Charge-tagged N-heterocyclic carbenes†‡
Yuri E.
Corilo
a,
Fabiane M.
Nachtigall
a,
Patricia V.
Abdelnur
a,
Gunter
Ebeling
b,
Jairton
Dupont
*b and
Marcos N.
Eberlin
*a
aThoMSon Mass Spectrometry Laboratory, Institute of Chemistry University of Campinas - UNICAMP, Campinas, SP-13083-970, Brazil. E-mail: eberlin@iqm.unicamp.br; Fax: (+) 55 19 3521 3073
bLaboratory of Molecular Catalysis, Institute of Chemistry, Federal university of Rio Grande do Sul - UFRGS, Porto Alegre, RS-91501-970, Brazil. E-mail: Jairton.dupont@ufrgs.br
First published on 18th July 2011
Abstract
The interception, formation and characterization of the first stable, long lived charge-tagged N-heterocyclic carbenes of the general type 4x+ (x = 1–3) and analogues is reported. Via ESI(+)-MS of solutions of bromine salts of doubly, triply and quadruply charged imidazolium ion IL (3.Brn, n = 2–4), the isolated 4x+ as well as charged aggregates [3.Br(n−x)]x+ likely to be participating in the [3.Br(n−x)]x+ ⇌ 4x+ + HBr solution equilibrium could be transferred and characterized in the gas phase. Mimicking the solution equilibrium, the gaseous [3.Br(n−x)]x+ were found to dissociate nearly exclusively viaHBr loss during thermal activationvia collisions to form gaseous 4x+, which were found to add to acrolein and acetone.
Introduction
Carbenes,1 due to their high reactivity, hypo-valent carbon and exotic electronic structures with low-lying singlet and triplet states, have long fascinated theoretical and experimental chemists. These highly reactive and transient species add to alkenes, insert to C–H bonds, and react with heteroatoms to yield ylides, and many reactions have also been found or designed to involve carbenes as intermediates. But due to their elusive nature, carbenes were for a long time inaccessible to experimental observation. Fortunately, however, owing to crossed resonance with the two vicinal ring nitrogens, long-lived N-heterocyclic carbenes such as those of the imidazolidene type 1 (Scheme 1) were discovered and isolated as the first stable carbenes.2N-heterocyclic carbenes have then been used as efficient catalysts,3 as ligands for transition metals,4 to stabilize highly reactive species,5 to activate small molecules,6 and as key reactants for catalytic organometallic transformations7 and many key organic reactions.8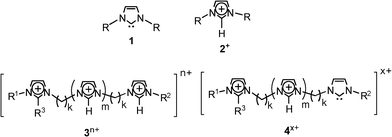 | ||
| Scheme 1 | ||
Mass spectrometry is inherently blind to neutrals9 hence simple carbenes have been investigated via gas phase MS experiments mainly in ionized forms10 such as Cl2C+. or ClHC−. or indirectly via neutralization-reionization MS (NRMS) experiments.11 To cure this blindness, an elegant MS strategy has been elaborated using charge tags and applied to handle gaseous radicals with distant charge sites.12 With the arrival of electrospray ionization (ESI),13 charge tags have also been used in solution to allow the favourable “fishing”14 of charge-tagged molecules directly from solution into the isolated gas phase environment for MS measurements and intrinsic reactivity investigations.15
Composed of organic cations and inorganic complex anions, room temperature ionic liquids (ILs) have attracted great attention in many fields such as synthesis, catalysis, electrochemistry, nanomaterials, processes of extraction and separation, and as a new class of materials and solvents for green chemistry.16 ILs based on singly charged 1,3-dialkylimidazolium ions 2+ (Scheme 1) have been one of the most thoroughly studied classes of such salts. Recently, multiply charged imidazolium ions of the general formula 3n+ (Scheme 1, Table 1) have also been prepared.17 We have then applied ESI(+)-MS to transfer from solution directly to the gas phase such multiply charged cations so as to use them as precursors of gaseous charge-tagged (di)-radicals.18
| 3 | R1 | R2 | R3 | m | k | n | [3a.Br(n−x)]x+ | m/z |
|---|---|---|---|---|---|---|---|---|
| a For 3i and 3j, a -(CH2)4- bridge connects the two central imidazolium rings. For m = 0 there is only one -(CH2)k- bridge connecting the imidazolium rings. | ||||||||
| 3a | Me | Me | H | 0 | 4 | 2 | [3a.Br]+ | 299 |
| 3b | Me | Me | H | 0 | 3 | 2 | [3b.Br]+ | 285 |
| 3c | Me | Me | H | 0 | 2 | 2 | [3c.Br]+ | 271 |
| 3d | Me | Me | Me | 0 | 2 | 2 | [3d.Br]+ | 285 |
| 3e | Me | Bun | H | 0 | 2 | 2 | [3e.Br]+ | 313 |
| 3f | Me | (CH2)2OMe | H | 0 | 3 | 2 | [3f.Br]+ | 315 |
| 3g | Me | Me | H | 1 | 2 | 3 | [3g.Br]2+ | 183 |
| 3h | Me | Me | H | 1 | 2 | 3 | [3h.Br2]+ | 445 |
| 3i a | Me | Me | H | 2 | 2 | 4 | [3i.Br2]2+ | 284 |
| 3j a | Me | Me | H | 2 | 2 | 4 | [3j.Br]3+ | 163 |
In imidazolium ions, the ring hydrogens are acidic, mostly particular the C2–H with relatively high pKa values (21–23) roughly intermediate between the acidities of acetone (pKa = 19.3) and ethyl acetate (pKa = 25.6).19 Depending on the nature of the anion, the C2–H easily exchanges with deuterium from D2O.20 This H/D exchange is believed to be promoted by the basic anion/solvent and to occur via N-heterocyclic carbenes, but such transient intermediates have never been directly observed.21N-heterocyclic carbenes are also invoked to stabilize metal complexes of ILs and metal nanoparticle based catalysts.22
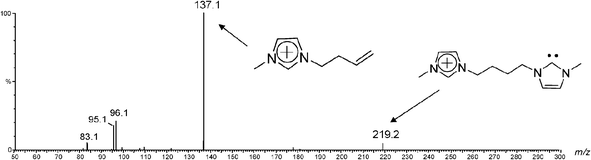
This work reports on the interception, formation and characterization of the first stable, long lived charge-tagged N-heterocyclic carbenes of the general type 4x+ (Scheme 1) and analogues via ESI(+)-MS of solutions of 3.Brn (Table 1). In solution, imidazolium ions are stabilized by intermolecular interactions with solvents and counter ions forming a large supramolecular network, which is preserved to some extent during ESI transfer to the gas phase and MS analysis.23 When methanolic solutions of IL salts 3a–j.Brn were subjected to ESI(+), as Fig. 1 exemplifies for 3a.Br2 (and Fig. S1‡ for 3f.Br2), we found that this gentle ionization technique was able to transfer to the gas phase, together with the free doubly charged imidazolium ion 3a2+ of m/z 110, the unique singly charged supramolecular species [3a.Br]+ detected mainly as its pair of 79Br and 81Br isotopologue ions of m/z 299 and 301.
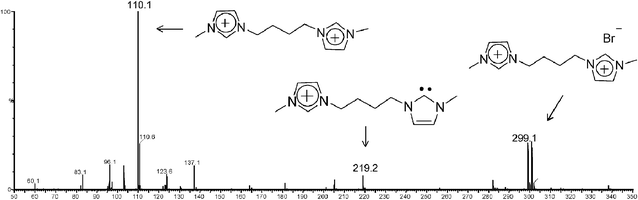 | ||
| Fig. 1 ESI(+)-MS of a methanolic solution of 3a.Br2. | ||
Yet more interestingly, another unique ion of m/z 219 was observed in Fig. 1, most likely the gaseous charge-tagged carbene 4a+ (Scheme 2). Therefore, it is likely that both [3a.Br]+ and 4a+ were “fished” by ESI(+) from the [3a.Br]+ ⇌ 4a+ + HBr solution equilibrium in which Br− abstracts the most acidic C2–H hydrogen. Such abstraction was also used in solution by Arduengo2 to form the first neutral N-heterocyclic carbene.
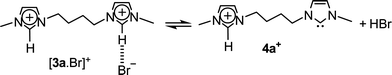 | ||
| Scheme 2 | ||
To confirm the charge-tagged carbene structures of these new species, ESI(+)-MS/MS experiments were performed, in which 4x+ as well as their counterparts [3.Br(n−x)]x+ were isolated and subjected to CID. Fig. 2 illustrates such spectra for 4a+, Fig. S2 for [3a.Br]+ whereas Fig. S3 compares those for 4a+, 4b+ and 4c+.‡
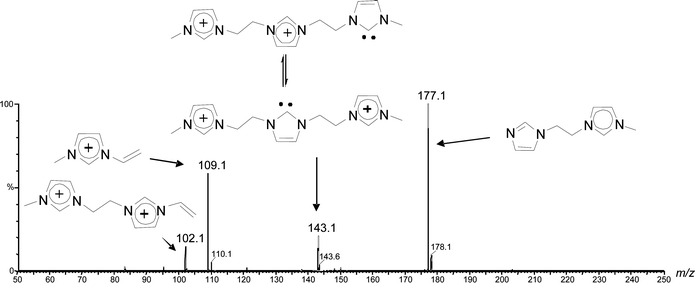
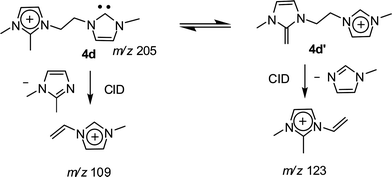
The dissociation chemistry observed for 4+ corroborates the charge-tagged carbene structures while revealing interesting intramolecular proton transfer equilibrium. The major dissociation channel of 4+ involves the loss of the neutral 1-methyl-imidazol of 82 Da via straightforward charge-induced dissociation that forms, after fast proton shift, the vinyl methyl imidazolium ion of m/z 109 (Scheme 3).
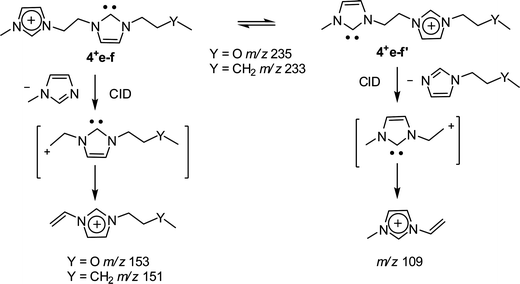 | ||
| Scheme 3 | ||
The operation of such characteristic, structurally diagnostic dissociation (Scheme 3) was corroborated when dissociating the asymmetrically substituted charge-tagged carbenes 4e+ (Fig. S4‡) and 4f+ (Fig. 3).
Dissociation of 4d+ (Fig. S5‡) was also revealing, as for the intrinsic acidity of imidazolium ions, since two dissociation routes were also observed (Scheme 4). These independent dissociations indicate fast intramolecular proton exchange likely involving the C2–H and C2–Me hydrogens. This intrinsic equilibrium, observed in the gas phase but also likely to occur in solution, further confirms that C2–H by C2-R replacement does not limit the acidity of imidazolium ions.24 Theoretical calculations at the Beck3LYP/6-31G+(d,p) level for the optimized gaseous structures of these new charge-tagged carbenes 4x+ reveal a stable and interesting gaseous structure in which the C2–H is involved in intramolecular H-bonding with the heterocyclic carbene center (Fig. 4).
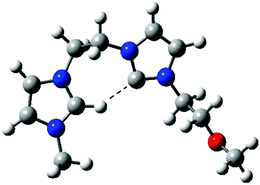 | ||
| Fig. 4 Beck3LYP/6-31G+(d,p) optimized structure of the charge-tagged carbene 4f+. | ||
 | ||
| Scheme 4 | ||
Calculations also indicate very small differences in intrinsic acidity (for instance 0.2–1.0 kcal mol−1 for 4f+, Fig. S6‡) for the two possible C2–H in the asymmetrically substituted charge-tagged carbenes. Interestingly, despite considerable intramolecular charge repulsion expected for such small species, ESI(+) was also able to transfer to the gas phase intact doubly and triply charged supramolecular aggregates such as [3g,i.Br2]2+ and [3j.Br]3+ (Table 1). Dissociation by HBr loss (Fig. 5) of these unique species seems to provide prompt access to several new multiply charged mono- and di-carbenes. The di-carbenes could undergo fast intramolecular coupling and this possibility is still to be fully scrutinized but their similar dissociation chemistry (Fig. S7‡) to that observed for the charge-tagged mono-carbenes seems to indicate that the N-heterocyclic carbene centers are preserved. Fig. S7 shows the ESI(+)-MS/MS for the dissociation of the doubly charged dicarbene4h+ and the triply charged monocarbene4j3+, which again corroborate their proposed and unprecedented structures.‡
![ESI(+)-MS/MS of a) [3h.Br2]+ of m/z 445 which forms by HBr loss the charge-tagged N-heterocyclic dicarbene4h+ of m/z 285 and b) [3g.Br]2+ of m/z 183 which forms the doubly charged N-heterocyclic monocarbene 4g2+ of m/z 143. Note that the structures shown are likely to be in equilibrium with isomers via intramolecular Br− or C2–H exchanges.](/image/article/2011/RA/c1ra00024a/c1ra00024a-f5.gif) | ||
| Fig. 5 ESI(+)-MS/MS of a) [3h.Br2]+ of m/z 445 which forms by HBr loss the charge-tagged N-heterocyclic dicarbene4h+ of m/z 285 and b) [3g.Br]2+ of m/z 183 which forms the doubly charged N-heterocyclic monocarbene 4g2+ of m/z 143. Note that the structures shown are likely to be in equilibrium with isomers via intramolecular Br− or C2–H exchanges. | ||
The same major dissociation routes observed for the singly charged carbenes4+ was also observed for the doubly (42+) and triply charged carbenes (43+) as Fig. 6 exemplifies for 4g2+. C-N cleavage of 4g2+ forms a singly charged imidazolium ion of m/z 109 but the counterpart neutral imidazole remains charge-tagged and is consequently detected by MS as the fragment ion of m/z 177 (Scheme S1‡).
Carbenes, in the singlet state, can react either as electrophiles or as nucleophiles depending on the nature of the counter reactant.25 With carbonyl compounds, carbenes readily acts as nucleophiles adding to the carbonyl carbon.25a To test the usefulness of such unique long lived, gaseous charge-tagged 4x+ species in assessing the intrinsic gas phase reactivity of N-heterocyclic carbenes, 4g2+ of m/z 143 was reacted with acrolein and acetone (Fig. 7). Although the cell conditions in the mass spectrometer used are not optimized for ion/molecule reactions, addition products with acrolein (m/z 172) and acetone (m/z 171) were indeed detected together with CID fragments of 4g2+ (Fig. 6).
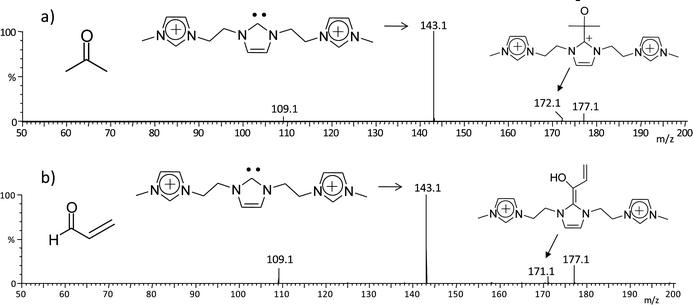 | ||
| Fig. 7 ESI(+)-MS/MS for ion/molecule reactions of the charge tagged N-heterocyclic carbene 4g2+ of m/z 143 with a) acetone and b) acrolein. | ||
Conclusions
The first stable charge tagged N-heterocyclic carbenes 4x+ (x = 1–3) as well as [3.Br(n−x)]x+ aggregates have been “fished”, likely from the [3.Br(n−x)]x+ ⇌ 4x+ + HBr solution equilibrium, and fully characterized in the gas phase. Analogous neutral carbenes for singly charged ILs have long been hypothesized to form from imidazolium ions in solution via C2–H abstraction by counter anions or bases, and the present finding supports this hypothesis. The dissociation of the [3.Br(n−x)]x+ aggregates (in which Br− works as the base) via thermal activation during collisions has also been found to mimic the solution equilibrium and to form gaseous 4x+ by HBr loss. The fishing directly from solution to the gas phase, isolation, and investigation of gas phase chemistry of long-lived charge-tagged N-heterocyclic carbenes is likely to provide insight into the intrinsic (solvent and counter ion free) reactivity of these key but elusive intermediates and their roles in IL solution chemistry, catalysis and metal coordination. The interception of 4x+ and the dominance of HBr loss (C2–H abstraction by Br−) for gaseous [3.Br(n−x)]x+ also indicates that, when imidazolium based ionic liquids are employed in solutions, even under relatively “neutral” conditions, the relatively stable N-heterocyclic carbenes are most likely to be formed with either detrimental or beneficial results. The presence of N-heterocyclic carbenes in imidazolium IL solutions is therefore most probably inevitable; hence, their participation should always be considered in processes performed in imidazolium IL or using ILs as reactants or catalysts.Experimental section
The imidazolium salts were prepared according to the procedures described in the literature.17–18 ESI mass and tandem mass spectra in the positive ion mode were acquired using a Waters Micromass (Manchester, UK) QTOF instrument with ESI-QTOF configuration having 5000 mass resolution and less than 50 ppm mass accuracy in the TOF mass analyzer. The following typical operating conditions were used: 3 kV capillary voltage, 40 V cone voltage, and desolvation gas temperature of 100 °C. ESI-MS/MS analyses were performed using 15–30 eV collision-induced dissociation (CID) of mass-selected ions with argon. Selection was generally performed by Q1 using a unitary m/z window, and collisions were performed in the rf-only hexapole collision cell, followed by mass analysis of product ions by the high resolution orthogonal-reflectron TOF analyzer. Reactions were performed by mass selection of the ion of interest and through low-energy (about 3 eV) collisions in the rf-only hexapole collision cell.Note added after first publication
This article replaces the version published on 18th July 2011, which contained errors in Schemes 2–4 and Fig. 6 and 7.Acknowledgements
The authors thanks CNPq, CAPES, FAPESP, INCT-MCT and FAPERGS for financial support.References
- (a) W. Kirmse, Carbene Chemistry, Academic Press, New York, 1971 Search PubMed; (b) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS; (c) H. W. Wanzlick, Angew. Chem., 1962, 74, 129 CrossRef CAS.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef CAS.
- N. Marion, S. Diez-Gonzalez and I. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS.
- (a) W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1291 Search PubMed; (b) O. Navarro, R. A. Kelly and S. P. Nolan, J. Am. Chem. Soc., 2003, 125, 16194 CrossRef CAS.
- (a) O. Back, M. A. Celik, G. Frenking, M. Melaimi, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2010, 132, 10262 CrossRef CAS; (b) O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369 CrossRef CAS.
- O. Back, G. Kuchenbeiser, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2009, 48, 5530 CrossRef CAS.
- V. Lavallo, A. El-Batta, G. Bertrand and R. H. Grubbs, Angew. Chem., Int. Ed., 2011, 50, 268 CrossRef CAS.
- (a) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534 CrossRef CAS; (b) R. Singh, R. M. Kissling, M. A. Letellier and S. P. Nolan, J. Org. Chem., 2004, 69, 209 CrossRef CAS; (c) D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743 CrossRef CAS; (d) M. S. Kerr, J. R. de Alaniz and T. Rovis, J. Am. Chem. Soc., 2002, 124, 10298 CrossRef CAS; (e) V. Nair, V. Sreekumar, S. Bindu and E. Suresh, Org. Lett., 2005, 7, 2297 CrossRef CAS; (f) H. Rigby and Z. Q. Wang, Org. Lett., 2002, 4, 4289 CrossRef.
- F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2011, 50, 5261 CrossRef CAS.
- (a) R. R. Julian, J. A. May, B. M. Stoltz and J. L. Beauchamp, Int. J. Mass Spectrom., 2003, 228, 851 CrossRef CAS; (b) S. M. Villano, N. Eyet, W. C. Lineberger and V. M. Bierbaum, J. Am. Chem. Soc., 2008, 130, 7214 CrossRef CAS.
- (a) F. A. Wiedmann, J. N. Cai and C. Wesdemiotis, Rapid Commun. Mass Spectrom., 1994, 8, 804 CrossRef CAS; (b) F. Turecek in Transient intermediates of chemical reactions by neutralization-reionization mass spectrometry, Topics in Current Chemistry, 2003, vol. 225, p. 77, Springer-Verlab, Berlin. Search PubMed; (c) D. J. Lavorato, T. K. Dargel, W. Koch, G. A. McGibbon, H. Schwarz and J. K. Terlouw, Int. J. Mass Spectrom., 2001, 210–211, 43 Search PubMed.
- K. G. Stirk and H. I. Kenttamaa, J. Am. Chem. Soc., 1991, 113, 5880 CrossRef CAS.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64 CAS.
- (a) C. Hinderling, C. Adlhart and P. Chen, Angew. Chem., Int. Ed., 1998, 37, 2685 CrossRef CAS; (b) C. Adlhart and P. Chen, Helv. Chim. Acta, 2000, 83, 2192 CrossRef CAS; (c) L. S. Santos, C. H. Pavam, W. P. Almeida, F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 4330 CrossRef CAS; (d) A. Sabino, A. Machado, C. Correia and M. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 2514 CrossRef CAS.
- (a) M. A. Schade, J. E. Feckenstem, P. Knochel and K. Koszinowski, J. Org. Chem., 2010, 75, 6848 CrossRef CAS; (b) P. Chen, Angew. Chem., Int. Ed., 2003, 42, 2832 CrossRef CAS.
- (a) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS; (b) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef.
- (a) J. L. Anderson, R. F. Ding, A. Ellern and D. W. Armstrong, J. Am. Chem. Soc., 2005, 127, 593 CrossRef CAS; (b) G. N. Sheldrake and D. Schleck, Green Chem., 2007, 9, 1044 RSC; (c) X. Han and D. W. Armstrong, Org. Lett., 2005, 7, 4205 CrossRef CAS.
- F. M. Nachtigall, Y. E. Corilo, C. C. Cassol, G. Ebeling, N. H. Morgon, J. Dupont and M. N. Eberlin, Angew. Chem., Int. Ed., 2008, 47, 151 CrossRef CAS.
- T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366 CrossRef CAS.
- (a) R. Giernoth and D. Bankmann, Eur. J. Org. Chem., 2008, 2881 CrossRef CAS; (b) J. D. Scholten and J. Dupont, Organometallics, 2008, 27, 4439 CrossRef CAS.
- J. Dupont and J. Spencer, Angew. Chem., Int. Ed., 2004, 43, 5296 CrossRef CAS.
- (a) L. S. Ott, M. L. Cline, M. Deetlefs, K. R. Seddon and R. G. Finke, J. Am. Chem. Soc., 2005, 127, 5758 CrossRef CAS; (b) J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780 RSC.
- (a) F. C. Gozzo, L. S. Santos, R. Augusti, C. S. Consorti, J. Dupont and M. N. Eberlin, Chem.–Eur. J., 2004, 10, 6187 CrossRef CAS; (b) B. A. D. Neto, L. S. Santos, F. M. Nachtigall, M. N. Eberlin and J. Dupont, Angew. Chem., Int. Ed., 2006, 45, 7251 CrossRef.
- J. D. Scholten, G. Ebeling and J. Dupont, Dalton Trans., 2007, 5554 RSC.
- (a) O. Illa, H. Gornitzka, V. Branchadell, A. Baceiredo, G. Bertrand and R. M. Ortuno, Eur. J. Org. Chem., 2003, 3147 CrossRef CAS; (b) K. A. Williams and C. W. Bielawski, Chem. Commun., 2010, 46, 5166 RSC.
Footnotes |
| † Dedicated to Dr Hilkka I. Kenttämaa for her pioneering work on gas phase charge-tagged radicals. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00024a |
| This journal is © The Royal Society of Chemistry 2011 |