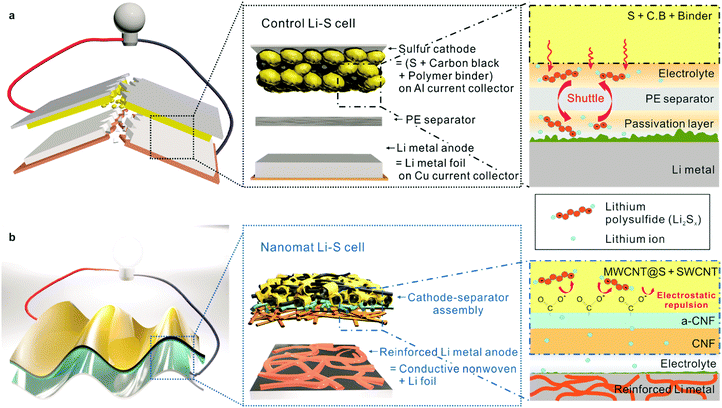
Nanomat Li–S batteries based on all-fibrous cathode/separator assemblies and reinforced Li metal anodes: towards ultrahigh energy density and flexibility†
Jung-Hwan
Kim
a,
Yong-Hyeok
Lee
a,
Sung-Ju
Cho
a,
Jae-Gyoung
Gwon
b,
Hye-Jung
Cho
b,
Minchul
Jang
c,
Sun-Young
Lee
*b and
Sang-Young
Lee
 *a
*a
aDepartment of Energy Engineering, School of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919, Republic of Korea. E-mail: syleek@unist.ac.kr
bDepartment of Forest Products, National Institute of Forest Science, Seoul, 02455, Republic of Korea. E-mail: nararawood@korea.kr
cFuture Technology Research Center, LG Chem., Seoul, 07796, Republic of Korea
First published on 14th August 2018
Abstract
Lithium–sulfur (Li–S) batteries have attracted considerable attention as a promising alternative to current state-of-the-art lithium-ion batteries (LIBs), however, their practical use remains elusive, which becomes more serious upon application to flexible/wearable electronics. Here, we demonstrate a new class of nanomat Li–S batteries based on all-fibrous cathode–separator assemblies and conductive nonwoven-reinforced Li metal anodes as an unprecedented strategy toward ultrahigh energy density and mechanical flexibility. Sulfur cathodes, which are fibrous mixtures of sulfur-deposited multi-walled carbon nanotubes and single-walled carbon nanotubes, are monolithically integrated with bi-layered (pristine cellulose nanofiber (CNF)–anionic CNF) paper separators, resulting in metallic foil current collector-free, all-fibrous cathode–separator assemblies. The cathode–separator assemblies, driven by their all-fibrous structure (contributing to three-dimensional bi-continuous electron/ion conduction pathways) and anionic CNFs (suppressing the shuttle effect via electrostatic repulsion), improve redox kinetics, cyclability and flexibility. Nickel-/copper-plated conductive poly(ethylene terephthalate) nonwovens are physically embedded into Li foils to fabricate reinforced Li metal anodes with dimensional/electrochemical superiority. Driven by the structural uniqueness and chemical functionalities, the nanomat Li–S cells provide exceptional improvements in electrochemical performance (the (cell-based) gravimetric/volumetric energy density = 457 W h kgcell−1/565 W h Lcell−1 and the cycling performance (over 500 cycles) under 110% capacity excess of the Li metal anode) and mechanical deformability (they even can be crumpled).
Broader contextThe forthcoming smart/ubiquitous energy era, which will find widespread use of the Internet of Things (IoTs), electric vehicles and flexible/wearable electronics, inspires the relentless pursuit of advanced power sources with high energy density, electrochemical sustainability and mechanical flexibility. Among the numerous energy storage systems reported to date, lithium–sulfur (Li–S) batteries have attracted considerable attention as a promising alternative that can outperform the current state-of-the-art lithium-ion batteries (LIBs) due to their high theoretical capacity and low cost and the natural abundance of environmentally benign sulfur active materials. Stimulated by these advantageous characteristics, Li–S batteries have been extensively investigated for various applications. Despite the enormous research efforts, however, the simultaneous fulfilment of high energy density, fast redox kinetics and long-term cycling performance, along with mechanical flexibility, still remains a formidable challenge in Li–S batteries. Here, we demonstrate a new class of nanomat Li–S batteries based on all-fibrous cathode–separator assemblies and conductive nonwoven-reinforced Li metal anodes as an unprecedented strategy toward ultrahigh energy density and mechanical flexibility. Benefiting from the structural uniqueness and chemical functionalities, the nanomat Li–S batteries provide exceptional improvements in electrochemical performance and mechanical flexibility, which have never been simultaneously reached with conventional Li–S battery technologies, to the best of our knowledge. We envision that the nanomat Li–S battery strategy presented herein holds promise as a versatile and scalable platform for development of high-performance flexible batteries. |
Introduction
The forthcoming ubiquitous energy era, which will find widespread use of the Internet of Things (IoTs), electric vehicles and flexible/wearable electronics, inspires the relentless pursuit of advanced power sources with high energy density, electrochemical sustainability and mechanical flexibility.1–3 Among various battery systems explored to reach this goal, lithium–sulfur (Li–S) batteries have received considerable attention as a promising alternative that can outperform the current state-of-the-art lithium-ion batteries (LIBs) due to their high theoretical energy density and low cost and the natural abundance of environmentally benign sulfur active materials.4–8Stimulated by these advantageous characteristics, Li–S batteries have been extensively investigated for applications in various fields, with a particular focus on sulfur cathodes. Many of the previous approaches9–11 were based on a combination of sulfur with carbonaceous conductive substances. However, the low electronic conductivity of sulfur, structural instability of sulfur cathodes and shuttle effect have not been yet fully resolved. Moreover, upon exposure to mechanical deformation, major components of sulfur cathodes tend to easily detach from metallic foil current collectors, resulting in rapid capacity loss and safety failure.
In addition to the sulfur cathodes, significant attention should also be paid to Li metal anodes. Unfortunately, Li metal anodes suffer from severe problems12–17 such as uncontrolled dendrite growth, poor Coulombic efficiency, large volume change and unstable interfaces with electrolytes. Note that these challenges become more serious as the Li source is limited (i.e., thin Li metal). In most Li–S batteries reported to date, thick Li metal foils5,6,18 are used as anodes to ensure electrochemical reliability of Li–S batteries. These thick Li metal anodes, however, have limitations in mechanical flexibility (due to stiffness and low fatigue resistance) and negatively affect the volumetric/gravimetric energy density of the resultant Li–S cells.
Despite the enormous research efforts, the simultaneous fulfilment of high energy density (notably, cell-based values are highly significant for commercial applications), fast redox kinetics and long-term cycling performance, along with mechanical flexibility, still remains a formidable challenge in Li–S batteries. Here, we demonstrate a new class of nanomat Li–S batteries based on all-fibrous cathode–separator assemblies and conductive nonwoven-reinforced Li metal anodes as an effective and scalable strategy to enable exceptionally higher energy density and mechanical flexibility. Sulfur cathodes, in which sulfur-deposited multi-walled carbon nanotubes (MWCNTs) (MWCNT@S) are intermingled with single-walled carbon nanotubes (SWCNTs), are monolithically integrated with bi-layered (a cellulose nanofiber (CNF) support layer and a negatively charged CNF (a-CNF) active layer) paper separators, leading to metallic foil current collector-free, all-fibrous cathode–separator assemblies. The all-fibrous structure of the cathode–separator assemblies, in combination with the a-CNFs bearing carboxylate groups, enables substantial improvement in redox kinetics and cyclability. Nickel (Ni)-/copper (Cu)-plated, conductive poly(ethylene terephthalate) (PET) nonwovens are physically embedded into Li metal foils as both a mechanical framework and porous current collector, resulting in reinforced Li metal anodes with dimensional/electrochemical superiority.
Benefiting from the structural uniqueness and chemical functionalities described above, the nanomat Li–S batteries achieve exceptional improvements in the (cell-based) energy density, cycling performance and mechanical deformability, which have never been simultaneously reached with conventional Li–S battery technologies, to the best of our knowledge.
Experimental
Preparation of MWCNT@S
Sulfur powder (Sigma-Aldrich) was mixed with MWCNTs (diameter = 9.5 nm, Nanocyl (Belgium)) in ethanol through ball milling for 2 h. The as-obtained mixture was heated in a Teflon-sealed flask at 155 °C for 2 h and then stored under vacuum for 0.5 h,9,10 yielding MWCNT@S. The sulfur content in MWCNT@S was estimated to be 63.5 wt% from the thermogravimetric analysis (TGA).Synthesis of a-CNFs
A pristine CNF suspension was obtained by repeated high-pressure homogenization of pretreated wood cellulose powder (average size ∼45 μm, KC Flock, Nippon Paper Chemicals) in water. Details of the preparation procedure of the CNF suspension were described in previous publications.19,20 2,2,6,6-Tetramethylpiperidin-1-oxyl radical (TEMPO)-mediated oxidation of the CNFs20–22 was conducted to generate negatively charged carboxylate groups.Fabrication of cathode–separator assemblies and reinforced Li metal anodes
Unitized cathode–separator assemblies were fabricated via a simple vacuum-assisted infiltration process.19,23 First, CNF and a-CNF suspensions (in IPA/water = 95/5 (v/v)) were sequentially poured onto filter paper positioned inside a Porcelain Buchner funnel and then subjected to vacuum infiltration, resulting in the formation of bi-layered CNF/a-CNF paper. Meanwhile, SWCNTs (diameter = 1.9 nm, MEIJO Nano Carbon Co. (Japan)) were mixed with the above-prepared MWCNT@S in ethanol through ultra-sonication for 1 h. The as-prepared sulfur cathode suspension was poured onto the a-CNF layer of the bi-layered paper using the same infiltration method, yielding a wet-state cathode–separator assembly. To promote the formation of a porous structure, the wet-state cathode–separator assembly was subjected to freeze drying, eventually producing a self-standing cathode–separator assembly with well-developed pore structure. The obtained cathode–separator assembly consisted of the MWCNT@S/SWCNT cathode and the bi-layered paper separator. The thickness of each layer in the cathode–separator assembly was controlled by varying the amount of each suspension. Assemblies with higher sulfur mass-loadings were fabricated by increasing the thickness of the cathode layer while holding the thickness of the paper separator constant. The Ni/Cu-plated conductive PET nonwoven (porosity ∼ 75% and median pore diameter ∼ 11 μm), the details of which are proprietary information, was obtained from LG Chem (Korea). The conductive PET nonwoven and the pristine Li metal foil were roll-pressed at room temperature, thus producing the reinforced Li metal anode (thickness = 55 μm).Structural/physicochemical characterization
The structure and physicochemical characteristics of the MWCNT@S were examined with field emission transmission electron microscopy (FE-TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) (JEM-2100, JEOL), X-ray diffraction (D/MAZX 2500V/PC, Rigaku) and TGA (SDT Q600, TA Instruments). The surface and cross-sectional morphologies of the electrodes were characterized by FE-SEM (S-4800, Hitachi) in conjunction with energy-dispersive X-ray spectroscopy (EDS). The zeta potentials of the pristine CNFs and a-CNFs were analysed with a Zetasizer Nano ZS (Malvern Instruments Inc.). The electronic conductivities of the electrodes were measured using the four-point probe technique (CMT-SR1000N, Advanced Instrument Tech). The mechanical flexibility tests were conducted using a universal testing machine (DA-01, Petrol LAB). The electrolyte wettability of the electrodes was estimated by measuring the electrolyte immersion-height. The effect of the separator on polysulfide migration was analysed by conducting the model experiment described below. A sample tube containing a solution of 0.1 M Li2S8 in 1,3-dioxolane (DOL)/1,2-dimethyoxyethane (DME) = 1/1 (v/v) (upper side) fitted with a hollow lid sealed with the separator was inserted into a centrifuge tube having a solvent mixture of DOL/DME = 1/1 (v/v) (bottom side). Diffusion of the polysulfides through the separators into the blank solvent was monitored as a function of time. Post-mortem analysis of the Li–S cells after the charge/discharge cycling tests was performed using inductively coupled plasma mass spectrometry (ICP-MS) (ELAN DRC-II, Perkin Elmer), and X-ray photoelectron spectroscopy (XPS) (ThermoFisher) with focused monochromatized Al Kα radiation (Alpha300s, Witec) with a 785 nm single-mode diode laser (Xtra, Toptica).Electrochemical characterization
The AC impedance of symmetric Li/Li cells (frequency range = 10−2 to 106 Hz at an amplitude of 10 mV) was examined using a potentiostat (VSP classic, Bio-Logic). The electrochemical performance of the Li–S cells was characterized using 2032-type coin and pouch-type cells. The control sulfur cathode (S/C composite (= 9/1 (w/w))/styrene butadiene rubber (SBR)–carboxymethyl cellulose (CMC) binder/Denka black carbon additive = 75/5/20 (w/w/w) on an Al foil current collector, areal sulfur loading = 3.0 mg cm−2) was prepared using a typical slurry casting method. A commercial polyethylene (PE) separator (thickness = 20 μm, F20BHE, Toray-Tonen) was used for the control Li–S cells. A liquid electrolyte of 1 M lithium bis (trifluoromethylsulfonyl) imide (LiTFSI) in DOL/DME = 1/1 (v/v) with 2 wt% LiNO3 additive was used. The electrolyte to sulfur (E/S) ratio for all cells examined herein was fixed at 15/1 [mL g−1]. The cell performance was investigated using a cycle tester (PNE Solution) at 25 °C under various charge/discharge conditions.Results and discussion
Structural features and multifunctionalities of nanomat Li–S batteries
Fig. 1 depicts structural features and the electrochemical/mechanical superiority of the nanomat Li–S battery over a conventional Li–S battery. The cathode–separator assemblies have the following advantages: (1) removal of heavy metallic foil current collectors in the sulfur cathodes leads to an increase in the volumetric/gravimetric energy density, (2) a-CNFs of the paper separator, which are prepared through 2,2,6,6-tetramethylpiperidin-1-oxyl radical (TEMPO)-mediated oxidation,21,24 mitigate the polysulfide migration-induced shuttle effect via electrostatic repulsion (i.e., the Donnan exclusion effect25–27), (3) the all-fibrous structure based on one-dimensional (1D) CNTs and CNFs creates three-dimensional (3D) bi-continuous electron/ion transport pathways, contributing to redox kinetics; and (4) the nanomat architecture, in combination with elimination of stiff metallic foil current collectors, provides mechanical compliance. The reinforced Li metal anodes have the following advantages: (1) a lower thickness compared to those (typically, thicker than 200 μm) of conventional Li metal foil anodes18,28 is beneficial for improving the gravimetric/volumetric energy density, (2) conductive PET nonwovens embedded into Li metal foils serve as both a “host/cage” for stable Li plating and a 3D porous current collector, thereby facilitating utilization of active Li and improving the Coulombic efficiency during charge/discharge cycling; and (3) the conductive PET nonwovens also act as a structural framework to ensure mechanical flexibility and dimensional stability of the reinforced Li metal anodes.Fabrication and characterization of the all-fibrous cathode–separator assembly
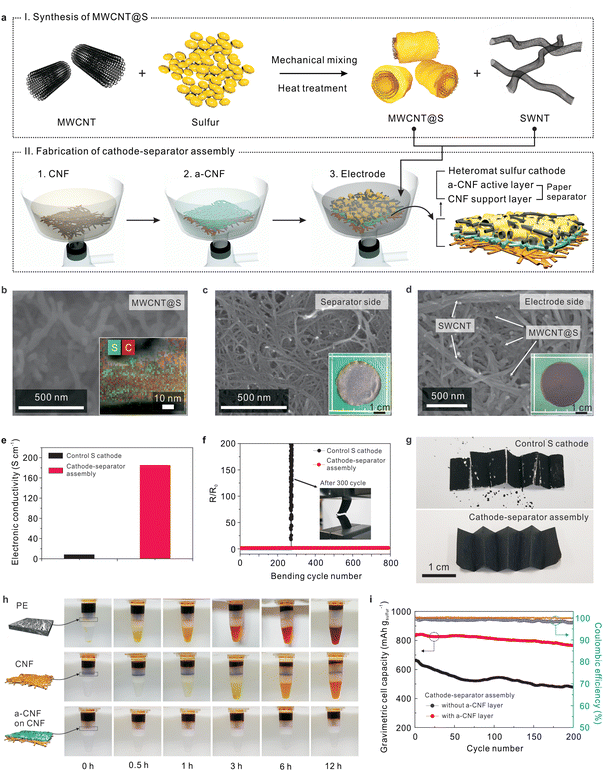
Stepwise fabrication of the unitized cathode–separator assemblies is described below. First, sulfur powder was mechanically mixed with MWCNTs and then subjected to melting-diffusion9,10 to synthesize MWCNT@S powder (Fig. 2a), in which the MWCNTs are conformally deposited with nanoscale-thick sulfur layers (Fig. 2b and Fig. S1a, ESI†). The combined diffraction peaks of MWCNTs and elemental sulfur verified the successful formation of MWCNT@S (Fig. S1b, ESI†). The sulfur content of MWCNT@S was estimated to be 63.5 wt% from the TGA measurement (Fig. S1c, ESI†).Using the as-synthesized MWCNT@S, the all-fibrous cathode–separator assemblies were fabricated through a simple vacuum-assisted infiltration process analogous to a traditional paper-making method.19,23 A CNF suspension (in a mixed solvent of isoprophylalcohol (IPA)/water (= 95/5 (v/v))) was prepared and then poured onto filter paper to fabricate a porous CNF layer. Subsequently, an a-CNF suspension (in a mixed solvent of IPA/water = 95/5 (v/v)) was poured onto the above-prepared CNF layer, resulting in a bi-layered (an a-CNF active layer on a CNF support layer) paper separator. An electrode suspension (MWCNT@S and SWCNTs in ethanol) was then introduced on top of the a-CNF active layer of the bi-layered paper separator using the same infiltration method. After undergoing freeze-drying, which is known to effectively prevent dense packing of CNFs,19 a self-standing cathode–separator assembly with well-developed pore structure was obtained. Cross-sectional scanning electron microscopy (SEM) images (Fig. S2, ESI†) showed that the heteromat sulfur cathode (∼30 μm) was seamlessly integrated with the paper separator (∼20 μm). From the TGA data (Fig. S3, ESI†) and information on initial composition ratios of mixtures, the weight-based composition ratio of the resultant cathode–separator assembly was estimated to be (sulfur/MWCNT/SWCNT)/(CNF/a-CNF) = (49.9/28.0/12.7)/(8.8/0.6).
Note that the cathode–separator assembly showed two different faces (black color for the sulfur cathode and white color for the paper separator) (insets of Fig. 2c and d). The surface morphology (Fig. 2c) of the paper separator (here, the CNF layer was examined) shows the formation of highly reticulated pore structure with submicron-sized pores (created between interconnected CNFs), which will act as ion-conducting channels after being filled with liquid electrolyte. Fig. 2d shows the morphology of the sulfur cathode in the cathode–separator assembly. The MWCNT@S powder was uniformly dispersed and spatially intermingled with well-interconnected SWCNT strands, yielding a heteromat-structured sulfur cathode. The MWCNTs (in the MWCNT@S) and SWCNTs are expected to act as short-range and long-range electronically conductive networks, respectively, and physical frameworks. In addition, interstitial voids formed between the 1D mixtures of MWCNT@S and SWCNTs were developed over a wide area of the sulfur cathode, thus enabling facile access of liquid electrolytes to the sulfur active materials.
A control sulfur cathode, the detailed information of which is described in the experimental section, was prepared using conventional components and the slurry casting method. Fig. 2e compares electronic conductivities of the heteromat sulfur cathode (in the cathode–separator assembly) and the control sulfur cathode. The heteromat sulfur cathode showed higher electronic conductivity than the control sulfur cathode, revealing the excellence of the MWCNT/SWCNT-mediated electronic networks. In addition, the cathode–separator assembly, driven by the well-developed pore structure and the polarity of the CNFs, facilitated capillary intrusion of the liquid electrolyte (Fig. S4, ESI†), leading to better electrolyte wettability of the heteromat sulfur cathode than the control sulfur cathode. The abovementioned results demonstrate the structural benefits of the cathode–separator assembly, i.e., the all-fibrous structure based on 1D CNTs/CNFs enables 3D bi-continuous electron/ion transport, which will contribute to enhanced redox kinetics of the sulfur active materials.
The mechanical flexibility of the cathode–separator assembly was examined by monitoring the change in electronic resistance during cycles of longitudinal compression (Fig. 2f). The electronic resistance of the cathode–separator assembly remained nearly constant, even after 800 bending cycles, whereas the control sulfur cathode showed mechanical breakdown after just 300 cycles. Moreover, the cathode–separator assembly was subjected to multiple folding cycles (Fig. 2g). Neither significant cracks nor defects were observed at the cathode–separator assembly, whereas the control sulfur cathode showed severe structural rupture and detachment of its components, particularly at the folded edges.
Fig. 2h shows the difference in the permeation behavior of polysulfides through various separators, in which a simple experimental set-up29 was exploited for visualization. The separators were positioned between a polysulfide solution (upper side, 0.1 M Li2S8 in DOL/DME = 1/1 (v/v)) and a solvent mixture of DOL/DME (bottom side). For the bi-layered paper separator, the bottom side of the permeation tube showed no appreciable color change after an elapsed time of 12 h, in contrast to the findings of the conventional PE and CNF separators, indicating the effective suppression of polysulfide crossover. This result was further confirmed by quantitatively analyzing the grey level25 of the solvent mixture in the bottom side (Fig. S5, ESI†). In comparison to the results of the PE and CNF separators, the grey level of the bi-layered paper separator remained nearly unchanged over 12 h, indicating the excellent capability of preventing polysulfide crossover.
This advantageous contribution of the bi-layered paper separator is mainly attributed to the anionic groups and unique morphology of the a-CNF layer. The presence of negatively charged carboxylate groups in the a-CNFs was verified by measuring their zeta potential (Fig. S6, ESI†). The a-CNFs show a more negative value (= −90.3 mV) than the pristine CNFs (= −25.4 mV), and are therefore expected to enable electrostatic repulsion of polysulfides possessing anionic characteristics. The a-CNF layer has a relatively dense morphology compared to the porous CNF layer (Fig. S7, ESI†), which may be beneficial for suppressing polysulfide migration and promoting intermolecular contact between polysulfides and a-CNFs. This morphological feature of the a-CNF layer is well consistent with those found in previously reported TEMPO-oxidized cellulose paper.21,22
Driven by the abovementioned structural/chemical uniqueness, the cathode–separator assembly containing the a-CNF layer showed better cycling performance (Fig. 2i and Fig. S8, ESI†) than a control sample (here, a cathode–separator assembly without the a-CNF layer). This result verifies that the a-CNF layer mitigates the crossover of polysulfides, eventually improving the capacity retention during cycling. Meanwhile, despite the advantageous contribution of the a-CNF layer, its relatively dense morphology may hamper the ionic flux through the layer, which in turn could negatively affect the electrochemical performance of the cells. To address this concern, various cathode–separator assemblies with a-CNF layers of different thicknesses were fabricated and their effect on the discharge rate capability of the Li–S cells was investigated (Fig. S9, ESI†). The optimum thickness of the a-CNF layer was found to be 1 μm based on the result of discharge rate capability.
Fabrication and characteristics of reinforced Li metal anodes
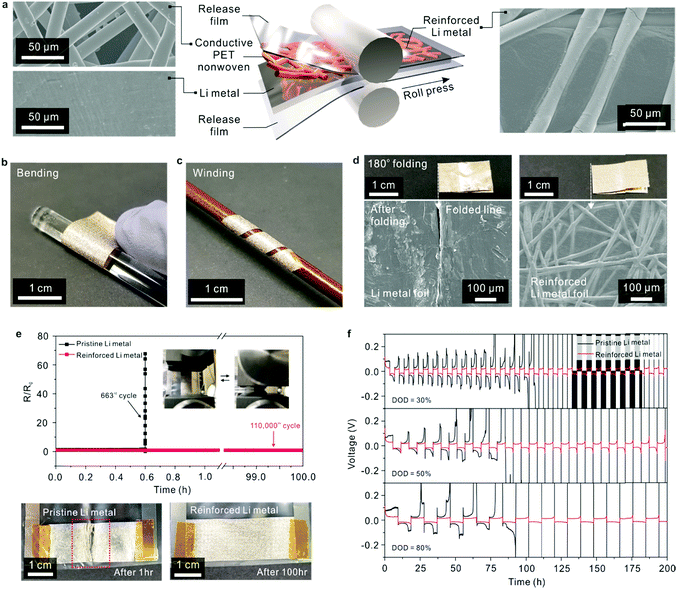
A reinforced Li metal electrode (thickness = 55 μm) was fabricated using a simple roll-pressing process at room temperature (Fig. 3a). Owing to the ductility of Li metal, the conductive PET nonwoven (Fig. S10, ESI†), details of which are provided in the experimental section, was successfully embedded into the Li metal foil. This result was further verified by examining the cross-sectional morphology (Fig. S11, ESI†) of the resultant reinforced Li metal. The reinforced Li metal was bendable and wound around a rod without any mechanical rupture (Fig. 3b and c). In addition, the reinforced Li metal was subjected to a repeated 180° folding–unfolding test (Fig. 3d). The reinforced Li metal maintained its structural integrity without any appreciable defects, in comparison to pristine Li metal with the same thickness (= 55 μm) which showed severe cracks along the folded lines.This mechanical flexibility of the reinforced Li metal was verified by measuring the variation in its electronic resistance during longitudinal compression cycles (Fig. 3e). The electronic resistance of the reinforced Li metal remained almost unchanged after 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles (over 100 h), in contrast to that of the pristine Li metal foil. The abovementioned results revealed that the conductive PET nonwoven played a decisive role in the exceptional mechanical deformability of the reinforced Li metal.
000 bending cycles (over 100 h), in contrast to that of the pristine Li metal foil. The abovementioned results revealed that the conductive PET nonwoven played a decisive role in the exceptional mechanical deformability of the reinforced Li metal.
The electrochemical sustainability of the reinforced Li metal was investigated using a symmetric Li/Li cell configuration (Fig. 3f), in which two identical reinforced Li metal electrodes were assembled. A control cell with two pristine Li metal electrodes was also fabricated for comparison. From the information on the theoretical capacity (3860 mA h g−1) of Li,12,15 the reinforced Li metal (areal mass of Li = 2.93 mg cm−2) is estimated to deliver an areal capacity of 11.30 mA h cm−2. Li plating/stripping of the Li metal electrodes was repeatedly conducted at various depth of discharge (DOD) values (= 30, 50 and 80%) and a fixed current density of 1.0 mA cm−2. The cell containing the pristine Li metal showed a large and irreversible increase in overpotential after a certain number of plating/stripping cycles, and its overpotential fluctuation became more extensive with increasing DOD value, similar to the results of previous studies.13,14,30 In comparison, the cells with the reinforced Li metal electrodes exhibited stable voltage profiles with negligible hysteresis and decent cyclability even at 80% DOD. This result indicates that the conductive PET nonwoven in the reinforced Li metal electrode could act as a Li host that accommodates the plating of Li, thus preventing random growth of Li dendrites and generation of isolated (so-called, “dead”14,30) Li. To better elucidate this advantageous effect of the reinforced Li metal electrodes, the cells (measured at 50% DOD) were disassembled after the plating/stripping test, and structural changes in their major components were examined (Fig. S12, ESI†). The reinforced Li metal electrode preserved its dimensional integrity, whereas the structure of the pristine Li metal electrode was heavily disrupted and adhered to the bottom can (i.e., a bottom current collector) of the coin cell or a separator. This result was further verified by analyzing SEM images of the disassembled Li metal electrodes. The SEM images of the disassembled Li metal electrodes showed that the pristine Li metal electrode (Fig. S13a, ESI†) was severely cracked and crushed into several pieces, which was commonly observed in previous work13,30 on Li metal electrodes. In contrast, the reinforced Li metal electrode (Fig. S13b, ESI†) exhibited a smooth and uniform morphology. We also measured the volume change of the Li metal electrodes after the plating/stripping cycles (conducted at 50% DOD). The thickness of the pristine Li metal was substantially increased from 55 to 128 μm (Fig. S13c, ESI†), revealing that its structure was less dense and some active Li may be detached due to random growth of Li. By comparison, the thickness of the reinforced Li metal was slightly increased (55 → 63 μm, Fig. S13d, ESI†), demonstrating the beneficial effect of the conductive PET nonwoven which can act as a kind of Li host13,14,30 to spatially accommodate the plating of Li. As another piece of evidence for the superiority of the reinforced Li metal anode, the EIS profiles of the symmetric Li/Li cells were monitored during the repeated plating/stripping tests. Comparison of the EIS profiles (Fig. S14a and b, ESI†) between the 1st and 20th cycles revealed that the reinforced Li metal electrode exhibited a decrease in the solid electrolyte interface resistance (RSEI) in contrast to the pristine Li metal electrode. Additionally, the reinforced Li metal electrode showed a lower charge transfer resistance (Rct) than the pristine Li metal. This improvement in the electrochemical resistances confirms the beneficial contribution of the conductive PET nonwovens, which was consistent with results13,14,30 of the porous conductive Li hosts.
Electrochemical performance and mechanical flexibility of nanomat Li–S cells
Both the cathode–separator assembly and reinforced Li metal anode in the nanomat Li–S cell do not contain traditional heavy metallic current collectors such as Al and Cu foils, which thus allows for a substantial reduction in the total areal weight under similar areal loadings of electrode active materials compared to the control Li–S cell (Fig. 4a). Consequently, the nanomat Li–S cell showed considerably higher gravimetric charge/discharge capacities (expressed based on the cell weight) than the control Li–S cell (Fig. 4b).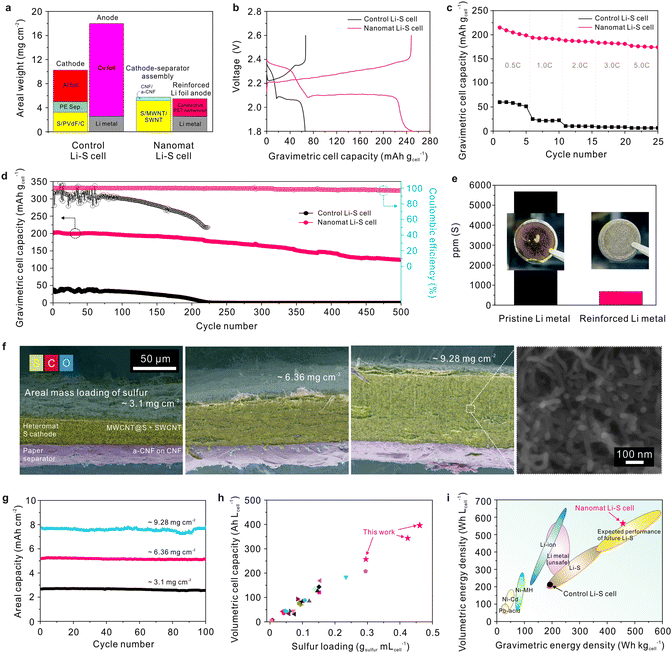 | ||
| Fig. 4 Electrochemical performance of the nanomat Li–S cells. (a) Comparison of the areal weight (mg cm−2) of the major components between the nanomat and control Li–S cells. (b) Galvanostatic charge/discharge profiles (at a constant charge/discharge current density of 0.2C/0.2C) of the nanomat and control Li–S cells, in which the specific gravimetric capacities (mA h gcell−1) are expressed based on the cell weight. (c) Discharge rate capabilities of the nanomat and control Li–S cells over a wide range of discharge current densities (0.2–5.0C) at a fixed charge current density of 0.2C in a voltage range of 1.8–2.6 V. (d) Cycling performance (at a charge/discharge current density = 1.0C/1.0C) of the control and nanomat Li–S cells. (e) Amount of polysulfides (measured using ICP-MS analysis) deposited on the reinforced Li metal anode (vs. the pristine Li metal anode) after the cycling test (500 cycles). The insets are photographs of the Li metal anodes. (f) SEM images (cross-sectional view) of the cathode–separator assemblies with different thicknesses/sulfur mass loadings (50 μm/3.10 mg cm−2, 95 μm/6.36 mg cm−2 and 135 μm/9.28 mg cm−2) under the fixed paper separator (thickness ∼ 20 μm). A high-magnification image of the thicker electrode (135 μm) is also provided. (g) Cycling performance (at a charge/discharge current density of 0.2C/0.2C) of nanomat Li–S cells fabricated with different cathode–separator assemblies as a function of sulfur mass loading. (h) Volumetric capacities (A h Lcell−1) of the nanomat Li–S cells as a function of sulfur loading per cell volume (gsulfur mLcell−1). The results of previously reported flexible Li–S cells (supplementary references SR1–SR24, ESI†) are also included for comparison. More details are provided in Table S1, ESI.† (i) Comparison of the volumetric (W h Lcell−1)/gravimetric (W h kgcell−1) energy densities: nanomat Li–S cells vs. different types of rechargeable power sources.35 | ||
Fig. 4c compares the discharge rate capability of the nanomat and control Li–S cells, in which the discharge current density was varied from 0.2 to 5.0C at a fixed charge current density of 0.2C. The nanomat Li–S cell showed higher discharge capacities (expressed as mA h gcell−1) than the control Li–S cell over a wide range of discharge current densities. A comparison of the sulfur mass-based discharge capacities (expressed as mA h gsulfur−1) is provided in Fig. S15a, ESI.† The higher discharge rate capability of the nanomat Li–S batteries was verified by the lower cell polarization and well-established voltage profiles (Fig. S15b and c, ESI†). This superiority of the redox kinetics is attributed to the nanomat-mediated 3D bi-continuous electron/ion conduction pathways in the cathode–separator assembly (specifically, the combined effect of the well-interconnected electronic networks based on MWCNTs/SWCNTs and the facile electrolyte accessibility driven by the all-fibrous structure/polar CNFs).
The cycling performance of the cells was examined at a charge/discharge current density of 1.0C/1.0C (Fig. 4d and Fig. S16, ESI†). The nanomat Li–S cell showed superior cycling stability during 500 cycles with 0.07% decay per cycle, compared to the control Li–S cell (= 0.2%). This decent cycling performance of the nanomat Li–S cell was verified by conducting post-mortem analysis (Fig. S17–S19, ESI†) after the cycling test (500 cycles). The sulfur cathode of the control Li–S cell was severely contaminated with dense resistive layers, similar to the previously reported results (Fig. S17a, ESI†).31,32 In comparison, the fibrous structure of the MWCNT@S/SWCNT cathode and the CNF/a-CNF paper separator was well maintained without morphological defects and disruptions (Fig. S17b, ESI†), thereby exhibiting long-term structural stability.
In addition, the surface (facing the Li metal anode) of the separators was investigated using XPS analysis (Fig. S18, ESI†). The CNF layer of the paper separator showed weaker peak intensities at both 162.3 and 163.9 eV than the PE separator. The S 2p3/2 peaks at 162.3 and 163.9 eV are known to represent lower-order polysulfides (Li2Sx(II)) and higher-order polysulfides or elemental sulfur (Li2Sx(I)), respectively.33,34 This result reveals that the CNF/a-CNF paper separator, due to the Donnan exclusion effect enabled by the a-CNF layer, effectively suppressed the migration of polysulfides towards the Li anodes. To further verify this beneficial effect, the absolute amount of polysulfides deposited on the Li anodes was quantitatively analyzed using inductively coupled plasma mass spectrometry (ICP-MS) and energy dispersive X-ray spectroscopy (EDS) techniques. A substantially lower sulfur content (Fig. 4e and Fig. S19, ESI†), along with a clean and smooth surface, was detected on the reinforced Li metal anode compared to the pristine Li metal one.
A variety of nanomat Li–S cells were fabricated as a function of the cathode thickness of the cathode–separator assembly with the same reinforced Li metal anode (thickness ∼ 55 μm). Fig. 4f shows that the cathode thickness (affecting the areal mass loading of sulfur) of the assemblies varied from 50 μm (sulfur loading = 3.10 mg cm−2) to 95 μm (= 6.36 mg cm−2) and 135 μm (= 9.28 mg cm−2) on the fixed paper separator (thickness ∼ 20 μm). Note that the nanomat structure of the sulfur cathode is well established in the through-thickness direction, even for the thicker cathode. The areal capacity of the nanomat Li–S cells tended to increase in proportion to the sulfur loading (Fig. 4g and Fig. S20, ESI†). Additionally, stable capacity retention was observed during cycling for all nanomat Li–S cells. The volumetric capacities of the nanomat Li–S cells were plotted as a function of sulfur loading per cell volume (gsulfur mLcell−1) and compared with those of previously reported flexible Li–S cells (supplementary references SR1–SR24, ESI†). These cell-based values are highly significant for commercial application of Li–S cells. Notably, the nanomat Li–S cells exhibited the higher volumetric cell capacities, which far exceeded those attained with conventional Li–S cell approaches (Fig. 4h and Table S1, ESI†). To underscore this advantageous effect of the nanomat Li–S cells, their gravimetric/volumetric energy densities (= 457 W h kgcell−1/565 W h Lcell−1) were compared with those35 of different types of rechargeable battery systems (Fig. 4i). The exceptionally higher energy densities of the nanomat Li–S cells demonstrate the viability and effectiveness of the unitized cathode–separator assembly and the reinforced thin Li metal anode.
Mechanical flexibility of nanomat Li–S cells under various deformation modes
The mechanical flexibility of the nanomat Li–S cells was investigated under various deformation modes. Fig. 5a shows the change in cell voltage (in the charged state) as a function of the number of bending cycles. The nanomat Li–S cell presented no significant loss in cell voltage during repeated bending cycles, whereas a sharp drop in cell voltage was observed for the control Li–S cell after just 400 cycles. In addition, the change in cell voltage (in the charged/deformed state) was monitored as a function of bending radius (Fig. 5b). The voltage of the nanomat Li–S cell was stably maintained, even at the smallest radius of 0.5 mm, in comparison to the control cell that showed severe voltage fluctuation. The excellent voltage stability of the nanomat Li–S cell described above reveals that the bi-layered paper separator in the cathode–separator assembly can effectively prevent the occurrence of leakage current between electrodes (that may trigger internal short-circuit failure of cells). This superior mechanical robustness of the nanomat Li–S cell was verified through in situ monitoring of the cell voltage during charge/discharge reaction upon repeated bending deformation (Fig. S21, ESI†). For the control Li–S cell, the gap distance between the cell components (i.e., cathode, separator and anode) continuously varied upon repeated bending, resulting in fluctuation in the charge/discharge voltages.19 By contrast, the nanomat Li–S cell, driven by the unitized cathode–separator assembly, effectively mitigated the bending-induced voltage fluctuation (∼3 mV vs. ∼15 mV for the control cell).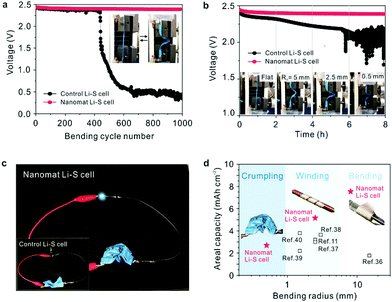 | ||
| Fig. 5 Mechanical flexibility of the nanomat Li–S cell (vs. the control Li–S cell) under various deformation modes. (a) Change in cell voltage (in the charged state) as a function of the bending cycle (bending radius = 2.5 mm and deformation rate = 500 mm min−1). (b) Change in cell voltage (in the charged and deformed state) as a function of the bending radius (= 0.5, 2.5 and 5.0 mm). (c) Photographs showing the operation of a green LED lamp connected to the nanomat Li–S cell (vs. the control Li–S cell (inset)) in the severely crumpled state. (d) Comparison of the mechanical flexibility between the nanomat Li–S cell and previously reported ones11,36–40 as a function of the bending radius. | ||
The nanomat Li–S cell successfully powered a light-emitting diode (LED) lamp even after being severely crumpled, whereas the control Li–S cell failed to power the LED lamp (Fig. 5c). The mechanical flexibility of the nanomat Li–S cell was highlighted through comparison with the previously reported results (Fig. 5d).11,36–40 Additionally, the analysis modes used to elucidate the mechanical deformability of the flexible Li–S cells were compared with those of previous publications (supplementary references SR1–SR24, ESI†) to support the comprehensive investigation of this study (Fig. S22, ESI†).
Conclusions
In summary, we have presented nanomat Li–S batteries with exceptionally higher energy density and mechanical flexibility. The nanomat Li–S batteries consisted of all-fibrous cathode–separator assemblies and conductive nonwoven-reinforced Li metal anodes. The all-fibrous structure (offering 3D bi-continuous electron/ion transport pathways) of the cathode–separator assemblies, in combination with the a-CNFs (mitigating the shuttle effect via Donnan exclusion), led to substantial improvements in the redox kinetics and cycling stability. The reinforced Li metal anode, in which the conductive PET nonwoven was embedded into the Li metal foil, significantly improved the thickness, flexibility and electrochemical stability/utilization of the Li metal. Benefiting from the structural uniqueness and chemical functionalities, the nanomat Li–S batteries provided significant improvements in the cell performance (specifically, the (cell-based) gravimetric/volumetric energy density = 457 W h kgcell−1/565 W h Lcell−1 and the cycling performance (over 500 cycles) under 110% capacity excess of the Li metal anode) and mechanical deformability (they even can be crumpled), which lie far beyond those attainable with conventional Li–S batteries. To the best of our knowledge, this is the first report of a Li–S battery that simultaneously achieved such (cell-based) high energy density and mechanical flexibility. We envision that the nanomat Li–S battery strategy presented herein holds promise as a versatile and scalable platform for development of advanced flexible batteries, which will lead us a step closer to the future smart/ubiquitous energy era.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Basic Science Research Program (2017M1A2A2087810 and 2018R1A2A1A05019733) and Wearable Platform Materials Technology Center (2016R1A5A1009926) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning. This work was also supported by the Industry Technology Development Program (10080540) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and the National Institute of Forest Science (FP 0400-2016-01).References
- L. Li, Z. Wu, S. Yuan and X.-B. Zhang, Energy Environ. Sci., 2014, 7, 2101–2122 RSC.
- X. Wang, X. Lu, B. Liu, D. Chen, Y. Tong and G. Shen, Adv. Mater., 2014, 26, 4763–4782 CrossRef CAS PubMed.
- H.-J. Peng, J.-Q. Huang and Q. Zhang, Chem. Soc. Rev., 2017, 46, 5237–5288 RSC.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- A. Manthiram, Y. Fu, S.-H. Chung, C. Zu and Y.-S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- A. Manthiram, S. H. Chung and C. Zu, Adv. Mater., 2015, 27, 1980–2006 CrossRef CAS PubMed.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1, 16132 CrossRef CAS.
- M. Liu, D. Zhou, Y.-B. He, Y. Fu, X. Qin, C. Miao, H. Du, B. Li, Q.-H. Yang and Z. Lin, Nano Energy, 2016, 22, 278–289 CrossRef CAS.
- Z. Yuan, H. J. Peng, J. Q. Huang, X. Y. Liu, D. W. Wang, X. B. Cheng and Q. Zhang, Adv. Funct. Mater., 2014, 24, 6105–6112 CrossRef CAS.
- H. S. Kang and Y. K. Sun, Adv. Funct. Mater., 2016, 26, 1225–1232 CrossRef CAS.
- Y. Mao, G. Li, Y. Guo, Z. Li, C. Liang, X. Peng and Z. Lin, Nat. Commun., 2017, 8, 14628 CrossRef PubMed.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS.
- S. S. Chi, Y. Liu, W. L. Song, L. Z. Fan and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1700348 CrossRef.
- Q. Li, S. Zhu and Y. Lu, Adv. Funct. Mater., 2017, 27, 1606422 CrossRef.
- P. Albertus, S. Babinec, S. Litzelman and A. Newman, Nat. Energy, 2018, 3, 16–21 CrossRef CAS.
- G. Zheng, S. W. Lee, Z. Liang, H.-W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu and Y. Cui, Nat. Nanotechnol., 2014, 9, 618–623 CrossRef CAS PubMed.
- Y. Liu, D. Lin, P. Y. Yuen, K. Liu, J. Xie, R. H. Dauskardt and Y. Cui, Adv. Mater., 2017, 29, 1605531 CrossRef PubMed.
- X.-B. Cheng, J.-Q. Huang and Q. Zhang, J. Electrochem. Soc., 2018, 165, A6058–A6072 CrossRef CAS.
- K.-H. Choi, S.-J. Cho, S.-J. Chun, J. T. Yoo, C. K. Lee, W. Kim, Q. Wu, S.-B. Park, D.-H. Choi and S.-Y. Lee, Nano Lett., 2014, 14, 5677–5686 CrossRef CAS PubMed.
- J.-H. Kim, M. Gu, D. H. Lee, J.-H. Kim, Y.-S. Oh, S. H. Min, B.-S. Kim and S.-Y. Lee, Nano Lett., 2016, 16, 5533–5541 CrossRef CAS PubMed.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- Z. Fang, H. Zhu, Y. Yuan, D. Ha, S. Zhu, C. Preston, Q. Chen, Y. Li, X. Han and S. Lee, Nano Lett., 2014, 14, 765–773 CrossRef CAS PubMed.
- S. Leijonmarck, A. Cornell, G. Lindbergh and L. Wågberg, J. Mater. Chem. A, 2013, 1, 4671–4677 RSC.
- Z. Wang, D. O. Carlsson, P. Tammela, K. Hua, P. Zhang, L. Nyholm and M. Strømme, ACS Nano, 2015, 9, 7563–7571 CrossRef CAS PubMed.
- J.-Q. Huang, Q. Zhang, H.-J. Peng, X.-Y. Liu, W.-Z. Qian and F. Wei, Energy Environ. Sci., 2014, 7, 347–353 RSC.
- W. Ahn, S. N. Lim, D. U. Lee, K.-B. Kim, Z. Chen and S.-H. Yeon, J. Mater. Chem. A, 2015, 3, 9461–9467 RSC.
- X. Yu, J. Joseph and A. Manthiram, Mater. Horiz., 2016, 3, 314–319 RSC.
- T. Tao, S. Lu, Y. Fan, W. Lei, S. Huang and Y. Chen, Adv. Mater., 2017, 29, 1700542 CrossRef PubMed.
- T. Z. Zhuang, J. Q. Huang, H. J. Peng, L. Y. He, X. B. Cheng, C. M. Chen and Q. Zhang, Small, 2016, 12, 381–389 CrossRef CAS PubMed.
- Q. Yun, Y. B. He, W. Lv, Y. Zhao, B. Li, F. Kang and Q. H. Yang, Adv. Mater., 2016, 28, 6932–6939 CrossRef CAS PubMed.
- H. Yao, K. Yan, W. Li, G. Zheng, D. Kong, Z. W. Seh, V. K. Narasimhan, Z. Liang and Y. Cui, Energy Environ. Sci., 2014, 7, 3381–3390 RSC.
- J.-H. Kim, G. Y. Jung, Y.-H. Lee, J.-H. Kim, S.-Y. Lee, S. K. Kwak and S.-Y. Lee, Nano Lett., 2017, 17, 2220–2228 CrossRef CAS PubMed.
- M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescu and G. Offer, Energy Environ. Sci., 2015, 8, 3477–3494 RSC.
- C. Zu and A. Manthiram, Adv. Energy Mater., 2014, 4, 1400897 CrossRef.
- M. Hagen, D. Hanselmann, K. Ahlbrecht, R. Maça, D. Gerber and J. Tübke, Adv. Energy Mater., 2015, 5, 1401986 CrossRef.
- G. Zhou, L. Li, D. W. Wang, X. Y. Shan, S. Pei, F. Li and H. M. Cheng, Adv. Mater., 2015, 27, 641–647 CrossRef CAS PubMed.
- P. Xiao, F. Bu, G. Yang, Y. Zhang and Y. Xu, Adv. Mater., 2017, 29, 1703324 CrossRef PubMed.
- M. Xiang, H. Wu, H. Liu, J. Huang, Y. Zheng, L. Yang, P. Jing, Y. Zhang, S. Dou and H. Liu, Adv. Funct. Mater., 2017, 27, 1702573 CrossRef.
- M. Yu, Z. Wang, Y. Wang, Y. Dong and J. Qiu, Adv. Energy Mater., 2017, 7, 1700018 CrossRef.
- C.-H. Chang, S.-H. Chung and A. Manthiram, Mater. Horiz., 2017, 4, 249–258 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1 and Fig. S1–S22. See DOI: 10.1039/c8ee01879k |
| This journal is © The Royal Society of Chemistry 2019 |