Antimicrobial and lubrication properties of 1-acetyl-3-hexylbenzotriazolium benzoate/sorbate ionic liquids†
Paramjeet Singh Bakshia,
Rashi Gusainab,
Manisha Dhawariac,
Sunil K. Sumanc and
Om P. Khatri*ab
aChemical Science Division, CSIR-Indian Institute of Petroleum, Dehradun – 248005, India. E-mail: opkhatri@iip.res.in; Fax: +91 135 2660 200
bAcademy of Scientific and Innovative Research (AcSIR), New Delhi, India
cBiofuels Division, CSIR-Indian Institute of Petroleum, Dehradun – 248005, India
First published on 4th May 2016
Abstract
Ionic liquids exhibit immense potential for a wide range of applications including biotechnology, medicinal chemistry and lubrication. Herein, 1-acetyl-3-hexylbenzotriazolium cation-based ionic liquids having benzoate and sorbate anions are prepared by chemical derivatization of benzotriazole. The antimicrobial activities of these ionic liquids are studied using the Escherichia coli and Rhodococcus erythropolis bacterial cultures and we monitored their growth inhibition property. Further, the biostatic properties are evaluated by the kinetic growth rate inhibition method. These ionic liquids exhibited improved antimicrobial activities compared to their precursors: benzotriazole, sodium benzoate and potassium sorbate. Furthermore, these ionic liquids as an additive to polyol lube base oil improved the lubrication property by reducing the friction and wear characteristics. Microscopic images along with elemental mapping of worn surfaces confirmed the formation of an ionic liquid-constituted tribo-chemical thin film, which protects the contact surfaces against the undesirable wear and reduces the friction.
Introduction
Ionic liquids, sterically hindered salts of organic cations and organic/inorganic anions, promise wide potential for several applications including catalysis, extraction, CO2 capture, lubrication, pharmaceutical ingredients, batteries, energy devices, medicinal chemistry etc.1–8 This is attributed to their remarkable and tunable physico-chemical properties like non-volatility, high conductivity, wide liquid range, negligible flammability, high thermal stability, inherent polarity and wide electrochemical window.9–12 Till now, most of the ionic liquids being studied for fundamental understanding to various applications are composed of imidazolium, ammonium, phosphonium and pyridinium cations and variable anions such as halides, phosphate, tetrafluoroborate (BF4−), hexafluorophosphate (PF6−), trifluoromethansulfonate (OTf−), bis(trifluoromethylsulfonyl)imide (NTf2−) and trifluorotris(pentafluoroethyl)phosphate (FAP−). Recently, the environmental toxicity of these ionic liquids and their harmful effects on aquatic life have attracted a lot of attention.13 As a result, environmentally-friendly and cost-effective ionic liquids are gaining immense interest.14High friction and wear owing to poor lubrication between the engineering bodies lead to energy and material losses. In this context, the inherent polarity of ionic liquids is believed to facilitate their interaction with engineering surfaces and provide enhanced lubrication properties by forming a tribo-chemical thin film between the interacting surfaces.15–20 In lubricant industries, resistant to microbial contamination is required to store and use the lubricants for the longer period. The ionic liquids with tunable biological activities are considered as active pharmaceutical ingredients and provide innovative solutions in medical treatment and delivery options.21 The various drugs available in the market are served as antimicrobial agents, however, the microbes tend to develop resistance against most of them. Currently, the antimicrobial agents that show high pharmaco-kinetics and low toxicity are showing large interest. Pernak et al. demonstrated antimicrobial activities of 3-alkoxymethyl-1-methylimidazolium ionic liquids and revealed that antimicrobial activities are greatly affected by alkyl chain length; longer chain length exhibited high anti-microbial activities compared to that of shorter chain substituent.22 The non-toxic and pharmaceutically acceptable lactate anion-based protic ionic liquids exhibited antimicrobial activities against the rods and the cocci.23 The ionic liquids, exhibiting the wide range of biological activities i.e. antimicrobial, antiseptic etc., can be used to overcome the problems of drug solubility as well as development of antibodies against the antimicrobial agents.24–26 Yoo et al. studied antimicrobial activity of imidazolium and pyrrolidinium ionic liquids and correlate the antimicrobial activities with the structural parameter, which further confirmed that long alkyl chain exhibits comparatively better antimicrobial acitivity.27
The azole compounds possess antimicrobial activities owing to their inherent pharmaceutical properties such as antiulcer,28 antifungal,29 anesthetic,30 antipsychotic,31 antiallergic32 etc. Benzotriazole, a heterocyclic organic compound, shows wide range of biological and pharmacological activities.33 It is composed of aromatic ring (π–π interaction) and triazole structure, which forms hydrogen and coordination bonds easily. Thereby benzotriazole derivatives can be easily bind to a variety of enzymes and receptors in biological system via diverse non-covalent interactions, resulting in a broad spectrum of biological activities.34 Herein, 1-acetyl-3-hexylbenzotriazolium cation having environmentally-friendly anions i.e. benzoate and sorbate are synthesized. The effect of benzoate and sorbate anion is studied for bacterial growth inhibition property against Gram positive and Gram negative bacteria. The biostatic properties of these ionic liquids are studied by kinetic growth rate inhibition method. Furthermore, the lubrication performance of both ionic liquids as additives to polyol ester lube base oil is demonstrated.
Experimental
Materials and chemicals
1,2,3-Benzotriazole (S.D Fine Chemicals, >98.5%), benzoic acid (Merck Chemical >99.5%), potassium sorbate (Sigma Aldrich 99%), 1-bromohexane (Sigma Aldrich 98%), acetyl chloride (Merck Chemical >98%), sodium hydroxide pellets (Fischer Scientific), Luria Bertani and agar (Hi Media, India) were used as received. Microbial culture Escherichia coli MTCC 443 and Rhodococcus erythropolis ATCC 53968 were used for antimicrobial activity. Double distil water and organic solvents of analytical grade were used without further purification.In this study, two ionic liquids viz. 1-acetyl-3-hexylbenzotriazolium benzoate (HAB-Bz) and 1-acetyl-3-hexylbenzotriazolium sorbate (HAB-Sb) were synthesized by following a two-step reaction. In the first step, 1-acetyl-3-hexyl benzotriazolium bromide (HAB-Br) as a cationic precursor was prepared via acetylation reaction by refluxing equimolar amount of 1,2,3-benzotriazole and acetyl chloride for 24 hours in the nitrogen atmosphere using toluene as a reaction medium. The developed product was collected by centrifugation at 5000 rpm and then washed several times with toluene to remove undigested ingredients and impurities. In the subsequent step, resulting material i.e. 1-acetyl benzotriazole was treated with 1-bromohexane for alkylation under nitrogen atmosphere at 65 °C for 24 hours to afford the HAB-Br. Finally, bromide anion of HAB-Br ionic liquid was replaced by benzoate and sorbate anions using sodium benzoate and potassium sorbate salts, respectively, and afforded the HAB-Bz and HAB-Sb ionic liquids. In this context, equimolar amount of HAB-Br was uninterruptedly stirred with aqueous solution of sodium benzoate and potassium sorbate salts for 12 hours to prepare the HAB-Bz and HAB-Sb ionic liquids, respectively. The synthesized ionic liquids were extracted out using dichloromethane (DCM) as an extracting solvent and then washed several times with distil water to remove the halide content and impurities. The DCM was then distilled under the reduced pressure using the rota-evaporator. Each ionic liquid was dried in the vacuum oven at 80 °C for 48 hours and then used for the characterization, antimicrobial and lubrication performance. The yields of HAB-Bz and HAB-Sb ionic liquids are found to be 78 and 85%, respectively, whereas the purity of these ionic liquids is in the range of 80–85%. These values are based on three independent batches preparation.
Characterization of ionic liquids
Synthesis and molecular structure of HAB-Bz and HAB-Sb ionic liquids were confirmed by Nuclear Magnetic Resonance (NMR: 1H and 13C) and Fourier Transform Infrared (FTIR) spectroscopic analyses. The NMR analyses are carried out using Bruker Av III 500 MHz spectrometer preparing 30% w/v solution of each ionic liquid in the CDCl3 solvent. The FTIR spectrum of each ionic liquid was collected using Nicolet 8700 Research spectrometer at resolution of 4 cm−1.Antimicrobial activities of ionic liquids
Antimicrobial activities of HAB-Bz and HAB-Sb ionic liquids were probed against Gram negative Escherichia coli (MTCC 443) and Gram positive Rhodococcus erythropolis (ATCC 53968) bacteria using the agar diffusion technique.35 Ampicillin was used for the comparison purpose. The well of 6.0 mm diameter was punched using sterilized tip and then culture of each microbial strain (100 μl, optical density: 0.8) was spread using sterile spreader. The ionic liquids blends in methanol were poured into the wells at different concentrations viz. 0.5, 1, 1.5, 2.5, 5, and 25 mg per well. The methanol was used as a control. The plates are incubated at 32 °C temperature and examined for inhibition zones as a function of incubation time. The areas of inhibition zone for 24 hours microbial growth were observed as clear zone around the well. The reported values are average of three independent measurements. The Luria Bertani broth having 2.5 mg of each ionic liquid was further used to examine the growth profile of bacterial cells in the presence of HAB-Bz and HAB-Sb ionic liquids. Each culture was incubated in a shaking incubator at 32 °C for 0 to 8 hours. The growth curves of bacterial cell cultures were attained by measuring the optical density (O.D.) at 600 nm. The reported values are an average of three independent measurements. These results were further compared with controlled experiment (without ionic liquid). The optical density was recorded using Eppendorf bio photometer.Lubrication properties of ionic liquids
Lubrication properties of ionic liquids as additives to polyol ester (pentaerythritol tetraoleate) lube base oil were probed in terms of coefficient of friction and wear scar width using ball-on-disc contact geometry. Polyol ester is a biodegradable synthetic lube base oil with four oleate alkyl chains. All tribological tests were conducted using CSM microtribometer (Model: NTR2) at 250 mN normal load and 50 rpm rotational speed. Each test was conducted for the 2000 cyclic laps. The changes in coefficient of friction with number of cyclic laps were recorded while the worn area of aluminium disc was probed by using field emission scanning electron microscope (FESEM; FEI Quanta 200). Further, elemental mapping of worn scar of aluminium disc was carried out using energy-dispersive X-ray (EDX) spectroscopy coupled to the FESEM to understand the nature of tribo-chemical thin film formed on the contact surfaces under tribo stress.Results and discussion
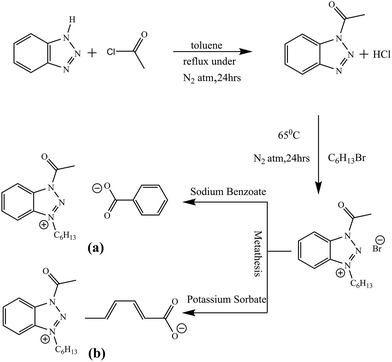
The HAB-Br salt, a cationic precursor to HAB-Bz and HAB-Sb ionic liquids was prepared by acetylation of 1,2,3-benzotriazole and then alkylation of developed product with 1-bromohexane as shown in Scheme 1. In the subsequent step, bromide anion of HAB-Br salt was replaced by benzoate and sorbate using sodium benzoate and potassium sorbate, respectively, and afforded HAB-Bz and HAB-Sb ionic liquids. The chemical characterization of each ionic liquid was carried out by FTIR and NMR analyses. Table 1 depicts the characteristics vibration modes of both ionic liquids along with their assigned functional groups. The vibrational modes at 3066 and 3030 cm−1 owing to![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H aromatic ring stretches, 1599, 1495, 1421 cm−1 attributed to C–C stretches, in the range of 800–600 cm−1 associated to the
C–H aromatic ring stretches, 1599, 1495, 1421 cm−1 attributed to C–C stretches, in the range of 800–600 cm−1 associated to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H (aromatic) bending and ring torsion modes, in the range of 3000–2850 cm−1 owing to asymmetric and symmetric stretches of methylene and methyl units and peaks at 1573 and 1326 cm−1 attributed to the asymmetric and symmetric stretches of COO− (carboxylate) group confirmed the presence of characteristics functionalities in the both ionic liquids. The detailed vibration spectra of both ionic liquids are shown in the Fig. S1 (ESI).† Furthermore, the molecular structure of each ionic liquid was examined by 1H and 13C NMR. The characteristics chemical shifts based on 1H and 13C NMR spectra of both ionic liquids are shown in Fig. S2 and S3 (ESI).† The chemical shifts based on 1H and 13C NMR confirmed the preparation of HAB-Bz and HAB-Sb ionic liquids.
C–H (aromatic) bending and ring torsion modes, in the range of 3000–2850 cm−1 owing to asymmetric and symmetric stretches of methylene and methyl units and peaks at 1573 and 1326 cm−1 attributed to the asymmetric and symmetric stretches of COO− (carboxylate) group confirmed the presence of characteristics functionalities in the both ionic liquids. The detailed vibration spectra of both ionic liquids are shown in the Fig. S1 (ESI).† Furthermore, the molecular structure of each ionic liquid was examined by 1H and 13C NMR. The characteristics chemical shifts based on 1H and 13C NMR spectra of both ionic liquids are shown in Fig. S2 and S3 (ESI).† The chemical shifts based on 1H and 13C NMR confirmed the preparation of HAB-Bz and HAB-Sb ionic liquids.
| HAB-Bz, cm−1 | HAB-Sb, cm−1 | Vibrational assignment |
|---|---|---|
| 3066 | 3066, 3030 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretch (aromatic) C–H stretch (aromatic) |
| 3000–2850 | 3000–2850 | –C–H stretches (methylene, methyl units) |
| 1690 | 1690 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch O stretch |
| — | 1642 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch (alkene) C stretch (alkene) |
| 1573, 1326 | 1573, 1325 | COO asym and sym stretches |
| 1599, 1495, 1421 | 1599, 1495, 1421 | C–C stretches (di-substituted aromatic ring) |
| 1452 | 1451 | C–H bending |
| 1291 | 1291 | C–O stretch |
| 1176 | 1176 | C–N stretch |
| 800–600 | 800–600 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H (aromatic) bending/ring torsion modes C–H (aromatic) bending/ring torsion modes |
The antimicrobial activities of HAB-Bz and HAB-Sb ionic liquids were studied against two types of bacteria i.e. Gram negative E. coli and Gram positive Rhodococcus erythropolis. The agar diffusion test was carried out to examine the effect of each ionic liquid on microbial inhibition. Table 2 represents the inhibitory observations and revealed that the inhibition zone increases with increasing concentration of ionic liquid in the diffusion well. Both ionic liquids show biological activities against Gram positive and Gram negative bacteria. The 0.5 to 1.5 mg concentration range of these ionic liquids are observed to be less effective for the Gram positive bacteria and inhibition zones could not increased significantly. Further, no perceptible inhibition zones were observed; when the concentrations of each ionic liquid are used below 0.5 mg. The higher dosing of ionic liquids (2.5 mg and above) showed remarkably improved antimicrobial activities and the zone of inhibition rises up to 11th folds compared to that of with lower concentration of ionic liquids. Hence, 2.5 mg of ionic liquids is considered as an optimum dose for both Gram positive and Gram negative microorganism. Ampicillin, a well-established antibiotic to prevent and treat a number of bacterial infections, was used for the comparison purpose. At all dosages, ampicillin showed better antimicrobial activities compared to that of HAB-Bz and HAB-Sb ionic liquids (Table 2). The ampicillin showed higher antimicrobial activities against Gram negative bacteria compared to Gram positive bacteria as illustrated by their inhibition zone (Table 2). Recently, Branco et al. demonstrated the antibacterial activity of ampicillin based ionic liquids and revealed more resistance towards Gram negative strains compared to Gram positive strains. Further, it was deduced that ampicillin-based ionic liquids exhibit higher potential for antibacterial activities compared to the sodium salt of ampicillin.36 The benzotriazole, sodium benzoate and potassium sorbate, the precursors to HAB-Bz and HAB-Sb ionic liquids exhibit antimicrobial activities.37–39 Therefore, the inhibitory effects of these precursors were probed against the Gram positive and Gram negative bacterial strains. The benzotriazole was able to create maximum 10 mm diameter of inhibition zone in similar experimental conditions, while sodium benzoate and potassium sorbate does not show any notable zone of inhibition on the tested organism with concentration 2.5 mg. These results suggest that HAB-Bz and HAB-Sb ionic liquids possess remarkably improved antimicrobial activities compared to that of constituent components. The biological properties of HAB-Bz and HAB-Sb ionic liquids against microorganism were attributed to the several factors including polarity provided by acetyl and hydrazine functionalities, hydrophobicity owing to hexyl ring in the cationic moiety and higher solubility in the test medium etc. It is believed that the interaction of the cationic moiety of these ionic liquids with electro-statically negatively charged cell wall leads to death of cells by leakage of intracellular substances and provides antimicrobial activities.40,41
| Sample | Strain | Microbial inhibition zone, mm | |||||
|---|---|---|---|---|---|---|---|
| 0.5 mg | 1.0 mg | 1.5 mg | 2.5 mg | 5 mg | 25 mg | ||
| HAB-Bz | Gram(+) | 2 | 1 | 2 | 19 | 21 | 22 |
| HAB-Bz | Gram(−) | 1 | 2 | 10 | 11 | 16 | 18 |
| HAB-Sb | Gram(+) | 0 | 1 | 2 | 17 | 20 | 23 |
| HAB-Sb | Gram(−) | 2 | 10 | 12 | 14 | 15 | 16 |
| Ampicillin | Gram(+) | 14 | 18 | 19 | 20 | 38 | 40 |
| Ampicillin | Gram(−) | 24 | 34 | 36 | 37 | 38 | 42 |
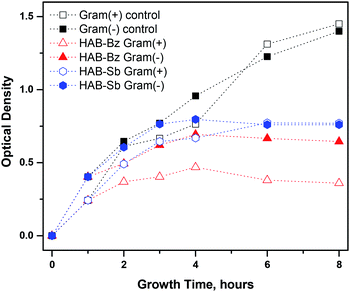
The effect of HAB-Bz and HAB-Sb ionic liquids on the bacterial growth patterns in the liquid culture medium was further studied based on optical density measurements. As shown in the Fig. 1, the optical density of bacterial cultures gradually increased for the initial four hours and then stabilized with further increasing of time for the bacterial growth. It is believed that after four hours these ionic liquids inhibit the bacterial growth, whereas in the control experiments (in absence of ionic liquids) increase of optical density suggested the gradual growth of microbes with time.
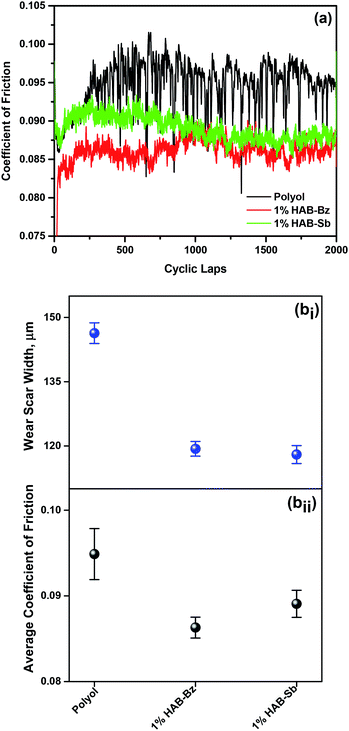
Furthermore, friction and wear properties of HAB-Bz and HAB-Sb ionic liquids as additives to polyol lube base oil were evaluated using steel-to-aluminium tribo-pair. Fig. 2a shows the changes in the coefficient of friction with respect to cyclic laps using 1 wt% blend of each ionic liquid (HAB-Bz and HAB-Sb) in the polyol ester. The average coefficient of friction with polyol ester was found to be 0.095 under a load of 250 mN. The use of HAB-Bz and HAB-Sb ionic liquids as additive to polyol lube base oil showed reduction of friction (∼10%) and wear (∼20%) (Fig. 2b(i and ii)). The polyol ester lube base oil has inherent lubricity owing to presence of four oleate functionalities, which prepare the low shear strength thin film under the tribo-stress and reduces the friction and wear. In spite of that, 1 wt% of HAB ionic liquid reduces the friction and wear properties of the polyol ester.
Fig. 3 shows microscopic images and corresponding elemental mapping of worn surfaces of aluminium discs lubricated with 1 wt% blend of HAB-Bz and HAB-Sb ionic liquids. The elemental distribution images of worn surfaces explicitly demonstrate the uniform distribution of nitrogen and carbon. In these ionic liquids, carbon and nitrogen are characteristics elements and are uniformly distributed on the worn areas. These results suggested the formation of a tribo-chemical thin film composed of ionic liquids that not only provides low shear strength to reduce the friction but also protects the surface against the wear. It is believed that the inherent polarity of ionic liquids facilitates their interaction with sliding metal interfaces under the tribo-stress and forms the tribo-chemical thin film of low shear strength.42,43
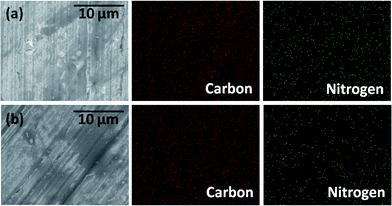 | ||
| Fig. 3 FESEM images and corresponding elemental distributions on the worn surfaces of aluminium discs lubricated with (a) 1 wt% of HAB-Bz and (b) 1 wt% HAB-Sb ionic liquid blends with polyol ester. | ||
Conclusion
Ionic liquids with tailorable structural parameters are gaining large interest for various applications such as active pharmaceutical ingredients, biological active materials, lubrication etc. Herein, 1-acetyl-3-hexylbenzotriazolium cation-based ionic liquids having two different anions i.e. benzoate and sorbate were selected for this study. The preparation and chemical composition of these ionic liquids were confirmed by FTIR and NMR analyses. The antimicrobial activities of these ionic liquids (HAB-Bz and HAB-Sb) were probed against Gram(+) and Gram(−) bacterial strains. Both ionic liquids exhibited higher antimicrobial activities compared to their precursors (benzotriazole, sodium benzoate and potassium sorbate). These results suggest that the conventional active pharmaceutical ingredients could provide improved antimicrobial activities, when they are synergistically converted into the ionic liquids. The ionic liquid as an antimicrobial agent promises its potential for biotechnology, medicinal chemistry and lubrication applications to fight against the microbial pathogen. From a lubrication perspective, HAB-Bz and HAB-Sb ionic liquids as additives to polyol lube base oil provide improved friction and wear properties compared to the polyol ester. The elemental mapping of worn surfaces confirmed the formation of ionic liquid-constituted tribo-chemical thin film, which reduces the friction and wear.Acknowledgements
We kindly acknowledge the Director CSIR-IIP for his kind permission to publish these results. Authors are thankful to Analytical Science Division of CSIR-IIP Dehradun for providing helps in various measurements. RG thanks the CSIR, Govt of India for the fellowship support.Notes and references
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- Q. Zhang, S. Zhang and Y. Deng, Green Chem., 2011, 13, 2619–2637 RSC.
- F. Zhou, Y. Liang and W. Liu, Chem. Soc. Rev., 2009, 38, 2590–2599 RSC.
- R. Gusain, P. Gupta, S. Saran and O. P. Khatri, ACS Appl. Mater. Interfaces, 2014, 6, 15318–15328 CAS.
- I. M. Marrucho, L. C. Branco and L. P. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527–546 CrossRef CAS PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley publication, 2nd edn, 2007 Search PubMed.
- A. Lewandowski and A. S. Mocek, J. Power Sources, 2009, 194, 601–609 CrossRef CAS.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis Jr., M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 CAS.
- O. Aschenbrenner, S. Supasitmongkol, M. Taylor and P. Styring, Green Chem., 2009, 11, 1217–1221 RSC.
- C. G. Cassity, A. Mirjafari, N. Mobarrez, K. J. Strickland, R. A. O'Brien and J. H. Davis, Chem. Commun., 2013, 49, 7590–7592 RSC.
- A. J. Carmichael and K. R. Seddon, J. Phys. Org. Chem., 2000, 13, 591–595 CrossRef CAS.
- O. Zech, A. Stoppa, R. Buchner and W. Kunz, J. Chem. Eng. Data, 2010, 55, 1774–1778 CrossRef CAS.
- D. J. Couling, R. J. Bernot, K. M. Docherty, J. K. Dixon and E. J. Maginn, Green Chem., 2006, 8, 82–86 RSC.
- F. I. Ali, S. A. Subhan, T. Y. Mujahid, S. Muhammad, A. Wahab, S. K. Ali and I. A. Hashmi, Int. J. Adv. Res., 2015, 3, 159–164 Search PubMed.
- M. D. Bermudez, A. E. Jimenez, J. Sanes and F. J. Carrion, Molecules, 2009, 14, 2888–2908 CrossRef CAS PubMed.
- I. Minami, Molecules, 2009, 14, 2286–2305 CrossRef CAS PubMed.
- C. Ye, W. Liu, Y. Chen and L. Yu, Chem. Commun., 2001, 21, 2244–2245 RSC.
- I. Otero, E. R. Lopez, M. Reichelt, M. Villanueva, J. Salgado and J. Fernandez, ACS Appl. Mater. Interfaces, 2014, 6, 13115–13128 CAS.
- R. Gusain, S. Dhingra and O. P. Khatri, Ind. Eng. Chem. Res., 2016, 55, 856–865 CrossRef CAS.
- R. Gusain and O. P. Khatri, RSC Adv., 2016, 6, 3462–3469 RSC.
- R. Ferraz, L. C. Branco, C. Prudencio, J. P. Noronha and Z. Petrovski, ChemMedChem, 2011, 8, 975–985 CrossRef PubMed.
- J. Pernak, K. Sobaszkiewicz and I. Mirska, Green Chem., 2003, 5, 52–56 RSC.
- J. Pernak, I. Goc and I. Mirska, Green Chem., 2004, 6, 323–329 RSC.
- J. N. Pendleton and B. F. Gilmore, Int. J. Antimicrob. Agents, 2015, 46, 131–139 CrossRef CAS PubMed.
- P. Borowiecki, M. M. Krawczyk, D. Brzezinska, M. Wielechowska and J. Plenkiewicz, Eur. J. Org. Chem., 2013, 4, 712–720 CrossRef.
- M. I. Hossain, M. El-Harbawi, Y. A. Noaman, M. A. B. Bustam, N. Banu, M. Alitheen, N. A. Affandi, G. Hefter and C. Y. Yin, Chemosphere, 2011, 84, 101–104 CrossRef PubMed.
- D. Demberelnyamba, K. S. Kim, S. Choi, S. Y. Park, H. Lee, C. J. Kim and I. D. Yoo, Bioorg. Med. Chem., 2004, 12, 853–857 CrossRef CAS PubMed.
- U. K. Junggren and S. E. Sjostrand, Eur. Patent EP0005129 B1, 1981.
- L. Zirngibl, Azoles, Antifungal Active Substances-Synthesis and Uses, Wiley-VCH Publications, 1997 Search PubMed.
- P. A. J. Janssen, C. J. E. Niemegeers, K. H. L. Schellekens and F. M. Lenaerts, Arzneim. Forsch., 1971, 21, 1234 CAS.
- M. Sato, M. Arimoto, K. Ueno, H. Kojima, T. Yamasaki, T. Sakurai and A. Kasahara, J. Med. Chem., 1978, 21, 1116 CrossRef CAS PubMed.
- H. Nakano, T. Inoue, N. Kawasaki, H. Miyataka, H. Matsumoto, T. Taguchi, N. Inagaki, H. Nagai and T. Satoh, Bioorg. Med. Chem., 2000, 8, 373 CrossRef CAS PubMed.
- B. V. Suma, N. N. Natesh and V. Madhavan, J. Chem. Pharm. Res., 2011, 3, 375–381 CAS.
- Y. Ren, L. Zhang, C. H. Zhou and R. X. Geng, Med. Chem., 2014, 4, 640–662 Search PubMed.
- A. W. Bauer, W. M. Kirby, J. C. Sherris and M. Turck, Am. J. Clin. Pathol., 1966, 45, 493–496 CAS.
- R. Ferraz, V. Teixeira, D. Rodrigues, R. Fernandes, C. Prudencio, J. P. Noronha, Z. Petrovski and L. C. Branco, RSC Adv., 2014, 4, 4301–4307 RSC.
- S. N. Swamy, Basappa, G. Sarala, B. S. Priya, S. L. Gaonkar, J. Shashidhara and K. S. Rangappa, Bioorg. Med. Chem. Lett., 2016, 16, 999–1004 CrossRef PubMed.
- S. S. Soebagyo and P. J. Stewart, Int. J. Pharm., 2005, 288, 263–271 CrossRef PubMed.
- R. J. Lambert and M. Stratford, J. Appl. Microbiol., 1999, 86, 157–164 CrossRef CAS PubMed.
- M. T. Garcia, I. Ribosa, L. Perez, A. Manresa and F. Comelles, Colloids Surf., B, 2014, 123, 318–325 CrossRef CAS PubMed.
- K. M. Docherty and C. F. Kulpa Jr., Green Chem., 2005, 7, 185–189 RSC.
- J. Qu, D. G. Bansal, B. Yu, J. Y. Howe, H. Luo, S. Dai, H. Li, P. J. Blau, B. G. Bunting, G. Mordukhovich and D. J. Smolenski, ACS Appl. Mater. Interfaces, 2012, 4, 997–1002 CAS.
- R. Gusain and O. P. Khatri, RSC Adv., 2015, 5, 25215–25221 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR, NMR (1H, 13C) results of ionic liquids. See DOI: 10.1039/c6ra09900a |
| This journal is © The Royal Society of Chemistry 2016 |