Selective concentration-dependent manipulation of intrinsic fluorescence of plasma proteins by graphene oxide nanosheets
Abstract
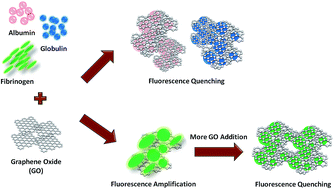
We investigate the molecular interactions between graphene oxide (GO) and blood plasma proteins, in particular, the influence of GO on the intrinsic fluorescence of these proteins. We observe that GO acts as an efficient quencher of the intrinsic fluorescence of albumin, globulin, and fibrinogen. Interestingly, we also note for the first time that, in addition to the robust fluorescence quenching, GO is capable of selectively amplifying the fluorescence emission of fibrinogen up to approximately 30% or 1.3 fold under certain concentrations but not those of albumin and globulin. We suggest that GO may possibly play a dual role in controlling the intrinsic fluorescence emission of the plasma proteins. Furthermore, this role switching may be influenced by the competition between the aggregation and encapsulation effects. We propose that the GO-induced intrinsic fluorescence quenching is driven by the physical encapsulation of the plasma proteins by GO nanosheets. Contrastingly, the GO-mediated fluorescence amplification is promoted by an aggregation of fibrinogen.

 Please wait while we load your content...
Please wait while we load your content...