Open Access Article
Open Access ArticleA periodic review of chemical and pharmacological profiles of Tubiechong as insect Chinese medicine
Jiayu Xie
ab,
Dapeng Zhang
c,
Cheng Liu
*b and
Lingchong Wang
 *a
*a
aSchool of Pharmacy, Nanjing University of Chinese Medicine, Nanjing City, Jiangsu Province 210023, P. R. China. E-mail: 20190441@njucm.edu.cn; 993wlc@njucm.edu.cn; Tel: (+86)-15050581339
bCentral Laboratory, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai City, 200062 P. R. China. E-mail: Liucheng0082010@163.com; Tel: (+86)-021-22233329
cThe First Affiliated Hospital of Guangzhou Medical University, Guangzhou City, 510120 P. R. China. E-mail: dapeng096@163.com
First published on 19th October 2021
Abstract
Tubiechong, in Chinese medicine, denotes the dried female insects of Eupolyphaga sinensis Walker (ESW) or Polyphaga plancyi Bolivar (PPB). As a traditional insect-type, in medicine, it has been historically utilized to treat bruises, fractures, amenorrhea, postpartum blood stasis, lumps and relieving pain. We herein have performed a systematic survey involving the chemical and biological studies in the past decades to reveal the value of such insect resources for their development and clinical utilization. Chemical studies indicated that Tubiechong generated many active compounds, including proteins, amino acids, peptides, fatty acids, alkaloids, nucleosides, polysaccharides, fat-soluble vitamins and mineral elements. Tubiechong or its extract has a wide range of activities including anticoagulation and anti-thrombosis, anti-tumor, antioxidant, immune regulation, blood lipid regulation and hepatoprotection. Finally, a periodic mini-review was conducted to summarize such chemical and pharmacological profiles of Tubiechong medicine. The active peptides in Tubiechong are majorly focused in this review and introduced as one important aspect since there is much literature and huge investigative interest in it. Traditional medical use of the insect was also stressed in this review associating with its disease-eliminating actions by promoting blood circulation or eliminating tissue-swelling pains, which might play important roles in anticancer practices or investigation. In accordance with the modern pharmacological progress, Tubiechong and its extracts indeed exerted antitumor actions through multiple pathways, such as interfering with tumor biological behaviors (growth, apoptosis, invasion, metastasis and angiogenesis), and regulating host immune function. To some extent, this knowledge would provide a basis for further research and application of Tubiechong medicine.
1 Introduction
It is determined that insects are critical sources of active agents for modern medicine. Many insects such as bees, flies, ants, cockroaches and blood-sucking insects are utilized to derive antibacterial, antitumor or antithrombotic agents. Human beings have utilized them as food, medicine, cosmetic and biochemical materials for ages for the reason that they are one of the largest resources of living organisms. In long-term practice, medicinal insects and their products have multiple applications. The most important of these is to treat or prevent various diseases, including infectious diseases, neoplasia, and miscellaneous disorders.1 Tubiechong is an acclaimed medicinal insect, and it is a dry female carcass of the Eupolyphaga sinensis Walker (ESW) or Polyphaga plancyi Bolivar (PPB). Between the two sources, ESW is more commonly applied than PPB due to the abundant resources. ESW, belonging to the family Corydiidae (Blattodea), is widely distributed in China,2 and it is a critical insect used in traditional Chinese medicine (TCM) to eliminate the stasis caused by low movement of blood in local tissue. Moreover, Tubiechong is known by the Chinese as one preferred drug to make fast healing of damaged tendons and reinforce bone strength. In TCM, Tubiechong is considered a natural health product and is expansively utilized to treat or prevent diseases, including bone injury, bruises, cancer, hepatic fibrosis, and immune-related diseases.3Tubiechong is one of the numerous insects that have been used in TCM for a long time. This medicine not only includes many nutrients but also has high medicinal value and health benefits.4 Modern pharmacological studies indicate that Tubiechong has anticoagulation, antithrombotic activity, hepatoprotective and antitumor effects, etc.5 In Southeast Asia, such as in China, Thailand, India and Malaysia, due to its special flavor and healthy effects, Tubiechong has been exploited as tonic and spice recently.1 Until now, it is still difficult to understand the therapeutic role of Tubiechong against various diseases completely, especially the chemical basis and action mechanism. Although, some extracts have been isolated from Tubiechong and proven to have pharmacological activities, and even several pure compounds with strong activities have been purified and identified for their absolute structures.3,6 Many research efforts are needed along the modern pharmacologic route to explore the chemical constitutes and biological actions of Tubiechong. We will retrospectively present the traditional usage, chemical compositions and pharmacological actions of medically relevant insects in this review. It is anticipated to give an integrated viewpoint on the chemical diversity and pharmacological actions of Tubiechong from ingredient-action relation in order to avoid unilateral conclusions such as those in most previous investigations. Some content might address the potential development and application of Tubiechong agents in the future. Some anticancer products of Tubiechong were cited in this review and their possible mechanisms through interfering with carcinogenesis or cancer progression were highlighted.
2 Traditional medical opinion and clinical application
In TCM, the use of Tubiechong was documented in many well-known medicinal works, such as Shennong's Herbal Classic, Synopsis of the Golden Chamber and Compendium of Materia Medica. It was believed to have abundant capabilities, e.g. renewing muscles and bones, boosting the immune response and removing blood stasis to stimulate blood circulation. Because of the light of TCM theories, those diseases are closely related to Qi stagnation and blood stasis in the organs. Therefore, Tubiechong was widely employed to treat many diseases, including bruises, fractures, amenorrhea, postpartum blood stasis, lumps and it was also used to control pain.7,8 These insect medicines also have many folk names, including Dibiechong, Tuyuan, Diwugui, Zhechong, Zhonghuazhendibie, Chouchongmu and Jieguchong. In TCM, folk physicians reckoned Tubiechong to be tasting salty and cold, and possibly showing slight toxicity to patients. After oral administration of decoction of Tubiechong, some Chinese folk physicians thought that the medicine would give blood meridians and distribute it into the liver channels. Thus, Tubiechong was widely applied to treat various painful diseases associated with liver damage.As for the traditional preparation methods, Tubiechong had different usages according to different indications, such as grinding, frying, water extraction, and rice wine extraction. Among them, the main method was extraction with water or rice wine whose alcohol content was less than 20%. Tubiechong is also an important part of many famous medicinal prescriptions and Chinese patent medicines. Expelling blood stasis decoction, also known as Xiayuxue Tang, was first recorded in the book Synopsis of the Golden Chamber, and consisted of three Chinese medicinal materials, Rhei Radix et Rhizoma, Persicae Semen, and Tubiechong. It had good clinical efficacy in treating hepatic fibrosis and cirrhosis.9 Similarly, a famous and classical Chinese herbal prescription, Dahuang Tubiechong pill (DHZCP), consisting of Tubiechong, Dahuang and other 10 herbs, was clinically utilized to treat hepatic diseases, gynecopathy and atherosclerosis in China for a long history.10 Chinese patent medicine is known as Huoxuezhitong capsule (HXZT) contains Tubiechong. HXZT capsule had the activities in activating the blood circulation and relieving pain, and it had been applied for osteoarthritis since 1974.11 Lumbago free capsules, also known as Yaotongning (YTN) capsules, were registered TCM preparations for lumbar, leg pain, rheumatoid arthritis (RA) treatment in China for decades. YTN was composed of 11 herbs and Tubiechong was of the most important composition for manufacturing and quality control, as well as the efficiency of clinic applications.12,13 Another Chinese patent medicine containing Tubiechong as major ingredients belong to aitongke capsules, which was claimed as an antitumor agent and is mainly used for patients with the advanced cancers. Chinese medicine practitioners thought aitongke was capable of extenuating tumor swelling, eliminating cancer pain, reducing hydrops, lifting leukocytes and inducing pus evacuation. Aitongke prescriptions were composed of gold scorpion, Tubiechong, aspongopus, rhubarb, ginseng, Gyrophora, astragali and so on.14
Overall, according to TCM theoretical records and traditional clinical practice, Tubiechong was considered an effective Chinese insect medicine to treat those diseases occurring in parenchymal tissues or malignant cancers through play actions on blood vessels. This hypothesis might lay guides for modern chemical and pharmacological researches on Tubiechong.
3 Chemical constituents of Tubiechong
Many types of compounds have been found and identified from Tubiechong (ESW and/or PPB), including a variety of active proteins, amino acids, peptides, fatty acids, alkaloids, nucleosides, polysaccharides, phenylpropanoids, pyrazines, fat-soluble vitamins, and mineral elements, and some of them can even be isolated and identified as new compounds.153.1 Proteins, amino acids, peptides
Tubiechong is an excellent source of high-quality proteins and is rich in amino acids.16 Amino acids, peptides, and proteins are commonly known as the major components of animal organisms, and most of them have been confirmed by modern studies as specific components of animal-derived biomass with unique nutritional and medicinal purposes. Tubiechong (ESW) has been found to contain 18 kinds of amino acids, which belong to regular amino acids and found in normal protein-constitutes. Among them, Gly, Ala, Pro, Tyr, Arg and Lys are the six most abundant amino acids.17 Another study showed that ESW had high contents of Arg, Ser and Ala residues, but little amounts of Gly and Iso residues. Moreover, the hydrophobic amino acid contents in ESW were relatively high, which accounted for nearly 46.7% of total amino acids. The abundance of hydrophobic amino acids could boost solubility in lipids and therefore enhance the activity of Tubiechong peptides product. One ESW crude peptide product named EPs was obtained by sequentially hydrolyzing with pepsin and trypsin, which was an effective antioxidant and could serve as a powerful treatment for skin photo-aging, and its further purified fractions A4 was confirmed to be more potent antioxidant activities.16 Tubiechong was also used to prepare antitumor protein products. Some researchers utilized salting-out, ultrafiltration, ion-exchange chromatography, hydrophobic chromatography, and gel filtration chromatographic techniques to purify antitumor proteins, such as EPS72 (ref. 3) and one unnamed glycoprotein product.18 EPS72 is a purified protein with 72 kDa of molecular weight and has potent anti-proliferative activities against A549 cancer cells.3 The glycoprotein has 41.3 kDa of molecular weight, and 10.5% carbohydrate content, and was capable of inhibiting Tea-8113 cells proliferation.18 EFP, a kind of fibrinolytic protein purified from ESW, is an anti-angiogenic agent. The anti-angiogenic proteins could strongly restrict the invasive growth and metastasis of malignant tumors. EFP was proved to inhibit the proliferation of MVEC (human microvascular endothelial cells).19 The subsequent study showed that EFP had an obvious antitumor effect on S180 and H22 cells in vivo.20 Tubiechong medicine is also suitable material to isolate proteinic products with various functions, such as fibrinolytic, plasminogen-activating, hypolipidemic and anti-microbial activities. A fibrinolytic protein named eupolytin1,21 was purified from ESW and was confirmed for its activity for inducing fibrinolysis and activating plasminogen. Additionally, scholars obtained a hypolipidemic peptide DP17 with 1.43 kDa of molecular weight from ESW through biomimetic enzymatic hydrolysis.22 In detail, following Table 1 summarizes all active peptide or protein products that have been prepared with Tubiechong recently and some critical information is also listed in the table.| Preparing method | Products | Identification | Characterizations | Bioactivities | Potential applications | Ref. |
|---|---|---|---|---|---|---|
| Hydrolyzation of ESW with pepsin and trypsin | EPs | Crude extract | With less than 3.3 kDa of Mw, fifteen amino acids (Asp, Glu, Ser, Gly, Thr, Ala, Arg, Tyr, Val, Met, Phe, Iso, Leu, Lys and Pro) in detection | Antioxidant | Protection against photo-aging skin | 16 |
| Hydrolyzation of ESW with pepsin and trypsin, gel filtration & ion exchange chromatography | A4 | Purified fraction | With less Mw than EPs | Antioxidant | Protection against photo-aging of skin | |
| Hydrolyzation of ESW with arazyme protease, ultrafiltration, DEAE-cellulose chromatography | F-I, F-II, F-III | Purified fractions | No date | Antioxidant | Antioxidant agent | 23 |
| Homogenization of ESW, salting out, ultrafiltration, ion exchange, hydrophobic & gel filtration chromatographies | EPS72 | Purified protein | 72 kDa of Mw, a band in SDS–PAGE | Antitumor, anti-proliferative effect on A549 (IC50, 18.76 μg mL−1) | Protection against tumor | 3 |
| Homogenization of ESW, savage deproteinization, dialysis, DEAE-cellulose chromatography | Unnamed | Purified glycoprotein | 41.3 kDa of Mw, | Antitumor, Antiproliferative effect on Tea-8113 | Protection against tumor | 18 |
| Homogenization of ESW, salting out, DEAE-cellulose & Sephadex G-75 column chromatographies | EFP | Purified fibrinolytic peptide | No date | Anti-proliferative effect on MVEC, induce apoptosis and cause cell cycle arrest at S and G2/M phases | Anti-angiogenic agent | 19 |
| Homogenization of ESW, dialysis, cationic exchange, gel filtration & anionic exchange chromatographies | Eupolytin1 | Purified protein | 26 kDa of Mw, with IVGGSDANIEDLPYQLSFETIDYDVAVARVATPFSYGSGVQQLQVVSVPIVSPQQCNNDYASDPCQGDSSGPLTVGGYPGVYSNVATLR in sequence | Fibrinolytic and plasminogen-activating (PA) activities | Anti-thrombosis agent | 21 |
| Homogenization of ESW, salting out, DEAE-cellulose chromatography; PAGE electrophoresis | EFF-1, EFF-2, EFF-3 | Purified protein | With 41 kDa, 32.9 kDa and 30.6 kDa of Mw, respectively | Fibrinolytic activities | Fibrinolytic enzyme | 24 |
| Homogenization of ESW; salting out; DEAE-cellulose & gel filtration chromatographies | Unnamed | Purified glycoprotein | 41.3 kDa of Mw, 10.5% of carbohydrate content, | Fibrinolytic activities | Fibrinolytic enzyme | 25 |
| Water extract and alcohol precipitate, ion exchange & gel filtration chromatographies, RP-HPLC | Fraction VI | Purified fraction | 3.8 kDa of Mw, 89.3% of protein content, two bands in SDS–PAGE | Fibrinolytic activities | Fibrinolytic enzyme | 26 |
| Hydrolyzation of ESW with pepsin and trypsin, ultrafiltration & nanofiltration, macroporous resin, Sephadex G-25 column chromatography, RP-HPLC | DP17 | Purified peptide | 1.43 kDa of Mw, with DAVPGAGPAGCHPGAGP in sequence, 8 beta sheets and 2 alpha sheets in configuration | Lipid accumulation reducing in liver tissues, reducing blood lipids | Hypolipidemic agent | 22 |
| Hydrolyzation of ESW with pepsin and trypsin, ultrafiltration | APE | Crude extract | 71.05 ± 3.10% of the protein content | Blood lipid reducing activity | Hypolipidemic agent | 27 |
| Homogenization of ESW, gel filtration chromatography, RP-HPLC | Unnamed | Purified peptide | ACDFQQCWVTCQRQYSINFISARCNGDSCVCTFRT in sequence | Antimicrobial | Antimicrobial agent | 28 |
Based on the currently available information, many protein products have been isolated from insect materials, and these, peptides are basically assumed to have strong potentials in development and application due to their strong activities and high content, as well as structural diversity. However, further development of peptide products is limited in their separation, purification and structural identification. The combination of minimal separation rate and the diversification of components has caused a serious lack of understanding of the structure and function of macromolecular components of Tubiechong. Overall, it is worthy to develop innovative methods and strategies on functional peptides for utilizing Tubiechong materials.
3.2 Polysaccharides
Polysaccharides are macromolecules gained from natural sources and might possess diverse structures and various biological activities.6 Tubiechong also contained polysaccharide ingredients to contribute to its medical effects in the clinic. However, there are relatively few studies on polysaccharide constituents of Tubiechong due to their low content and difficulty in extraction. In spite of such a dilemma, a polysaccharide fraction named ESPS had been purified from ESW using ion exchange and gel chromatography. Its structure characterization and physiological effect (antitumor) were further explored. The structural determinations indicated that the mean weight (Mw) of ESPS was 21.4 kDa. The main chain of ESPS was mainly composed of →4)-α-D-Glcp-(1→ and →3)-β-D-Galp-(1→. Moreover, the residues and their side chains are joined to the main chain through the O-6 atom of glucose and O-4 and O-6 atoms of Gal residue. Additionally, its monosaccharide composition included fucose (Fuc), rhamnose (Rha), xylose (Xyl), arabinose (Ara), galactose (Gal) and glucose (Glc), and their molar ratios were 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4
7.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.3
9.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.9
13.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.5
26.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39.7, respectively. The possible fragments constituting absolute polysaccharide structures of the ESPS are presented in Fig. 1, which could be used for further structure speculation. In evaluation, ESPS enhanced lymphocyte activity in vitro, mainly to the NK cells. Thereby, it could promote lymphocyte proliferation and inhibit liver cancer cell growth. Moreover, ESPS stimulated immunity and effectively inhibited H22 cell growth in H22-bearing mice. The results revealed that ESPS, the polysaccharide product in ESW had high anti-hepatocellular carcinoma activity and was a potential immunotherapy candidate for the treatment of liver cancer.6
39.7, respectively. The possible fragments constituting absolute polysaccharide structures of the ESPS are presented in Fig. 1, which could be used for further structure speculation. In evaluation, ESPS enhanced lymphocyte activity in vitro, mainly to the NK cells. Thereby, it could promote lymphocyte proliferation and inhibit liver cancer cell growth. Moreover, ESPS stimulated immunity and effectively inhibited H22 cell growth in H22-bearing mice. The results revealed that ESPS, the polysaccharide product in ESW had high anti-hepatocellular carcinoma activity and was a potential immunotherapy candidate for the treatment of liver cancer.6
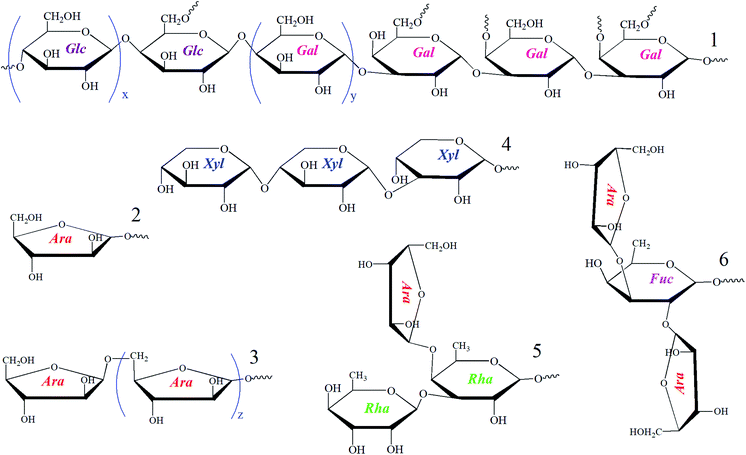 | ||
| Fig. 1 Sugar chain fragments found in the structural elucidation of ESPS polysaccharide isolated from ESW. | ||
3.3 Fatty acids
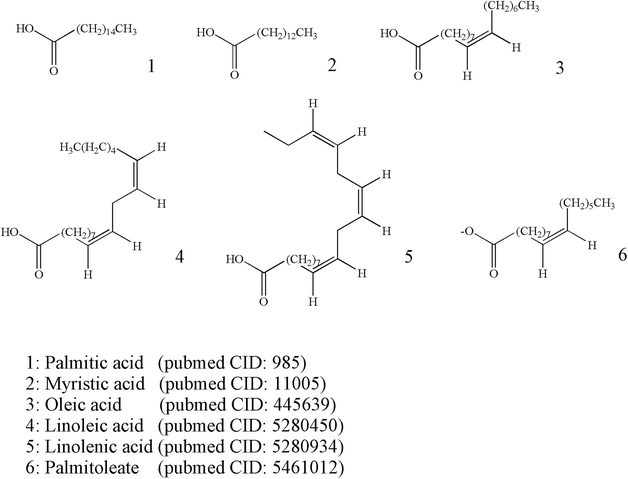
The content of fatty acids in ESW is relatively high. Among all lipids that are extractable from the insect, unsaturated fatty acids accounted for 75% of the total, and essential fatty acids linoleic acid accounted for 28.5% of unsaturated fatty acids. ESW is rich in essential fatty acids such as linolenic acid. The tonic effect of Tubiechong may be due to the essential fatty acids as nutritional ingredients in combination with the essential amino acids and some vitamins.1 The chemical analysis uncovered the presence of 6 lipids components occurring as saturated and polyunsaturated fatty acids that accounted for 97.55% of fatty acids, indicating the high nutritional value of Tubiechong. Tubiechong oily extract consisted of a significant amount of saturated and unsaturated fatty acids. The six highest content fatty acids in Tubiechong oily extract were identified as palmitic acid (21.70%), cis-9-oleic acid (40.78%), cis-9,12-linoleic acid (21.86%), cis-9-palmitoleate (9.86%), cis-9,12,15-linolenate (1.69%) and myristate (1.67%). The extract containing a high amount of oleic and polyunsaturated fatty acids may have potential in treating tumors owing to oleic and polyunsaturated fatty acids produced anti-inflammatory, antitumor and cardioprotective effects.1 The chemical structures of these major fatty acid molecules identified from Tubiechong extract are indicated in Fig. 2.3.4 Nucleosides
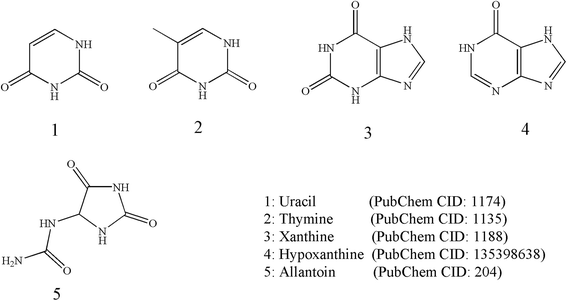
Tubiechong contains nucleosides although there are a few literature reports on them. However, there are several reports on its metabolites and analogs. The nucleoside analogs mainly found in Tubiechong are uracil, allantoin and hypoxanthine. Among these nucleoside analogs, allantoin is even recognized as an active component and quality marker of Tubiechong by Chinese Pharmacopoeia. Allantoin can be separated from the n-hexane extract and n-butanol of extract ESW.29 Moreover, the contents of uracil, xanthine, hypoxanthine and uridine from ESW were evaluated using RP-HPLC.30 Another HPLC fingerprint analysis for ESW showed that there were seven characteristic peaks in the HPLC fingerprint of ESW, one of which was allantoin. The contents of allantoin were proportional to the analgesic effect of ESW.31,32 In addition, other scholars also isolated thymine from the ethyl acetate part of the 70% acetone extract of ESW.33 The chemical structures of these nucleoside analogs founded in ESW are presented in Fig. 3.3.5 Phenylpropanoids
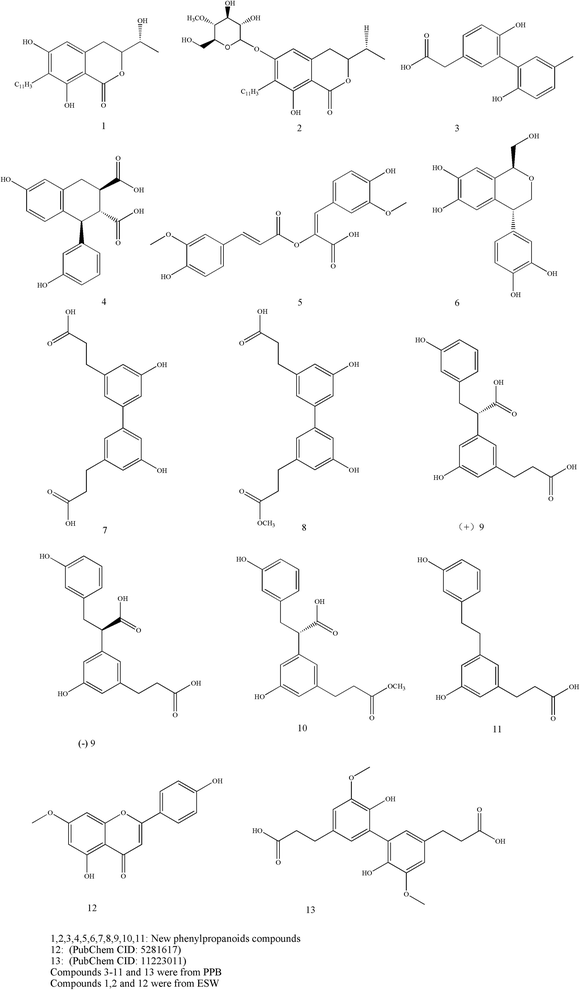
Phenylpropanoids are an important group of natural products and are known to have multiple effects including antimicrobial, antioxidant, anti-inflammatory, antidiabetic and antitumor activities. In structure, those compounds are generally connected three straight-chain carbons with a benzene ring together. The classification includes simple phenylpropanoids, coumarins, lignans, lignins and flavonoids. Researchers recently confirmed that Tubiechong contained many types of phenylpropanoids compounds. According to their reports, at least thirteen phenylpropanoids compounds, including neolignans 3–11, 13,34 isocoumarins 1, 2 (ref. 35) and a flavonoid (genkwanin) 12,36 were found in Tubiechong materials. The neolignans 3–11 and 13 were isolated from PPB and belonged to new compounds,34 while isocoumarins and genkwanin were found in ESW.36,37 The chemical structures of these compounds are presented in Fig. 4. In addition, these insect-derived phenylpropanoids were also assayed in biological activities associated with clinic usages of Tubiechong. Most phenylpropanoids, covering compounds 1–11, possessed strong anti-inflammatory activities. It was also evidenced that the phenylpropanoids 5 and 7 could disrupt Smad activation and inhibit renal fibrosis. Genkwanin has proved to be a potential antitumor candidate in breast carcinoma therapy.38 Studies by some other scholars have shown that genkwanin also has anti-inflammatory activities.39,40 It is common for phenylpropanoids to have such activities and there are many reports on them. For example, several important natural phenylpropanoids have significant antitumor and immunomodulatory activity on colorectal cancer in the APC (Min/+) (multiple intestinal neoplasia) mice.41 As far as the antitumor actions are concerned, phenylpropanoids could provoke apoptosis in breast cancer cells through reactive oxygen species and mitochondrial-dependent pathways.42 Moreover, studies demonstrated that phenylpropanoids induced cell apoptosis and thus exhibited strong cytotoxicity to liver cancer cell lines.43 Apart from those, phenylpropanoids were thought to play immunostimulatory and antioxidant roles in exhibiting their antitumor abilities. Overall, the diversity and abundance of phenylpropanoids decided that Tubiechong would have huge potentials in development as therapeutics agents against inflammatory and/or cancerous diseases.3.6 Alkaloids
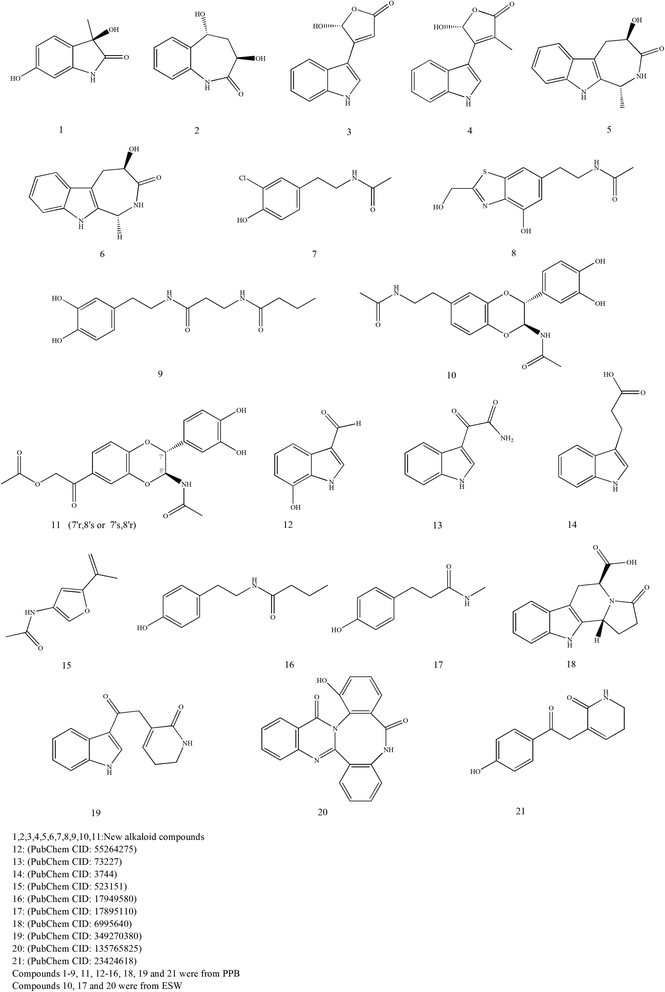
Alkaloids are a large group of basic nitrogen-containing compounds that exist in organisms. Tubiechong contains a number of alkaloids. Pharmacodynamic experiments showed that the total alkaloids of Tubiechong could directly dilate blood vessels to reduce peripheral resistance and cardiac load. It could improve the tolerance of the myocardium and brain to ischemia and reduce the oxygen consumption of the heart and brain tissue.25 Currently, twenty-one alkaloids are found in Tubiechong medicine. The chemical structures of these molecules are presented in Fig. 5. On the one hand, about 18 alkaloids were separated from the ethanol extract of whole bodies of PPB.44,45 Among them, eight chemicals were known alkaloids and recognized, such as alkaloids 12–16, 18–19, and 21. Other ten alkaloids might be new compounds after being verified in the Pubchem database (https://pubchem.ncbi.nlm.nih.gov/), which indicated as compounds 1–9, and 11. The PPB-derived alkaloids were investigated for their renal protective activities against virus attraction and tuberculosis. Surprisingly, most of them had strong activities. On the other hand, only three alkaloids had been reported in ESW, including compounds 10, 17, and 20.33,35 Compound 10 might be a new compound and was extractable from the insect. Alkaloids 17 and 20 were isolated from the ethyl acetate extract of ESW and had some cytotoxicity to 10 types of cancer cells in vitro. The potential effects of those alkaloids compounds can be evidenced by many other individual pharmacological investigations. For example, some scholars have found that indole-3-glyoxylamide has anti-tumor46 and antiviral activities.47 Indole propionic acid has anti-inflammatory and antioxidant properties.48 The analogs can even promote human and murine intestinal homeostasis,49 restrain gut dysbiosis and endotoxin leakage to alleviate steatohepatitis in rats.50 These pieces of evidence have supported strong activities and wide range of potential medical applications of Tubiechong.3.7 Other constituents
Tubiechong also contains fat-soluble vitamins, mineral elements, steroids, pyranzines and alcohols. Vitamins play an important role in anti-oxidation and preventing fat peroxidation. Meanwhile, 4 trace element contents of Ca, Mg, Zn and Mn had also been found in ESW.51 Two steroids, including beta-sitosterol29 and cholesterol36 were reported in ESW along with octacosanol and butyl alcohol.29 Furthermore, some pyrazines were also found in Tubiechong, including plancypyrazine A isolated from PPB45 and 2-methyl-6-(2′3′4′-trihydroxybutyl) pyrazine isolated from ESW.33 In addition, there was a phthalide derivative isolated from ethanol extract of PPB.45 Apart from those, some ketones were also found in Tubiechong, such as 6,8-dihydroxy-3,7-dimethyl-3,4-dihydro-1H-isochromen-1-one.33 3-Hydroxypyridine was found in Tubiechong as well.33 There was also a lactone compound found in PPB. The lactone ring was combined with a benzene ring and glucose was connected to the benzene ring.454 Pharmacological activities of Tubiechong
Modern pharmacological studies have confirmed that Tubiechong and its extracts have a wide range of pharmacological activities such as thrombolysis, anticoagulant, anti-tumor, immunomodulatory, anti-oxidative and hepatoprotective.52 Some important activities are summarized in Table 2.| Pharmacological activity | Testing substance | Tested living system/organ/cell | Dose and administration | Results | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Anti-tumor activity | ESW 70% ethanol extract | Cultured A549 cells; cultured HUVECs; | 0.1–0.8 mg mL−1 for 48 h | Inhibited cell proliferation (IC50 of HUVECs was 0.34 mg mL−1; IC50 of A549 was 0.27 mg mL−1) and migration | Inhibited the autophosphorylation of KDR, downregulate the activation of AKT and (ERK)1/2 | 5 |
| ESW 70% ethanol extract (ESWE) | Cultured MDA-MB-435s and MDA-MB-231 cells | 0.4–1.6 mg mL−1 for 24 h | Inhibited cell proliferation (with 0.564 and 0.724 mg mL−1 of IC50, respectively) and migration | Inhibition the expression of MAPK signaling and related metastasis factors | 7 | |
| MDA-MB-231 xenograft mice model | 200, 400 mg kg−1 for 14 days | Tumor growth inhibition | ||||
| ESW 70% ethanol extract (ESEE) | K562 | 0.05–0.2 mg mL−1 for 48 h | Tumor growth inhibition, inducing G2–M phase arrest | Down-regulating phosphorylation of EGFR, AKT and ERK1/2 | 53 | |
| Tumor (S180)-bearing mice | 100, 200 and 400 mg kg−1 for 10 days | Tumor growth inhibition | ||||
| ESW 70% ethanol extract (ESWE) | SMMC-7721, BEL-7402 and HepG2 cells; | 0.05, 0.1, and 0.2 mg mL−1 for 48 h | Inhibited cell proliferation (with 0.13 mg mL−1, 0.14 mg mL−1 and 0.67 mg mL−1 of IC50, respectively) | Inhibited growth and metastasis signaling (the PKC, AKT, MAPK signaling and related metastasis signaling) | 54 | |
| SMMC-7721 xenograft in athymic mice | 400 mg kg−1 for 10 days | Tumor growth inhibition | ||||
| ESW 95% ethanol extract (ESEE) (mainly fatty acids) | Tumor (H22)-bearing mice | 31–124 mg kg−1 for 14 days | Tumor growth inhibition, promoting TNF-α and IFN-γ production and inducing apoptosis | Via increase of Bax/Bcl-2 ratio and activation of caspases-3 | 1 | |
| Polysaccharide from ESW(ESPS) | Spleen lymphocytes | 50, 100 and 200 μg mL−1 for 48 h | Enhanced lymphocyte activity, promoted lymphocyte proliferation | Enhancement of lymphocyte cytotoxicity | 6 | |
| NK cells | 100, 200 and 400 μg mL−1 for 48 h | Enhanced NK cytotoxicity | ||||
| Tumor (H22)-bearing mice | 5, 10 and 20 mg kg−1 once every two days for 15 days | Tumor growth inhibition | ||||
| Purified protein EPS72 | A549 cells | 5 and 40 μg mL−1 for 48 h | Inhibited cell proliferation (with 18.76 μg mL−1 of IC50), restrained cell migration and invasion | Inhibited cell adhesion to fibronectin and collagen IV, down-regulated the expression of β1-integrin | 3 | |
| ESW 80% ethanol extract (ESWE) | PC3 cells | 0.25 and 0.5 mg mL−1 for 24 h | Inhibited the growth, migration and invasion of PC3 cells | MMP-2 and MMP-9 expression inhibition | 55 | |
| ESW 95% ethanol extract (ESE) | HepG2 and SGC-7901 cells | 0.1–0.535 μg mL−1 for 48 h | Inhibited cell proliferation (with 0.90 and 0.11 μg mL−1 of IC50, respectively), induce HepG2 cell apoptosis | No mention | 56 | |
| Protein of ESW (EFP) containing serum | A549 cells | Serum drug from 0.73–2.90 mg kg−1 for 48 h | Tumor proliferation inhibition, inducing apoptosis | Increasing the ratio of Bax/Bcl-2 | 57 | |
| ESW fibrinolyric protein (EFP) drug serum | HepG2 and MCF-7 cells | Serum drug from 1.25 to 5 mg kg−1 for 48 h | Tumor proliferation inhibition, inhibiting angiogenesis | Down-regulating the expression of VEGF and bFGF | 58 | |
| Serum containing ESW | HepG2 cells | 20% ESW serum for 72 hours | Tumor proliferation inhibition | Inducing G0–G1 phase arrest | 59 | |
| Purified protein from ESW | Tea-8113 cells | 0.010–0.0902g mL−1 for 72 h | Tumor proliferation inhibition | No mention | 18 | |
| Neolignans from PPB | K562, A549, and Huh7 cells | 2.5–40 μM for 48 h | Phenylpropanoids compound 9 inhibited cell proliferation of Huh-7 cells (with 23.2 μM and 27.1 μM of IC50, respectively), and inhibited cell proliferation of K562, A549 | No mention | 34 | |
| Anti- thrombogenic and anticoagulant activities | Three kinds (EFF-1, EFF-2 and EFF-3) of fibrinolytic factors from ESW | Fibrin plate experiment in vitro | 20 μL per well | Activated plasminogen | No mention | 24 |
| Purified protein from ESW (eupolytin1) | Arteriovenous shunt rat models | 0.06 μmol kg−1 | Reduced thrombus weight | No mention | 21 | |
| Fraction from ESW (fraction VI) | Fibrin plate experiment in vitro | 5–20 μL per well | Degraded fibrin and activated the plasminogen | No mention | 26 | |
| ESW fibrinolytic protein (EFP) | Carrageenan-induced thrombosis model mice | 4,8,16 g kg−1 d−1 for 10 days | Reduce the length of thrombus | No mention | 60 | |
| Immunomodulatory activity | Decoction of ESW | Healthy mice | 1.89–7.56 g kg−1 d−1 to mice for 4 weeks | Enhanced the carbon expurgatory index and phagocytic index | No mention | 61 |
| Papain-hydrolyzed peptides of ESW | Healthy mice | 0.3 g kg−1 d−1 to mice for 10 days | Increased index of thymus and spleen, enhanced the phagocytic function of macrophage and promoted the level of IL-2 in serum | No mention | 62 | |
| ESW lyophilized powder (ESL) | Immunosuppressed mice induced by cyclophosphamide | 0.5, 1.0 and 2.0 g kg−1 d−1 for 14 days | Increased the immune organ index, mononuclear macrophages function and the level of NK cell | Down-regulated the phosphorylation of JNK and the Bax/Bcl-2 ratio | 2 | |
| Hepatoprotective activity | ESW polypeptides | CCl4-induced chronic liver injury mice | 50, 100 and 200 mg kg−1 d−1 for 6 weeks | Attenuates CCl4-induced chronic liver injury in mice | Down-regulated the expression of Bax, caspase-3,α-SMA and TGF-β1 | 63 |
| Anti-oxidative and anti-aging activities | Arazyme enzymatic hydrolysis peptides of ESW | D-Galactose-induced aging model mice | 300 mg kg−1 d−1 for 6 weeks | Scavenge free radicals in vitro, reduced the content of MDA, increased the activities of CAT and GSH-Px and T-SOD in liver | No mention | 64 |
| Polypeptide extracts of ESW | D-Galactose-induced aging model mice | 0, 40, 80, 160 mg kg−1d for 20 days | Enhanced the anti-stress and antioxidative capacity, delayed the oxidative aging | Initiating Nrf2-ARE antioxidant signaling pathway | 65 | |
| Peptides from the enzymatic hydrolysate of ESW(F-I, F-II, and F-III) | Free radicals in vitro | F-II (10 mg mL−1, 0.7 mg mL−1, 1.8 mg mL−1 for O2-˙,OH˙,and DPPH˙ respectively | Scavenge free radicals in vitro | No mention | 23 | |
| Enzymatic hydrolysis products of ESW (EPs) | UV radiation-induced skin photoaging mice | Daubed with 140 μL of EPs at a dose of 25, 50 and 75 mg mL−1 per day for 8 weeks | Improved UV irradiation-induced damage of skin texture and morphology | Enhanced the activities of SOD, CAT and GPH-Px, increased the contents of HYP, and reduced the content of MDA in skin | 16 |
4.1 Anti-tumor activity
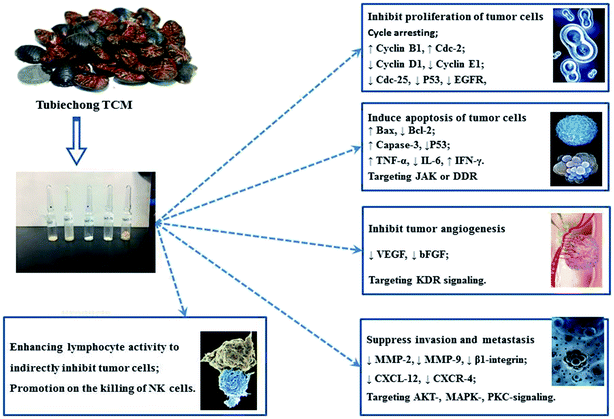
According to TCM theories, the occurrence and progression of cancerous diseases are closely related to the Qi stagnation and blood stasis in the organs. Therefore, TCM physicians even believed that the medicines were very useful for the therapy and prevention of tumors if they had the capability of improving blood circulation and eliminating blood stasis.1 Obviously, Tubiechong belongs to such medicines according to its traditional use and many anti-tumor ingredients.First, some proteins prepared from ESW have been proved to have anti-tumor activities58 due to their anti-angiogenesis effect.19,66 At present, there are many research works paying attention to the anti-tumor effect of Tubiechong and its extracts. Some researchers have estimated the antitumor and immunomodulatory of ESEE (ESW ethanol extract) in hepatocarcinoma H22 bearing mice. After implanting H22 tumor cells, ICR mice were treated with ESEE for 14 consecutive days at doses of 31, 62 and 124 mg kg−1. Oral administration of ESEE could inhibit tumor growth, enhance Th1 type cytokine production (TNF-α and IFN-γ) and induce apoptosis of hepatocarcinoma by increasing the ratio of Bax/Bcl-2 and activating caspases-3.1 In addition, some people elucidated that a product extracted from ESW with 70% ethanol and named ESWE had an anti-proliferation and anti-invasion effect on breast cancer.7 ESWE was capable of inhibiting breast cancer growth, migration, and invasion. The mechanism underlying the above effects was that ESWE could weaken the activity of ERK1/2 and down-regulate the expression of CXCR4, MMP2, and MMP9. Another study indicated that 70% ethanol extract of ESW significantly restrained A549 cell migration in a time and dose-dependent manner and inhibited human umbilical vein endothelial cell proliferation, migration and tube formation. Furthermore, it effectively suppressed blood vessel formation in the established tissue model for angiogenesis. In addition, 70% ethanol extract of ESW was proved to inhibit the autophosphorylation of KDR, and down-regulate the subsequent activation of AKT and extracellular signal-regulated kinase (ERK)1/2 in A549 cells.5 Apart from these, a polysaccharide (ESPS) obtained from ESW by ion-exchange chromatography and gel chromatography was also proved to have strong antitumor activity. ESPS-enhanced lymphocyte activity in vitro, mainly NK cells. Thus, it could stimulate lymphocyte proliferation and inhibit liver cancer cell growth.6 Similarly, PPB and its extracts have anti-tumor activity as well. Some researchers have disclosed that compound plancyol A and pyrazine compound plancypyrazine A isolated from PPB could inhibit JAK3 kinase with IC50 values of 12.6 and 5.0 μM, respectively. Besides, plancyol A expressed inhibitory activity towards DDR1 kinase with an IC50 value of 4.87 μM.45 JAK kinase is related to cancer. Thus, these results showed their potential in anti-tumor applications. In general, Tubiechong and its extracts exert anti-tumor actions through multiple ways as shown in Fig. 6, such as interfering with tumor biological behavior (growth, differentiation, apoptosis, invasion, metastasis and angiogenesis), and regulating the host's anti-tumor response. Among all aspects, the more prominent antitumor roles of Tubiechong and its derived products lie in interfering with tumor cell proliferation, invasion and metastasis by inducing apoptosis, cell cycle arrest and inhibiting angiogenesis.
4.2 Anti-thrombogenic and anticoagulant activities
The incidence of thrombotic disorders such as cerebral stroke, myocardial infarction, and venous thromboembolism is rapidly increasing throughout the world. Thrombolytic therapy is an acknowledged approach to treat these disorders. All thrombolytic agents in current clinical usage are plasminogen activators (PAs), which require plasminogen to achieve thrombosis. Although effective, PAs uniformly increase the risk of bleeding complications, especially intracranial hemorrhage, and there is no laboratory test to avoid such bleeding.21 As reported in Compendium of Materia Medica, Tubiechong has been majorly applied as traditional anti-thrombosis medicine without bleeding risk for a long time.21 Three kinds (EFF-1, EFF-2 and EFF-3) of fibrinolytic factors from ESW were isolated by salting out, DEAE-cellulose column and preparative PAGE electrophoresis. Their activities as plasminogen activators were 171.3 U mg−1, 234.0 U mg−1 and 148.5 U mg−1,24 respectively. Another purified protein from ESW named eupolytin1, a bi-functional anti-thrombosis protein that not only possessed a direct-acting fibrinolytic activity but also had plasminogen-activating activity. Thus, it had anti-thrombosis activity in vivo. The thrombus weight was reduced to 1 ± 0.2 mg by administration of 0.06 μmol kg−1 of eupolytin1.21 In addition, a sample was obtained from ESW by water extraction and alcohol sinking, which was purified by ion-exchange column, gel filtration column and RP-HPLC, when fraction VI was obtained. Moreover, the experimental results of activity indicate that fraction VI had the ability to degrade fibrin directly and active the plasminogen.26 Apart from ESW, neolignans, isolated from PPB, could play potential therapeutic roles in the inhibition of renal fibrosis by the disruption of Smad activation.34 Besides, other researchers have found that alkaloid compounds 4, 13, and 21 might have benefits in the treatment of renal fibrosis with different and unique mechanisms through Smad or non-Smad pathways.444.3 Immunomodulatory activity
Tubiechong has the immunoregulatory effect, which could contribute to its ability to develop and utilize as a health food resource.67 Administration of the decoction of ESW in water at doses of 1.89–7.56 g kg−1 d−1 to mice for 4 weeks observably enhanced the carbon expurgatory index and phagocytic index of mice.68 The papain-hydrolyzed peptides of ESW could elevate immune function in the mice model. In vivo tests demonstrated that ESW-derived peptide could significantly increase the index of the thymus and spleen of mice, enhance the phagocytic function of macrophage and promote the level of IL-2 in serum.69 Another study elucidated that ESW lyophilized powder (ESL) had immuno-enhancement effects in immunosuppressed mice induced by cyclophosphamide. In brief, results suggested that ESL could modulate oxidative systems and innate immune cells, thereby effectively improving immune functions. ESL markedly increased the immune organ index, mononuclear macrophage function and the level of NK cells.2 Moreover, many research works70 showed that ESW could boost the immune function in rats of yin-deficiency and fire-hyperactivity syndrome with chronic blood stasis. Meanwhile, another study showed that ESW (25 g kg−1) could enhance the immune function of mice since it could increase RBC-C3bR rosette rate in a mouse model with blood-deficiency. Besides, it could correct the mice body weight loss induced by cyclophosphamide and increase the spleen thymus index.714.4 Hepatoprotective activity
The liver is the metabolic center and participates in many important physiological functions. As the most common pathological process of various liver diseases, liver injury can develop into serious diseases such as hepatic cirrhosis, hepatic fibrosis, hepatic carcinoma, and cause harm to human health.Research has shown that drugs that could improve blood circulation and remove blood stasis were used in model rats to treat mild chronic hepatic damage induced by CCl4.72 Tubiechong is part of those drugs, it enters the liver meridian, and its hepatoprotective effect has been recorded from ancient times on. Tubiechong is one of the main components of DHZCP. Some reports showed that it could alleviate hepatic fibrosis by decreasing the secretion of TNF-α and IL-13 by the downregulation of p38 and ERK phosphorylation.7 Moreover, DHZCP significantly declined the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid, laminin, type IV collagen and procollagen III, and reversed hepatic fibrosis in a rat model.73 Another study suggested that DHZCP conferred protection against alcoholic liver fibrosis (ALF) injury in mice by suppressing the generation of collagen 1 (COL-1) and down-regulating apoptosis of liver cells as a result of adjusting the levels of inflammatory factors.74,75 These results indicated that Tubiechong was used for the treatment of liver diseases in ancient times. In addition, some researchers have investigated the antioxidative protective effects of ESW polypeptides on CCl4-induced chronic liver injury in mice.63 No microscopic abnormalities were found in the liver cells of the normal group. In the model group, the hepatocytes showed hyperemia, necrosis and fibrosis, and the hepatic lobules had disappeared. The morphological structures of the hepatocytes in the ESW peptide group were damaged, but increasing the ESW peptide dose significantly lessened the degree of the injury, and the liver and spleen indices of these mice were significantly lower than those of the model group. Compared with the model group, the serum AST, ALT activities and the malondialdehyde (MDA) content in the livers of the mice in the ESW peptide group decreased significantly. Additionally, the antioxidant enzyme (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px)) activities were remarkably improved, the inflammatory factor (IL-6, TNF-a, iNOS) protein and gene expression levels were significantly decreased, and the proapoptotic factor (Bax, Bcl-2, caspase-3) and fibrosis factor (alpha-SMA, TGF-beta) gene levels were significantly decreased and positively correlated with the ESW polypeptide dose. It suggested that ESW peptides could attenuate CCl4-induced chronic liver injury in mice, and these protective effects might be related to the antioxidant activities of the peptide products.
4.5 Anti-oxidative and anti-aging activities
Tubiechong and its extracts have antioxidant activity.76–78 Some scholars have disclosed that arazyme enzymatic hydrolysis peptides of ESW could scavenge hydroxyl, superoxide anion and DPPH free radicals with IC50 of 0.40, 8.73 mg mL−1 and 1.32 mg mL−1, respectively. Compared to the aging model group, the peptides significantly reduced the content of MDA, increased the activities of CAT and GSH-Px and hydroxyl radical scavenging rate in both plasma and liver, and also enhanced the activity of T-SOD in liver.64 The results of another study revealed that polypeptide extracts of ESW enhanced the anti-stress and antioxidative capacities in D-galactose-induced mouse models of oxidative aging by initiating the Nrf2-ARE antioxidant signaling pathway, therefore, delayed oxidative aging in mice.65 Other scholars investigated the separation of antioxidant peptides from the enzymatic hydrolysate of ESW and the antioxidant activities of peptides. Three fractions, including F-I, F-II, and F-III, were obtained using DEAE-Sephadex A50 chromatography, of which, the fraction F-II exhibited the highest O2-˙, OH˙, and DPPH˙ scavenging capabilities, reaching 86.83% (10 mg mL−1), 92.28% (0.7 mg mL−1), and 68.06% (1.8 mg mL−1), respectively.234.6 Other activities
Tubiechong also has analgesic activity and can be a treatment for arthralgia79 and avascular necrosis of femoral head.80 Besides, ESW could regulate blood lipids. ESW freeze-dried powder could inhibit the rise of the blood lipid of rabbits that were fed with a high-fat diet.81 Other scholars found that ESW could also reduce the cholesterol levels of hypercholesterolaemia rabbits.82 Another active peptide of ESW (APE) could significantly reduce the serum lipid index in model rats and improve the degree of liver pathological changes.27 Another study demonstrated that ESW could raise plasma HDL-C/TC ratio and increase lecithin-cholesterol acyltransferase activity, decrease plasma HDL3-C level and decelerate the progress of atherosclerosis to a certain degree.83 In addition, ESW could also reduce the levels of blood sugar in type 2 diabetes rat models.84 Apart from this, the substances in ESW have antibacterial activity.85 Also, other extracts from ESW could enhance the production of polysaccharides,86 ganoderic acid87 and triterpenoids88 in Ganoderma lucidum.5 Summary and outlook
Tubiechong is an important insect medicine in TCM and has been extensively used to treat several illnesses. It has been used to treat bruises, fractures, amenorrhea, postpartum blood stasis, lumps and relieving pain. In this review, we summarize the knowledge on traditional uses, chemical composition and pharmacological activities of such medicine. Our review has confirmed the diversity of its chemical composition and verified that the insects contained many active small molecular substances and macromolecular ingredients such as proteins, peptides and polysaccharides. However, gaps exist between the applications and scientific studies on Tubiechong. There is basically a lack of direct biological activity assays performed on single compounds isolated from Tubiechong. Most pharmacological studies employed crude extracts or isolated products of Tubiechong as testing samples. In a coincidence, chemical information and structural characterization of products of Tubiechong are relatively very limited as well. A lack of chemical analysis of the assessed extract is a relative insufficiency in current studies, which makes the main active ingredients to be unknown. The functional material basis is still unclear and on the consideration of its safety and effectiveness in clinical application and industrial developments. It is urgent to conduct deeper chemical-combined biological studies on the two insect medicines than before.On the other hand, for traditional medicines, we need to give more attention to its traditional usage, including traditional preparation methods and medical applications. Current investigations on Tubiechong were mostly focused on 70% or 95% alcohol extracts of the insect with little attention to aqueous extracts and low-concentration ethanol extracts, which were its main traditional preparation methods. Therefore, in order to ensure its efficacy, we are supposed to carry out research on Tubiechong based on following the traditional preparation methods. Understanding traditional medical applications of Tubiechong is also important for new drug discovery and development because it can make us easy to know the aim and therefore perform the pre-clinical and clinical investigations.
Tubiechong has multiple pharmacological actions and therapeutic behavior based on both traditional medicine and scientific practices, such as thrombolysis, anticoagulant, anti-tumor, immunomodulation, damage-repairing and hepatoprotection. However, pharmacological studies are performed only in animals or cell models. There is currently some suspicion regarding the data to support the therapeutic roles of Tubiechong in the clinic, and thus well-designed experiments and clinical trials are critical to confirm the safety and efficacy of the insect for human beings. Future integrated and profound studies on the chemical composition, pharmacological, and toxicological aspects of Tubiechong are essential to understand the therapeutic roles of such insect-based Chinese medicine.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was mainly supported by The National Natural Science Foundation of China (no. 81673788, 81873136 (to C. L).References
- G.-F. Ge, C.-H. Yu, B. Yu, Z.-H. Shen, D.-L. Zhang and Q.-F. Wu, J. Ethnopharmacol., 2012, 141, 178–182 CrossRef CAS PubMed
.
- H. Liu, Y. Yan, F. Zhang and Q. Wu, Immunol. Invest., 2019, 48, 844–859 CrossRef CAS PubMed
.
- F.-X. Wang, N. Wu, J.-T. Wei, J. Liu, J. Zhao, A.-g. Ji and X.-K. Lin, Biochem. Cell Biol., 2013, 91, 244–251 CrossRef CAS PubMed
.
- B. Dai, Y. Zhan, J. Qi and Y. Zhang, Environ. Toxicol. Pharmacol., 2014, 37, 1177–1185 CrossRef CAS PubMed
.
- B. Dai, J. Qi, R. Liu and Y. Zhang, Mol. Med. Rep., 2014, 10, 1590–1596 CrossRef CAS PubMed
.
- X. Xie, W. Shen, Y. Zhou, L. Ma, D. Xu, J. Ding, L. He, B. Shen and C. Zhou, Int. J. Biol. Macromol., 2020, 162, 31–42 CrossRef CAS PubMed
.
- Y. Zhan, H. Zhang, R. Liu, W. Wang, J. Qi and Y. Zhang, Integr. Cancer Ther., 2016, 15, 102–112 CrossRef CAS PubMed
.
- L. Sun, Z. He, M. Zhao, R. He and Y. Feng, Environ. Entomol., 2018, 40, 23–29 Search PubMed
.
- D.-Q. Zhang, Y. Xu, Y.-P. Mu, W. Liu and P. Liu, Chin. J. Exp. Tradit. Med. Formulae, 2018, 24, 219–224 Search PubMed
.
- Y.-H. Zhang, J.-T. Liu, B.-Y. Wen and N. Liu, J. Ethnopharmacol., 2009, 124, 125–129 CrossRef PubMed
.
- L.-J. Ju, P.-P. Hu, P. Chen, X. Xue, Z.-Q. Li, F.-Y. He, Z.-X. Qiu, J. Cheng and F. Huang, Biomed. Pharmacother., 2020, 129, 110471 CrossRef CAS PubMed
.
- X.-F. Wang, Chin. Herb. Med., 2019, 50, 2224–2228 Search PubMed
.
- L. J. Ni, N. N. Wang, L. G. Zhang, Y. Z. Guo and W. Z. Shi, J. Ethnopharmacol., 2016, 179, 420–431 CrossRef CAS PubMed
.
- B. Xu, G. H. Zhu, J. Xia and S. H. Li, J. Clin. Rehabil. Tissue Eng. Res., 2007, 11, 2253–2256 Search PubMed
.
- Y. Lu and P. Jiang, Zhongguo Zhongyao Zazhi, 1992, 17, 487–489 CAS
.
- N. Zhang, Y. Zhao, Y. Shi, R. Chen, X. Fu and Y. Zhao, Biomed. Pharmacother., 2019, 112, 108636 CrossRef CAS PubMed
.
- P. Cai, D. Wan, J. Xiao, S.-H. Zhang and G.-X. Cai, Chin. Tradit. Pat. Med., 2011, 33, 1645–1648 CAS
.
- Z. Dong-mei, L. I. Sui-jing and H. A. N. Yali, Lishizhen Med. Mater. Med. Res., 2009, 20, 778–779 Search PubMed
.
- L. I. XingNuan and H. A. N. YaLi, Acta Zool. Sin., 2007, 53, 135–142 Search PubMed
.
- H. Liu, Y.-L. Han, H. Ding and Z.-W. Li, Lishizhen Med. Mater. Med. Res., 2010, 21, 2140–2142 CAS
.
- H. Yang, Y. Wang, Y. Xiao, Y. Wang, J. Wu, C. Liu, H. Ye, F. Li, H. Yu and R. Lai, PLoS One, 2011, 6, e17519 CrossRef CAS PubMed
.
- S. Jiang, P.-P. Dong, H.-R. Li, J. Xu, H.-J. Li, Y.-Y. Yu, L. Dai, P. Gao, S.-P. Wang and J.-Y. Zhang, China J. Chin. Mater. Med., 2020, 45, 5265–5272 Search PubMed
.
- M.-C. Wang, Y.-L. Jin and M.-Z. Piao, J. Chin. Inst. Food Sci. Technol., 2012, 12, 34–38 CAS
.
- Y.-L. Han and Z.-W. Li, Chin. Med. Mater., 2006, 29, 765–767 CAS
.
- Y.-L. Han and Z.-W. Li, Chin. J. Biotechnol., 2006, 22, 639–643 CAS
.
- S. M. Wang, X. L. Zhao, B. X. Wang, Q. L. Zhou, Z. Y. Liu, R. G. An, Z. Q. Liu and S. Y. Liu, Chin. J. Anal. Chem., 2005, 33, 1385–1388 CAS
.
- S.-P. Wang, S. Jiang, Y.-M. Zhao, Y. Lin, J.-Y. Zhang and L. Dai, Chin. Pharmacol. Bull., 2020, 36, 621–626 Search PubMed
.
- Z.-C. Liu, K.-H. Yuan, R.-P. Zhang, X.-C. Ren, X.-L. Liu, S.-H. Zhao and D.-K. Wang, J. Venomous Anim. Toxins, 2016, 22 DOI:10.1186/s40409-016-0058-7
.
- Y. Lu and P. Jiang, China J. Chin. Mater. Med., 1992, 17, 487–512 CAS
.
- L. Liu, R. Jin and G. Xu, China J. Chin. Mater. Med., 1999, 24, 73–124 CAS
.
- Z. Chen, W.-T. Chen, W.-H. Luo and X.-L. Bi, Chin. Tradit. Pat. Med., 2016, 38, 1074–1077 Search PubMed
.
- X.-L. Wang, Q. Li, B.-H. Li, Y. Hui, Y.-Y. Chen and K.-S. Bi, Chin. Herb. Med., 2016, 47, 1780–1784 CAS
.
- J.-S. Wang, Y. Nian, F.-L. Zhang and H. Li, J. Beijing Univ. Tradit. Chin. Med., 2016, 39, 850–854 Search PubMed
.
- H. J. Zhu, T. Xu, Y. M. Yan, Z. C. Tu and Y. X. Cheng, Nat. Prod. Res. Dev., 2020, 11, 1–12 Search PubMed
.
- H. L. Jiang, X. H. Luo, X. Z. Wang, J. L. Yang, X. J. Yao, P. Crews, F. A. Valeriote and Q. X. Wu, Fitoterapia, 2012, 83, 1275–1280 CrossRef CAS PubMed
.
- X. Q. Jin, M. M. Yan, E. X. Huang, Y. J. Xu, Y. J. Gu, D. B. Cui, S. Y. Lin and D. M. Xu, China J. Chin. Mater. Med., 1993, 18, 355–356 CAS
.
- H.-L. Jiang, X.-H. Luo, X.-Z. Wang, J.-L. Yang, X.-J. Yao, P. Crews, F. A. Valeriote and Q.-X. Wu, Fitoterapia, 2012, 83, 1275–1280 CrossRef CAS PubMed
.
- Y. Li, J. Hong, H. Li, X. Qi, Y. Guo, M. Han and X. Wang, Drug Delivery, 2017, 24, 1491–1500 CrossRef CAS PubMed
.
- Y. Bao, Y. W. Sun, J. Ji, L. Gan, C. F. Zhang, C. Z. Wang and C. S. Yuan, Phytomedicine, 2019, 63, 153036 CrossRef CAS PubMed
.
- Y. Gao, F. Liu, L. Fang, R. Cai, C. Zong and Y. Qi, PLoS One, 2014, 9, e96741 CrossRef PubMed
.
- X. Wang, Z. J. Song, X. He, R. Q. Zhang, C. F. Zhang, F. Li, C. Z. Wang and C. S. Yuan, Int. Immunopharmacol., 2015, 29, 701–707 CrossRef CAS PubMed
.
- A. Hematpoor, M. Paydar, S. Y. Liew, Y. Sivasothy, N. Mohebali, C. Y. Looi, W. F. Wong, M. S. Azirun and K. Awang, Chem.-Biol. Interact., 2018, 279, 210–218 CrossRef CAS PubMed
.
- Z. Y. Cheng, G. D. Yao, R. Guo, X. X. Huang and S. J. Song, Bioorg. Med. Chem. Lett., 2017, 27, 597–601 CrossRef CAS PubMed
.
- H. J. Zhu, T. Xu, Y. M. Yan and Y. X. Cheng, Bioorg. Chem., 2020, 104, 104258 CrossRef CAS PubMed
.
- H. J. Zhu, Y. M. Yan, Z. C. Tu, J. F. Luo, R. Liang, T. H. Yang, Y. X. Cheng and S. M. Wang, Fitoterapia, 2016, 114, 163–167 CrossRef CAS PubMed
.
- H. E. Colley, M. Muthana, S. J. Danson, L. V. Jackson, M. L. Brett, J. Harrison, S. F. Coole, D. P. Mason, L. R. Jennings, M. Wong, V. Tulasi, D. Norman, P. M. Lockey, L. Williams, A. G. Dossetter, E. J. Griffen and M. J. Thompson, J. Med. Chem., 2015, 58, 9309–9333 CrossRef CAS PubMed
.
- M. J. Thompson, J. C. Louth, S. Ferrara, F. J. Sorrell, B. J. Irving, E. J. Cochrane, A. J. Meijer and B. Chen, ChemMedChem, 2011, 6, 115–130 CrossRef CAS PubMed
.
- D. A. Negatu, M. Gengenbacher, V. Dartois and T. Dick, Front. Microbiol., 2020, 11, 575586 CrossRef PubMed
.
- E. E. Alexeev, J. M. Lanis, D. J. Kao, E. L. Campbell, C. J. Kelly, K. D. Battista, M. E. Gerich, B. R. Jenkins, S. T. Walk, D. J. Kominsky and S. P. Colgan, Am. J. Pathol., 2018, 188, 1183–1194 CrossRef CAS PubMed
.
- Z. H. Zhao, F. Z. Xin, Y. Xue, Z. Hu, Y. Han, F. Ma, D. Zhou, X. L. Liu, A. Cui, Z. Liu, Y. Liu, J. Gao, Q. Pan, Y. Li and J. G. Fan, Exp. Mol. Med., 2019, 51, 1–14 CAS
.
- J.-F. Chen, C. Yang and Y. Zhang, Chin. J. Spectrosc. Lab., 2010, 27, 2009–2011 CAS
.
- Y.-F. Yang, Chin. Med. Mater., 2002, 25, 150–152 Search PubMed
.
- B. Dai, Y. Zhan, J. Qi and Y. Zhang, Environ. Toxicol. Pharmacol., 2014, 37, 1177–1185 CrossRef CAS PubMed
.
- Y. Zhang, Y. Zhan, D. Zhang, B. Dai, W. Ma, J. Qi, R. Liu and L. He, Sci. Rep., 2014, 4, 5518 CrossRef CAS PubMed
.
- Y. Zhang, J. Yu, H. Luo and R.-H. Dai, Nat. Prod. Res. Dev., 2020, 32, 1051–1056 Search PubMed
.
- G. Ge, C. Yu and Q. Wu, China J. Tradit. Chin. Med. Pharm., 2013, 28, 826–828 CAS
.
- Z. Lin, Y. Han and B. Chen, Shizhen Guoyi Guoyao, 2013, 24, 807–810 CAS
.
- F.-C. Cao, Y.-l. Han, B. Chen, M.-X. Huang, H. Ding, L. Yu and Y. Ye, Lishizhen Med. Mater. Med. Res., 2011, 22, 1827–1829 CAS
.
- W. Zhang, X. Zou, X.-P. Qian, L.-X. Yu and B.-R. Liu, Tradit. Chin. New Drug Res. Clin. Pharmacol., 2007, 18, 257–259 Search PubMed
.
- X.-N. Li and Y. Han, Pharmacol. Clin. Chin. Mater. Med., 2008, 24, 44–46 Search PubMed
.
- Q.-F. Tang, Y. Dai and X.-L. Liu, Chin. Bull. Entomol., 2011, 48, 156–159 CAS
.
- D. Liu, X.-N. Li, Z.-J. Qin, W. He and Y. Zhao, Chin. Med. Mater., 2012, 35, 1382–1385 CAS
.
- D. Liu, S. Cao, Y. Liu, J. Liu, Z. Wang, Y.-H. Zhang and H. Shen, Acta Lab. Anim. Sci. Sin., 2020, 28, 73–80 Search PubMed
.
- M.-Z. Piao, M.-C. Wang and X.-D. Wang, Food Sci., 2013, 34, 242–245 CAS
.
- C.-G. Gu, Y.-H. Zhang, R.-R. Bai, M.-J. Tian and H. Shen, Acta Lab. Anim. Sci. Sin., 2014, 22, 66–74 CAS
.
- F.-C. Cao, Y.-L. Han, B. Chen, H. Liu, H. Ding, M.-X. Huang and Y. Ye, Chin. Med. Mater., 2011, 34, 676–679 CAS
.
- Q.-F. Tang, Y. Dai and X.-L. Liu, Afr. J. Biotechnol., 2010, 9, 8682–8686 Search PubMed
.
- Q. Tang, Y. Dai and X. Liu, Chin. J. Appl. Entomol., 2011, 48, 156–159 CAS
.
- D. Liu, X. Li, Z. Qin, W. He and Y. Zhao, J. Chin. Med. Mater., 2012, 35, 1382–1385 CAS
.
- Y.-F. Yang, S.-Q. Wang, M.-J. Feng, A.-H. Ning and C.-H. Huang, Chin. J. Cell. Mol. Immunol., 2005, 21, 53–56 Search PubMed
.
- Y.-F. Yang, M.-S. Peng and Y.-W. Yang, Chin. J. Immunol., 2003, 19, 686–689 Search PubMed
.
- F. Xie, X. Li, K. Sun, Y. Chu, H. Cao, N. Chen, W. Wang, M. Liu, W. Liu and D. Mao, J. Tradit. Chin. Med., 2001, 21, 225–231 CAS
.
- H. B. Cai, X. G. Sun, Z. F. Liu, Y. W. Liu, J. Tang, Q. Liu, B. M. Ji, Y. H. Song, Y. C. Zhou, M. H. Yang and Z. P. Lv, J. Ethnopharmacol., 2010, 132, 157–164 CrossRef PubMed
.
- Y.-F. Deng, L. Qu, Y. Song and Y. Jiang, J. Beijing Univ. Tradit. Chin. Med., 2015, 38, 420–425 Search PubMed
.
- W.-C. Zhong, C.-Y. Zhou, L. Gao, Z.-P. Lu and S.-H. Huang, Chin. Tradit. Pat. Med., 2017, 39, 2475–2480 Search PubMed
.
- M.-R. Xie, Q.-M. Li, S.-Q. Qiu, X.-l. Qi and H. Shen, Acta Zoonutr. Sin., 2018, 30, 1726–1735 Search PubMed
.
- M.-R. Xie, S.-Q. Qiu and H. Shen, Acta Lab. Anim. Sci. Sin., 2016, 24, 427–431 CAS
.
- M.-Z. Piao, J. Ning and F.-W. Wang, Sci. Technol. Food Ind., 2010, 31, 205–208 Search PubMed
.
- G.-L. Wu, T.-Y. Li and Y.-S. Fan, China J. Chin. Mater. Med., 2019, 44, 845–848 Search PubMed
.
- Z.-X. Qi, S.-Q. Li and T. Yu, J. Clin. Rehabil. Tissue Eng. Res., 2013, 17, 2597–2602 Search PubMed
.
- X.-J. Bai, H.-J. Ren, Z.-R. Luo, H. Wang, Z.-Z. Zhao and X.-B. Leng, J. Northeast Agric. Univ., 2014, 45, 71–75 CAS
.
- Z. Zhang, E.-Z. Jiang, F.-Y. Ning, Z.-H. Du and X.-J. Bai, J. Northeast Agric. Univ., 2018, 49, 52–58 Search PubMed
.
- W. Wang, J. Wang, D. Zhao, H. Liu, W. Zhou and K. Chen, China J. Chin. Mater. Med., 1991, 16, 299–320 CAS
.
- Z. Jing and G.-X. Xu, J. Fourth Mil. Med. Univ., 2009, 30, 465–467 Search PubMed
.
- X.-M. Suo, X.-J. Lu, J.-Z. Dong, J. Li, P. Song, R. Zhao and L.-Y. Fu, Chin. J. Biol. Control, 2007, 23, 64–67 Search PubMed
.
- G.-Q. Liu and K.-C. Zhang, Appl. Biochem. Biotechnol., 2007, 74, 572–577 CAS
.
- G.-Q. Liu, X.-L. Wang, Y.-G. Zhang, Y.-H. Wu, W.-J. Han and H.-Y. Zhang, Afr. J. Biotechnol., 2010, 9, 6129–6134 CAS
.
- G.-Q. Liu, H.-X. Xiao, X.-L. Wang, Y. Zhao, Y.-G. Zhang and G.-P. Ren, Appl. Biochem. Biotechnol., 2011, 165, 87–97 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |