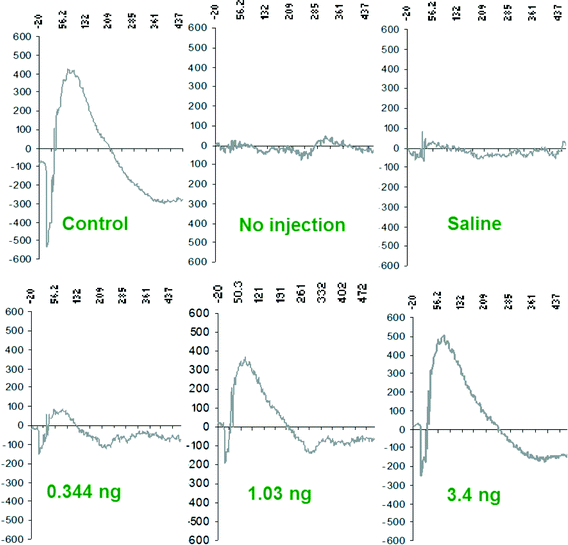 | ||
| Fig. 6 CeNP provide concentration dependent protection of retinal function from light damage. Waveform ERG data. See text for details. [Modified from ref. 4]. | ||
Inherited retinal degeneration
The light damage model exhibits many features of AMD but in keeping with our overarching hypothesis that most eye diseases involve ROS as a major connection between the primary defect and downstream effects, we next examined a mouse model with hereditary eye disease. The tubby mouse has inherited retinal degeneration with an early onset and continuous progression to blindness. Our data demonstrated89 that systemic delivery of CeNP, via intracardial injections, protect the tubby retina by decreasing ROS, up-regulating the expression of neuroprotection-associated genes; down-regulating apoptosis signaling pathways and/or up-regulating survival signaling pathways to slow photoreceptor degeneration over a two week period. We next tested88 the duration of the effects of intravitreal injection of CeNP at Postnatal day (P)7 when examined at P28, P49, P80 and P120. The expression of antioxidant associated genes and photoreceptor-specific genes was significantly up regulated, the mislocalization of rod and cone opsins was decreased, and retinal structure and function were protected.AMD and diabetic retinopathy
Many neurodegenerative diseases are known to occur and progress because of oxidative stress, the presence of ROS in excess of the cellular defensive capabilities. AMD and Diabetic Retinopathy (DR) share oxidative stress as a common node upstream of the blinding effects of these diseases. Knockout of the very low density lipoprotein receptor (vldlr) gene results in a mouse that develops pathological intraretinal and subretinal neovascular lesions within the first month of life and is an excellent model for a “wet” form of AMD called retinal angiomatous proliferation (RAP). We found90 that a single intravitreal injection of nanoceria (172 nanograms) into the vldlr−/− eye inhibited: the rise in ROS in the retina; increases in vascular endothelial growth factor (VEGF) in the photoreceptor layer; and the formation of intraretinal and subretinal neovascular lesions. Of more therapeutic significance, injection of nanoceria into older mice resulted in the regression of existing vascular lesions indicating that the pathologic neovessels require the continual production of excessive ROS. Our data demonstrate the unique ability of nanoceria to prevent downstream effects of oxidative stress in vivo and support their therapeutic potential for treatment of neurodegenerative diseases such as AMD and DR.Therapeutic duration of nanoceria and pathways affected
To determine the long-term therapeutic effects and mechanisms of nanoceria action on regression of existing pathologic neovascularization in vldlr−/− mouse, young adult mice were injected at P28 and their therapeutic function analyzed through P70 (ref. 153). Multiple parameters for nanoceria effects were examined including: regression of existing abnormal blood vessels, reduction of vascular leakage, down-regulation of the expression VEGF, acrolein, glial fibrillary acidic protein (GFAP) and caspase 3 as well as the up-regulation of the expression of rod- and cone-opsin genes and the pro-apoptotic ASK1-P38/JNKeNFekB signaling pathway. Our data (Fig. 7) demonstrated that a single intravitreal injection of nanoceria in P28 vldlr−/− mice produced sustained regression of existing oxidative stress-induced neovascularizations, prevented blood vessel leakage and inhibited apoptosis via down-regulation of the ASK1-P38/JNK-NF-kB signaling pathway through P70. The nanoceria do not destroy all ROS and, in the case of the vldlr−/− mouse, they do not eliminate all VEGF or have any effect on normal retinal vasculature. This is important because VEGF is also a neuroprotectant and is necessary for intracellular signaling.CeNP effects on oxidative stress and inflammation
AMD is accompanied by an inflammatory immune response without the presence of any bacterial or viral agent and is referred to as sterile inflammation.154 In our study,118 we examined the effect of nanoceria on expression of 88 major cytokines in the retinas of vldlr−/− mice using a PCR array. A single intravitreal injection of nanoceria at P28 caused inhibition of pro-inflammatory cytokines and pro-angiogenic growth factors including Tslp, Lif, Il3, Il7, VEGFa, Fgf1, Fgf2, Fgf7, Egf, Efna3, Lep, and up-regulation of several cytokines and anti-angiogenic genes in the vldlr−/− retina within one week. An Ingenuity Pathway Analysis software search for biological functions, pathways, and interrelationships between gene networks identified many genes whose activities are involved in cell signaling, cellular development, growth and proliferation, and tissue development. Western blot data analysis revealed that nanoceria inhibit the activation of ERK 1/2, JNK, p38 MAP kinase, and Akt. These data indicate that nanoceria may represent a novel therapeutic strategy to treat AMD, RAP, and other neurodegenerative diseases.Ocular oncology
Most recently, using a mouse model155 which develops bilateral retinoblastomas, we demonstrated that a single injection of nanoceria inhibited the growth of the retinoblastoma tumors.156 For this experimental paradigm, after bilateral tumors had developed, the left eye was injected with saline and the right eye with nanoceria in saline and mice were euthanized at one, two and three weeks later. The tumor area was determined on histological serial sections through the entire eye and the total tumor volume in each eye was calculated by summing all of the areas. CeNP injected eyes had 50% less tumor volume over three weeks indicating that CeNP inhibit tumor growth. VEGF in the CeNP injected eyes was also found to be less than half the amount in the saline injected eyes. Histological analysis also showed that most CeNP injected eyes had no metastasis from the posterior chamber of the eye to the anterior chamber whereas all saline or uninjected eyes showed tumor invasion of the anterior chamber. Longitudinal studies on individual mice using small animal Magnetic Resonance Imaging (MRI) confirmed the conclusion from the histological data that the volume of the tumors was half as large in the CeNP injected eyes as in the saline injected eyes. However, most intriguing from a therapeutic perspective, the MRI data also demonstrated that the tumors present at the time of CeNP injection decreased by 43% over the three week period and that the blood flow in the tumor of the nanoceria injected eyes was 53% less than that in the saline injected eyes156 suggesting that one of the mechanisms by which nanoceria shrink retinoblastomas is by decreasing their access to oxygen and nutrients.Summary
For all models of eye disease we have studied, the most common feature is the production of excess ROS and this step appears to be a very early change with multiple downstream signaling pathways being subsequently affected. However, the pattern of change in gene expression appears to be unique for each mutation and/or insult (e.g., light damage) and nanoceria simply reduce the ROS to normal levels in those areas (location, location, location) most affected such as the mitochondria and rod/cone outer segments. When the ROS levels are returned to near normal by nanoceria, there are additional pathways which are affected by these changes. We (unpublished observations) and others have found that simply penetrating the eye with a needle or injecting saline, results in the upregulation of numerous neuroprotective genes and the downregulation of pro-apoptotic genes. However, for rats injected with saline, cDNA microarrays showed that the gene changes induced by saline were transient and returned to normal levels after 24 hours whereas in the eyes of the nanoceria injected rats, these changes continued to be up/down regulated when measured at 48 and 72 hours. These data indicate that the neuroprotective response of the retina to injury is maintained and amplified by nanoceria suggesting that nanoceria would have an additive or synergistic effect when used in a combinatorial manner with other agents which provide a “preconditioning effect”. Such systems are currently being evaluated. Nanoceria are catalytic antioxidants with broad spectrum effects which suggest they may be therapeutic for multiple types of ocular diseases.Conclusions
It is clear from the depth and scope of the studies reviewed in this critical review that nanoceria is emerging as an anti-inflammatory material. The synthesis and characterization of many different types of nanoceria also show that the chemistry of the surface of these nanoparticles is quite varied, and that interactions with biologically relevant molecules such as proteins, anions and lipids are likely to alter the behaviour of nanoceria in vivo. Many questions still remain as to the natural protein corona that builds on nanoceria in vivo, and the role it plays in maintaining catalytic activity of nanoceria. Undoubtedly the study of these very intriguing nanoparticles will continue at the interface of biology, biomedical science and material science for years to come.Acknowledgements
JFM work was supported by NIH NEI grant COBRE-P20 RR017703, P30-EY 12190, R21EY018306, R01EY18724, R01EY022111; National Science Foundation: CBET-0708172; Foundation Fighting Blindness.This article is a product of a workshop on nanoceria held November 2, 2013 at the Fess Parker Doubletree Resort, Santa Barbara, CA, made possible by financial support from the Sustainable Nanotechnology Organization, The Tracy Farmer Institute for Sustainability and the Environment, the Department of Pharmaceutical Sciences, Associate Dean of Research of the College of Pharmacy and Office of the Vice President for Research, University of Kentucky.
Notes and references
- L. M. Cook, J. Non-Cryst. Solids, 1990, 120, 152–171 CrossRef CAS.
- K. Reed, A. Cormack, A. Kulkarni, M. Mayton, D. Sayle, F. Klaessig and B. Stadler, Environ. Sci.: Nano, 2014, 1, 390–405 RSC.
- H. Jung, D. B. Kittelson and M. R. Zachariah, Combust. Flame, 2005, 142, 276–288 CrossRef CAS PubMed.
- R. W. Tarnuzzer, J. Colon, S. Patil and S. Seal, Nano Lett., 2005, 5, 2573–2577 CrossRef CAS PubMed.
- J. Chen, S. Patil, S. Seal and J. F. McGinnis, Nat. Nanotechnol., 2006, 1, 142–150 CrossRef CAS PubMed.
- C. A. Cohen, M. D. Kurnick and B. A. Rzigalinski, Free Radical Biol. Med., 2006, 41, S20–S20 Search PubMed.
- B. A. Rzigalinski, K. Meehan, R. M. Davis, Y. Xu, W. C. Miles and C. A. Cohen, Nanomedicine, 2006, 1, 399–412 CrossRef CAS PubMed.
- D. Schubert, R. Dargusch, J. Raitano and S. W. Chan, Biochem. Biophys. Res. Commun., 2006, 342, 86–91 CrossRef CAS PubMed.
- M. Das, S. Patil, N. Bhargava, J.-F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS PubMed.
- R. A. Yokel, S. Hussain, S. Garantziotis, P. Demokritou, V. Castranova and F. R. Cassee, Environ. Sci.: Nano, 2014, 1, 406–428 RSC.
- K. Reed, A. Cormack, A. Kulkarni, M. Mayton, D. Sayle, F. Klaessig and B. Stadler, Environ. Sci.: Nano, 2014, 1, 390–405 RSC.
- E. Grulke, K. Reed, M. Beck, X. Huang, A. Cormack and S. Seal, Environ. Sci.: Nano, 2014, 1, 429–444 RSC.
- T. Cedervall, I. Lynch, S. Lindman, T. Berggård, E. Thulin, H. Nilsson, K. A. Dawson and S. Linse, Proc. Natl. Acad. Sci. U.S.A., 2007, 104, 2050–2055 CrossRef CAS PubMed.
- M. P. Monopoli, C. Aberg, A. Salvati and K. A. Dawson, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- C. D. Walkey and W. C. Chan, Chem. Soc. Rev., 2012, 41, 2780–2799 RSC.
- D. Walczyk, F. B. Bombelli, M. P. Monopoli, I. Lynch and K. A. Dawson, J. Am. Chem. Soc., 2010, 132, 5761–5768 CrossRef CAS PubMed.
- P. P. Karmali and D. Simberg, Expert Opin. Drug Delivery, 2011, 8, 343–357 CrossRef CAS PubMed.
- N. L. Anderson and N. G. Anderson, Mol. Cell. Proteomics, 2002, 1, 845–867 CAS.
- A. L. Barrán-Berdón, D. Pozzi, G. Caracciolo, A. L. Capriotti, G. Caruso, C. Cavaliere, A. Riccioli, S. Palchetti and A. Laganà, Langmuir, 2013, 29, 6485–6494 CrossRef PubMed.
- D. Dell'Orco, M. Lundqvist, C. Oslakovic, T. Cedervall and S. Linse, PLoS One, 2010, 5, e10949 Search PubMed.
- S. Tenzer, D. Docter, J. Kuharev, A. Musyanovych, V. Fetz, R. Hecht, F. Schlenk, D. Fischer, K. Kiouptsi, C. Reinhardt, K. Landfester, H. Schild, M. Maskos, S. K. Knauer and R. H. Stauber, Nat. Nanotechnol., 2013, 8, 772–781 CrossRef CAS PubMed.
- H. Zhang, K. E. Burnum, M. L. Luna, B. O. Petritis, J. S. Kim, W. J. Qian, R. J. Moore, A. Heredia-Langner, B. J. Webb-Robertson, B. D. Thrall, D. G. Camp 2nd, R. D. Smith, J. G. Pounds and T. Liu, Proteomics, 2011, 11, 4569–4577 CrossRef CAS PubMed.
- T. Cedervall, I. Lynch, M. Foy, T. Berggard, S. C. Donnelly, G. Cagney, S. Linse and K. A. Dawson, Angew. Chem., Int. Ed., 2007, 46, 5754–5756 CrossRef CAS PubMed.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. Baldelli Bombelli and K. A. Dawson, J. Am. Chem. Soc., 2011, 133, 2525–2534 CrossRef CAS PubMed.
- C. Rocker, M. Potzl, F. Zhang, W. J. Parak and G. U. Nienhaus, Nat. Nanotechnol., 2009, 4, 577–580 CrossRef PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. Puntes, ACS Nano, 2010, 4, 3623–3632 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. F. Puntes, Small, 2011, 7, 3479–3486 CrossRef CAS PubMed.
- S. Tenzer, D. Docter, S. Rosfa, A. Wlodarski, J. Kuharev, A. Rekik, S. K. Knauer, C. Bantz, T. Nawroth, C. Bier, J. Sirirattanapan, W. Mann, L. Treuel, R. Zellner, M. Maskos, H. Schild and R. H. Stauber, ACS Nano, 2011, 5, 7155–7167 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, H. Guo, A. Emili and W. C. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS PubMed.
- J. H. Shannahan, J. M. Brown, R. Chen, P. C. Ke, X. Lai, S. Mitra and F. A. Witzmann, Small, 2013, 9, 2171–2181 CrossRef CAS PubMed.
- Z. J. Deng, G. Mortimer, T. Schiller, A. Musumeci, D. Martin and R. F. Minchin, Nanotechnology, 2009, 20, 455101 CrossRef PubMed.
- G. Caracciolo, D. Pozzi, A. Capriotti, C. Cavaliere and A. Laganà, J. Nanopart. Res., 2013, 15, 1–11 CrossRef.
- M. A. Dobrovolskaia, A. K. Patri, J. Zheng, J. D. Clogston, N. Ayub, P. Aggarwal, B. W. Neun, J. B. Hall and S. E. McNeil, Nanomedicine, 2009, 5, 106–117 CrossRef CAS PubMed.
- R. E. Serda, J. Gu, R. C. Bhavane, X. Liu, C. Chiappini, P. Decuzzi and M. Ferrari, Biomaterials, 2009, 30, 2440–2448 CrossRef CAS PubMed.
- C. C. Fleischer and C. K. Payne, J. Phys. Chem. B, 2012, 116, 8901–8907 CrossRef CAS PubMed.
- A. Bajaj, B. Samanta, H. Yan, D. J. Jerry and V. M. Rotello, J. Mater. Chem., 2009, 19, 6328–6331 RSC.
- I. Stayton, J. Winiarz, K. Shannon and Y. Ma, Anal. Bioanal. Chem., 2009, 394, 1595–1608 CrossRef CAS PubMed.
- D. Zhang, O. Neumann, H. Wang, V. M. Yuwono, A. Barhoumi, M. Perham, J. D. Hartgerink, P. Wittung-Stafshede and N. J. Halas, Nano Lett., 2009, 9, 666–671 CrossRef CAS PubMed.
- R. Tedja, M. Lim, R. Amal and C. Marquis, ACS Nano, 2012, 6, 4083–4093 CrossRef CAS PubMed.
- J. T. Quik, I. Lynch, K. Van Hoecke, C. J. Miermans, K. A. De Schamphelaere, C. R. Janssen, K. A. Dawson, M. A. Stuart and D. Van De Meent, Chemosphere, 2010, 81, 711–715 CrossRef CAS PubMed.
- Y. Zhu, W. Li, Q. Li, Y. Li, Y. Li, X. Zhang and Q. Huang, Carbon, 2009, 47, 1351–1358 CrossRef CAS PubMed.
- J. Shi, H. L. Karlsson, K. Johansson, V. Gogvadze, L. Xiao, J. Li, T. Burks, A. Garcia-Bennett, A. Uheida, M. Muhammed, S. Mathur, R. Morgenstern, V. E. Kagan and B. Fadeel, ACS Nano, 2012, 6, 1925–1938 CrossRef CAS PubMed.
- D. Guarnieri, A. Guaccio, S. Fusco and P. Netti, J. Nanopart. Res., 2011, 13, 4295–4309 CrossRef CAS.
- C. C. Fleischer, U. Kumar and C. K. Payne, Biomater. Sci., 2013, 1, 975–982 RSC.
- W. Hu, C. Peng, M. Lv, X. Li, Y. Zhang, N. Chen, C. Fan and Q. Huang, ACS Nano, 2011, 5, 3693–3700 CrossRef CAS PubMed.
- A. Lesniak, A. Salvati, M. J. Santos-Martinez, M. W. Radomski, K. A. Dawson and C. Åberg, J. Am. Chem. Soc., 2013, 135, 1438–1444 CrossRef CAS PubMed.
- A. Lesniak, F. Fenaroli, M. P. Monopoli, C. Aberg, K. A. Dawson and A. Salvati, ACS Nano, 2012, 6, 5845–5857 CrossRef CAS PubMed.
- C. Ge, J. Du, L. Zhao, L. Wang, Y. Liu, D. Li, Y. Yang, R. Zhou, Y. Zhao, Z. Chai and C. Chen, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16968–16973 CrossRef CAS PubMed.
- C. Li, S. Bolisetty, K. Chaitanya, J. Adamcik and R. Mezzenga, Adv. Mater., 2013, 25, 1010–1015 CrossRef CAS PubMed.
- K. Furumoto, K. Ogawara, S. Nagayama, Y. Takakura, M. Hashida, K. Higaki and T. Kimura, J. Controlled Release, 2002, 83, 89–96 CrossRef CAS.
- H. Harashima, K. Sakata, K. Funato and H. Kiwada, Pharm. Res., 1994, 11, 402–406 CrossRef CAS.
- M. J. Hsu and R. L. Juliano, Biochim. Biophys. Acta, 1982, 720, 411–419 CrossRef CAS.
- O. Lunov, T. Syrovets, C. Loos, J. Beil, M. Delacher, K. Tron, G. U. Nienhaus, A. Musyanovych, V. Mailander, K. Landfester and T. Simmet, ACS Nano, 2011, 5, 1657–1669 CrossRef CAS PubMed.
- S. M. Moghimi, A. C. Hunter and J. C. Murray, Pharmacol. Rev., 2001, 53, 283–318 CAS.
- X. R. Xia, N. A. Monteiro-Riviere and J. E. Riviere, Nat. Nanotechnol., 2010, 5, 671–675 CrossRef CAS PubMed.
- A. Gessner, A. Lieske, B. R. Paulke and R. H. Muller, J. Biomed. Mater. Res., Part A, 2003, 65, 319–326 CrossRef PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- D. E. Owens 3rd and N. A. Peppas, Int. J. Pharm., 2006, 307, 93–102 CrossRef PubMed.
- J. Satulovsky, M. A. Carignano and I. Szleifer, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9037–9041 CrossRef CAS PubMed.
- C. H. Choi, C. A. Alabi, P. Webster and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1235–1240 CrossRef CAS PubMed.
- R. Gref, M. Luck, P. Quellec, M. Marchand, E. Dellacherie, S. Harnisch, T. Blunk and R. H. Muller, Colloids Surf., B, 2000, 18, 301–313 CrossRef CAS.
- J. Kreuter, Adv. Drug Delivery Rev., 2001, 47, 65–81 CrossRef CAS.
- H. R. Kim, K. Andrieux, S. Gil, M. Taverna, H. Chacun, D. Desmaele, F. Taran, D. Georgin and P. Couvreur, Biomacromolecules, 2007, 8, 793–799 CrossRef CAS PubMed.
- J. Kreuter, D. Shamenkov, V. Petrov, P. Ramge, K. Cychutek, C. Koch-Brandt and R. Alyautdin, J. Drug Targeting, 2002, 10, 317–325 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, F. Song, R. Liu, H. Guo, D. W. H. Olsen, Y. Cohen, A. Emili and W. C. W. Chan, ACS Nano, 2014, 8, 2439–2455 CrossRef CAS PubMed.
- I. Fridovich, J. Biol. Chem., 1997, 272, 18515–18517 CrossRef CAS PubMed.
- B. Kalyanaraman, Redox. Biol., 2013, 1, 244–257 CrossRef CAS PubMed.
- A. Karakoti, S. Singh, J. M. Dowding, S. Seal and W. T. Self, Chem. Soc. Rev., 2010, 39, 4422–4432 RSC.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058, 10.1039/b615134e.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- A. S. Karakoti, S. Singh, A. Kumar, M. Malinska, S. V. Kuchibhatla, K. Wozniak, W. T. Self and S. Seal, J. Am. Chem. Soc., 2009, 131, 14144–14145 CrossRef CAS PubMed.
- V. Singh, S. Singh, S. Das, A. Kumar, W. T. Self and S. Seal, Nanoscale, 2012, 4, 2597–2605 RSC.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753 CrossRef CAS PubMed.
- T. Naganuma and E. Traversa, Biomaterials, 2014, 35, 4441–4453 CrossRef CAS PubMed.
- A. Y. Estevez, S. Pritchard, K. Harper, J. W. Aston, A. Lynch, J. J. Lucky, J. S. Ludington, P. Chatani, W. P. Mosenthal, J. C. Leiter, S. Andreescu and J. S. Erlichman, Free Radic. Biol. Med., 2011, 51, 1155–1163 CrossRef CAS PubMed.
- M. Ganesana, J. S. Erlichman and S. Andreescu, Free Radic. Biol. Med., 2012, 53, 2240–2249 CrossRef CAS PubMed.
- T. X. Sayle, M. Molinari, S. Das, U. M. Bhatta, G. Mobus, S. C. Parker, S. Seal and D. C. Sayle, Nanoscale, 2013, 5, 6063–6073 RSC.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- S. I. Liochev, Free Radical Biol. Med., 2013, 60, 1–4 CrossRef CAS PubMed.
- S. S. Lee, W. Song, M. Cho, H. L. Puppala, P. Nguyen, H. Zhu, L. Segatori and V. L. Colvin, ACS Nano, 2013, 7, 9693–9703 CrossRef CAS PubMed.
- K. L. Heckman, W. DeCoteau, A. Estevez, K. J. Reed, W. Costanzo, D. Sanford, J. C. Leiter, J. Clauss, K. Knapp, C. Gomez, P. Mullen, E. Rathbun, K. Prime, J. Marini, J. Patchefsky, A. S. Patchefsky, R. K. Hailstone and J. S. Erlichman, ACS Nano, 2013, 7, 10582–10596 CrossRef CAS PubMed.
- J.-D. Cafun, K. O. Kvashnina, E. Casals, V. F. Puntes and P. Glatzel, ACS Nano, 2013, 7, 10726–10732 CrossRef CAS PubMed.
- J. M. Dowding, T. Dosani, A. Kumar, S. Seal and W. T. Self, Chem. Commun., 2012, 48, 4896–4898 RSC.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed.
- J. M. Dowding, S. Das, A. Kumar, T. Dosani, R. McCormack, A. Gupta, T. X. T. Sayle, D. C. Sayle, L. von Kalm, S. Seal and W. T. Self, ACS Nano, 2013, 7, 4855–4868 CrossRef CAS PubMed.
- J. M. Dowding, S. Seal and W. T. Self, Drug Delivery Transl. Res., 2013, 3, 375–379 CrossRef CAS PubMed.
- X. Cai, S. A. Sezate, S. Seal and J. F. McGinnis, Biomaterials, 2012, 33, 8771–8781 CrossRef CAS PubMed.
- L. Kong, X. Cai, X. Zhou, L. L. Wong, A. S. Karakoti, S. Seal and J. F. McGinnis, Neurobiol. Dis., 2011, 42, 514–523 CrossRef CAS PubMed.
- X. Zhou, L. L. Wong, A. S. Karakoti, S. Seal and J. F. McGinnis, PLoS One, 2011, 6, e16733 CAS.
- J. Colon, L. Herrera, J. Smith, S. Patil, C. Komanski, P. Kupelian, S. Seal, D. W. Jenkins and C. H. Baker, Nanomedicine, 2009, 5, 225–231 CrossRef CAS PubMed.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- S. Das, S. Singh, J. M. Dowding, S. Oommen, A. Kumar, T. X. Sayle, S. Saraf, C. R. Patra, N. E. Vlahakis, D. C. Sayle, W. T. Self and S. Seal, Biomaterials, 2012, 33, 7746–7755 CrossRef CAS PubMed.
- S. Chigurupati, M. R. Mughal, E. Okun, S. Das, A. Kumar, M. McCaffery, S. Seal and M. P. Mattson, Biomaterials, 2013, 34, 2194–2201 CrossRef CAS PubMed.
- A. S. Karakoti, O. Tsigkou, S. Yue, P. D. Lee, M. M. Stevens, J. R. Jones and S. Seal, J. Mater. Chem., 2010, 20, 8912–8919 RSC.
- R. Davan, R. Prasad, V. S. Jakka, R. Aparna, A. Phani, B. Jacob, P. C. Salins and D. Raju, J. Bionanosci., 2012, 6, 78–83 CrossRef CAS PubMed.
- A. Cimini, B. D'Angelo, S. Das, R. Gentile, E. Benedetti, V. Singh, A. M. Monaco, S. Santucci and S. Seal, Acta Biomater., 2012, 8, 2056–2067 CrossRef CAS PubMed.
- B. DAngelo, S. Santucci, E. Benedetti, S. Di Loreto, R. Phani, S. Falone, F. Amicarelli, M. P. Ceru and A. Cimini, Curr. Nanosci., 2009, 5, 167–176 CrossRef CAS.
- K. L. Heckman, W. DeCoteau, A. Estevez, K. J. Reed, W. Costanzo, D. Sanford, J. C. Leiter, J. Clauss, K. Knapp, C. Gomez, P. Mullen, E. Rathbun, K. Prime, J. Marini, J. Patchefsky, A. S. Patchefsky, R. K. Hailstone and J. S. Erlichman, ACS Nano, 2013, 7, 10582–10596 CrossRef CAS PubMed.
- C. K. Kim, T. Kim, I.-Y. Choi, M. Soh, D. Kim, Y.-J. Kim, H. Jang, H.-S. Yang, J. Y. Kim, H.-K. Park, S. P. Park, S. Park, T. Yu, B.-W. Yoon, S.-H. Lee and T. Hyeon, Angew. Chem., 2012, 124, 11201–11205 CrossRef.
- L. Alili, M. Sack, A. S. Karakoti, S. Teuber, K. Puschmann, S. M. Hirst, C. M. Reilly, K. Zanger, W. Stahl, S. Das, S. Seal and P. Brenneisen, Biomaterials, 2011, 32, 2918–2929 CrossRef CAS PubMed.
- L. Alili, M. Sack, C. von Montfort, S. Giri, S. Das, K. S. Carroll, K. Zanger, S. Seal and P. Brenneisen, Antioxid. Redox Signaling, 2013, 19, 765–778 CrossRef CAS PubMed.
- S. Giri, A. Karakoti, R. P. Graham, J. L. Maguire, C. M. Reilly, S. Seal, R. Rattan and V. Shridhar, PLoS One, 2013, 8, e54578 CAS.
- K. Chaudhury, K. N. Babu, A. K. Singh, S. Das, A. Kumar and S. Seal, Nanomedicine, 2013, 9, 439–448 CrossRef CAS PubMed.
- S. Patil, A. Sandberg, E. Heckert, W. Self and S. Seal, Biomaterials, 2007, 28, 4600–4607 CrossRef CAS PubMed.
- S. Singh, A. Kumar, A. Karakoti, S. Seal and W. T. Self, Mol. BioSyst., 2010, 6, 1813–1820 RSC.
- S. M. Hirst, A. Karakoti, S. Singh, W. Self, R. Tyler, S. Seal and C. M. Reilly, Environ. Toxicol., 2013, 28, 107–118 CrossRef CAS PubMed.
- R. A. Yokel, M. T. Tseng, M. Dan, J. M. Unrine, U. M. Graham, P. Wu and E. A. Grulke, Nanomedicine, 2013, 9, 398–407 CrossRef CAS PubMed.
- I. Celardo, J. Z. Pedersen, E. Traversa and L. Ghibelli, Nanoscale, 2011, 3, 1411–1420 RSC.
- I. Celardo, M. De Nicola, C. Mandoli, J. Z. Pedersen, E. Traversa and L. Ghibelli, ACS Nano, 2011, 5, 4537–4549 CrossRef CAS PubMed.
- M. De Nicola, G. Gualandi, A. Alfonsi, C. Cerella, M. D'Alessio, A. Bergamaschi, A. Magrini and L. Ghibelli, Biochem. Pharmacol., 2006, 72, 1405–1416 CrossRef CAS PubMed.
- L. Ghibelli, C. Fanelli, G. Rotilio, E. Lafavia, S. Coppola, C. Colussi, P. Civitareale and M. R. Ciriolo, FASEB J., 1998, 12, 479–486 CAS.
- M. T. Kuo, Antioxid. Redox Signaling, 2009, 11, 99–133 CrossRef CAS PubMed.
- D. Gonzalez-Flores, M. De Nicola, E. Bruni, F. Caputo, A. B. Rodriguez, J. A. Pariente and L. Ghibelli, Mol. Cell. Biochem., 2014, 397, 245–253 CrossRef CAS PubMed.
- J. Colon, N. Hsieh, A. Ferguson, P. Kupelian, S. Seal, D. W. Jenkins and C. H. Baker, Nanomedicine, 2010, 6, 698–705 CrossRef CAS PubMed.
- J. Niu, K. Wang and P. E. Kolattukudy, J. Pharmacol. Exp. Ther., 2011, 338, 53–61 CrossRef CAS PubMed.
- S. M. Hirst, A. S. Karakoti, R. D. Tyler, N. Sriranganathan, S. Seal and C. M. Reilly, Small, 2009, 5, 2848–2856 CrossRef CAS PubMed.
- S. V. Kyosseva, L. Chen, S. Seal and J. F. McGinnis, Exp. Eye Res., 2013, 116, 63–74 CrossRef CAS PubMed.
- S. Das, J. M. Dowding, K. E. Klump, J. F. McGinnis, W. Self and S. Seal, Nanomedicine, 2013, 8, 1483–1508 CrossRef CAS PubMed.
- J. M. Perez, A. Asati, S. Nath and C. Kaittanis, Small, 2008, 4, 552–556 CrossRef CAS PubMed.
- M. Safi, H. Sarrouj, O. Sandre, N. Mignet and J. F. Berret, Nanotechnology, 2010, 21, 145103 CrossRef CAS PubMed.
- F. Caputo, M. De Nicola and L. Ghibelli, Biochem. Pharmacol., 2014 DOI:10.1016/j.bcp.2014.08.015.
- A. Briggs, S. Corde, S. Oktaria, R. Brown, A. Rosenfeld, M. Lerch, K. Konstantinov and M. Tehei, Nanomedicine, 2013, 9, 1098–1105 CrossRef CAS PubMed.
- G. F. Tomaselli and A. S. Barth, Nat. Med., 2010, 16, 648–649 CrossRef CAS PubMed.
- G. Forte, F. Carotenuto, F. Pagliari, S. Pagliari, P. Cossa, R. Fiaccavento, A. Ahluwalia, G. Vozzi, B. Vinci, A. Serafino, A. Rinaldi, E. Traversa, L. Carosella, M. Minieri and P. Di Nardo, Stem Cells, 2008, 26, 2093–2103 CrossRef CAS PubMed.
- L. Shao, H. Li, S. K. Pazhanisamy, A. Meng, Y. Wang and D. Zhou, Int. J. Hematol., 2011, 94, 24–32 CrossRef CAS PubMed.
- A. Mohyeldin, T. Garzon-Muvdi and A. Quinones-Hinojosa, Cell Stem Cell, 2010, 7, 150–161 CrossRef CAS PubMed.
- C. Mandoli, F. Pagliari, S. Pagliari, G. Forte, P. Di Nardo, S. Licoccia and E. Traversa, Adv. Funct. Mater., 2010, 20, 1617–1624 CrossRef CAS.
- W. F. B. Boron and L. Emile, Medical Physiology: a cellular and molecular approach, Saunders/Elsevier, 2009 Search PubMed.
- J. Niu, A. Azfer, L. M. Rogers, X. Wang and P. E. Kolattukudy, Cardiovasc. Res., 2007, 73, 549–559 CrossRef CAS PubMed.
- S. S. Hardas, D. A. Butterfield, R. Sultana, M. T. Tseng, M. Dan, R. L. Florence, J. M. Unrine, U. M. Graham, P. Wu, E. A. Grulke and R. A. Yokel, Toxicol. Sci., 2010, 116, 562–576 CrossRef CAS PubMed.
- P. L. Rodriguez, T. Harada, D. A. Christian, D. A. Pantano, R. K. Tsai and D. E. Discher, Science, 2013, 339, 971–975 CrossRef CAS PubMed.
- R. A. Yokel, T. C. Au, R. MacPhail, S. S. Hardas, D. A. Butterfield, R. Sultana, M. Goodman, M. T. Tseng, M. Dan, H. Haghnazar, J. M. Unrine, U. M. Graham, P. Wu and E. A. Grulke, Toxicol. Sci., 2012, 127, 256–268 CrossRef CAS PubMed.
- S. S. Hardas, R. Sultana, G. Warrier, M. Dan, R. L. Florence, P. Wu, E. A. Grulke, M. T. Tseng, J. M. Unrine, U. M. Graham, R. A. Yokel and D. A. Butterfield, Neurotoxicology, 2012, 33, 1147–1155 CrossRef CAS PubMed.
- M. Dan, M. T. Tseng, P. Wu, J. M. Unrine, E. A. Grulke and R. A. Yokel, Int. J. Nanomed., 2012, 7, 4023–4036 CrossRef CAS PubMed.
- A. Albanese, P. S. Tang and W. C. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- M. T. Tseng, X. Lu, X. Duan, S. S. Hardas, R. Sultana, P. Wu, J. M. Unrine, U. Graham, D. A. Butterfield, E. A. Grulke and R. A. Yokel, Toxicol. Appl. Pharmacol., 2012, 260, 173–182 CrossRef CAS PubMed.
- C. S. Lustgarten, R. G. Cuddihy, B. B. Boecker and N. Huber, et al., Biliary excretion of 144Ce after inhalation of 144Ce citrate in rats and Syrian hamsters, Lovelace Foundation for Medical Education and Research, 1976 Search PubMed.
- P. W. Durbin, M. H. Williams, M. Gee, R. H. Newman and J. G. Hamilton, Proc. Soc. Exp. Biol. Med., 1956, 91, 78–85 CrossRef CAS.
- W. Wang, S. K. Singh, N. Li, M. R. Toler, K. R. King and S. Nema, Int. J. Pharm., 2012, 431, 1–11 CrossRef CAS PubMed.
- J. Uetrecht and D. J. Naisbitt, Pharmacol. Rev., 2013, 65, 779–808 CrossRef PubMed.
- K. Babin, F. Antoine, D. M. Goncalves and D. Girard, Toxicol. Lett., 2013, 221, 57–63 CrossRef CAS PubMed.
- A. Asati, S. Santra, C. Kaittanis and J. M. Perez, ACS Nano, 2010, 4, 5321–5331 CrossRef CAS PubMed.
- S. R. Ting, J. M. Whitelock, R. Tomic, C. Gunawan, W. Y. Teoh, R. Amal and M. S. Lord, Biomaterials, 2013, 34, 4377–4386 CrossRef CAS PubMed.
- M. S. Lord, M. Jung, W. Y. Teoh, C. Gunawan, J. A. Vassie, R. Amal and J. M. Whitelock, Biomaterials, 2012, 33, 7915–7924 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, L. Madler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS PubMed.
- D. Roos and M. de Boer, Clin. Exp. Immunol., 2014, 175, 139–149 CrossRef CAS PubMed.
- S. Hussain, F. Al-Nsour, A. B. Rice, J. Marshburn, B. Yingling, Z. Ji, J. I. Zink, N. J. Walker and S. Garantziotis, ACS Nano, 2012, 6, 5820–5829 CrossRef CAS PubMed.
- A. Nylander and D. A. Hafler, J. Clin. Invest., 2012, 122, 1180–1188 CrossRef CAS PubMed.
- H. Lassmann and J. van Horssen, FEBS Lett., 2011, 585, 3715–3723 CrossRef CAS PubMed.
- E. Mix, H. Meyer-Rienecker, H. P. Hartung and U. K. Zettl, Prog. Neurobiol., 2010, 92, 386–404 CrossRef PubMed.
- L. L. Wong, S. M. Hirst, Q. N. Pye, C. M. Reilly, S. Seal and J. F. McGinnis, PLoS One, 2013, 8, e58431 CAS.
- X. Cai, S. Seal and J. F. McGinnis, Biomaterials, 2014, 35, 249–258 CrossRef CAS PubMed.
- S. L. Doyle, M. Campbell, E. Ozaki, R. G. Salomon, A. Mori, P. F. Kenna, G. J. Farrar, A. S. Kiang, M. M. Humphries, E. C. Lavelle, L. A. O'Neill, J. G. Hollyfield and P. Humphries, Nat. Med., 2012, 18, 791–798 CrossRef CAS PubMed.
- J. Zhang, B. Schweers and M. A. Dyer, Cell Cycle, 2004, 3, 952–959 CAS.
- K. E. Klump, X. Cai, R. Towner, S. Seal, M. Dyer and J. McGinnis, FASEB J., 2013, 27, 1088.1016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4en00138a |
| This journal is © The Royal Society of Chemistry 2015 |