Bioorthogonal nanozymes: an emerging strategy for disease therapy
Zheao
Zhang
a and
Kelong
Fan
 *abc
*abc
aCAS Engineering Laboratory for Nanozyme, Key Laboratory of Protein and Peptide Pharmaceutical, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, P. R. China. E-mail: fankelong@ibp.ac.cn
bNanozyme Medical Center, School of Basic Medical Sciences, Zhengzhou University, Zhengzhou 450052, China
cUniversity of Chinese Academy of Sciences, Beijing 101408, China
First published on 6th December 2022
Abstract
Transition metal catalysts (TMCs), capable of performing bioorthogonal reactions, have been engineered to trigger the formation of bioactive molecules in a controlled manner for biomedical applications. However, the widespread use of TMCs based biorthogonal reactions in vivo is still largely limited owing to their toxicity, poor stability, and insufficient targeting properties. The emergence of nanozymes (nanomaterials with enzyme-like activity), especially bioorthogonal nanozymes that combine the bioorthogonal catalytic activity of TMCs, the physicochemical properties of nanomaterials, and the enzymatic properties of classical nanozymes potentially provide opportunities to address these challenges. Thus, they can be used as multifunctional catalytic platforms for disease treatment and will be far-reaching. In this review, we first briefly recall the classical TMC-based bioorthogonal reactions. Furthermore, this review highlights the diverse strategies for manufacturing bioorthogonal nanozymes and their potential for therapeutic applications, with the goal of facilitating bioorthogonal catalysis in the clinic. Finally, we present challenges and the prospects of bioorthogonal nanozymes in bioorthogonal chemistry.
1. Introduction
Bioorthogonal chemistry has facilitated a significant revolution in the development of chemistry and biology since its introduction by Bertozzi and co-workers in 2003, which has greatly inspired scientists using exogenous chemical methods to explore intrinsic life processes that cannot be visualized by conventional approaches.1 Over the past 20 years, bioorthogonal chemistry as an efficient toolbox has proven its usability in many biomedical applications, which is mainly attributed to its selectivity, biocompatibility, and little influence on normal physiological processes.2 The emergence of bioorthogonal catalysis meets an ever-increasing need for high-performance biocompatible chemical reactions to execute complicated tasks in living cells and organisms. It mainly involves the following types of reactions including the Staudinger ligation, copper catalyzed azide–alkyne cycloaddition (CuAAC), strain promoted azide–alkyne cycloaddition (SPAAC), the inverse electron-demand Diels–Alder (IEDDA) cycloaddition reaction, transition metal-catalyzed bioorthogonal reactions, photoinducible bioorthogonal reactions, etc.3–5 From the perspective of chemical bond transformation in reactions, these bioorthogonal reactions can also be classified into two major categories, which are the bond formation reactions taking place between two mutually reactive bioorthogonal pairs and the bond cleavage reactions that emerged as an alternative approach to uncage bioactive agents from their inactive state.6–8Noteworthily, transition metal catalysts (TMCs) with enzyme-like substrate specificity are widely considered to be an excellent tool for promoting bioorthogonal catalytic reactions and enhancing reaction rates in biological environments.9 Until now, metal complexes and metal nanoparticles focused on TMCs, including ruthenium (Ru), palladium (Pd), iridium (Ir), platinum (Pt), copper (Cu), iron (Fe) and gold (Au), have exhibited outstanding physical and chemical properties that are not possessed by natural enzymes. Furthermore, they have developed to be a competitive alternative for producing in situ the required bioactive agents with spatial and temporal control and catalyzing new-to-nature reactions in biological settings.10,11 For example, in the classical CuAAC reaction, the first introduction of Cu(I) catalysts into bioorthogonal reactions not only increased the reaction rate and improved the yield of reactions between azides and alkyne, but also made these reaction conditions milder and more compatible, which significantly boosted the interest in investigating in vivo physiological processes using TMC-based bioorthogonal catalysis.12,13 Apart from the widely used Cu(I) catalyst, another breakthrough in the exploitation of TMCs was that Eric Meggers and co-workers reported for the first time the Ru-catalyzed release of amines from the respective allyl carbamates in living mammalian cells.14 Although TMCs have facilitated the applications of organic reactions in living organisms, given the complexity of biological systems and the toxicity of TMCs, the construction of high-efficiency and low-toxicity TMCs delivery systems to overcome physiological barriers is of equal importance. In living settings, the presence of components such as glutathione and thiol-containing substrates that can inactivate TMCs is very challenging.15 In general, applying TMCs to living cells faces many unresolved problems related to their biocompatibility, as well as their stability and catalytic reactivity in an aqueous environment.
Recently, nanozymes as a new type of nanomaterial with enzymatic activity have been coming to the forefront and exhibiting their positive potential in various fields, extending from sensing, detection, and imaging to disease therapy.16 Since the discovery of the peroxidase-like activity of Fe3O4 by Yan's group,17 the concept of nanozymes has been used to describe nanomaterials that possess enzyme-like activities. The enzyme-like activities demonstrated by nanozymes are mainly focused on peroxidase (POD), oxidase (OXD), catalase (CAT) and superoxide dismutase (SOD). With the multiple enzyme-mimicking activities of nanozymes, it is always possible to find the matching nanozymes with therapeutic effects for different diseases. Thus, inspired by the concept of nanozymes, when bioorthogonal catalysis encounters nanozymes, it is very promising to develop more effective bioorthogonal catalytic reactions relying on multifunctional TMC-based bioorthogonal nanozymes with the ability to provide control of bioorthogonal reactions. By leveraging the nanotechnology, the developed bioorthogonal nanozymes not only possess the bioorthogonal catalytic activity of the TMCs on their own, but can also be engineered for a variety of functions, such as better stability and water solubility, enhanced cellular uptake, precise targeting property, environmental stimuli responsiveness and natural enzyme-like activity.18–20 One of the more illustrative examples was reported by Rotello and coworkers. They fabricated the attractive cucurbit[7]uril-gated bioorthogonal nanozymes by incorporating TMCs into the hydrophobic monolayer of Au nanoparticles, which could effectively activate the release of fluorescent molecules and drugs from their corresponding pro-fluorophores and pro-drugs within living cells.21 It is important to note that these bioorthogonal nanozymes exhibit the ability to provide a universal platform for bioorthogonal catalysis-mediated clinical applications.
Thus, in this review, we first summarize TMC-mediated bioorthogonal catalytic reactions, so as to facilitate the design and in situ activation of pro-drugs and pro-fluorophores. Then, we highlight a few strategies for the preparation of multifunctional bioorthogonal nanozymes to pave a well-guided path for the advancement of bioorthogonal nanozymes in living organisms (Table 1). Finally, we describe the achievements and breakthroughs of bioorthogonal nanozymes in therapeutic applications (Scheme 1), and prospect the potential and challenges of bioorthogonal nanozymes in clinical applications.
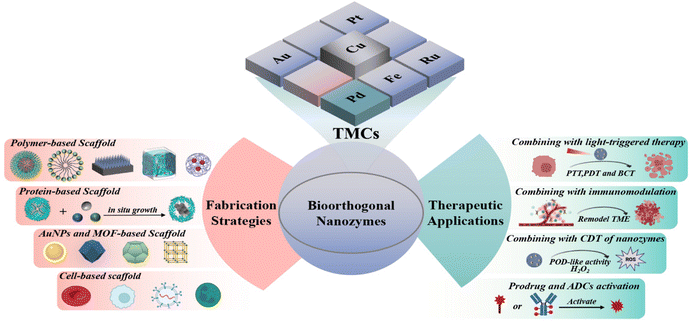 | ||
| Scheme 1 A schematic illustration for the fabrication of bioorthogonal nanozymes and their applications in disease treatment. | ||
| Transition metal catalysts (TMCs) | Nano-scaffold | TMC-based biorthogonal nanozymes | Applications | Ref. |
|---|---|---|---|---|
| [Fe(TPP)]Cl | A quaternary ammonium poly(oxanorbornene imide) | Bioorthogonal polyzyme loading [Fe(TPP)]Cl | Treatment of bacterial biofilms | 22 |
| [Cp*Ru(cod)Cl] | Poly(oxanorbornene imide) (PONI) polymers | Bioorthogonal polyzyme loading [Cp*Ru(cod)Cl] | Activation of prodrugs for anti-cancer therapy | 23 |
| Ru(bpy)3 | Single-chain nanoparticle | Ru(bpy)3-containing RuSCNP | Intracellular catalysis | 24 |
| Pd metal nanoparticles | Ferritin nanocages (FTn) | Pd nanozymes | Activation of pro-drugs for anti-cancer therapy | 25 |
| [Cp*Ru(cod)Cl] | Gold nanoparticles (AuNPs) | Bioorthogonal nanozyme loading [Cp*Ru(cod)Cl] | Intracellular activation of pro-Dox for anti-cancer therapy | 26 |
| Iron(III) tetraphenylporphyrin (FeTPP) | Gold nanoparticles (AuNPs) | Thermoresponsive bio-orthogonal nanozymes loading FeTPP | For antimicrobial therapy | 27 |
| [CpRu(8HQ) (allyl)PF6] | Gold nanoparticles (AuNPs) | Protein corona-based bioorthogonal nanozyme | Intracellular proteolysis for the generation of therapeutic | 28 |
| Cu nanoparticles | Zr metal–organic framework (MOF) | Triphenyl phosphonium (TPP) decorated MOF-Cu | Drug synthesis in mitochondria for tumor therapy | 29 |
| Cu2+ ions | Zeolitic imidazolate framework-8 (ZIF-8) MOF | Cu/Zn bimetallic MOF nanoparticles | Intracellular drug synthesis for anti-cancer therapy | 30 |
| Pd nanocubes | ZIF-8 MOF | Core–shell Pd/ZIF-8 | Multifunctional nanoreactors in biological settings | 31 |
| Pd nanoparticles | Amine-functionalized UiO-66 MOF | AS1411@Pd@UiO-66 | Activation of pro-drugs for anti-cancer therapy | 32 |
| Ultrathin Pd nanosheets | Exosome from A549 cell | Pd-ExoA549 | Bioorthogonal in situ activation of the anti-cancer drug | 33 |
| FeTPP | AuNPs | RBC-hitchhiked nanozymes | The treatment of bacterial infections | 34 |
| (1,1′-Bis(diphenylphosphino)ferrocene)palladium(II) dichloride | AuNPs | Bioorthogonal nanozymes-loaded macrophage | Targeting and killing cancer cells | 35 |
| Pd nanoparticles | Large-pore mesoporous silica nanoparticles (MSNs) | Neutrophil membrane coating MSN-Pd/CD@Neu | For anti-inflammation therapy | 36 |
| Pd nanoclusters | Au nanorods | BSA–Pd–Au nanorods | Tri-modality therapy of PTT–BCT–CDT for cancer therapy | 37 |
| Cu nanoparticles | Mesoporous carbon nanospheres (MCNs) | MCNs–Cu | In situ drug synthesis and photothermal treatment of tumor | 38 |
| Ultra-small Pd nanoparticles | MoS2 nanoflowers | MoS2@Pd | Reprogramming TME and treating tumor | 39 |
| Cu nanoparticles | Template of bacteria | Antibody bioorthogonal catalyst | For killing bacteria through in situ drug synthesis | 40 |
2. A brief historical overview of TMCs for bioorthogonal chemistry
With the recent developments, an increasing focus has been placed on TMC-mediated bioorthogonal uncaging and ligation reactions. As one of the cores in bioorthogonal catalysis, TMCs possess the ability to enhance catalytic reaction rates in living organisms and are widely popular by virtue of their high catalytic efficiency. It offers the possibility of allowing lower concentrations of reactants to perform reactions that cannot be completed by natural enzymes at a specific site and on a practical time scale.22,41 TMCs exhibit potential advantages for various applications in complex aqueous media, even in biologically relevant environments. Thus, in this section, some classical bioorthogonal reactions catalyzed by TMCs will be reviewed. Moreover, another important purpose of historical reviewing is to better combine the promising nanozymes with bioorthogonal catalysis and to promote the fabrication of biocompatible nanozymes based on TMCs, taking the merits of them to develop bioorthogonal nanozymes for biological research and biomedical applications that will be more in line with the intended goals.When it comes to classical metal-mediated bioorthogonal catalytic reactions, the focus is automatically on the copper(I)-promoted azide–alkyne cycloaddition (CuAAC). In Fig. 1a and b, since it was first proposed to use copper(I) as a transition metal catalyst for 1,3-dipolar cycloaddition between terminal alkynes and azides to synthesize [1,2,3]-triazole in mild environments,12,42 CuAAC served as an ideal complement to the click reaction family and facilitated the development of TMC-based bioorthogonal chemistry in biomedical applications such as in situ synthesis of drugs,29 disease therapy,43 bioengineering,44etc. A universal feature of these various applications relying on the CuAAC reaction is that the investigators usually employ different approaches to avoid the most worrying drawbacks of the CuAAC reaction, that is, the instability and toxicity of Cu(I) catalysts in vivo.45 The toxicity of Cu(I) ions is a major factor limiting their wider applications in biological environments and is also a problem that the researchers are committed to addressing. For this reason, lots of efforts are made to develop safer and more biocompatible TMCs. For example, Qu's group reported a promising bioorthogonal catalytic system based on Cu nanoparticles (CuNPs). CuNPs exhibited improved biosafety compared to free Cu(I). In this catalytic system, NIR light irradiation effectively promoted the conversion of Cu(0) to Cu(I) and increased the local temperature (Fig. 1c). Then, the locally elevated temperature further accelerated the production of Cu(I), thus collectively boosting the CuAAC reaction process.38 This strategy largely minimized the toxicity associated with Cu(I) while ensuring that the prodrug could be effectively activated in the living environment and exerted promising antitumor effects, opening a prospective path for the development of innovative alternative bioorthogonal catalysis.
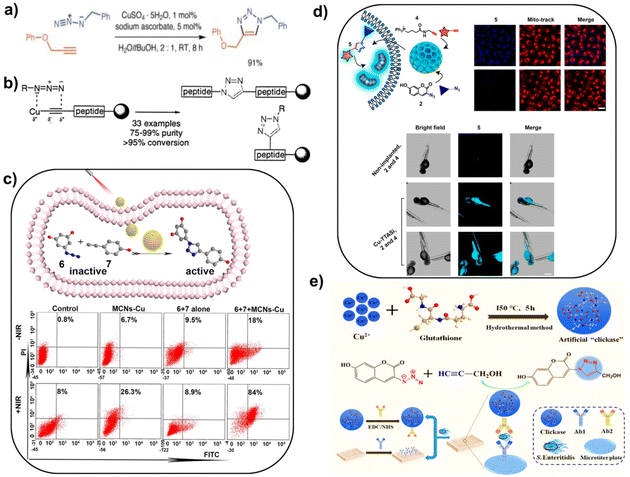 | ||
| Fig. 1 Cu-mediated bioorthogonal catalytic reactions. (a) Cu(I) catalyzed ligation reaction between phenyl propargyl ether and benzylazide and the yield of 1,4-disubstituted triazole was 91%. This figure has been reproduced from ref. 42 with permission from John Wiley & Sons, copyright 2019. (b) Cu(I)-mediated 1,3-dipolar cyclization of alkynes with azides to furnish analogs of peptidetriazoles or N-substituted histidines. This figure has been reproduced from ref. 12 with permission from the American Chemical Society, copyright 2002. (c) Resveratrol analogue was produced with NIR irradiation in HeLa cells. The apoptosis assay of HeLa cells was conducted by flow cytometry. Data are expressed as mean ± SD (n = 3). This figure has been reproduced from ref. 38 with permission from the American Chemical Society, copyright 2020. (d) Fluorescent molecule 5 with the ability to target mitochondria was synthesized in cells via the Cu-TTASi-catalyzed reaction of precursors 2 and 4. The confocal images of MCF-7 cells (scale bar: 50 μm) and Cu-TTASi-implanted zebrafish embryos (scale bar: 500 μm) were presented. The red indicated the mitochondria stained with Mito-Tracker Red. The fluorescent product 5 appeared blue. This figure has been reproduced from ref. 51 with permission from the American Chemical Society, copyright 2021. (e) Schematic diagram of preparing artificial “clickase” and its applicability in the catalytic click immunoassay towards S. enteritidis. This figure has been reproduced from ref. 50 with permission from the American Chemical Society, copyright 2021. | ||
In addition, another alternative way to optimize the poor properties of Cu(I) is taking advantage of reductants or a ligand with the function of stabilizing Cu(I), which can effectively avoid toxicity and the undesired byproducts formed by direct exposure of Cu(I) to the physiological environment.46,47 For example, Wan et al. developed a highly sensitive and accurate method for detecting pathogenic bacteria, which was dependent on the dual signal amplification effects of DNAzymes and Cu3(PO4)2-induced CuAAC. In this electrochemical biosensor, sodium ascorbate as a reductant plays a key role, where it was responsible for reducing Cu(II) ions released from dissolved Cu3(PO4)2 to Cu(I). Subsequently, the formed Cu(I) could effectively produce an electrical signal by triggering the CuAAC reactions between azide-functionalized ssDNA DNAzymes and alkyne-functionalized ssDNA anchored on the electrode surface.48 Along this direction, Wang et al. built a catalytic system combining CuSO4 and sodium borohydride (CuAAC-Bor). Cu(II) in this system was reduced in situ by sodium borohydride to Cu nanoclusters (CuNCs) that exhibited stronger efficiency in immobilizing the peptides on Ti implants. In contrast to the CuSO4/sodium ascorbate catalytic system, CuSO4/sodium borohydride catalysts greatly improved the peptide immobilization efficiency. Moreover, the fabricated peptide-engineered implants not only demonstrated superior advantages in inhibiting five types of clinical bacteria in vitro, but also promoted angiogenesis and osteogenesis in vivo, rendering CuAAC reactions more clinically valuable.44
Apart from that, Cu(I)-stabilizing ligands also contribute to preventing the oxidation and disproportionation of Cu(I). Just as reported, tannic acid (TA) and NaBH4 functioned as a green stabilizer and a reductant and were used to synthesize a range of transition metal nanoparticles (TMNPs) including CuNPs, PdNPs, AuNPs and AgNPs. The as-prepared small-sized CuNPs catalyzed a highly efficient CuAAC reaction between azides and alkynes with a satisfactory yield.49 Moreover, there are other substances that serve as stabilizers of Cu(I) in the CuAAC reaction. For instance, as shown in Fig. 1e, Li and co-workers developed an artificial “clickase” (CCN) through a simple hydrothermal method using CuSO4 and the stabilizer glutathione (GSH). Benefitting from the stabilizing effect of GSH, CCN clickases had a positive effect in promoting the CuAAC reaction process, showing high analytical performances for the catalytic click immunoassay.50 In a proof-of-concept study, N(C3N3)3 derivatives and the Cu(I)-stabilizing ligand were integrated into mesoporous organosilicon nanoparticles to prepare Cu-TTASi nanoreactors for the CuAAC “click” reactions with high selectivity and reactivity (Fig. 1d). The implications of tris(triazolylmethyl)amine (TTA)-bridged organosilane as a stabilizing ligand for CuAAC chemistry lie in the fact that it optimized the catalytic properties and improved the biocompatibility and safety of the biorthogonal catalyst, providing a bright future for bioorthogonal reactions in pharmacy.51
In addition to the above-mentioned Cu, which is most widely used in bioorthogonal catalysis, further breakthroughs in the development of high performance TMCs were obtained by introducing palladium (Pd)-catalyzed bioorthogonal chemistry. In particular, as shown in Fig. 2a, Pd catalyzed Suzuki–Miyaura coupling reactions offer a new methodology for forming carbon–carbon and carbon–heteroatom bonds in the synthesis of pharmaceuticals and organic compounds, and protein modification.52–54 The impacts of Pd-mediated reactions on the development of bioorthogonal catalysis and pharmaceuticals are far-reaching and significant to date, such as precisely controlling the activity of anti-cancer drugs in a targeted site and decreasing the systemic side effects (Fig. 2b).55 Pd can act as both a homogeneous catalyst (metallic compounds of Pd) for bioorthogonal catalysis and a non-homogeneous catalyst (Pd nanoparticles) for bond breaking and formation (depropargylation and Suzuki–Miyaura cross-coupling reaction). In addition, Pd NPs were found to be more biocompatible and have a higher catalytic activity than the ionic form of Pd compounds.3,8,56 Of the numerous studies, Bradley's pioneering work has contributed to the use of Pd0-catalysts in bioorthogonal reactions for in situ labelling of cellular structures and pro-drug activation. For example, in 2011, Bradley and co-workers reported the preparation of Pd0 microspheres that effectively crossed cell membranes and completed a series of Pd0-catalysed reactions including allylcarbamate cleavage and the formation of C–C bonds, which was the first intracellular Pd0 catalyst used to synthesize exogenous materials with virtually no toxicity.57 This approach is viewed as an invaluable complement to the toolkit of catalytic reactions that can be conducted within cells. Building on this foundation, in combination with rapid progress in nanotechnology, a variety of Pd catalysts including Pd complexes and Pd nanocatalysts have emerged for bioorthogonal chemical reactions within biological environments. For instance, it was reported that the commercial and water soluble Na2PdCl4 complexes were reduced to active biocompatible Pd0 species by sodium ascorbate under intracellular conditions, which exhibited excellent performance in triggering bioorthogonal bond cleavage reactions and promoting the release of the compounds (such as fluorophores and cytotoxic drugs) from the corresponding precursors.58 Moreover, the Pd nanocatalyst is generally applied to complete targeted bioorthogonal catalytic reactions with minimal adverse effects. Salvador Pané and co-workers developed a hybrid nanowire composed of Fe and Pd (FePd NWs) with catalytic ability to activate Pro-5-FU into 5-FU and first showed the therapeutic potential of nontoxicity nanorobots in Pd-mediated bioorthogonal organometallic (BOOM) reactions for disease chemotherapy.59 In addition, the modification of TMCs with a targeting peptide is generally a common and effective method to increase the targeting ability of TMCs. For example, in Zou's work, RGD-functionalized Pd(II) selectively enriched within tumor cells and then triggered the transmetallation reactions of organogold(I) into active gold(I) species. With the help of Pd(II), the formed active gold(I) not only was potentially active for bioorthogonal catalysis but also inhibited the proliferation of cancer cells and presented the ability of anti-angiogenesis in zebrafish models.60 In another interesting study, Qu's group delicately integrated bioorthogonal catalysis with ROS-mediated therapy for anti-bacterial infection using novel antimicrobial bioorthogonal nanozymes (PdCu-urchin) with a special surface topology. The mechanisms by which the PdCu-urchin nanozyme acted as an antibacterial agent involved two parts: on the one hand, PdCu-urchin allowed the in situ synthesis of antibacterial active substances through a Cu-catalyzed CuAAC reaction; on the other hand, Pd with the peroxidase-like enzyme activity could effectively produce high-toxicity ROS in the presence of H2O2 for synergistically killing bacteria.61 Importantly, this strategy offered new ideas for the applications of bioorthogonal nanozymes in antibacterial therapy by combining the different catalytic properties of Cu and Pd catalysts. It appears that the collaborative use of TMCs with both non-natural enzyme activity and natural enzyme-like catalytic activity will compensate for the shortcomings of single catalysts, as well as bring some innovations and breakthroughs in the biomedical applications of TMC-mediated bioorthogonal catalysis.
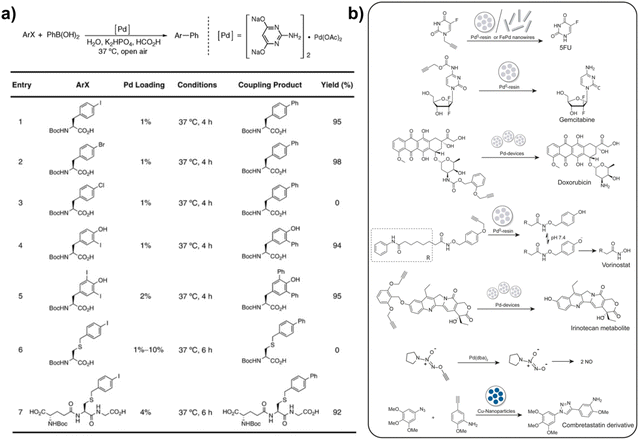 | ||
| Fig. 2 Pd-catalyzed bioorthogonal reactions. (a) Pd-pyrimidine catalyzes cross-coupling reactions. This figure has been reproduced from ref. 53 with permission from the American Chemical Society, copyright 2009. (b) Pd and Cu mediate the catalytic deprotection of prodrugs. This figure has been reproduced from ref. 55 with permission from John Wiley & Sons, copyright 2019. | ||
The TMC ruthenium (Ru) also occupies a place in catalyzing bioorthogonal reactions. In 2006, Craig Streu and Eric Meggers reported the [Cp*Ru(cod)Cl] complex (Cp* represents pentamethylcyclopentadienyl, cod means 1,5-cyclooctadiene) that catalyzed the cleavage of allylcarbamates in biological environments (such as in living cells) as evidenced by the strong green color from the uncaged rhodamine 110, demonstrating the bioorthogonality of Ru and inspiring the pursuit of Ru-based bioorthogonal reactions to explore life processes.14 The researchers found that the yield of Ru-mediated catalytic reactions was virtually unaffected by air and water, which showed excellent tolerance. On the basis of this study, Eric Meggers et al. further designed an efficient [CpRu(QA-NMe2)(allyl)]PF6/allyl carbamate catalytic system for the activation of anticancer prodrugs, where the prodrug of doxorubicin, N-(allyloxycarbonyl)doxorubicin, was efficiently converted to doxorubicin in the cellular cytoplasm and induced apoptosis of cells, presenting the potential of Ru-mediated cancer therapy at that time.62 There are now a number of reports that do realize meaningful pharmaceutical applications using Ru-based multifunctional bioorthogonal catalysts. For example, it was found that cationic Ru(II) complexes more efficiently catalyzed cycloaddition reactions between thioalkynes and azides (RuAtAC) in comparison with the previously reported neutral [Cp*RuCl(COD)] complex in biological environments, such as PBS and cell lysates, which demonstrated excellent bioorthogonality, chemoselectivity, and biocompatibility (Fig. 3a). In this study, the [Cp*Ru(II)arene] sandwich complexes presented the photoactivated properties and controlled the production of active Ru(II) species under the irradiation of a lamp at 365 nm, providing strong support for light and Ru(II) complex co-mediated bioorthogonal catalytic reactions for further biological applications.63 As illustrated by the work of Chao and co-workers, they effectively labeled tumor cells via Ru(II) complex-catalyzed bioorthogonal cycloaddition reactions between an alkyne group and the tagged azide group on the tumor cell membrane. The produced cycloaddition product functioned as a photosensitizer, which could destroy the tumor cell membrane under two-photon irradiation and trigger cell apoptosis. This methodology that combined bioorthogonal labeling with photodynamic therapy is a promising way to avoid the difficulties encountered in the treatment of triple-negative breast cancer, such as the lack of a suitable target receptor.64 Ru-based bioorthogonal catalysts can be used not only in the form of complexes for therapeutic applications, but also in combination with nano-scaffolds to construct bioorthogonal nanozymes with excellent biodegradability, as in the study of Vincent M. Rotello.65 He developed a novel bioorthogonal nanozyme (ZnS_NZ) by incorporating Ru-based [Cp*Ru(cod)Cl] complexes into the monolayers of ZnS nanoparticles (ZnS NPs), which endowed the [Cp*Ru(cod)Cl] catalyst with better catalytic activity, biocompatibility, and biodegradability (Fig. 3b). ZnS NPs, as a nano-scaffold, not only provided excellent stability with the catalyst, but also participated in the bioorthogonal transformation as a cofactor to facilitate the catalytic process. More importantly, the prodrug (pro-Mit) of mitoxantrone (Mit) could be uncaged by ZnS_NZ in situ to form its active form, showing effective killing ability against cancer cells and offering a valuable opportunity for clinical translation of bioorthogonal catalysis.
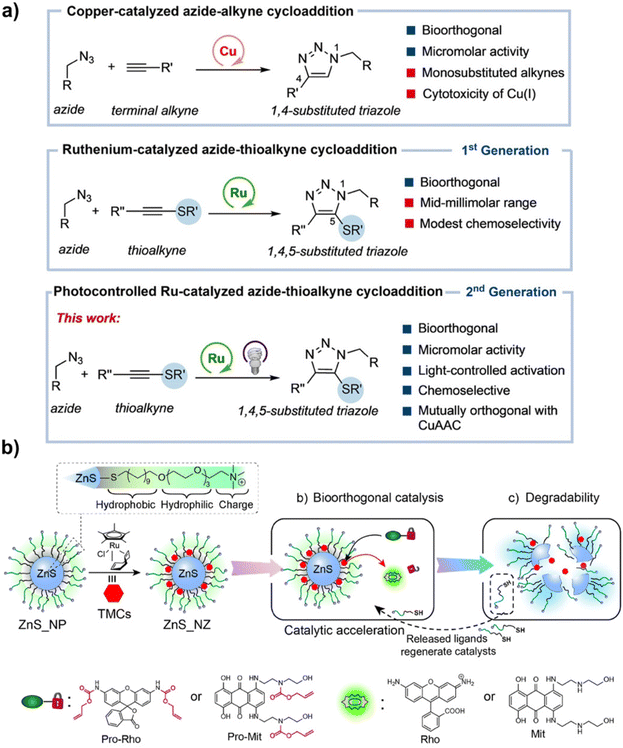 | ||
| Fig. 3 Ru-catalyzed bioorthogonal reactions. (a) The advantages and limitations of Cu and Ru-catalyzed azide–alkyne cycloaddition reactions. This figure has been reproduced from ref. 63 with permission from John Wiley & Sons, copyright 2021. (b) The preparation of ZnS_NZ with accelerated catalytic activity, biocompatibility, and biodegradability. This figure has been reproduced from ref. 65 with permission from the American Chemical Society, copyright 2022. | ||
In addition, other TMCs including Au, Pt, and Fe also show bioorthogonal catalytic ability to achieve the release of prodrugs or pro-fluorophores under intracellular conditions. For instance, Yoon's group reported for the first time that rhodamine-alkyne derivative was used as an “off–on” type fluorescent probe to specifically detect Au3+. The addition of Au3+ induced the conversion of propargylamide moiety to oxazolecarbaldehyde and produced an obvious color change from colorless to pink.66 Furthermore, Vidal's group designed gold(I) chloride complexes with water compatible ligands that demonstrated effective bioorthogonal catalytic activity for mild intramolecular alkyne hydroarylation.67 In addition, Au, similar to Pd, can act as a non-homogeneous catalyst to mediate bioorthogonal catalytic reactions. Sun and co-workers found that Pt(II) effectively triggered the bioorthogonal cleavage of O/N-propargyl in a wide range of molecules. On this basis, they rationally designed bioorthogonal cleavage reaction-catalyzed prodrug that was able to specifically enter cancer cells and generate synergistic tumor suppression.68 Moreover, in 2012, Sasmal's group discovered that [Fe(TPP)Cl] efficiently catalyzed the reduction of aromatic azides to the respective amines in physiological environments and even in living cells.69 Moreover, in recent years, Fe(TPP)Cl catalysts have demonstrated their successful applications in disease therapy, which will be described in the next sections in detail.
From a brief review of general bioorthogonal TMCs, we summarize the advantages of TMCs in catalyzing bioorthogonal reactions for various applications, while there is still room for improvement in some aspects of TMCs such as (1) inherent toxicity, (2) physicochemical stability in living environments, (3) efficiency of uptake by cells, (4) targeting properties to the aimed site, etc. To tackle these challenges, TMCs require a difficult balance between catalytic activity, stability, and biosafety.
Nanozymes as a novel class of nanomaterials with enzyme-like catalytic activity bring a paradigm shift for improving the bioorthogonal catalytic performance of TMCs. Due to their nanoscale size and excellent physicochemical properties, nanozymes hold great potential in the treatment of various diseases. Therefore, a nanozyme with bioorthogonal catalytic activity is necessarily developed to overcome the challenges faced by TMCs in vivo. From a certain standpoint, when TMCs are loaded into nanomaterials scaffold or prepared into nanoparticles, this whole can be considered as a new category of nanozymes with bioorthogonal catalytic activity, called bioorthogonal nanozymes. On the one hand, the formation of bioorthogonal nanozymes provides a protective environment for TMCs and reduces their non-specific exposure in vivo, ensuring their catalytic activity at the targeted site. On the other hand, using different nano-scaffolds, the formed bioorthogonal nanozymes can confer smarter properties to the TMCs, such as precise targeting ability, photothermal properties, and environmental responsiveness. Moreover, TMCs in many reported nanozymes also exhibited natural enzyme-like activity, such as POD-like activity, for achieving synergistic therapy for diseases. Thus, in the following sections, we will summarize some attractive strategies for constructing TMC-based bioorthogonal nanozymes with excellent biocompatibility, safety, active targeting ability and permeability of cell membranes, facilitating the availability of bioorthogonal nanozymes in biomedical applications that is suitable for clinical translation.
Thus, in the following sections, we will summarize some of the attractive strategies for constructing TMC-based bioorthogonal nanozymes with excellent biocompability, safety, active targeting ability and enhanced permeability of cell membranes, offering the potential of bioorthogonal nanozymes in facilitating bioorthogonal catalysis in biomedical applications that is suitable for clinical translation.
3. The development of TMC-based bioorthogonal nanozymes
At present, to meet the needs of bioorthogonal catalytic reactions mediated by TMCs in various scenarios, it is no longer enough to use naked TMCs alone, but preferably to build multifunctional TMC-based bioorthogonal nanozymes with good stability, water solubility, and in vivo environmental responsiveness. In this section, we will focus on the different approaches for preparing bioorthogonal nanozymes with desirable physicochemical properties, promoting the establishment of a nanozyme-based bioorthogonal catalysis platform for various demands in biomedicine. Some representative strategies are proposed to develop bioorthogonal nanozymes using polymer and protein nanocages, gold nanoparticles and metal–organic frameworks, as well as cell-based carriers as scaffolds.3.1 Polymer scaffolds and protein nanocages for TMC-based bioorthogonal nanozymes
The direct usage of naked TMCs in the living environment always raises common concerns, mainly including poor stability, loss of catalytic activity and toxicity to tissues and organs.70,71 These issues that need to be addressed constitute a major driving force for developing TMC-based bioorthogonal nanozymes, encouraging researchers to design effective strategies that can ameliorate the catalytic properties of TMCs.In this regard, a variety of formulations consisting of degradable and biocompatible polymers become attractive candidates for constructing bioorthogonal nanozymes. Rotello and co-workers have contributed much effort in this field. For example, as shown in Fig. 4a, they reported a polymeric nanoparticle that could encapsulate TMCs into a protective hydrophobic core to form bioorthogonal “polyzymes”. This designed bioorthogonal nanozyme was composed of two parts: the hosting polymer with an alkyl chain and a cationic terminal group, as well as the loaded catalyst ([Fe(TPP)]Cl). By virtue of the protective effect and positive surface charge of the polymer, [Fe(TPP)]Cl exhibited improved stability, catalytic activity, and permeability to biofilms, which effectively activated the pro-antibiotic and exerted therapeutic effects against bacterial infections.22 Moreover, they fabricated another “polyzyme” through employing poly(oxanorbornene imide) as a polymeric scaffold to encapsulate [Cp*Ru(cod)Cl] catalysts. Compared to the free Ru catalyst, the catalytic activity of the polymeric bioorthogonal nanozyme was significantly improved in serum, which hopefully overcame the main obstacle to the application of TMCs. Importantly, allyloxycarbonyl-protected anticancer drug mitoxantrone (pro-Mit) was effectively decaged in the presence of bioorthogonal nanozyme and released the active mitoxantrone that inhibited the growth of cancer cells.23 In addition, Chen and coworkers prepared cross-linked copper-containing single chain nanoparticles (SCNPs) that encapsulated a tris(bipyridine)ruthenium (Ru(bpy)3) complex (RuSCNPs). The reported RuSCNPs with a cationic surface was capable of binding and delivering exogenous β-galactosidase (βGal) into the cell. In addition, the internalized βGal and RuSCNP were located in endosomes, which could be regarded as artificial organelles and acted as “intracellular drug factories” to produce active drugs and fluorescent agents through tandem catalysis (Fig. 4c). This provided a useful platform for the combined applications of bioorthogonal nanozymes and natural enzymes in the treatment of diseases.24 Taken together, the polymeric nanoparticles offer superior support for TMC-based bioorthogonal nanozymes.
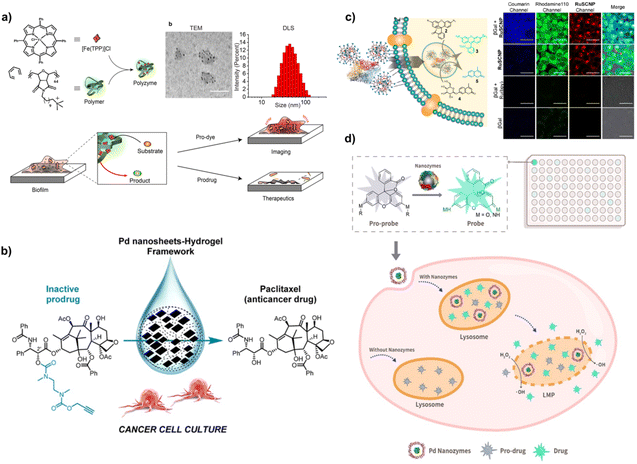 | ||
| Fig. 4 Polymer and protein nanocages as scaffolds for bioorthogonal nanozymes. (a) The fabrication of polyzyme, and the results of its morphology and size were measured by TEM and DLS. Scale bar: 100 nm. And polyzyme was used to bioorthogonally activate the prodrug and pro-dye. This figure has been reproduced from ref. 73 with permission from the American Chemical Society, copyright 2020. (b) Pd nanosheets-hydrogel bioorthogonal framework catalyzed the deprotection reaction of paclitaxel prodrug in cancer cells. This figure has been reproduced from ref. 72 with permission from the American Chemical Society, copyright 2020. (c) The co-delivery of RuSCNP and βGal and dual catalysis for pre-dyes in HeLa cells. This figure has been reproduced from ref. 24 with permission from the American Chemical Society, copyright 2020. (d) HFn protein-based nanozyme platform to encapsulate TMCs and bioorthogonal nanozyme induced lysosomal membrane leakage for drug delivery. This figure has been reproduced from ref. 25 with permission from the Ivyspring International Publisher, copyright 2022. | ||
Furthermore, inspired by bioorthogonal catalysis in biomedicine that can modulate drug activity in situ, Asier Unciti-Broceta and co-workers for the first time developed agarose and alginate hydrogel-entrapped Pd nanosheets for the selective activation of the paclitaxel prodrug (Pro-PTX) under physiological conditions. Benefitting from the biodegradability and non-thrombogenic properties of FDA-approved agarose and alginate, this Pd nanosheets–hydrogel bioorthogonal framework achieved controlled release of the Pd catalyst without influences on its catalytic activity, and rapidly mediated the dealkylation of Pro-PTX to release PTX in cancer cell culture, which further enriched the toolkit of bioorthogonal catalysis (Fig. 4b).72
Recently, Huang's group constructed a ferritin (FTn) nanocage-based bioorthogonal nanozyme platform in which most of the TMCs (Co, Fe, Mn, Rh, Ir, Pt, Au, Ru and Pd) were incorporated into the FTn nanocage, well illustrating the synergistic effects of bioorthogonal catalysis and enzyme-like catalysis of nanozymes in disease treatment (Fig. 4d).25 In this study, a series of bioorthogonal nanozymes were synthesized through in situ growth of metal nanoclusters in the inner cavity of FTn. The results of fluorescence measurements showed that only Pd nanozymes exhibited excellent bioorthogonal catalytic activity for the activation of the pro-fluorescent dye by cleaving the propargyl ether bond, which presented the enzyme-mimicking activity of mutant P450BM3. In addition, the Pd nanozyme also possessed peroxidase (POD)-like activity under acidic conditions (pH 5.0) and catalyzed the conversion of intracellular H2O2 to free radicals, demonstrating the ability to induce lysosomal membrane leakage. Owing to the presence of ferritin-based Pd nanozyme mainly in lysosomes after entering cells via receptor-mediated endocytosis, Pd nanozymes with P450BM3-like activity and POD-like activity could not only promote lysosomal membrane rupture to achieve the lysosomal escape, but also could intracellularly generate active drugs in situ through Pd-mediated bioorthogonal catalytic reactions. Given the catalytic potential of Pd nanozymes in disease therapy, in the “all-in-one” system, Pd nanozymes and hydroxycamptothecin with a propargyl ether group (pro-HCPT) were co-loaded into the hydrophilic and hydrophobic layers of liposomes (Lipo-Pd-pHCPT) with RGD modification respectively. After systemic administration, Lipo-Pd-pHCPT had a significant advantage in tumor growth inhibition compared to other groups, which was attributed to the effective catalytic activity of the Pd nanozymes, the specific targeting ability and the prolonged in vivo circulation time. Taken together, ferritin based Pd nanozymes represent an attractive direction in biomedicine and will inspire more efforts to design bioorthogonal nanozymes with multiple enzymatic activities for drug development.
3.2 AuNPs and metal organic frameworks (MOFs) for TMC-based bioorthogonal nanozymes
Engineered gold nanoparticles (AuNPs) represent a versatile platform for developing bioorthogonal catalytic nanozymes. In particular, the recent studies performed by Rotello provided a promising direction for the fabrication of multifunctional nanozymes using AuNPs for disease therapy. Here are several examples to illustrate the availability of AuNPs in facilitating the construction of bioorthogonal nanozymes.For instance, Rotello's group designed a surface-engineered bioorthogonal nanozyme via loading with TMCs in a hydrophobic monolayer of AuNPs, which could be utilized to activate pro-fluorophores and prodrugs either intracellularly or extracellularly. The developed bioorthogonal nanozymes composed of AuNPs and the functionalized ligand including a hydrophobic segment for encapsulation of [Cp*Ru(cod)Cl], a tetraethylene glycol spacer for improving biocompatibility and a terminal interacting unit for cellular localization. As such, cationic Pos-NZ and zwitterionic Zw-NZ nanozymes were prepared by the respective modification of positively charged quaternary ammonium ligands and zwitterionic sulfobetaine moieties. The experimental results showed that cationic Pos-NZ exhibited a high level of cellular uptake as proven by the high contents of Au and Ru catalysts inside the cells compared to the neutral Zw-NZ, which provided the basis for activation of prodrugs and profluorophores in a controlled spatial manner.26 In addition, they developed another bioorthogonal nanozyme featured an on–off gated thermal response, demonstrating the effective thermo-regulated antibacterial therapeutic effects through the activation of antibiotic-based prodrugs. As shown in Fig. 5c, this thermoresponsive nanozyme system (Fe-NZ) was successfully prepared by integrating iron(III) tetraphenylporphyrin (FeTPP) into the hydrophobic monolayer of AuNPs, in which the different assembly states of FeTPP at high and low temperatures play a major role in the thermally responsive bioorthogonal catalytic activity. When at low temperatures, FeTPP preferred to exist as a compact assembly, greatly limiting the contact of the catalytically active center with the substrate. In contrast, with the increase of temperature, the aggregated FeTPP gradually redispersed in the monolayer of AuNPs, and its catalytic activity was restored. The thermoresponsive behavior of Fe-NZ presented great potential for eradicating bacterial biofilms in biological environments, which could be reflected by the significant inhibitory effects on biofilms co-treated with pro-Mox and Fe-NZ_37 at 37 °C.27 In addition, Rotello's group found that the soft or hard protein corona could be formed on the surface of AuNPs embedded with Ru catalyst (bioorthogonal nanozymes), which mainly depended on the hydrophilic or hydrophobic nature of the polymer modified on the outer layer of the AuNPs. As shown in Fig. 5a, the hard corona formed on the bioorthogonal nanozymes (called Corona-NZ1) resulted in Corona-NZ1 aggregation and the loss of catalytic activity. However, for the soft corona on the bioorthogonal nanozymes (called Corona-NZ2), it had little effect on the bioorthogonal catalytic activity, which could be attributed to the fact that the soft corona did not induce the agglomeration of Corona-NZ2. Given that this bioorthogonal nanozyme exhibited contrasting catalytic activities with different protein corona, the authors proposed to regulate the intracellular activation of bioorthogonal nanozymes by controlling the formation of the protein corona. Notably, the inhibition of the catalytic activity could be reversed via the hydrolysis of the protein corona by endogenous proteases in the endosome and lysosome, which right contributed to the intracellular in situ activation of the Corona-NZ1 activity. In the corresponding experiments, the points of the authors were verified. The results showed that removing the protein corona by proteolysis well reactivated the catalytic activity of Corona-NZ1 and Corona-NZ2, and this conclusion could also be further confirmed by the fact that these nanozymes did not effectively catalyze the decaging of the pro-rhodamine in the presence of the protease inhibitor cocktail. This study combined the co-regulation of protein corona and bioorthogonal catalytic activity, largely avoiding off-target effects and providing insights into the path to in vivo delivery of bioorthogonal nanozymes and in situ activation of catalytic activity.28
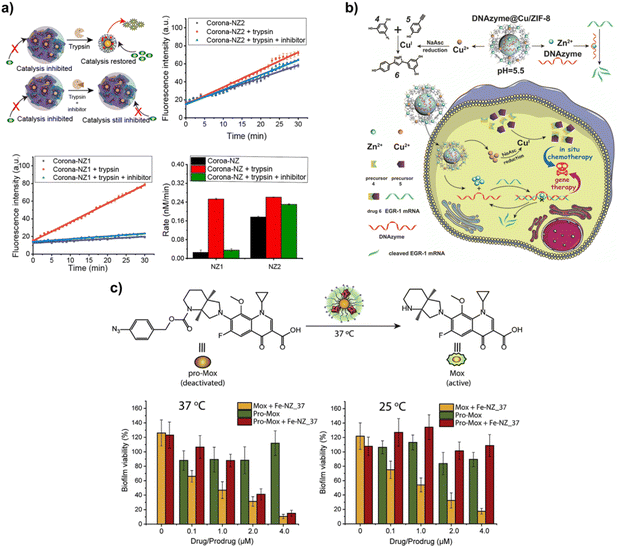 | ||
| Fig. 5 AuNPs and MOF as scaffolds for bioorthogonal nanozymes. (a) Bioorthogonal catalytic activity of nanozymes was inhibited by hard protein corona and was restored by corona proteolysis. This figure has been reproduced from ref. 28 with permission from the American Chemical Society, copyright 2020. (b) DNAzyme@Cu/ZIF-8 for the synergistic treatment via CuAAC reaction-catalyzed chemotherapy and mRNA cleavage reaction-mediated gene therapy. This figure has been reproduced from ref. 30 with permission from John Wiley & Sons, copyright 2021. (c) Thermoresponsive Fe-NZ activated antimicrobial pro-Mox at 37 °C and effectively inhibited the biofilm viability. Each experiment was replicated 5 times. Error bars represent standard deviations of the measurements. This figure has been reproduced from ref. 27 with permission from Elsevier, copyright 2020. | ||
Except for this well-studied AuNPs, metal–organic frameworks (MOFs) have also exerted as an attractive approach to prepare MOF-based nanozymes and is gradually becoming a key part in catalysis and drug delivery depending on their well-organized porous structure, high specific surface area, and various sites available for functionalized modifications.74 In view of this, MOFs are undoubtedly an excellent candidate for encapsulating TMCs to fabricate bioorthogonal nanozymes, helping to improve the stability and targeted ability of TMCs and protecting them from inactivation in a living environment. Qu's group provided meaningful research. They synthesized triphenylphosphonium (TPP)-decorated MOF-Cu bioorthogonal nanozymes with the catalytic activity for the CuAAC reaction. Ultra-small Cu nanoparticles formed by reduction within the Zr MOF could catalyze the CuAAC reaction in water, PBS and culture media and further mediate the conversion of the pro-fluorescent molecules into their parent form, as evidenced by the increased fluorescence intensity. TPP enabled MOF-Cu bioorthogonal nanozymes to be competent to target intracellular mitochondria and helped to in situ synthesize drugs (resveratrol) at the mitochondria, which could cause mitochondrial damage and promote the apoptosis of tumor cells, such as MCF-7 cells. More importantly, MOF-Cu nanozymes demonstrated significant therapeutic efficiency through CuAAC-mediated in situ drug synthesis in a mouse tumor model.29 Following this foundation, Qu and co-workers further developed a multifunctional bioorthogonal nanozyme system (DNAzyme@Cu/ZIF-8) for synergistic anti-cancer treatment through combining in situ drug synthesis-based chemotherapy with DNAzyme-mediated gene therapy. In more detail, DNAzyme@Cu/ZIF-8 was prepared via a one-step method in which Cu(NO3)2, Zn(NO3)2, 2-methylimidazole, and DNAzyme were mixed in methanol together and stirred. Due to the pH-responsive nature of ZIF-8, the produced DNAzyme@Cu/ZIF-8 could dissociate and release Zn2+, Cu2+ and DNAzyme in lysosomes after being endocytosed by tumor cells. The released Cu2+ was transformed into Cu+ in the presence of sodium ascorbate and then catalyzed the CuAAc reaction to synthesize resveratrol inside cells, and Zn2+ acted as a cofactor to cooperate with DNAzyme for the cleavage reaction of human early growth response-1 (EGR-1) mRNA (Fig. 5b). Overall, DNAzyme@Cu/ZIF-8 bioorthogonal nanozyme platform more preferentially accumulated at tumor sites through the EPR effect and performed strong inhibitory efficacy against tumor growth and metastasis with the synergistic treatment of chemotherapy and gene therapy, which effectively facilitated the eradication of tumor.30
In an effort to explore the applicability of MOFs as scaffolds in encapsulating other TMCs except for Cu catalysts, Pablo del Pino and co-workers designed an amphiphilic polymer PMA (PMA refers to poly[isobutylene-alt-maleic anhydride]-graft-dodecyl) modified core–shell Pd/ZIF-8 bioorthogonal nanoreactors with a size of 250 nm, demonstrating the favorable aqueous compatibility, colloidal stability, and long-lasting bioorthogonal catalytic activity for the uncaging of phenol-derived pro-fluorophores. Due to its high porosity allowing the substrates and products to flow in a diffusive manner without interfering with the inner core Pd catalyst, ZIF-8 decorated with PMA played a major role in improving the catalytic activity of Pd and making the Pd/ZIF-8 a recyclable catalyst. It is in this way that the authors envisioned the Pd/ZIF-8 possessing the potential to develop into recurrent intracellular nanoreactors. The Pd-NPs could be effectively incorporated in 3D tumor spheroids to form 3D tissue-like catalytic models that conducted Pd-mediated bioorthogonal reactions when incubated with profluorophores in a recurrent manner. This strategy achieved for the first time TMCs to complete bioorthogonal reactions within a biological tissue, which established the basis for the building and development of “catalytic cell or tissue nano-implants” in the future.31 Moreover, by taking advantage of the facile modifiability of the MOF surface, Wang and coworkers reported a DNA-based aptamer (AS1411) modified UiO-66 MOF with Pd nanoparticle deposition (AS1411@Pd@UiO-66), which specifically delivered Pd catalyst into the targeted cancer cells for intracellular bioorthogonal catalysis. The introduction of the AS1411 aptamer resulted in better uptake of AS1411@Pd@UiO-66 by cancer cells with high expression of nucleolin, and the uptaken AS1411@Pd@UiO-66 was responsible for regulating the intracellular protein stability and activity through performing a deprotection reaction of caged 4-hydroxytamoxifen (Proc-4-OHT) to generate its parent drug. Furthermore, the cellular study revealed the MAPK/ERK signaling of cancer cells could be controlled via AS1411@Pd@UiO-66-mediated bioorthogonal activation of Proc-4-OHT, which was viewed as a promising approach to manipulate cell fate and treat cancer.32 Collectively, MOFs provide lots of convenience and advantages for developing versatile TMC-based bioorthogonal nanozymes. Thus, it is also worthwhile to proceed with the exploration.
3.3 Cell-based carriers for TMC-based bioorthogonal nanozymes
With the growing applications of TMC-based bioorthogonal catalysis in tumor treatment, it is necessary to design bioorthogonal nanozymes that are controllably localized at the target disease site, which is a key strategy to improve the therapeutic efficacy and decrease off-target effects. Thanks to the cell-specific targeting features, long circulation times, low immunogenicity, and the capacity to load and transport diverse cargoes,75,76 cell-based carriers are gradually becoming attractive to advance the use of bioorthogonal nanozymes in biomedicine.33,77 Following, respective examples will be presented to illustrate the catalytic activities of cell structure-based bioorthogonal nanozymes in the living setting and their corresponding applications.To expand on exosomes, exosomes as a cell-derived nano-delivery vehicle have received increasing focus owing to their diverse origins and a range of functionality.78 For example, Unciti-Broceta and co-workers designed a mild chemical approach to in situ directly synthesize Pd nanosheets inside A549 cancer cell-derived exosomes (Pd-ExoA549) through using carbon monoxide (CO) as a gaseous reductant, while not affecting the integrity and functionality of exosomes. A series of experimental results revealed that the Pd nanosheets were successfully synthesized within the exosomes with an average Pd content of 0.64 μg per μg of Pd-ExoA549 protein, and the membrane proteins of the exosomes remained intact. The Pd-ExoA549, as the bioorthogonal nanozymes with an average diameter of 100–140 nm, were incubated with a non-fluorescent compound for evaluating the catalytic activity of Pd encapsulated into exosomes. After incubation, a significant fluorescent signal was observed, and the Pd-ExoA549-mediated catalytic process was in line with the classical Michaelis–Menten growth curve. More importantly, to further investigate the homologous targeted ability and the catalytic activity of Pd-ExoA549 within the intracellular environment, the authors performed cell-based assays to verify whether the Pd-ExoA549 could specifically target its progenitor A549 cell, as well as promote uncaging reaction inside the targeted cell. The results indicated that Pd-ExoA549 was more preferentially distributed in its parent A549 cell when incubating U87 cells and A549 cells separately, and the Pd-ExoA549 could be internalized into A549 cells. This proof-of-concept research convincingly described the potential of bioorthogonal nanozymes that were formulated from cancer derived-exosomes encapsulated Pd catalysts in the treatment of cancer and paved the way for exosome-mediated targeted delivery of TMCs, which greatly improved the biocompatibility of TMCs and decreased the dose-dependent toxicity of anticancer drugs.33 While cell-targeting exosomes are certainly beneficial for producing multifunctional bioorthogonal nanozymes, from the perspective of clinical applications, it is necessary to further evaluate the physicochemical and biologic properties of exosome-based bioorthogonal nanozymes, including loading capacity, catalytic activity, in vivo fate, etc.
In an opinion similar to the concept of using exosomes to develop bioorthogonal nanozymes, there are also some innovative investigations using intact cells as scaffolds for building bioorthogonal nanozymes. For instance, Rotello's group developed a novel bioorthogonal nanozyme delivery system in which nanozymes could hijack erythrocytes in vivo and bind to the surface of the erythrocyte via electrostatic interactions. The yielded red blood cell (RBC)-hitchhiked nanozymes (RBC-NZs) could be further precisely delivered to the targeted sites of bacterial infection and accumulated at the infection area according to the sensitivity of erythrocytes to bacterial toxins and the hemolysis of the erythrocytes in the presence of bacterial toxins. These bioorthogonal nanozymes could be fabricated by loading FeTPP into the hydrophobic alkyl chain of AuNPs. And a library of nanozymes (NZ 1–9) with different surface charges, hydrophobicity and aromatic properties was further constructed to screen for the most potent NZs to hijack red blood cells. The results revealed that NZ 1 could solidly bind to the erythrocyte membrane without obvious toxicity to erythrocytes and did also not impair the expression of key CD47 biomarkers that contributed to ensuring the long circulation properties of erythrocytes. Erythrocyte hemolysis at the site of bacterial infection could promote the release of nanozymes, activate the aryl azide protected moxifloxacin and decrease the viability of pathogenic biofilms without influencing non-pathogenic biofilms. Thus, the strategy used in the state-of-the-art study provided an innovative alternative to specifically deliver TMCs to the targeted site by taking advantage of the natural transportation properties of RBC in vivo, paving the way for designing biomimetic bioorthogonal nanozymes in a wide of diseases.34 In another study reported by Rotello and co-workers, they employed macrophage-based delivery system to design a targeted bioorthogonal nanozyme with the ability to spontaneously migrate to the tumor regions due to the macrophage chemoattractants secreted by many types of cancers. First, the nanozyme TTMA-NZ was prepared by incorporating Pd catalysts into the hydrophobic alkane interior of cationic AuNPs functionally modified with thioalkyl tetra(ethylene glycol) trimethylammonium (TTMA). The resulting nanozyme TTMA-NZ were then incubated with macrophages for 24 h to promote the internalization of TTMA-NZ, and the uninternalized TTMA-NZ adsorbed on the surface of macrophages were washed away using PBS for developing TTMA-NZ-bearing macrophages (RAW_NZ). Pd catalysts loaded RAW_NZ preserved their catalytic activity within macrophages and RAW_NZ demonstrated the efficient chemotactic migration towards colony stimulating factor 1, which indicated the excellent targeting efficiency of macrophage-based bioorthogonal nanozymes compared to free TTMA-NZ. Crucially, RAW_NZ exerted dose-dependent toxicity on HeLa cells by catalyzing the deprotection reaction of propargyl-protected 5-fluorouracil (pro-5FU), which was important to develop bioorthogonal nanozymes with high biosafety.35
Cell membrane coating strategy also occupies a position in promoting site-specific bioorthogonal catalytic reactions and developing bioorthogonal nanozymes. In contrast to the cell carriers and exosomes described above, using cell membranes to encapsulate TMCs not only inherits the desired function of the parent cell, but also makes the process of fabrication simpler and more feasible. For example, in the work of Qu's group, cell membranes derived from neutrophils were used to prepare biomimetic bioorthogonal nanozymes for inflammation targeted therapy through Pd-catalyzed asymmetric transfer hydrogenation (ATH) reaction to produce chiral ibuprofen (IBU) in situ. Towards the goal of applying targeted bioorthogonal catalysis for the synthesis of chiral drugs, the authors first constructed Pd-deposited mesoporous silica nanoparticles (MSN-Pd/CD) with the modification of cinchonidine (CD), followed by cell membranes of neutrophils coating on the surface of MSN-Pd/CD (MSN-Pd/CD@Neu). The spontaneous migration of neutrophils to the inflammation site endowed the MSN-Pd/CD@Neu with the ability to specifically target the inflammation area. In addition, to selectively deliver the prodrugs (HCOONa and preIBU) to the targeted site, MSN-prodrugs@Neu were produced by coating the cell membrane from neutrophils on the MSN containing HCOONa and preIBU. The results of in vivo experiments suggested that MSN-Pd/CD@Neu and MSN-prodrugs@Neu were able to efficiently target inflammatory sites and synthesize S-ibuprofen (S-IBU) at the targeted sites through ATH reactions, thus significantly inhibiting the production of ROS and PGE2 levels in LPS-induced inflamed paw model (Fig. 6).36 The developed cell membrane-based bioorthogonal nanozymes containing Pd catalysts, as a novel “drug factory”, brings new perspectives to targeted bioorthogonal chemistry.
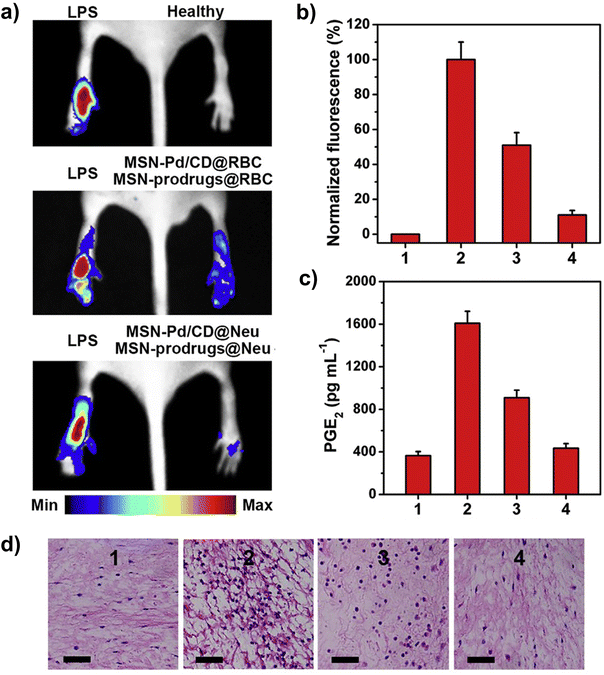 | ||
| Fig. 6 Cell-based carriers for bioorthogonal nanozymes. (a and b) ROS imaging of LPS-induced inflamed paws and normalized fluorescence intensities of ROS probe DCFH-DA. (c) PEG2 (prostaglandin E2) level after treatment with MSN-Pd/CD@Neu and MSN-prodrugs@Neu. (d) H&E staining results of inflamed paws. Scale bars: 40 μm. Error bars indicate ±SD. This figure has been reproduced from ref. 36 with permission from the Elsevier, copyright 2020. | ||
Taken together, in this section, we introduce a variety of strategies for the development of bioorthogonal nanozymes and the preliminary effectiveness they have obtained in the applications of bioorthogonal catalysis in vivo. Constructing TMC-based bioorthogonal nanozymes enables TMCs to smartly exert catalytic activity in a spatiotemporally controlled manner, which to a large extent overcomes the concerns about stability, targeting ability, and toxicity that have plagued practical bioapplications. More importantly, some TMCs such as PdNPs also exhibit other enzyme-like activities such as peroxidase activity, which effectively combines natural and non-natural enzymatic activities in one and establishes a foundation for more active therapeutic effects on diseases. In this regard, the development of bioorthogonal nanozymes with multi-enzyme activities in combination with other enzymatic activity of TMCs (such as peroxidase, superoxide dismutase or catalase) represents a potential opportunity in the treatment of disease.
4. TMC-based bioorthognal nanozymes for therapeutic applications
The ultimate frontier for bioorthogonal nanozymes that perform in a biological setting will be working as expected in living animals and even in humans. The benefits derived from bioorthogonal nanozymes in biomedical applications are considered, as such, recent investigations have made achievements in the availability of nanozyme-mediated bioorthogonal reactions to activate prodrugs and antibody–drug conjugates (ADCs), as well as combine with photothermal or immunotherapy for the treatment of disorders, which shows the therapeutical implications of nanozyme-catalyzed bioorthogonal reactions. In this section, we will mainly summarize the therapeutic applications of the TMC-based bioorthogonal nanozymes, providing a valuable and innovative direction for improving the clinical translation of bioorthogonal nanozymes in the real world.4.1 Bioorthogonal nanozyme mediated prodrug activation for cancer therapy
First of all, nanozyme-catalyzed prodrug activation strategies for disease treatment are presented, which is considered as the important usefulness of bioorthogonal catalysis. For example, in the recent work reported by Qu and co-workers, they designed a DNA-based bioorthogonal platform composed of single-stranded DNA and CuNPs to complete prodrug activation for tumor therapy in living systems. The single-stranded DNA consisted of three parts including thymine-rich regions as a template for the formation of CuNPs, the aptamer for specific targeting of cancer cells and the linker for connecting the above two parts. The results proved that different lengths of a thymine-rich portion (T20, T30, and T40) influenced the catalytic behavior of the formed DNA-based CuNPs (Apt-Cu20, Apt-Cu30, and Apt-Cu40). Among them, Apt-Cu30 exhibited the best bioorthogonal catalytic activity reflected by the effective transformation of the precursor. Moreover, the linker sequence of DNA promoted the contact between CuNPs and their substrates by forming hairpin-like structures, hydrogen bonds, and hydrophobic interactions, enhancing the catalytic activity. Due to the modification of aptamer (MUC1 aptamer and AS1411 aptamer), MApt-Cu30 and AApt-Cu30 could respectively target MUC1-positive and nucleolin-positive cancer cells and be efficiently taken up, which represented the potential of aptamer-modified DNA-based CuNPs as a universal platform for targeting therapy. Based on these inspiring foundations, the targeted catalytic behavior of MApt-Cu30in vivo was examined. The nude mice bearing MCF-7 tumors were treated with prodrugs and MApt-Cu30 showed the smallest size of tumors and possessed a low rate of tumor growth, which illustrated that MApt-Cu30 with an increased accumulation at the tumor site catalyzed more conversion of prodrugs to active drugs (Fig. 7a). The proof-of-concept study gave a great demonstration of nanocatalyst-mediated bioorthogonal prodrug activation for cancer treatment, hopefully for practical use.79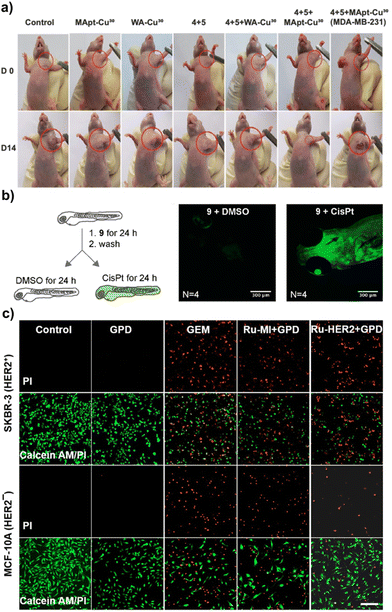 | ||
| Fig. 7 Bioorthogonal nanozymes for tumor therapy. (a) Representative images of the nude mice bearing MCF-7 tumor before (D0) and after treatment (D14) in different groups. This figure has been reproduced from ref. 79 with permission from Springer Nature, copyright 2022. (b) The confocal images showed that CisPt catalyzed the activation of the fluorogenic probe 9 in zebrafish larvae. This figure has been reproduced from ref. 82 with permission from the American Chemical Society, copyright 2020. (c) The fluorescence images of SKBR-3 and MCF-10A in a co-cultured cell model after different group treatments. Green indicated calcein AM, and red indicated PI. Scale bar: 200 μm. This figure has been reproduced from ref. 84 with permission from John Wiley & Sons, copyright 2022. | ||
In another study, they designed a novel AS1411 decorated DNA-based CuNPs bioorthogonal nanozymes (DNAzyme) with peroxidase-mimicking activity and bioorthogonal catalytic activity, providing an attractive approach for synergistic cancer targeted therapy. In detail, the main difference between this resulted DNAzyme and the DNA-based CuNPs nanozymes reported by their group above was that the DNAzyme was prepared with the incorporation of hemin, which endowed it with peroxidase-like activity and the capability to produce ROS for killing cancer cells. More importantly, the DNAzyme exhibited self-amplifying properties, as evidenced by the fact that ROS generated via peroxidase-like activity in the presence of H2O2 could facilitate the transformation of CuNPs into bioorthogonal Cu(I) for the CuAAC reaction. Thus, this DNA-based nanozyme system demonstrated a remarkable dominance in tumor growth inhibition with negligible toxicity through synergistic prodrug activation and chemodynamic therapy.80
Drum's group described a bioorthogonal nanozyme (tES-HRP) that consisted of iron(III) as a catalytic center, horseradish peroxidase (HRP) as a stabilizer of iron(III) and a thermostable exoshell (tES) as a protective shell for encapsulating HRP-stabilized iron(III). tESs feature high thermal stability and have four 4.5 nm pores for permitting free diffusion of substrates and products. When the tES-HRP nanozyme was co-administered with indole-3-acetic acid (IAA), the reduction of iron(III) to iron(II) could cause the oxidative decarboxylation of IAA to produce active substances and accompanied by the formation of free radicals, both of which effectively promoted the apoptosis of cancer cells. In the mouse model of triple-negative breast cancer, obvious tumor suppressive effects were observed with the treatment of tES-HRP and IAA, revealing the feasibility of bioorthogonal nanozyme-catalyzed prodrug activation in cancer therapy.81 Actually, the bioorthogonal prodrug activation strategy is not only feasible for the in situ synthesis of the protected chemotherapeutic drugs, but also it holds good promise for mediating the on-target release of drugs from the antibody–drug conjugate (ADC) on demand. For instance, as shown in Fig. 7b, Bernardes and co-workers developed cisplatin (CisPt)-mediated bioorthogonal cleavage of ADC to release the drug in cancer cells. Given that cisplatin (CisPt) is a commonly used anticancer agent in clinical practice, the exploitation of the Pt complex-mediated uncaging reaction of ADC is of clinical significance in cancer treatment.82 Although marketed ADCs currently have great progress in the clinic, they are still limited by some shortcomings such as the non-specific release of cytotoxic drugs, instability of linkers and low loading efficiency.83
Considering these, in contrast to the classical ADCs, Mao and co-workers first developed the anti-HER2 affibody-ruthenium bioorthogonal catalysts (Ru-HER2) by using a maleimide–thiol coupling reaction to attach Ru(II) to anti-HER2 affibody, which could bioorthogonally catalyze the decaging reaction of N-allyloxycarbonyl-caged prodrugs in a precisely targeted manner (Fig. 7c). Leveraging the ability to target HER2 receptors and active prodrug, Ru-HER2 exhibited significant cytotoxicity to HER2 positive tumor cells. In addition, Ru-HER2 played a role in inhibiting the proliferation of cancer cells through HER2 signaling blockade and DNA damage, which exerted a synergistic effect with chemotherapy. These synergistic anti-cancer effects also were confirmed in a zebrafish xenograft model.84
4.2 Bioorthogonal nanozymes mediated photothermal and catalytic cancer therapy
Bioorthogonal nanozymes not only can achieve therapeutic goals by synthesizing drugs at the disease site, but also more effectively combine photothermal and catalytic therapy to perform synergistic therapeutic functions.85 For example, PdNPs as popular bioorthogonal nanozymes possess many intrinsic excellent properties, such as physical, mechanical, and optical properties, which is undoubtedly a valuable opportunity for exploiting the unique features of PdNPs in biomedical applications.86 Ding and co-workers developed a multifunctional bioorthogonal nanozyme platform (BSA–Pd–Au NRs) that integrated photothermal therapy (PTT), prodrug activation, and enzyme-like activity mediated catalytic therapy in one unit for the synergistic treatment of cancer, tackling the bottlenecks of oncology therapy. BSA–Pd–Au NRs were prepared through the deposition of Pd on the end of Au NRs, on which a thin BSA shell was modified for improving the biocompatibility and safety. As such, with the BSA modification and deposition of Pd, the optical absorption of Au NRs shifted from NIR-I (950 nm for Au NRs) to NIR-II (1050 nm for BSA–Pd–Au NRs), which contributed to penetrating deeper tumor tissue and improve the efficacy of PTT. In addition, Pd–Au NRs showed dual-catalytic activities with bioorthogonal activity and POD-like activity, as respectively evidenced by the uncaging reactions of caged pro-5-Fu and pro-resorufin as well as catalyzing the generation of free radicals and causing TMB oxidation. The results of in vitro anti-tumor study suggested that co-treatment of BSA–Pd–Au NRs and pro-5-Fu had the best inhibition effect on cell viability under 1064 nm laser irradiation for 2 min. Similar results were also obtained in vivo study, where PTT in combination with bioorthogonal catalytic therapy (BCT) and chemodynamic therapy (CDT) not only induced the apoptosis of tumor cells through thermal energy, but also enhanced the catalytic efficiency of BSA–Pd–Au NRs to produce more hydroxyl radicals and 5-Fu for further inhibiting tumor growth and metastasis. Three therapeutic modalities of PTT–BCT–CDT provided a promising paradigm in resolving the dilemmas (such as inadequate tumor suppression) encountered in single PTT therapy and driving the clinical application of bioorthogonal nanozymes.37Moreover, to develop a promising alternative to enhance the activity of CuNPs in the CuAAC reaction, Qu and coworkers constructed an attractive bioorthogonal nanozyme (MCNs-Cu) through in situ growing CuNPs on the mesoporous carbon nanospheres (MCNs). The experimental results about the catalytic activity of MCNs-Cu in the CuAAC reaction showed that the catalytic efficiency of MCNs-Cu was dual-promoted by the produced ROS and heat energy converted from light energy under NIR light irradiation. The generated ROS could effectively oxidize Cu(0) to Cu(I) which accelerated the CuAAC reaction. In addition, MCNs-Cu with the high photothermal conversion efficiency (50.6%) further elevated the local temperature for promoting the whole reaction. Thanks to the photothermal and photodynamic properties of MCNs-Cu nanozymes, they have demonstrated good therapeutic potential in in vivo studies. In the mouse tumor model, the growth of tumors treated with prodrugs + MCNs-Cu + NIR were obviously suppressed and there was a significant increase in the number of apoptotic tumor cells. This work provided a comprehensive explanation of the synergistic effects of bioorthogonal nanozyme-based photothermal and photodynamic therapy in anti-cancer.38
4.3 Bioorthogonal nanozymes mediated cancer immunotherapy
Bioorthogonal nanozyme-mediated bioorthogonal chemistry has also demonstrated great promise in cancer immunotherapy. Tumor immunotherapy efficacy is often compromised by low immune response efficiency and an immunosuppressive microenvironment. Given that, a strategy to exploit the advantages of bioorthogonal chemistry to develop bioorthogonal nanozyme-mediated cancer immunotherapy was proposed. The researchers constructed a palladium bioorthogonal nanozyme (MoS2@Pd-Man) with mannose (Man) functionalization and cysteine modification, which not only could reshape the immune microenvironment (TME) through in situ the synthesis of the FDA approved histone deacetylase inhibitor vorinostat, but also could imitate peroxidase activity to catalyze the production of ROS from H2O2 and further kill cancer cells. The Man modified on the surface of MoS2@Pd-Man was able to specifically bind to the mannose receptor (CD206) overexpressed on the M2-like macrophages, achieving site-specific bioorthogonal uncaging reactions to release vorinostat (Fig. 8a). On the one hand, the produced vorinostat was used to re-educate M2-like macrophages and regulate macrophage polarization from M2 to M1, thereby remodeling the TME for anti-cancer therapy (Fig. 8b). On the other hand, compared to MoS2, MoS2 with deposition of PdNPs showed the stronger POD-like activity as proven by the lower Michaelis–Menten constant value and higher maximum initial velocity value, which was beneficial to increase oxidative stress level and cause tumor death (Fig. 8c). Furthermore, the therapeutic effectiveness of MoS2@Pd-Man was further evaluated in CT26 tumor model. The mice receiving Pro-vorinostat and MoS2@Pd-Man possessed the smallest tumor volumes in contrast to the other groups. And the results of in vivo reshaping TME indicated that the CD86 expression of TAMs from tumor tissues was markedly increased in Pro-vorinostat + MoS2@Pd-Man group, which was attributed to the effective activation of Pro-vorinostat and the enhanced ROS levels. Collectively, this study reveals that bioorthogonal nanozymes with bioorthogonal catalytic activity and natural enzyme-like activity are extremely prospective for cancer immunotherapy and can integrate multi-synergistic benefits into a single system, which represents a new approach for cancer immunotherapy.39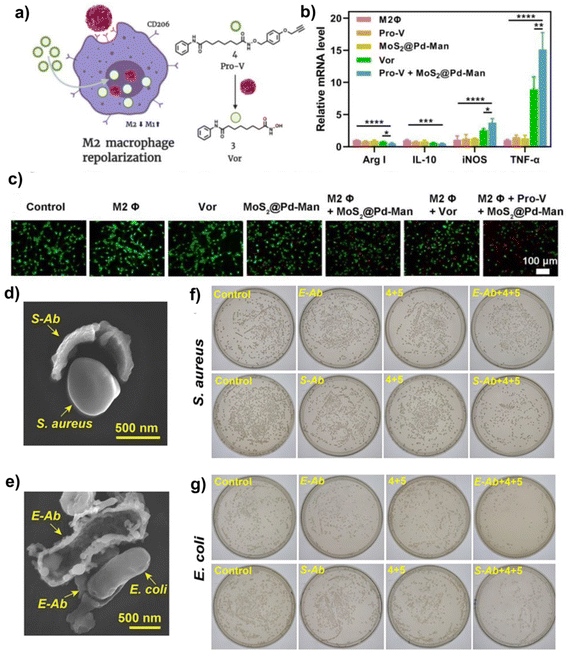 | ||
| Fig. 8 Bioorthogonal nanozymes for anti-tumor immunotherapy and anti-bacterial therapy. (a and b) In situ synthesis of vorinostat induced M2 macrophage repolarization and qPCR results demonstrated the mRNA levels of markers after macrophages were treated with different groups (**P < 0.01, ***P < 0.001, and ****P < 0.0001). (c) Live/dead staining of CT26 cells treated with different groups. This figure has been reproduced from ref. 39 with permission from Elsevier, copyright 2022. (d and e) SEM identified the bacterial recognition ability of the antibody-like TMCs catalysts. (f and g) Plate count method showed shape-dependent antimicrobial activity of the “antibody” bio-orthogonal catalysts that could in situ produce drugs from precursor molecules 4 and 5. This figure has been reproduced from ref. 40 with permission from the American Chemical Society, copyright 2021. | ||
Recently, in valuable research about revealing the antitumor immune function of pyroptosis, Liu and Shao et al. designed a bioorthogonal catalytic system where phenylalanine trifluoroborate (Phe-BF3) could enter the tumor cells and specifically catalyze the release of the active gasdermin from the Au nanoparticle-conjugated gasdermin.87 Although TMC-based nanozymes were not used as catalysts in this bioorthogonal catalytic system, this work reflected the importance of bioorthogonal catalysis in the study of biological processes such as cell death and immunity, as well as provided the new directions for applying bioorthogonal chemistry to cancer immunotherapy. Therefore, here a detailed description of how the bioorthogonal catalytic approach could be used to investigate pyroptosis-induced anti-tumor immunotherapy will be presented.
In this study, the authors attached GSDMA3(N + C) (gasdermin A3 (GSDMA3) with the N and C domains) to 60 nm Au nanoparticles by the triethylsilyl (TES) ether linker, forming NP-GSDMA3 that was sensitive to the catalysis of Phe-BF3. When incubated with Phe-BF3, NP-GSDMA3 could effectively release GSDMA3(N + C) that promoted the formation of pores on the liposome membranes. In addition, in vitro cellular assay results further showed that cells (HeLa, EMT6 and 4T1) co-treated with NP-GSDMA3 and Phe-BF3 presented the pyroptotic cell morphology, and statistically about 40% of HeLa, 35% of EMT6 and 20% of 4T1 cells suffered pyroptosis. In the 4T1 tumor model, NP-GSDMA3 and Phe-BF3 were observed to be co-distributed at the tumor region, and the 4T1 mice showed a significant reduction in tumor volume after three rounds of treatment with Phe-BF3 and NP-GSDMA3. Although the tumor was extensively suppressed, not all the tumor cells underwent pyroptosis; in contrast, only a small percentage of tumor cells was propidium iodide-positive pyroptotic cells. Meanwhile, after treatment with NP-GSDMA3 and Phe-BF3, the failure to observe tumor regression in athymic Nu/Nu mice without mature T cells provided further evidence that the immune system played an important role in pyroptosis-mediated tumor inhibition. Indeed, it found an increase in the number of CD4+, CD8+ and natural killer cells, but a decrease in the number of monocytes, neutrophil, and myeloid-derived suppressor cell populations. Taken together, the activation of gasdermin could be used as a promising approach to amplify anti-tumor immunity responses. The bioorthogonal catalytic system is a powerful tool to understand the biological process and can be applied synergistically with other modalities for therapeutic applications. It is believed that it is very instructive to establish bioorthogonal nanozyme systems for anti-cancer immunity.
4.4 Bioorthogonal nanozymes for anti-bacterial therapy
In addition to cancer treatment, bioorthogonal nanozymes also play a role in anti-bacterial therapy by in situ activating pro-antibiotics. Limited by the poor membrane penetration of antibiotics, it remains a challenge to treat intracellular bacterial infections. Rotello et al. fabricated a targeted bioorthogonal nanozyme (Man-NZ) by integrating FeTPP into AuNPs with mannose functionalization. Mannose promoted the cellular uptake of Man-NZ by macrophages through specific binding to its receptor (CD206) expressed on macrophages. Followed by uptake, Man-NZ was capable of efficiently catalyzing the uncaging reaction of pro-ciprofloxacin into ciprofloxacin for killing pathogens Salmonella infected in macrophages. This bioorthogonal strategy could not only activate pro-antibiotics within the targeted cell, but also could avoid the side effects of antibiotics on the beneficial bacteria in vivo and reduce the occurrence of bacterial resistance.88Alternatively, to recognize and capture specific bacteria, Qu and co-workers synthesized the artificial antibody-like bioorthogonal catalysts through bacterial imprinting technology. The designed antibody-like bioorthogonal catalysts had the imprinted matching morphology of the targeted bacteria and could specifically capture bacteria in an antigen–antibody binding manner. And importantly, these bioorthogonal catalysts were capable of in situ synthesis of antibacterial drugs via CuAAC reaction within the captured bacteria, showing precise and effective antimicrobial effects. In this study, the spherical Staphylococcus aureus (S. aureus) and rod-shaped Escherichia coli (E. coli) were used as template bacteria to prepare antibody-like bioorthogonal catalyst with SiO2 shell and the reduced Cu in its inner surface, named respectively as S-Ab and E-Ab. When S-Ab and E-Ab were mixed with S. aureus and E. coli, they could recognize and target their template bacteria, which was shown in the scanning electron microscopy (SEM) results (Fig. 8d and e). After targeting the aimed bacteria, the antibody bioorthogonal catalyst produced effectively an antimicrobial active drug, helping to inhibit the bacterial growth (Fig. 8f and g). In a S. aureus infected wound model, the wounds treated with S-Ab and prodrug were almost healed compared to the control group. This research combined bioorthogonal catalysis with imprinted artificial antibodies to develop a novel strategy for specific antimicrobial therapy, providing considerable design space for bioorthogonal catalysis-mediated disease treatment.40
Recently, Qu's group has very creatively developed a NIR light-controllable bioorthogonal motor catalyst (CNC-Cu) that was composed of carbonaceous nanocalabash (CNC) as an inner core and CuNPs modified on the outside surface of CNC, which had great promise in synthesizing active drugs via CuAAC reactions in the deeper biofilms for eradicating the shielded bacteria. Under NIR light irradiation, thanks to a gradual increase in temperature gradient within 100 ns and the generated quite a strong net force inside the CNC-Cu motor, the created CNC-Cu exhibited excellent photothermal effects and autonomous motion behaviors, which was particularly beneficial to enter the more complex biological environment and further perform bioorthogonal catalytic functions. It could be clearly observed that the CNC-Cu motor moved rapidly and deeply penetrate the biofilm just like an active swimmer and inhibited the growth of biofilms through in situ catalyzing the synthesis of active molecules. To elevate the in vivo antibiofilm effects of CNC-Cu mediated bioorthogonal catalysis, they constructed an implant-related periprosthetic infection (PPI) mouse model. In the PPI model, compared to the other groups, there was a significant healing tendency in the wound tissues of PPI mice treated with prodrugs 4 and 5 + CNC-Cu + NIR, as evidenced by the results of H&E staining and virtually no residual bacteria colonizing on the implanted catheters. As a proof of concept, CNC-Cu bioorthogonal motor effectively broaden the application scenario of bioorthogonal nanozymes and improved the effectiveness of bioorthogonal catalysis-mediated disease treatment.89
In general, bioorthogonal nanozymes provide a key tool for applying bioorthogonal catalytic systems for therapeutic applications. The engineered bioorthogonal nanozymes endow TMCs with excellent attributes such as targeting ability, controllable dosage, and biosafety to achieve the intended therapeutic goals. The combination of the nanozyme-mediated prodrug activation strategy with other therapeutic means including photothermal therapy, photodynamic therapy, chemodynamic therapy, and immunotherapy gradually develops into a versatile therapeutic platform for disease therapy. Moving forwards, from a clinical application point of view, nanozyme-mediated bioorthogonal catalytic systems hold great promise to make human health better.
5. Conclusion and perspectives
In this review, we first recalled a brief history of TMCs (mainly including Cu, Pd, Ru, Au and Fe) in the development of bioorthogonal catalytic chemistry, reflecting the importance of bioorthogonal TMCs in facilitating new-to-nature reactions, as well as the breadth of application prospects. While they can trigger catalytic reactions that cannot be catalyzed by natural enzymes, the performance behavior of TMCs within the biological environment is still affected by their inherent properties such as dosage toxicity, instability, poor cell membrane penetration, etc. Therefore, toward the objective of making TMCs more compatible with the intended use in vivo, we highlight the construction of multifunctional bioorthogonal nanozyme systems using polymers, protein nanocages, MOFs, AuNPs, and cell unity as scaffolds to overcome the biological barriers encountered by ‘naked’ TMCs. Finally, the therapeutic applications of bioorthogonal nanozymes are further presented, which shows promising prospects in the development of synergistic therapies to boost the clinical translation of nanozyme-mediated bioorthogonal chemistry. It is hoped that the bioorthogonal nanozyme strategy proposed in this review will drive the development of new disease therapies and make bioorthogonal chemistry more widely available.Nanozymes, a new type of nanomaterial with enzyme-like activity, show promising applications in many fields and present great potential to expand the modalities of disease treatment. Nanozymes can compensate for functions that natural enzymes do not possess, especially those chemical reactions that cannot be catalyzed by natural enzymes under complex environmental conditions. Furthermore, TMC-mediated bioorthogonal catalysis has good potential for catalyzing chemical reactions that cannot be accomplished under natural conditions and can further produce pharmacologically active substances in situ for therapeutic effects in a biological environment. However, TMCs need to be able to catalyze organic reactions safely and efficiently in vivo at low doses on demand, which make the applications of these effective TMCs in a biological setting still face many challenges. As such, it also remains attractive to the interest of many researchers. Therefore, designing and synthesizing smart bioorthogonal nanozymes that respond to the stimuli of the internal and external environments can break through both the physiological barriers and the limitations of TMCs themselves. Importantly, bioorthogonal nanozyme-mediated in situ on-demand synthesis of drugs maximizes the pharmaceutical efficacy while reducing its toxic side effects. The development of bioorthogonal nanozymes allows the catalytic activity of TMCs to be precisely regulated and can provide a more precise way to treat diseases in the future.
Despite all this progress, we are now still in the early stage of the journey towards the realistic biomedical applications of bioorthogonal catalytic chemistry based on bioorthogonal nanozymes. On this long road, bioorthogonal nanozymes still face many challenges. Bioorthogonal nanozymes, as a new type of nanozyme, are mainly characterized by both bioorthogonal catalytic activity which is not possessed by natural enzymes, and enzyme-like activity similar to that of natural enzymes such as POD. Hence, in the future, we should put more focus on the characterization of the catalytic behavior, catalytic reaction mechanism and kinetic characteristics of the bioorthogonal nanozymes. The factors affecting the catalytic activity of bioorthogonal nanozymes, such as the type of modified ligands, surface charge, etc., should also be investigated in depth to explore the conformational relationships of bioorthogonal nanozymes. Well-defined conformational relationships of bioorthogonal nanozymes can provide better guidance for disease treatment. In addition, to ensure bioorthogonal nanozymes to be better applied in the real world, more efforts should also be made on their batch-to-batch stability, and feasibility of mass production, which will effectively guarantee the stable and controllable quality of bioorthogonal nanozymes and provide a basis for safe and effective treatment.
Secondly, it is essential to note that a consensus in the use of bioorthogonal nanozymes in complex physiological systems is that biosafety is critical. High safety is the cornerstone of pharmaceutical applications of bioorthogonal nanozymes in a living environment. In fact, TMC-bioorthogonal nanozymes enable TMCs to primarily accumulate at the targeted site, reduce the dosage of TMCs without affecting their catalytic activities in vivo and avoid the non-specific distribution of TMCs and damage to normal tissues. Although bioorthogonal nanozymes did not show obvious toxicity to major organs as described above, their long-term toxicity was not examined, which deserves further elucidation because long-term toxicity plays a decisive role in whether bioorthogonal nanozymes can be applied in clinical treatment.
Thirdly, it is important to highlight that bioorthogonal nanozymes, like most chemotherapeutic drugs, will undergo the same processes including distribution, absorption, metabolism, and excretion in the body once they enter the circulation system via intravenous injection. This in vivo process is the focus of research about the bio-fate of bioorthogonal nanozymes in the body, and it is also the major challenge for the practical use of bioorthogonal nanozymes. However, to view it another way, the challenges imply opportunities and directions for development.
Fourthly, in this review, inspired by emerging nanozymes with natural enzyme-like activity, the described bioorthogonal nanozymes that mainly represent nanomaterials with bioorthogonal catalytic activity are fabricated by nanotechnological methods to encapsulate bioorthogonal TMCs in nanomaterial scaffolds. However, with the development of bioorthogonal catalysis and nanozymes, it is found that TMCs exerting bioorthogonal catalytic activity also have the ability to mimic natural enzyme activity. As mentioned above, Pd and Fe also exhibited POD-like activity when catalyzing the deprotection of prodrugs, which promoted the generation of ROS and played a co-therapeutic role with chemotherapeutic drugs. Therefore, this is certainly a good opportunity to develop novel nanozymes that incorporate both natural and non-natural enzymatic activities, not just those with only bioorthogonal catalytic activity or enzyme-like activity, which will open up exciting further new therapy approaches for cancer and other diseases. It is believed that a few exciting applications based on bioorthogonal nanozymes will come as a surprise and move towards the clinic.
Author contributions
K. F. and Z. Z. conceived the project. K. F. and Z. Z. wrote the manuscript. All authors approved the final version.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 82122037, 31900981), the National Key Research and Development Program of China (no. 2021YFC2102900), the CAS Interdisciplinary Innovation Team (JCTD-2020-08), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2019093).References
- H. C. Hang, C. Yu, D. L. Kato and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14846–14851 CrossRef CAS PubMed
.
- A. Battigelli, B. Almeida and A. Shukla, Bioconjugate Chem., 2022, 33, 263–271 CrossRef CAS PubMed
.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. Am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Nat. Rev. Methods Primers, 2021, 1, 30–52 CrossRef CAS PubMed
.
- R. E. Bird, S. A. Lemmel, X. Yu and Q. A. Zhou, Bioconjugate Chem., 2021, 32, 2457–2479 CrossRef CAS PubMed
.
- V. Sabatino, V. B. Unnikrishnan and G. J. L. Bernardes, Chem Catal., 2022, 2, 39–51 CrossRef
.
- T. Liang, Z. Chen, H. Li and Z. Gu, Trends Chem., 2022, 4, 157–168 CrossRef CAS
.
- E. Latocheski, G. M. Dal Forno, T. M. Ferreira, B. L. Oliveira, G. J. L. Bernardes and J. B. Domingos, Chem. Soc. Rev., 2020, 49, 7710–7729 RSC
.
- J. Li and P. R. Chen, Nat. Chem. Biol., 2016, 12, 129–137 CrossRef CAS PubMed
.
- M. O. N. van de L'Isle, M. C. Ortega-Liebana and A. Unciti-Broceta, Curr. Opin. Chem. Biol., 2021, 61, 32–42 CrossRef PubMed
.
- W. Wang, X. Zhang, R. Huang, C. M. Hirschbiegel, H. Wang, Y. Ding and V. M. Rotello, Adv. Drug Delivery Rev., 2021, 176, 113893–113909 CrossRef CAS PubMed
.
- J. G. Rebelein and T. R. Ward, Curr. Opin. Biotechnol, 2018, 53, 106–114 CrossRef CAS PubMed
.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed
.
- J. Idiago-Lopez, E. Moreno-Antolin, J. M. de la Fuente and R. M. Fratila, Nanoscale Adv., 2021, 3, 1261–1292 RSC
.
- C. Streu and E. Meggers, Angew. Chem., 2006, 118, 5773–5776 CrossRef
.
- P. Destito, C. Vidal, F. Lopez and J. L. Mascarenas, Chem. – Eur. J., 2021, 27, 4789–4816 CrossRef CAS PubMed
.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC
.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed
.
- S. Fedeli, J. Im, S. Gopalakrishnan, J. L. Elia, A. Gupta, D. Kim and V. M. Rotello, Chem. Soc. Rev., 2021, 50, 13467–13480 RSC
.
- X. Zhang, R. Huang, S. Gopalakrishnan, R. Cao-Milan and V. M. Rotello, Trends Chem., 2019, 1, 90–98 CrossRef CAS PubMed
.
- L. Taiariol, C. Chaix, C. Farre and E. Moreau, Chem. Rev., 2022, 122, 340–384 CrossRef CAS PubMed
.
- G. Y. Tonga, Y. Jeong, B. Duncan, T. Mizuhara, R. Mout, R. Das, S. T. Kim, Y. C. Yeh, B. Yan, S. Hou and V. M. Rotello, Nat. Chem., 2015, 7, 597–603 CrossRef CAS PubMed
.
- R. Huang, C. H. Li, R. Cao-Milan, L. D. He, J. M. Makabenta, X. Zhang, E. Yu and V. M. Rotello, J. Am. Chem. Soc., 2020, 142, 10723–10729 CrossRef CAS PubMed
.
- X. Zhang, R. F. Landis, P. Keshri, R. Cao-Milan, D. C. Luther, S. Gopalakrishnan, Y. Liu, R. Huang, G. Li, M. Malassine, I. Uddin, B. Rondon and V. M. Rotello, Adv. Healthc. Mater., 2021, 10, e2001627 CrossRef PubMed
.
- J. Chen, K. Li, J. S. L. Shon and S. C. Zimmerman, J. Am. Chem. Soc., 2020, 142, 4565–4569 CrossRef CAS PubMed
.
- Z. Sun, Q. Liu, X. Wang, J. Wu, X. Hu, M. Liu, X. Zhang, Y. Wei, Z. Liu, H. Liu, R. Chen, F. Wang, A. C. Midgley, A. Li, X. Yan, Y. Wang, J. Zhuang and X. Huang, Theranostics, 2022, 12, 1132–1147 CrossRef CAS PubMed
.
- R. Das, R. F. Landis, G. Y. Tonga, R. Cao-Milan, D. C. Luther and V. M. Rotello, ACS Nano, 2019, 13, 229–235 CrossRef CAS PubMed
.
- R. Cao-Milán, S. Gopalakrishnan, L. D. He, R. Huang, L.-S. Wang, L. Castellanos, D. C. Luther, R. F. Landis, J. M. V. Makabenta, C.-H. Li, X. Zhang, F. Scaletti, R. W. Vachet and V. M. Rotello, Chem, 2020, 6, 1113–1124 Search PubMed
.
- X. Zhang, Y. Liu, S. Gopalakrishnan, L. Castellanos-Garcia, G. Li, M. Malassine, I. Uddin, R. Huang, D. C. Luther, R. W. Vachet and V. M. Rotello, ACS Nano, 2020, 14, 4767–4773 CrossRef CAS PubMed
.
- F. Wang, Y. Zhang, Z. Liu, Z. Du, L. Zhang, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2019, 58, 6987–6992 CrossRef CAS
.
- Z. Wang, J. Niu, C. Zhao, X. Wang, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2021, 60, 12431–12437 CrossRef CAS PubMed
.
- R. Martinez, C. Carrillo-Carrion, P. Destito, A. Alvarez, M. Tomas-Gamasa, B. Pelaz, F. Lopez, J. L. Mascarenas and P. del Pino, Cell Rep. Phys. Sci., 2020, 1, 100076–100093 CrossRef PubMed
.
- X. Chen, W. Cai, J. Liu, L. Mao and M. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 10117–10124 CrossRef CAS
.
- M. Sancho-Albero, B. Rubio-Ruiz, A. M. Pérez-López, V. Sebastián, P. Martín-Duque, M. Arruebo, J. Santamaría and A. Unciti-Broceta, Nat. Catal., 2019, 2, 864–872 CrossRef CAS PubMed
.
- A. Gupta, R. Das, J. M. Makabenta, A. Gupta, X. Zhang, T. Jeon, R. Huang, Y. Liu, S. Gopalakrishnan, R. C. Milan and V. M. Rotello, Mater. Horiz., 2021, 8, 3424–3431 RSC
.
- R. Das, J. Hardie, B. P. Joshi, X. Zhang, A. Gupta, D. C. Luther, S. Fedeli, M. E. Farkas and V. M. Rotello, JACS Au, 2022, 2, 1679–1685 CrossRef CAS PubMed
.
- Z. Du, C. Liu, H. Song, P. Scott, Z. Liu, J. Ren and X. Qu, Chem, 2020, 6, 2060–2072 CAS
.
- L. Zhang, W. Wang, M. Ou, X. Huang, Y. Ma, J. Tang, T. Hou, S. Zhang, L. Yin, H. Chen, Y. Hou and Y. Ding, Nano Res., 2022, 15, 4310–4319 CrossRef CAS
.
- Y. You, F. Cao, Y. Zhao, Q. Deng, Y. Sang, Y. Li, K. Dong, J. Ren and X. Qu, ACS Nano, 2020, 14, 4178–4187 CrossRef CAS PubMed
.
- Y. Wei, S. Wu, Z. Liu, J. Niu, Y. Zhou, J. Ren and X. Qu, Mater. Today, 2022, 56, 16–28 CrossRef CAS
.
- J. Niu, L. Wang, T. Cui, Z. Wang, C. Zhao, J. Ren and X. Qu, ACS Nano, 2021, 15, 15841–15849 CrossRef CAS PubMed
.
- B. Lozhkin and T. R. Ward, Bioorg. Med. Chem., 2021, 45, 116310–116325 CrossRef CAS PubMed
.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed
.
- Y. Sun, X. Jiang, Y. Liu, D. Liu, C. Chen, C. Lu, S. Zhuang, A. Kumar and J. Liu, J. Inorg. Biochem., 2021, 225, 111599–111609 CrossRef CAS PubMed
.
- G. Hu, J. Chen, Y. Fan, H. Zhou, K. Guo, Z. Fang, L. Xie, L. Wang and Y. Wang, Chem. Eng. J., 2022, 427, 130918–130927 CrossRef CAS
.
- L. Gaetke, Toxicology, 2003, 189, 147–163 CrossRef CAS PubMed
.
- J. Clavadetscher, S. Hoffmann, A. Lilienkampf, L. Mackay, R. M. Yusop, S. A. Rider, J. J. Mullins and M. Bradley, Angew. Chem., Int. Ed., 2016, 55, 15662–15666 CrossRef CAS PubMed
.
- D. C. Kennedy, C. S. McKay, M. C. Legault, D. C. Danielson, J. A. Blake, A. F. Pegoraro, A. Stolow, Z. Mester and J. P. Pezacki, J. Am. Chem. Soc., 2011, 133, 17993–18001 CrossRef CAS PubMed
.
- H. Wei, S. Bu, W. Zhang, L. Ma, X. Liu, Z. Wang, Z. Li, Z. Hao, X. He and J. Wan, Analyst, 2021, 146, 4841–4847 RSC
.
- F. Liu, X. Liu, F. Chen and Q. Fu, J. Colloid Interface Sci., 2021, 604, 281–291 CrossRef CAS PubMed
.
- X. Zhang, Y. Wu, J. Chen, Y. Yang and G. Li, Anal. Chem., 2021, 93, 3217–3225 CrossRef CAS PubMed
.
- Z. Gao, Y. Li, Z. Liu, Y. Zhang, F. Chen, P. An, W. Lu, J. Hu, C. You, J. Xu, X. Zhang and B. Sun, Nano Lett., 2021, 21, 3401–3409 CrossRef CAS PubMed
.
- M. C. D'Alterio, E. Casals-Cruanas, N. V. Tzouras, G. Talarico, S. P. Nolan and A. Poater, Chem. – Eur. J., 2021, 27, 13481–13493 CrossRef PubMed
.
- J. M. Chalker, C. S. Wood and B. G. Davis, J. Am. Chem. Soc., 2009, 131, 16346–16347 CrossRef CAS PubMed
.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed
.
- S. Alonso-de Castro, A. Terenzi, J. Gurruchaga-Pereda and L. Salassa, Chem. – Eur. J., 2019, 25, 6651–6660 CrossRef CAS PubMed
.
- Y. Li and H. Fu, ChemistryOpen, 2020, 9, 835–853 CrossRef CAS PubMed
.
- R. M. Yusop, A. Unciti-Broceta, E. M. Johansson, R. M. Sanchez-Martin and M. Bradley, Nat. Chem., 2011, 3, 239–243 CrossRef CAS PubMed
.
- J. Konc, V. Sabatino, E. Jimenez-Moreno, E. Latocheski, L. R. Perez, J. Day, J. B. Domingos and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2022, 61, e202113519 CrossRef CAS PubMed
.
- M. Hoop, A. S. Ribeiro, D. Rösch, P. Weinand, N. Mendes, F. Mushtaq, X.-Z. Chen, Y. Shen, C. F. Pujante, J. Puigmartí-Luis, J. Paredes, B. J. Nelson, A. P. Pêgo and S. Pané, Adv. Funct. Mater., 2018, 28, 1705920–1705927 CrossRef
.
- Y. Long, B. Cao, X. Xiong, A. S. C. Chan, R. W. Sun and T. Zou, Angew. Chem., Int. Ed., 2021, 60, 4133–4141 CrossRef CAS PubMed
.
- J. Niu, C. Zhao, C. Liu, J. Ren and X. Qu, Chem. Mater., 2021, 33, 8052–8058 CrossRef CAS
.
- T. Volker, F. Dempwolff, P. L. Graumann and E. Meggers, Angew. Chem., Int. Ed., 2014, 53, 10536–10540 CrossRef PubMed
.
- A. Gutierrez-Gonzalez, P. Destito, J. R. Couceiro, C. Perez-Gonzalez, F. Lopez and J. L. Mascarenas, Angew. Chem., Int. Ed., 2021, 60, 16059–16066 CrossRef CAS PubMed
.
- M. Lin, S. Zou, X. Liao, Y. Chen, D. Luo, L. Ji and H. Chao, Chem. Commun., 2021, 57, 4408–4411 RSC
.
- X. Zhang, S. Lin, R. Huang, A. Gupta, S. Fedeli, R. Cao-Milan, D. C. Luther, Y. Liu, M. Jiang, G. Li, B. Rondon, H. Wei and V. M. Rotello, J. Am. Chem. Soc., 2022, 144, 12893–12900 CrossRef CAS PubMed
.
- M. J. Jou, X. Chen, K. M. Swamy, H. N. Kim, H. J. Kim, S. G. Lee and J. Yoon, Chem. Commun., 2009, 14, 7218–7220 Search PubMed
.
- C. Vidal, M. Tomas-Gamasa, P. Destito, F. Lopez and J. L. Mascarenas, Nat. Commun., 2018, 9, 1913–1921 CrossRef PubMed
.
- T. Sun, T. Lv, J. Wu, M. Zhu, Y. Fei, J. Zhu, Y. Zhang and Z. Huang, J. Med. Chem., 2020, 63, 13899–13912 CrossRef CAS PubMed
.
- P. K. Sasmal, S. Carregal-Romero, A. A. Han, C. N. Streu, Z. Lin, K. Namikawa, S. L. Elliott, R. W. Koster, W. J. Parak and E. Meggers, ChemBioChem, 2012, 13, 1116–1120 CrossRef CAS PubMed
.
- K. S. Egorova and V. P. Ananikov, Organometallics, 2017, 36, 4071–4090 CrossRef CAS
.
- R. Huang, C. M. Hirschbiegel, X. Zhang, A. Gupta, S. Fedeli, Y. Xu and V. M. Rotello, ACS Appl. Mater. Interfaces, 2022, 14, 31594–31600 CrossRef CAS PubMed
.
- A. M. Perez-Lopez, B. Rubio-Ruiz, T. Valero, R. Contreras-Montoya, L. Alvarez de Cienfuegos, V. Sebastian, J. Santamaria and A. Unciti-Broceta, J. Med. Chem., 2020, 63, 9650–9659 CrossRef CAS PubMed
.
- R. Huang, C. H. Li, R. Cao-Milán, L. D. He, J. M. Makabenta, X. Zhang, E. Yu and V. M. Rotello, J. Am. Chem. Soc., 2020, 142, 10723–10729 CrossRef CAS PubMed
.
- Q. Wang and D. Astruc, Chem. Rev., 2020, 120, 1438–1511 CrossRef CAS PubMed
.
- Y. Liang, L. Duan, J. Lu and J. Xia, Theranostics, 2021, 11, 3183–3195 CrossRef CAS PubMed
.
- W. Liu, Q. Yan, C. Xia, X. Wang, A. Kumar, Y. Wang, Y. Liu, Y. Pan and J. Liu, J. Mater. Chem. B, 2021, 9, 4459–4474 RSC
.
- V. Sebastian, M. Sancho-Albero, M. Arruebo, A. M. Perez-Lopez, B. Rubio-Ruiz, P. Martin-Duque, A. Unciti-Broceta and J. Santamaria, Nat. Protoc., 2021, 16, 131–163 CrossRef CAS PubMed
.
- J. Lee, J. H. Lee, K. Chakraborty, J. Hwang and Y. K. Lee, RSC Adv., 2022, 12, 18475–18492 RSC
.
- Y. You, Q. Deng, Y. Wang, Y. Sang, G. Li, F. Pu, J. Ren and X. Qu, Nat. Commun., 2022, 13, 1459–1469 CrossRef CAS PubMed
.
- Y. You, H. Liu, J. Zhu, Y. Wang, F. Pu, J. Ren and X. Qu, Chem. Sci., 2022, 13, 7829–7836 RSC
.
- S. Sadeghi, N. D. Masurkar, G. Vallerinteavide Mavelli, S. Deshpande, W. Kok Yong Tan, S. Yee, S. A. Kang, Y. P. Lim, E. Kai-Hua Chow and C. L. Drum, ACS Nano, 2022, 16, 10292–10301 CrossRef CAS PubMed
.
- B. L. Oliveira, B. J. Stenton, V. B. Unnikrishnan, C. R. de Almeida, J. Conde, M. Negrao, F. S. S. Schneider, C. Cordeiro, M. G. Ferreira, G. F. Caramori, J. B. Domingos, R. Fior and G. J. L. Bernardes, J. Am. Chem. Soc., 2020, 142, 10869–10880 CrossRef CAS PubMed
.
- Z. Su, D. Xiao, F. Xie, L. Liu, Y. Wang, S. Fan, X. Zhou and S. Li, Acta Pharm. Sin. B, 2021, 11, 3889–3907 CrossRef CAS PubMed
.
- Z. Zhao, X. Tao, Y. Xie, Q. Lai, W. Lin, K. Lu, J. Wang, W. Xia and Z. W. Mao, Angew. Chem., Int. Ed., 2022, 61, e202202855 CAS
.
- L. Ricciardi and M. La Deda, SN Appl. Sci., 2021, 3, 372–385 CrossRef CAS
.
- T. T. V. Phan, T. C. Huynh, P. Manivasagan, S. Mondal and J. Oh, Nanomaterials, 2020, 10, 66–84 CrossRef CAS PubMed
.
- Q. Wang, Y. Wang, J. Ding, C. Wang, X. Zhou, W. Gao, H. Huang, F. Shao and Z. Liu, Nature, 2020, 579, 421–426 CrossRef CAS PubMed
.
- J. Hardie, J. M. Makabenta, A. Gupta, R. Huang, R. Cao-Milan, R. Goswami, X. Zhang, P. Abdulpurkar, M. E. Farkas and V. M. Rotello, Mater. Horiz., 2022, 9, 1489–1494 RSC
.
- C. Liu, J. Niu, T. Cui, J. Ren and X. Qu, J. Am. Chem. Soc., 2022, 144, 19611–19618 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |