 Open Access Article
Open Access ArticleRecent advances in hydrogel-based flexible strain sensors for harsh environment applications
Miaoyu
Li
ab,
Jie
Pu
c,
Qinghe
Cao
 c,
Wenbo
Zhao
c,
Yong
Gao
c,
Ting
Meng
c,
Jipeng
Chen
c and
Cao
Guan
c,
Wenbo
Zhao
c,
Yong
Gao
c,
Ting
Meng
c,
Jipeng
Chen
c and
Cao
Guan
 *ac
*ac
aInstitute of Flexible Electronics and Intelligent Textile, Xi'an Polytechnic University, Xi'an 710048, P. R. China
bSchool of Textile Science and Engineering, Xi'an Polytechnic University, Xi'an 710048, P. R. China
cInstitute of Flexible Electronics, Northwestern Polytechnical University, Xi'an 710072, P. R. China. E-mail: iamcguan@nwpu.edu.cn
First published on 8th October 2024
Abstract
Flexible strain sensors are broadly investigated in electronic skins and human–machine interaction due to their light weight, high sensitivity, and wide sensing range. Hydrogels with unique three-dimensional network structures are widely used in flexible strain sensors for their exceptional flexibility and adaptability to mechanical deformation. However, hydrogels often suffer from damage, hardening, and collapse under harsh conditions, such as extreme temperatures and humidity levels, which lead to sensor performance degradation or even failure. In addition, the failure mechanism in extreme environments remains unclear. In this review, the performance degradation and failure mechanism of hydrogel flexible strain sensors under various harsh conditions are examined. Subsequently, strategies towards the environmental tolerance of hydrogel flexible strain sensors are summarized. Finally, the current challenges of hydrogel flexible strain sensors in harsh environments are discussed, along with potential directions for future development and applications.
1. Introduction
Flexible electronics technology holds revolutionary significance across various eras, including the information and artificial intelligence era.1–3 In particular, wearable sensors converting physical stimuli into electrical signals to monitor human states and surrounding information, play a crucial role in developing the medical monitoring equipment, soft robotics, electronic skins, and human–machine interaction.4–6 Among them, flexible strain sensors have garnered significant attention due to their high sensitivity, wide strain sensing range, and fast response time.7–11 Unlike conventional sensors that incorporate rigid semiconductor chips and circuit boards, flexible strain sensors can be bent, twisted, stretched, and compressed while maintaining conductive paths under large and small deformations.12,13 Furthermore, flexible strain sensors address the limitations of slow response times and non-portability associated with conventional rigid sensors. Additionally, they facilitate the detection of glucose14 and sweat,15 deoxyribonucleic acid (DNA) analysis,16etc., thereby driving a technological revolution in next-generation sensors.17,18Currently, research on flexible strain sensors focuses on constructing efficient and stable conductive networks to produce significant responses under minor external deformations.19 In recent years, conductive composite elastomers and metal percolation networks have been commonly used to fabricate flexible strain sensors.20,21 However, these sensors often compromise wearing comfort due to their high mechanical stiffness. Conductive hydrogels, as a typical wet-soft material, exhibit significant potential for flexible electronics due to their biomimetic structures, favorable mechanical properties, and excellent biocompatibility.22,23 Most conductive hydrogels are fabricated by incorporating conductive fillers (such as carbon nanotubes,24 graphene,25–27 polymers,28,29 natural fibers,30,31 and metal particles,32–34) into cross linked non-conductive polymer matrices. The polymer matrix provides stable three-dimension support, while conductive fillers construct a conductive pathway.35 When stretched, this pathway converts mechanical deformations into electrical signals, resulting in variations in resistance and conductivity. Therefore, by altering the conductive fillers, cross-linkers, and intermolecular interactions, the network structure, electrical properties, and mechanical properties of hydrogels can be effectively tuned over a wide range.36,37 Furthermore, designing and optimizing a three dimensional network structure hydrogel sensor with self-healing and adhesive properties can closely conform to the skin surface, detecting subtle physiological signals and enabling real-time monitoring of human conditions.38
Although significant progress has been made in enhancing the performance of hydrogel-based flexible strain sensors, their practical application in extreme environments such as polar regions, deserts, and deep seas is severely limited by the water states within the hydrogel (Fig. 1). A comprehensive understanding of the challenges faced by hydrogel flexible strain sensors under harsh conditions is crucial for developing sensors capable of withstanding various extreme environmental conditions (Fig. 2). For example, at extremely low temperatures, the free water molecules within the hydrogel freeze and solidify, causing a loss of flexibility and resulting in brittle deformation, which degrades performance or leads to failure.39 In extremely high-temperature environments, thermal expansion, deformation, and leakage of conductive fillers reduce sensing sensitivity and cause signal drift.40 Furthermore, rapid transitions between high and low temperatures require exceptional environmental adaptability to maintain performance. Additionally, high humidity causes strong interactions between water molecules and hydrogen bonds, leading to swelling, short-circuiting of the conductive network, and a reduction or loss of sensing performance.41 Conversely, in extremely dry environments, hydrogels can experience structural collapse and damage due to water loss. Finally, extreme mechanical stress and corrosive environments can severely impact the integrity and functionality of hydrogel-based flexible strain sensors.42–44
 | ||
| Fig. 1 Schematic overview of hydrogel-based flexible strain sensors in harsh environment applications. | ||
This review focuses on the key challenges, current research status, and future prospects of hydrogel flexible strain sensors under harsh environmental conditions. First, we summarize the key challenges faced by these sensors in extreme environments, including freezing, water loss, swelling, cracking, and corrosion due to extreme temperatures and humidity levels. Then, we systematically discuss and review modification strategies, such as improvement of hydrogel network structure, introduction of functional additives, and interfacial modification. Finally, we propose potential perspectives to accelerate the widespread use of hydrogel-based flexible strain sensors. We hope this review will provide valuable insights for researchers looking for novel approaches to enhance the long-term stability and application of hydrogel-based flexible strain sensors under harsh conditions.
2. Extreme temperature conditions
2.1 Extremely low-temperature conditions
One principal concern for the extensive deployment of hydrogel flexible strain sensors is their temperature tolerance. In a previous study, the researchers proposed three different states of water within a flexible strain sensor hydrogel. They are free water, weakly bound water, and strongly bound water.45 Among these, free water is the main component of the hydrogel network, whereas weakly bound water and strongly bound water are present in much smaller quantities. At low temperatures, free water primarily engages in hydrogen bonding and hardly interacts with the polymer network of the hydrogel. Although weakly bound and strongly bound water exhibit stronger interactions with the hydrogel network that can effectively inhibit ice crystal formation, their concentrations are too low to prevent freezing.46 Consequently, the hydrogel network often freezes at extremely low temperatures. As the quantity of free water molecules decreases sharply, the rate of electron/ion mobility within the hydrogel network is reduced, leading to variations in resistance and slower reconstruction of reversible dynamic bonds.47 Furthermore, the tunnelling effect is constantly weakened due to the disruption of electron/ion transport channels caused by the crystallization or phase transition of free water molecules. This leads to a reduction in the number of conductive pathways and an increase in the spacing between conduction particles, thereby causing a continuous increase in resistance and degradation of the sensing performance.44,48–51To reduce or eliminate the impact of low-temperature conditions and enhance the anti-freeze performance of the hydrogel-based flexible strain sensor, various strategies have been explored. Based on previous studies, researchers have introduced soluble salts, polyol solutions, and ionic liquids into the hydrogel network. These additives stabilize hydrogel sensors at low temperatures by disrupting the hydrogen bonds between water molecules within the hydrogel network and enhancing the interactions between hydrogel networks.
It is well-known that the Arctic and Antarctic ocean waters do not freeze at extremely low temperatures.58 That should be attributed mainly to the strong hydration effects caused by complex media containing high concentrations of salt (NaCl) and polyionic substrates.59,60 Inspired by this phenomenon,Wang and co-authors prepared a dual-network hydrophobic association hydrogel (PAMD–NaCl) by suit thermally initiated polymerization of sodium dodecyl sulfate (SDS), acrylamide (AM), and divinylbenzene (DVB) in saturated sodium chloride (NaCl) solution by using a one-step method (Fig. 3a).61 The hydrogel shows excellent anti-freeze properties due to the strong hydration effect between free water and NaCl (Fig. 3b).62,63 Furthermore, the NaCl environments inhibit hydrophobic cross-linking and improve ionic conductivity during hydrogel polymerization, resulting in soft hydrogels with excellent feature resistance, and high sensor performance compared to a nonsaline environment at low temperatures (Fig. 3c). The hydrogels exhibit excellent flexibility, stretchability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200%), and desirable ion conductivity (106 mS cm−1) even at extremely low temperatures. Based on their flexibility, the hydrogels exhibit high sensitivity even in tiny motions. Moreover, excellent freeze resistance can effectively reduce the impact of low-temperature freezing on the adhesion of hydrogel flexible strain sensors. Furthermore, the ionic conductivity of the hydrogel sensor was effectively improved by adding FeCl3 to the hydrogel network. Meanwhile, the hydrogen bonding between water molecules inhibits the formation of ice crystals and promotes the application of hydrogel strain sensors at low temperatures.64
200%), and desirable ion conductivity (106 mS cm−1) even at extremely low temperatures. Based on their flexibility, the hydrogels exhibit high sensitivity even in tiny motions. Moreover, excellent freeze resistance can effectively reduce the impact of low-temperature freezing on the adhesion of hydrogel flexible strain sensors. Furthermore, the ionic conductivity of the hydrogel sensor was effectively improved by adding FeCl3 to the hydrogel network. Meanwhile, the hydrogen bonding between water molecules inhibits the formation of ice crystals and promotes the application of hydrogel strain sensors at low temperatures.64
 | ||
| Fig. 3 Soluble salt modification strategies under extremely low temperature conditions. (a) Schematic diagram showing the manufacturing process of the salt-containing PAMD–NaCl anti-freeze hydrogel. (b) DSC trace of PAMD–NaCl hydrogels. (c) Sensing properties of the PAMD–NaCl anti-freeze hydrogel at 25 °C and −20 °C. Reproduced from ref. 61. Copyright 2023, The American Chemical Society. (d) DSC curve of the PAC–CGO0.2–Na90,360 hydrogel (0.2 is the weight percentage of CGO (wt%), 90 is the incubation time (min) of the hydrogel immersed in the NaCl solution, and 360 is the concentration of the NaCl solution (g L−1), respectively). (e) Gauge factor of PAC–CGO0.2–Na90,360 hydrogels under different strains. (f) Cyclic tensile tests (200%). (g) Schematic illustration of the fabrication process of the anti-freezing PAC–CGO–Na nanocomposite hydrogel. Reproduced from ref. 62. Copyright 2022, Elsevier. | ||
Compared to the traditional method of adding salt to the pre-gel precursor solution, the salt percolation strategy allows high concentrations of salt to penetrate into the interior of the hydrogel network through a concentration gradient and does not hinder the polymerization of the hydrogel.65 Based on this, the hydrogel with a hybrid network structure (PAC–CGO) consists of polyacrylamide and chitosan. The incorporation of a high concentration of NaCl provides the (PAC–CGO–Na) hydrogel with strong hydration, and the Na+ and Cl− ions not only effectively enhanced the polymer structure and improved conductivity but also endowed hydrogel networks with excellent anti-freezing properties (Fig. 3g).62 Compared to hydrogel networks without the introduction of NaCl, the PAC–CGO–Na hydrogel can achieve no freezing at −56.8 °C (Fig. 3d). Furthermore, sensing performance and flexibility are essential indicators of hydrogel flexible strain sensors. The PAC–CGO–Na hydrogel as a flexible strain sensor can be twisted and bent. In a low-temperature environment, it has the advantages of high sensitivity (gauge factor ∼ 6.67), fast response (≈120 ms), and a wide strain sensing range (0–1216%) (Fig. 3e and f). Moreover, the strain sensor also exhibited excellent stability in cyclic tensile measurements with a strain of 200%. Surprisingly, the gauge factor (GF) of hydrogel-based flexible strain sensors remains at a high retention value of over 90.0% with small or large strains.
Moreover, Wu and co-authors obtained DN hydrogels with carrageenan and polyacrylamide and soaked them in LiBr solutions. The higher content of LiBr (10–50 wt%) lowers the freezing point.65 In contrast to the previous studies, Li+ and Br− tend to form a stable cluster with several free water molecules to reduce the number of hydrogen bonds to suppress ice crystallization.66 Even at extremely low temperatures (−78.5 °C), the hydrogel retains excellent softness and sensor performance. The authors have introduced other salts (LiCl, MgCl2, and KCl) that show a low freezing point, although higher than that of LiBr. These results proved the correctness of the strategy to improve the anti-freeze performance of the hydrogel by adding soluble salts. Based on the above, the hydrogel flexible strain sensors can detect a wide strain sensing range from 0.1 to 180% at low temperatures. The low limit of detection enables extremely high sensitivity at tiny stretches.
For example, Sun and co-authors were inspired by catechol to prepare organohydrogels (Ls–Cu@WG–PHEAA) for use as skin strain sensors. The polymerization of HEAA (N-hydroxyethyl acrylamide) in the glycerol–water binary phase was achieved by establishing an autocatalytic system with sodium lignosulfonate and copper(II) ions (Ls–Cu2+) (Fig. 4a),74 by controlling the ratios of water (Wx) to glycerol (Gy) to improve anti-freeze properties. By introducing non-crystallized glycerol into the hydrogel network with water as the cosolvent, the hydrogel exhibited no freezing point even at extremely low temperatures (Fig. 4b and c). Additionally, a higher content of glycerol creates a unique polyhydroxy structure that tends to form intermolecular hydrogen bonds with water molecules and the PHEAA chain, which disrupts the formation of crystal lattices of ice crystals and inhibits the growth of ice crystals. Furthermore, the increased intermolecular sacrificial bonds between Ls–Cu2+–PHEAA chain glycerol form a strong physical cross-linking. This can realize effective energy dissipation during stretching and provide outstanding tolerance to high compressive deformation.75,76 At low temperature (−20 °C), Ls–Cu@WG–PHEAA hydrogel flexible strain sensors have relatively high sensitivity, especially at large strains. The flexible strain sensor exhibits highly sensitive sensing performance (a value of ΔR/R0 from 0 to 112%), when the different bending angles increase from 0° to 90° (Fig. 4d). With a similar strategy, Liu and co-authors proposed an interfacial engineering strategy in which tannic acid (TA, containing a high density of hydroxyl groups), encapsulated MXene to form a stable TA@MXene nanostructure.77 They introduced TA@MXene into the poly network (hydroxyethyl acrylate) (PHEA) in glycerol/water binary solvent to obtain a hydrogel with excellent properties. The introduction of glycine (Gly) contributed to the mechanical and anti-freeze performances. The weaker hydrogen bond cross-links with the polymer chain due to the introduction of glycerol to adapt to large strains. This inhibited the formation of ice crystals by forming hydrogen with water molecules. Even at low temperatures (−40 °C), the hydrogel used for the strain sensor exhibited large stretchability (>500%) with low hysteresis (<3%). Other researchers have similarly enabled strain sensing under extreme conditions (70 °C and −80 °C) by making hydrogels electrically conductive and mechanically stable via water/glycerol binary solvents.78
 | ||
| Fig. 4 Polyol solvent modification strategies under extremely low temperature conditions. (a) Schematic diagram of the water–glycerol binary phase anti-freeze organic hydrogel fabrication process. (b) The WxGy hydrogels at −50 °C. (c) DSC curves of the W1G2 hydrogel. (d) Monitoring the relative resistance variations of a finger by using an anti-freezing hydrogel strain sensor. Reproduced from ref. 74. Copyright 2021, Royal Society of Chemistry. (e) Schematic illustration of the fabrication of anti-freeze hydrogel MNOH. (f) Conductivity of MNOH and MNH at low temperature. (g) Tensile stress–strain curves of MNOH at 20 °C and −40 °C. (h) Gauge factor of MNOH hydrogels under different strains. Reproduced from ref. 67. Copyright 2019, Wiley-VCH. | ||
Compared to the direct-addition method, the solvent replacement method does not require consideration of the impact of the added amount on the polymerization of hydrogels. The anti-freeze MXene nanocomposite hydrogel (MNOH) was prepared by immersing the MXene nanocomposite hydrogel in an ethylene glycol (EG) solution to displace a portion of the water molecules (Fig. 4e).67 The formation of hydrogen bonds between water molecules and EG after solvent replacement inhibits the formation of ice crystal lattices and enables conductivity at low temperatures. Besides, the hydrogen bonds endowed the hydrogel with excellent stretching (≈980%, −40 °C) (Fig. 4f and g). Surprisingly, the kinetic cross-linking between PVA and tetrahydroxyboronic acid ions offers a dual effect that the supramolecular interactions between EG, PVA, and MXene provide them with excellent self-healing capabilities at sub-zero temperatures. The flexible strain sensor was prepared by using an MNOH hydrogel to monitor human activities with remarkable sensitivity (GF ∼ 44.85) and environmental tolerance over a wide strain sensing range (50–350% strain at −40 °C) (Fig. 4h).
To widen the application of ionic liquid modification strategies in the direction of the anti-freeze hydrogel flexible strain sensor, researchers have taken several measures. Dual network hydrogels were prepared by using starch and polyvinyl alcohol (PVA). The ratio of ionic liquid ([AMim]Cl)/glycol/water ternary solvent system regulated the comprehensive performance of the hydrogels (Fig. 5a).81 The hydrogel exhibits excellent tensile mechanical and anti-freeze properties because the [AMim]+ and hydroxyl groups interact with non-covalent bonding, and strong hydrogen bonds are formed between Cl− and hydroxyl hydrogen. Due to the synergistic action of ionic liquids and EG, hydrogen bonds are formed with water molecules to prevent ice crystallization and lower the freezing point to −128.9 °C (Fig. 5b). Besides, ionic liquids create an ion-rich environment that enhances the ionic conductivity of the hydrogel at low temperature (Fig. 5c) and disrupt bacterial cell membranes through electrostatic interactions, endowing the hydrogel with antibacterial properties.82,83 The excellent anti-freezing properties and sensitivity of these hydrogels enable them to be used as flexible strain sensors for detecting body signals. The strain sensor demonstrates exceptional sensitivity (GF ∼ 0.99 to 3.28) across a wide strain sensing range (below 55% to 650%–1000%) (Fig. 5d). Moreover, the sensing performance is still stable at extremely low temperatures compared to room temperature (Fig. 5e). The hydrogels prepared by using an ionic liquid modification strategy exhibit excellent performance when used as flexible strain sensors due to the properties of ionic liquids. Recently, Liu and co-authors prepared a hydrogel with a binary solvent system of ionic liquid (1-ethyl-3-methylimidazolium chloride, [EMIm]Cl) and water (Fig. 5h).84 First, the binary solvent system exhibits the electrochemical properties of an ionic liquid to ensure the ionic conductivity (0.28 S m−1 at 25 °C) of the hydrogel flexible strain sensor (Fig. 5f).85 Furthermore, the hydrogen bonds between the polymer chain, water, and ionic liquid could improve the comprehensive mechanical properties of strain sensors. They exhibited excellent tensile properties (strain > 1800%), durability (1000 times at 100% strain), and satisfactory sensitivity (gauge factor ∼ 2.15 at 200% strain) (Fig. 5g). Finally, the hydrogen bonds between the ionic liquid and water molecules hinder the formation of ice to lower the freezing point, which ensured the stability of the sensor at low temperatures (−20 °C). Furthermore, the sensor can accurately recognize small deformations such as swallowing, and has a wide application prospect (Fig. 5i and j).
 | ||
| Fig. 5 Ionic liquid modification strategies under extremely low temperature conditions. (a) Schematic of the SAEP hydrogel (with starch, [AMim]Cl, EG (ethylene glycol), and (PVA) polyvinyl alcohol). (b) DSC curves of hydrogels and the anti-freeze mechanism. (c) Conductivity of the SAEP hydrogel at −80 °C (d) Relative resistance of the hydrogel under 0–1000% strain. (e) Sensing relative resistance changes of the SAEP hydrogel (at a strain of 100%) at different temperatures (25 °C, 25 °C after 24 h, −20 °C, and −80 °C). Reproduced from ref. 81. Copyright 2023, Elsevier. (f) Conductivity of the ionic hydrogel at −20, 0, and 25 °C. (g) Gauge factor of the anti-freezing ionic hydrogel strain sensor. (h) Schematic illustration of the preparation process of the ionic hydrogel. (i) Resistance signal changes to elbow bending at −20 °C and 25 °C. (j) Monitoring the relative resistance variations of swallowing using an anti-freezing hydrogel strain sensor. Reproduced from ref. 84. Copyright 2024, The American Chemical Society. | ||
Recently, Liu and co-authors developed a poly(hydroxyethyl-L-glycol)-based polymer cross-linker (MC) and used it to improve the properties of the hydrogel (Fig. 6a).86 Compared to traditional short-chain cross-linkers, MC features a long chain that may change the network. When used in hydrogel based flexible strain sensors, the polar groups (hydroxyl and amide groups) in MC can form strong hydrogen bonds with water molecules, thereby lowering the freezing point.87 An increased content of MC (0.5–2 wt%) results in a lower freezing point (−25.9 to ∼−75.9 °C) (Fig. 6b). Furthermore, the hydrogel exhibits high sensitivity (GF ∼ 1.81–2.55) in a wide range of strain (5–200%) (Fig. 6c and d). When the finger bends at the same angle with different rates, the sensor consistently obtains almost the same value for ΔR/R0, confirming its stability (Fig. 6e).
 | ||
| Fig. 6 Other modification strategies under extremely low temperature conditions. (a) The fabrication process of the MC cross-linked hydrogel. (b) DSC curve of anti-freeze hydrogels. (c) Gauge factor of the anti-freezing hydrogel at different strains. (d) Relative resistance change of the hydrogel during consecutive loading–unloading tests at different strains (5, 20, 50, 100, and 200%). (e) Hydrogel strain sensors are used to monitor finger binding at different speeds. Reproduced from ref. 86. Copyright 2021, The American Chemical Society. (f) Schematic illustration of the preparation process of the MC-cross-linked hydrogels. (g) DSC curves of hydrogels with different AFPs contents. (h) ΔR/R0 and GF of a hydrogel sensor over a wide range of 0–1000%. Resistance variations of the hydrogel: (i) bending the wrist and (j) bending a finger. Reproduced from ref. 88. Copyright 2021, Elsevier. | ||
In nature, many kinds of biocompatible materials can effectively reduce hydrogel freezing points. Biocompatible anti-freeze hydrogel strain sensors were constructed by integrating natural fish anti-freeze proteins (AFPs) in a hydrogel network consisting of a chemical cross-linker (acrylamide/sodium methacrylate) and a physical cross-linker (polyvinyl alcohol) (Fig. 6f).88 The anti-freeze proteins can adjust the ice crystallization process by selectively bonding to the surface of ice crystals and can also interact with the hydrogel network or various materials through non-covalent bonds benefiting from the multiple amino acid groups.89,90 Overall, the ice growth prevented by AFPs provided the anti-freeze properties of hydrogel strain sensors, and the freezing point decreased with the increased content of APFs (Fig. 6g). Surprisingly, the hydrogel strain sensors with AFPs provide excellent sensitivity (GF ∼ 2.1–12.9) and fast response (0.17 s) in the field of wide strain sensing (0.3–1000%) (Fig. 6h). The advantages of low hysteresis and high durability enable monitoring of different human movement states (Fig. 6i and j). Inspired by plants, Liu and co-authors utilized amphoteric betaine hydrochloride (BH) with hydrated lithium chloride (LiCl) to prepare an ionic co-hybrid hydrogel (PBLL), which is used as a flexible strain sensor.91 Due to the strong hydrophilicity of BH, the strong interaction between BH and water prevents water molecules from arranging in an orderly manner at low temperatures, thus preventing the formation of ice crystals. At extremely low temperatures (−80 °C), the PBLL flexible strain sensor exhibited high conductivity (1.9 S m−1) and rapid response and recovery times.
2.2 Extremely high-temperature conditions
In equatorial regions, maximum temperatures can reach 55 °C. Although some of the issues faced by hydrogel flexible strain sensors at low temperatures may be mitigated or solved at high temperatures, new problems emerge. High temperatures can alter the lattice structure and tunnelling distance, affecting the sensor's resistance and potentially causing signal shifts or instability.50,92–94 Additionally, elevated temperature affects electron/ion mobility within the hydrogel network. The increased collision frequency of electrons and ions in the conductive network at high temperatures leads to higher thermal kinetic energy, which in turn alters electron energy levels and affects electron transitions.95Most recently, Li and co-authors prepared an organic hydrogel with excellent properties for flexible strain sensors by mixing cellulose nanofibers (CNFs), tannic acid (TA), polyvinyl alcohol (PVA), and NaCl in a water–glycerol (Gly) binary solvent (Fig. 7a).98 In the hydrogel network, improved mechanical properties are attributed to the salting-out effect of NaCl on chain entanglement. Under extreme temperature conditions, the synergistic effect of sodium chloride (salt) and glycerol effectively inhibits the evaporation and freezing of water molecules within the hydrogel network. As a result, the hydrogel retains its soft and moist characteristics when twisted at high temperatures, thereby significantly enhancing its sensing performance (Fig. 7b). For practical applications, hydrogel sensors exhibit excellent electrical conductivity (0.86 S m−1), strain sensitivity (GF ∼ 8.54), and stability under different deformations (Fig. 7c and d). The sensor accurately detects minor/major deformations, enabling the monitoring and sensing of the human movement status, especially at extremely high temperatures (60 °C) (Fig. 7e). Slightly differently, cross-linked poly(acrylic acid-co-acrylamide) hydrogels (P(AA-co-Am)) with silica nanoparticles were prepared by photopolymerization.99 The hydrogel achieved excellent comprehensive performance after being immersed in calcium chloride (CaCl2) solutions. This is attributed to the capability of Ca2+ ions to bind with water, resulting in the formation of hydrated ions that prevent the evaporation of water molecules at elevated temperatures, consequently enhancing the ionic conductivity of the hydrogel network.100 The strain sensor exhibits low hysteresis (<7% at 400% strain), high durability, and cycle stability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 100% strain). Additionally, the sensor exhibits a remarkable ability to precisely recognize and categorize 15 sign language gestures, thereby enabling their integration and utilization within the advanced domain of human–computer interaction technologies. A dual-network organohydrogel was prepared based on hyaluronic acid and polyacrylic acid–acrylamide for multifunctional strain sensors by introducing glycerol into the organohydrogel network via a solvent substitution strategy.101 Due to the water-locking effect of glycerol and the tough polymer backbone, the resulting organohydrogel exhibits stable stretchability and temperature tolerance, enabling stable operation over the range of −30 to 60 °C. In addition, it has excellent flexibility and stable electrical conductivity.
000 cycles at 100% strain). Additionally, the sensor exhibits a remarkable ability to precisely recognize and categorize 15 sign language gestures, thereby enabling their integration and utilization within the advanced domain of human–computer interaction technologies. A dual-network organohydrogel was prepared based on hyaluronic acid and polyacrylic acid–acrylamide for multifunctional strain sensors by introducing glycerol into the organohydrogel network via a solvent substitution strategy.101 Due to the water-locking effect of glycerol and the tough polymer backbone, the resulting organohydrogel exhibits stable stretchability and temperature tolerance, enabling stable operation over the range of −30 to 60 °C. In addition, it has excellent flexibility and stable electrical conductivity.
 | ||
| Fig. 7 Salt and polyol solvent and hydrogel network modification strategies under extremely high temperature conditions. (a) Schematic illustration of the fabrication process of the PVA/CNF/TA/Gly/NaCl hydrogels. (b) Organic-hydrogels in different environments (24 h). (c) Relative resistance of the sensor at different tensile strains. (d) Relative resistance changes at different strains. (e) Monitoring the relative resistance changes of finger movement signals after 12 h at 60 °C. Reproduced from ref. 98. Copyright 2022, Springer. (f) Schematic diagram of the DN organic hydrogels. (g) Resistance changes of the hydrogel of the flexible strain sensor under 100% strain at different temperatures (−18 °C, 25 °C, 40 °C, and 80 °C). (h) Resistance changes in the hydrogel during cyclic stretch-flexion of the wrist joint. Reproduced from ref. 102. Copyright 2021, Elsevier. | ||
Apart from the established methods, stable operation of hydrogel flexible strain sensors at high temperatures can also be achieved via dual-network (DN) hydrogel construction. A binary solvent of potassium chloride and glycerol–water was introduced into the DN hydrogels polymerized using glutaraldehyde cross-linked polyvinyl alcohol (PVA–GA) and poly(acrylic acid-co-acrylamide) (P(AA-co-Am)) (Fig. 7f).102 During the cross-linking process of the dual network, glycerol easily forms hydrogen bonds with the polymer chains, thereby inducing physical cross-linking.103 Glycerol can form hydrogen bonds with water molecules, effectively inhibiting the evaporation of water molecules at high temperatures.104 Furthermore, the interactions between physical and chemical cross-linking in DN networks exhibit a stabilized storage modulus (G′) across a wide range of temperatures (from −20 °C to 100 °C). The flexible strain sensor utilizing the DN hydrogel has outstanding temperature tolerance (from −18 to 80 °C), high stretchability (>2000%), stability (in different temperature environments), and sensitivity, making it ideal for applications in monitoring human motions. Stable sensing performance and high sensitivity enable accurate feedback on movements such as wrist bending, thereby contributing to the widespread application of sensors under high-temperature conditions (Fig. 7g and h).
From this perspective, the coating strategy effectively prevents direct exposure of the hydrogel to air and mitigates the rate of moisture loss. Lu and co-authors prepared a multilayer structured flexible strain sensor with an inner layer of ionic hydrogel (S-PAM). This layer was composed of a semi-interpenetrating network created with sodium carboxymethyl cellulose (CMC), lithium chloride (LiCl), and polyacrylamide (PAM) (Fig. 8a). In the inner layer of the hydrogel network, strong bonds between water molecules and Li+ and Cl− ions are difficult to disrupt, effectively inhibiting water evaporation at elevated temperatures. The outer layer was fully encapsulated with polydimethylsiloxane (PDMS).105 The sialon groups on the silane coupling agent condense between the inner hydrogel and the outer elastomer to form a siloxane bond. This bond enhances the adhesion between the inner and outer interfaces while also providing the sensors with excellent mechanical properties and water retention at high temperatures. The strain sensors constructed from a hybrid of hydrogels and elastomers demonstrate high sensitivity (gauge factor ∼ 3.8) over a wide temperature range (−20 °C to 60 °C) (Fig. 8b and c). Similarly, an encapsulated hydrogel with superior water retention properties was developed through a double-layer hydrophobic coating. This coating incorporates (3-aminopropyl)triethoxysilane as a chemical binder, thereby facilitating the stable operation of hydrogel strain sensors at elevated temperatures.106
 | ||
| Fig. 8 Other modification strategies under extremely high temperature conditions. (a) Preparation of hydrogel–elastomer (S-PAM/S-PDMS) hybrids. (b) Conductivities of the hybrids in various environments. (c) Cycling stability tests of ΔR/R0 in different environments. Reproduced from ref. 105. Copyright 2022, Wiley-VCH. (d) Schematic illustration of the preparation process of the PDGR (PVA/DMSO/RGO/GO) hydrogel. (e) Monitoring the relative resistance changes of leg movement. Reproduced from ref. 107. Copyright 2021, Royal Society of Chemistry. | ||
In addition to the aforementioned modification strategies, doping can effectively alter the internal network structure of the hydrogel, thereby enhancing its temperature tolerance. Reduced graphene oxide (RGO) and graphene oxide (GO) nanosheets were incorporated into porous, physically cross-linked polyvinyl alcohol (PVA) hydrogel networks using a dimethyl sulfoxide (DMSO)/water binary solvent system (Fig. 8d).107 The interaction of DMSO with polymer chains in the hydrogel network and the strong interaction between GO and PVA chains established a more intact hydrogel network to confer long-term environmental stability. The honeycomb-like micro-network structure and the uniformly dispersed conductive fillers (GO/RGO), which are rich in functional groups, confer the hydrogel network with exceptional stretchability (3.1 MPa at 600% strain) and excellent conductivity. Hydrogels as multifunctional sensors exhibit excellent sensitivity in a wide temperature range (from −30 °C to 60 °C) to monitor physiological signals (Fig. 8e).
3. Extreme humidity conditions
3.1 Extremely high humidity conditions
In high humidity areas, the hydrophilic groups of the polymer in the hydrogel network absorb water from the environment and swell. Swelling phenomena compromise the integrity of the network structure, degrade mechanical properties, and lead to distortion or interruption of sensor signals.108–112 Moreover, a concentration gradient develops between the conductive network of the hydrogel sensor and the high-humidity environment. In a high humidity environment, the electrons/ions in the hydrogel network can leak by diffusion across the interface, which leads to short-circuiting of the conductive network in the sensor.113,114For example, hydrogels with a dense hydrophobic network structure were developed through hydrophobic and electrostatic interactions between the cationic surfactant N-hexa-decyltrimethylammonium chloride (CTAC) and poly(acrylate-methacrylate octadecyl ester) [P(AA–SMA)], as well as double cross-linking and incorporation of CMC–Na (Fig. 9a).116 In the hydrogel network, the hydrophobic long-chain alkyl groups in CTAC interact electrostatically with the –COO groups on the P(AA–SMA) chain, serving as cross-linking points that effectively enhance the anti-swelling properties.117,118 Additionally, the hydrophobic SMA was grafted onto the hydrophilic PAA chain to form a double cross-linked network, thereby increasing the cross-link density and enhancing resistance to swelling. Notably, the mechanical properties remained exceptional after immersion in water for 7 days (Fig. 9b). Furthermore, the hydrogel exhibited outstanding anti-swelling properties across various liquid environments (Fig. 9c). The hydrogel was fabricated into flexible strain sensors that exhibit high sensitivity in the wide range of 0–400% (GF ∼ 1.55–5.03). Moreover, hydrophobic interfaces can disrupt the hydrated layer on the surface of hydrogels, facilitating the formation of strong connections between the target interfaces. Thus, hydrogels are well-suited for real-time underwater sensing applications (Fig. 9d and e). Similarly, based on the principle that myristyl methacrylate forms hydrophobic chain segments through electrostatic interactions within cetyltrimethylammonium bromide micelles, Du and co-authors fabricated hydrogels exhibiting enhanced anti-swelling properties (Fig. 9f).119 Additionally, the entanglement of chitosan chains within the hydrogel network, combined with electrostatic interactions, endows excellent anti-swelling and mechanical properties to the hydrogels by forming robust hydrogen bonds with phytic acid. In hydrogel networks, hydrophilic-hydrophobic copolymers with non-uniform hydrophobic structures can locally adsorb water. Meanwhile, dynamic hydrophobic association (HA) induced by electrostatic interactions can effectively modulate the hydrophobic structural domains, thereby inhibiting water penetration.120 The hydrogel exhibited negligible swelling after 15 days of immersion in water (Fig. 9g). The well-ordered hydrophobic structure, achieved through electrostatic interactions, afforded hydrogel high elongation (1500%) and exceptional elastic recovery behavior (elastic recovery up to 95%). Underwater, the hydrogel-based flexible strain sensors exhibit excellent sensitivity (gauge factor ∼ 1.45) over a strain range of 0–500% (Fig. 9h and i). These sensors, prepared from the hydrogel, are employed for monitoring human signals. The distinct step signals corresponding to finger bending, as illustrated in Fig. 9j, enable practical applications in underwater signaling and sensing.
 | ||
| Fig. 9 Hydrophobic network modification strategies under extremely high humidity conditions. (a) Preparation of the anti-swelling P(AA–SMA)–CMC–Na hydrogel. (b) Image of the P(AA–SMA)–CMC–Na hydrogel after 15 days of immersion in water. (c) Swelling curves of hydrogels immersed in water (pH = 3, 5, 7, 9, 11) for 15 days. (d) Relative resistance variation (ΔR/R0) of the P(AA–SMA)–CMC–Na hydrogel at 0–400% strain and the homologous gauge factor (GF). (e) Relative resistance changes for finger bending in seawater. Reproduced from ref. 116. Copyright 2023, Royal Society of Chemistry. (f) Schematic of the preparation process of constructing multiple physically (hydrogen bonds, electrostatic interaction, hydrophobic association) interacted HA hydrogels. (g) Swelling behavior of hydrogels immersed in water for 15 days. (h) Relative resistance changes under different strains (100–500%). (i) Sensing properties of the hydrogel-based strain sensor underwater. (j) Monitoring the relative resistance changes of finger bending underwater. Reproduced from ref. 119. Copyright 2024, The American Chemical Society. | ||
Hydrogels with fully hydrophobic structures can be achieved for flexible strain sensing by polymerizing hydrophobic monomers (tert-butyl acrylate, t-BuA) within hydrophobic ionic liquids (1-butyl-3-methylimidazolium bis(trifluoromethane sulfonyl)imide, [BMIm]TFSI).121 In liquid environments, a dense hydrophobic interface is formed by the aggregation of hydrophobic polymers within the hydrogel network. This structure effectively blocks the cross-interface diffusion of water molecules, thereby realizing the anti-swelling function of ionic liquid gels. Consequently, these hydrogels enhance the range of applications for flexible strain sensors, particularly for high-humidity sensing.
To resist the swelling behavior of hydrogels used as flexible strain sensors in high humidity environments, Sun and co-authors developed an excellent anti-swelling hydrogel (PAM–ALG–PPy) by incorporating hydrogen, coordination bonds, and cation–π interactions (Fig. 10a).122 The multi-network hydrogel was synthesized via a two-step polymerization process, comprising the free radical polymerization of a pristine solution of acrylamide (AM) and sodium alginate (ALG), followed by the in situ polymerization of pyrrole (Py) within the hydrogel network. Hydrogels with significant tensile strength properties (1.63 MPa) and elongation at break (453%) were synthesized using a supramolecular strategy. As a flexible strain sensor, the hydrogel exhibits high conductivity (2.16 S m−1), a wide strain sensing range (0–400%), and exceptional sensitivity (gauge factor ∼ 4.1). The flexible strain sensors exhibit outstanding resistance to swelling, with the hydrogels maintaining virtually unchanged dimensions after 20 days of immersion in deionized water and seawater. These properties enable the sensor to facilitate real-time monitoring of underwater motion and communication (Fig. 10b and c).
 | ||
| Fig. 10 Other modification strategies under extremely high humidity conditions. (a) Interactions between polymer networks in PAM–ALG–PPy. (b) Images of the PAM–ALG–PPy hydrogel after immersion in water for 20 days. (c) Monitoring the relative resistance changes of underwater motion. Reproduced from ref. 122. Copyright 2023, Wiley-VCH. (d) Schematic illustration of the fabrication process of the DN–FT–HCl (hydrogels obtained by HCl treatment after freeze/thaw treatment of copolymers consisting of PVA, SBMA and HEMA) hydrogel. (e) Swelling behavior of hydrogels immersed in seawater. (f) Relative resistance changes of DN–FT–HCl hydrogel sensors for underwater motion detection. Reproduced from ref. 123. Copyright 2022, Wiley-VCH. | ||
In addition to exploiting the metal ion coordination within the hydrogel network to achieve anti-swelling properties, an anti-swelling hydrogel flexible strain sensor was fabricated by polymerizing [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (SBMA) and 2-hydroxyethyl methacrylate (HEMA) copolymer networks into polyvinyl alcohol (PVA) microcrystalline domains (Fig. 10d).123 The formation of PVA microcrystalline domains was promoted by a freezing/thawing treatment, which provided cross-linking bonds for the amorphous hydrogel network, thereby enhancing the mechanical strength of the hydrogel.124 The incorporation of the amphiphilic ion SBMA creates ion migration channels that facilitate bulk ion transport, enhancing conductivity. Specifically, hydrochloric acid protonates the negatively charged SO3− groups in SBMA, resulting in electrostatic repulsion between cations and the elimination of water molecules. This reduction in osmotic pressure improves the anti-swelling properties of the hydrogel. As an underwater strain sensor, the hydrogel exhibits negligible swelling even when immersed in water or seawater. The sensitivity (gauge factors ∼ 1.434, 2.448, and 3.356) remains satisfactory over a wide strain sensing range (0–100%, 100–200%, and 200–300%), even during immersion in water or seawater (Fig. 10e). Moreover, when subjected to loading and unloading, the sensor exhibits notably short response (130 ms) and recovery times (200 ms).
After 1000 cycles under 50% strain, the hydrogel sensor exhibited zero degradation. Based on the above properties, the sensor is used to recognize different ranges of strain, including head raising, arm swinging, and finger bending, facilitating real-time motion monitoring in underwater environments (Fig. 10f).
3.2 Extremely dry conditions
The water molecules can easily evaporate from the hydrogel network under extremely dry conditions. The hydrogel network does not retain enough strongly bound water to sustain its wet-soft properties, resulting in diminished flexibility of the strain sensor.125–128 Furthermore, the evaporation of water molecules results in a depletion of charge carriers within the hydrogel network129,130 The deterioration in the conductivity of the hydrogel network leads to a subsequent degradation in sensing performance.In order to solve the above problems, the content of free water in the hydrogel network can be reduced or locked. An anti-drying hydrogel (SPGLH) was developed by using the synergistic water retention effect of glycerol and lithium chloride (Fig. 11a).131 In the hydrogel network, Li+ ions formed hydrated complexes, while glycerol established hydrogen bonds with water molecules, resulting in physical entanglement of the polymer.97,132–134 In addition, the introduction of LiCl contributed to the conductivity of SPGLH (Fig. 11b). Compared to the unmodified hydrogel sensors, the SPGLH sensor retained stretchability and adhesion due to excellent anti-drying properties when left at 22 °C/11% RH for 1 h (Fig. 11c). It can operate continuously for 40 hours at 20% relative humidity and maintains stable sensing capability at different humidity levels (10–70% RH) as a flexible strain sensor. Flexible strain sensors are capable of monitoring daily human body movements and enabling human–computer interactions to address real-time sensing requirements. Similarly, the hydrogels with anti-swelling properties were synthesized by homogeneously dispersing the nanofillers TA@HAP NWS, AlCl3, and PVA in a binary solvent of ethylene glycol (EG) and water (W).135 The binary system of EG and water mitigates the evaporation of water in dry environments. The fabrication of flexible strain sensors with a wide linear sensing range (350%) and high sensitivity (GF ∼ 2.84) by using hydrogels is an essential contribution to the future of multifunctional ionic hydrogel sensors.
 | ||
| Fig. 11 Modification strategies under extremely dry conditions. (a) Preparation of hydrogels with water retention properties. (b) The conductivity of SPH, SPGH, SPLH, and SPGLH hydrogels. (c) Water-retention properties of the SPH and SPGLH sensors under 22 °C/11% RH. Reproduced from ref. 131. Copyright 2023, Elsevier. (d) Preparation of C-PAM hydrogels with core–shell structures. (e) Schematic diagram of the anti-drying hydrogel. (f) Cyclic tensile test of the C-PAM hydrogel at 100% strain before and after 7 days of storage under ambient conditions. Reproduced from ref. 137. Copyright 2023, Royal Society of Chemistry. | ||
Furthermore, coatings as protection layers on the hydrogel surface can prevent water evaporation from the hydrogel network, thereby physically mitigating water loss and enhancing water retention properties.136 The hydrophobic hydrogels (C-PAM) were fabricated by coating the surface of micelle-crosslinked polyacrylamide (PAM) hydrogels with a poly(butyl acrylate) emulsion (Fig. 11d).137 The coatings formed a dense hydrophobic layer on the surface of the hydrogel, which largely hindered the diffusion of water molecules in the porous structure of the hydrogel (Fig. 11e).138,139 Excellent anti-drying properties are evidenced by a weight retention of 84 ± 0.45% even after 7 days at room temperature. A highly stable ΔR/R0 was observed at 100% tensile strain before drying and after 7 days of drying under 100 cycles of continuous cyclic loading, which shows the potential for long-term applications (Fig. 11f). Surprisingly, the dynamic micellar cross-linking in hydrogel networks provided an extremely high working range (elongation at break over 5000%). In practical applications, the sensor exhibits high application potential with the advantages of rapid, accurate, and repeatable detection of human motion.
4. Other extreme conditions
4.1 Extreme mechanical strength conditions
One principal factor for the extensive deployment of hydrogel flexible strain sensors is their mechanical strength. High mechanical stress can cause deformation and cracking of the flexible substrate of the sensor, as well as insufficient bonding at the interface between the flexible substrate and the conductive material, leading to slippage.140,141 Under high mechanical stretch conditions, hydrogel strain sensors suffer from hysteresis due to the irreversible energy dissipation of viscoelastic elastomers.142,143 The resistance change dominated by the tunnelling effect leads to a signal drift hysteresis. As the tensile strain increases, the conductive particles become more widely separated, thereby reducing the number of effective conductive pathways and ultimately leading to sensor failure.144 In addition, continuous loading–unloading of the stresses applied to the flexible strain sensors and cracks along the perpendicular direction of stretching lead to irreversible cracking of the interconnections between the conductive lamellae, which results in a complete disruption of the conductive pathway.145,146Most recently, a dual-network hydrogel was constructed by introducing a rigid cellulose/Zn2+/Ca2+ network into a flexible polyvinyl alcohol (PVA)/borax network (Fig. 12a).149 Among them, PVA/borax hydrogels are synthesized by the formation of dynamic borate bonds between borate ions and two diol units, which have high stretchability.150–152 The entanglement of flexible and rigid networks is achieved by adjusting the cross-link density of the hydrogel network. The interaction between the rigid and flexible networks generates an additional energy dissipation mechanism to improve the stretching resistance of the hydrogel further (Fig. 12b).153,154 Furthermore, the coordination of metal ions with tetrahedral borate ions, B(OH)4−, provides the hydrogel with conductivity.155 The hydrogel was assembled as a strain sensor, and demonstrates high sensor sensitivity (GF ∼ 0.7–1.04) over a wide strain sensing range (0–600%) and stability under multiple tensile cycling conditions (Fig. 12d). Moreover, it demonstrated a reasonable corresponding response time (124 ms) and recovery time (187 ms) during the sensing process. The assembled hydrogel sensors can be used for long-term real-time signal monitoring of different human motions. For example, the electrical signals are regularly reproducible at different angles of knee bending (Fig. 12c).
 | ||
| Fig. 12 DN hydrogel modification strategies under extreme mechanical strength conditions. (a) Schematic synthesis of double-network PVA/cellulose hydrogels. (b) Stretching mechanism of hydrogel strain sensors. (c) Relative resistance changes for knee squatting. (d) Relative resistance of the hydrogel strain sensor through 335 continuous cycles of stretching and recovery. Reproduced from ref. 149. Copyright 2024, Elsevier. (e) Schematic illustration of the preparation process of the PAM/SA/MXene hydrogel. (f) Schematic diagram of the PAM/SA/MXene hydrogel during the stretching and release process. Reproduced from ref. 156. Copyright 2022, Royal Society of Chemistry. | ||
Similarly, highly stretchable MXene composite dual-network hydrogels were synthesized by introducing electrically conductive MXene nanosheets into polyacrylamide (PAM) and sodium alginate (SA) hydrogel networks (Fig. 12e).156 The high electrical conductivity and sensitivity of hydrogels are attributed to the formation of a three-dimensional conductive network structure, which is facilitated by the uniform distribution of hydrophilic MXene nanosheets. Meanwhile, the supramolecular interactions between the dual-network matrices improved the mechanical properties of the hydrogels effectively, which resulted in excellent tensile properties (2000%) and tensile cycling stability. In a high mechanical stretching environment, chemical bonds within the brittle network fall victim to energy depletion. The interpenetrating ductile network imparts excellent tensile properties to the hydrogel, which overcomes the lack of tensile properties of single network hydrogels (Fig. 12f).157 The hydrogel was assembled as a flexible strain sensor, and the GF values are 0.81 and 1.4 when the strain is in the range of 0–100% and 100–2000%, respectively. Moreover, the relative resistance of the hydrogel sensor increased significantly with strain and immediately decreased upon release. This provided fast response (0.75 s) and recovery (0.81 s) performance. The excellent durability of the strain sensor is exhibited by loading and unloading 300 times at 50% tensile strain with the relative resistance value remaining stable. The flexible strain sensors based on double-network structure hydrogels are enabled to monitor the human body movement in real-time, thereby significantly enhancing the potential for these sensing devices in health monitoring applications.
Dual network (DN) hydrogels consisting of (sodium alginate–Zn) SA–Zn and P(AA–AM) (acroleic acid–acrylamide) were prepared by a one-step redox reaction method to improve the mechanical properties, and graphene oxide (GO) was modified by using polydopamine@silver nanoparticles (PDA@Ag) to improve the sensitivity.158,159 In addition, the construction of the dual network has endowed the hydrogel network with high stretchability for continuous strain sensing. The GO/PDA@Ag/SA–Zn hydrogel strain sensor was prepared with an ultra-low strain detection limit (0.1%), a good gauge factor (GF ∼ 8.29), and high stretchability (600%). Based on the above characteristics, hydrogel strain sensors can be used for continuous monitoring of full-size human body movements, and can be used as a speech interface to recognize the ten types of commonly used words (with 100% accuracy).
Compared to dual network hydrogels, the acid-induced cross-linking of metal ions within the hydrogel network generates sacrificial ionic bonds that possess energy-dissipating capabilities. Additionally, the acid facilitates the uniform release and distribution of calcium ions, which endows the hydrogel with enhanced mechanical tensile properties. Besides, with optimum calcium ion concentration, larger cross-link density and smaller pores can effectively avoid stress concentration and crack extension. Thus, hydrogels with strong mechanical properties were obtained by introducing Ca2+ into sodium alginate/polyacrylamide hydrogels and using acid-induced homogeneous release of calcium ions (Fig. 13a).162 The utilization of hydrogels for flexible sensors provided a wide strain sensing range (0.03–1800%), fast response time (∼0.02 s), high sensitivity (GF ∼ 8.9), and durability (500 cycles at a strain of 50%) (Fig. 13b). Hydrogel-based flexible strain sensors can be utilized to monitor both human movement and mouse activities.
 | ||
| Fig. 13 Metal ion modification strategies under extreme mechanical strength conditions. (a) Schematic diagram of acid-triggered Ca2+ release and SA network cross-linking. (b) Relative resistance changes to tensile strain in Ca2+/SA/PAM–DN hydrogels. Reproduced from ref. 162. Copyright 2023, Royal Society of Chemistry. (c) Schematic illustration of the fabrication of the PSM–DN hydrogels. (d) Relative resistance change of the PSM–Ca2+ hydrogel based on minor strain. (e) Resistance changes of the PSM–Ca2+ hydrogel strain sensor for swallowing movements. (f) Relative resistance change of the PSM–Ca2+ hydrogel based on large strain and GF. Reproduced from ref. 163. Copyright 2023, The American Chemical Society. | ||
In order to develop hydrogels with higher sensitivity and excellent mechanical properties, a strategy to improve the mechanical strength of MXene-based dual-network hydrogels through metal ion coordination effects was proposed. In the dual-network hydrogel, MXene nanosheets provide high conductivity, while the strong coordination bonds between metal ions (Ca2+) and sodium alginate (SA) construct the secondary network (Fig. 13c).163 During the process of stretching, the metal ionic ligand bonds act as energy dissipating sacrificial bonds and the metal–ligand covalent bonds and MXene nanosheets work together to inhibit the inter-sliding between the entangled molecular chains in the hydrogel. Furthermore, the hydrogen-bonded coordination and covalent bonding in the hydrogel network contribute to the molecules recovering to their original state, which significantly improves the durability of the hydrogel. The hydrogel was utilized to fabricate multifunctional sensors, which demonstrated high sensitivity to minor and major strains. Within small strain ranges, the sensor maintains stable signals even under repeated stretching, thereby enabling precise detection and monitoring of human swallowing movements (Fig. 13d and e). Moreover, the resistance value of hydrogel strain sensors exhibited almost no variation during the continuous tensile cycle test (400 cycles) at 90% strain, which indicated the stability and durability of the sensor (Fig. 13f). Wu and co-authors designed a bilayer-structured polyacrylamide–acrylic acid/Zr4+ (P(AAm-co-AAc)/Zr4+) hydrogel with a sandwich pattern made of liquid metal (LM). The moderate water content of the hydrogel matrix is controlled by using carboxyl–Zr4+ coordination bonds. It makes the flexible strain sensor highly sensitive and adaptable to high mechanical stretching.164
Conventional hydrogels typically exhibit monofunctionalization and fragile mechanical properties. The semi-interpenetrating network structure of a hydrogel with multiple cross-linking points was constructed by using carboxymethyl cellulose (CMC) with rigid chains and polyacrylic acid (PAA) with flexible chains, which conferred the hydrogel with excellent mechanical stretching properties (Fig. 14a).165 The chemical cross-linking of the polyacrylic acid molecular chain with Al3+ leads to metal coordination interaction, which forms multiple cross-linking points. This synergy enhances the conductivity of both electrons and ions, endowing the hydrogel with excellent conductivity (40.1 mS cm−1), sensitivity (GF ∼ 3.81), and mechanical stretchability (Fig. 14b). During the stretching process, the carboxymethylcellulose network with rigid chains may undergo internal fracturing, thereby shielding the polyacrylic acid flexible network from damage by reducing stress concentration. This mechanism provides the hydrogel with remarkable tensile properties (1904%) and toughness (1109.8 kJ m−3). The interaction fatigue test was performed with 200 loading–unloading cycles at 50% strain. After the cycles, ΔR/R0 remained almost unchanged, demonstrating the excellent stability and durability of the hydrogel sensor (Fig. 14d). In addition, non-covalent interactions such as hydrogen bonding, metal coordination, hydrophobic interactions, and π–π stacking exist between the hydrogel and the object surfaces, ensuring excellent adhesion of the flexible strain sensors under high mechanical stretching conditions. Based on the above properties, hydrogels used to prepare the strain sensors can be used for real-time monitoring of small muscle changes, information recognition, and transmission. Based on the above characteristics, the strain sensors prepared by using hydrogels can be used not only for real-time monitoring of small changes in muscles, but also for information identification and transmission. Wearable devices for personalized health monitoring and information encryption have a wide range of applications (Fig. 14c).
 | ||
| Fig. 14 Other modification strategies under extreme mechanical strength conditions. (a) Schematic illustration of the fabrication of the PDA–CNTs/CMC/PAA hydrogel. (b) Resistance and sensitivity changes of stretch deformation of PDA–CNTs/CMC/PAA hydrogels. (c) Relative resistance changes for knee bending. Reproduced from ref. 165. Copyright 2023, Royal Society of Chemistry. (d) The strain sensor loaded and unloaded for 200 cycles repeatedly at 50% tensile strain. (e) Preparation of PVA hydrogels based on the Hofmeister effect. Relative resistance variation of the hydrogel sensors with (f) tensile strains from 30% to 150%. (g) Gauge factor (GF) of the sensor. Reproduced from ref. 166. Copyright 2024, Elsevier. (h) Schematic diagram of stretching recovery of nanoparticle SiO2-g-PAAm hydrogels. (i and j) The relative resistance changes of the sensor in different positions during one complete basketball shooting behavior. Reproduced from ref. 170. Copyright 2021, The American Chemical Society. | ||
Compared to biomaterials, achieving a balance between strength and fatigue resistance is challenging due to the relatively low mechanical properties of hydrogels. To address this challenge, mechanically tunable hydrogels with excellent mechanical properties and electrical conductivity were fabricated by synergistically inducing the conformational modification of the molecular chains using solvent exchange and the Hofmeister effect (Fig. 14e).166 In the hydrogel network, the Hofmeister effect (salting-out effect) can trigger aggregation and rearrangement of PVA chains. In addition, the cooperative action of the salting-out effect through the entanglement of polymer chains induces increased crystallinity, which leads to the formation of a dense polymer network with a physically cross-linked microcrystalline structure to improve the mechanical stretching properties of hydrogels.167 The application of hydrogels in flexible strain sensors leads to excellent overall mechanical properties with a superior stretchability of 1252.3 ± 116%, an extraordinarily high strength of 26.4 ± 1.6 MPa, and high sensitivity (GF ∼ 0.95) under 150% strain (Fig. 14f and g). The sensors enable real-time monitoring of human motion (nodding, lifting, bending of the wrist, etc.) and can also serve as sensing elements for human–computer interaction.
Nanocomposites are a typical functional material. The formation of hydrogels has the function of physical or chemical cross-linking agent, which endows the hydrogel with excellent mechanical as well as electrical conductivity.168,169 Under high tensile strain, the nanomaterials efficiently transfer and relieve the loads, which endows the hydrogel with higher fracture strength, elongation, and compressive strain. The core–shell hybridized nanoparticle SiO2-g-PAAM (PAAc (polymer acrylic acid) matrix and the PAAm (polymer acrylamide) chains grafted onto the SiO2 core were used as physical cross-linking agent centers. The dense dynamic hydrogen bonding between the PAAm ectodomain and the PAAc matrix can enable reversible destruction and reconstruction, de-dissipating large amounts of energy, and can also confer hydrogel flexibility and self-healing properties (Fig. 14h). Due to these unique properties, hydrogels exhibit excellent stretchability (1600%) and self-healing capabilities. The sensors fabricated with the prepared hydrogels exhibited high sensitivity (GF ∼ 5.86) in the strain range of 50% to 500%. Based on the excellent performance, the sensors can be used to monitor a wide range of strains in human movement (e.g., basketball shooting).170 This research provides a reference for hydrogel sensors with excellent tensile and sensing properties (Fig. 14i).
4.2 Strong corrosion conditions
Hydrogel flexible strain sensors often fail to maintain structural integrity in strongly corrosive environments, as highly corrosive solvents typically cause swelling of the polymer matrix and weaken the interactions between molecular chains.171,172 For example, strong acidic solvents can break the hydrogen bonds in hydrogels that rely on physical cross-linking leading to the decomposition of the matrix material.173 This degradation can erode the conductive network, potentially affecting or altering the electron/ion transport process.174 Furthermore, the performance of flexible strain sensing is compromised due to increased swelling and loss of mechanical properties in such corrosive environments.175To address this issue, the swelling resistance of hydrogels in strong corrosive solutions was effectively improved by introducing strong chemical bonds and graphene oxide (GO) nanolayers. In addition, the chemical functionalization was effectively promoted by GO surface functional groups, which further improved the corrosion inhibition and mechanical stretching properties of the hydrogel (Fig. 15a).176–178 The hydrogels were immersed in different polar liquids and exhibited excellent anti-swelling behavior in most solutions (Fig. 15b).179 Flexible strain sensors prepared from hydrogels maintain structural integrity even after 400 days of immersion in strongly acidic solutions. Remarkably, the sensor even succeeds in enabling accurate and stable sensitivity for tensile strains in a variety of solvents such as water, strongly alkaline solutions, isopropanol, dimethyl sulfoxide, and cyclohexane. Nevertheless, hydrogel sensors combine high stretchability (800%), wide sensing range (0.5–800%), fast response time, high sensitivity (GF ∼ 2.17–4.96), and excellent durability (4000 cycles at 30% strain). The sensors can be used directly to detect human motion signals, including joint movements and facial expressions.
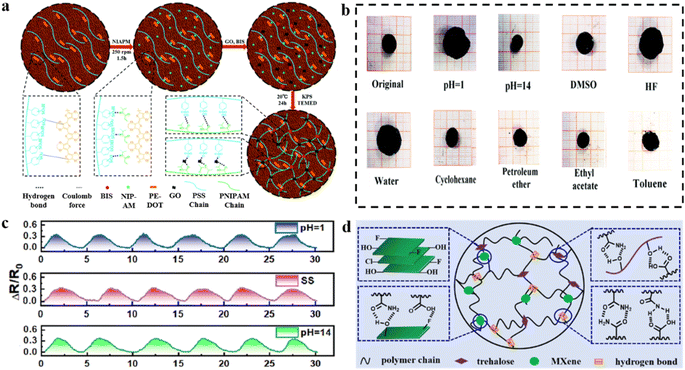 | ||
| Fig. 15 Modification strategies under strong corrosion conditions. (a) Preparation process of corrosion-resistant hydrogels. (b) Swelling behavior of hydrogels in solutions of different polarities. Reproduced from ref. 179. Copyright 2021, Royal Society of Chemistry. (c) Relative resistance changes of hydrogel strain sensors in solution (pH = 1, pH = 14). (d) Preparation of corrosion-resistant (PMATMx) hydrogels. Reproduced from ref. 180. Copyright 2022, Royal Society of Chemistry. | ||
Encapsulation is one of the most important strategies to ensure that electronic devices are protected from damage caused by corrosive environments. By incorporating MXenes into the hydrogel network, conductivity is enhanced through their role as a conductive filler, and mechanical properties are improved by increasing the physical cross-linking points within the network.180 Moreover, the hydrophobic fluorine groups in MXenes modulate the hydrophobicity and increase the bonding strength of the hydrogel to the encapsulation layer (PDMS) (Fig. 15d). Encapsulated hydrogel–elastomer hybrid materials with core–shell structures were developed for use as flexible strain sensors with the advantages of high sensitivity (GF is 2.21, 4.38, and 2.15 in the strain range of 0–60%, 60–120%, and 120–150%, respectively), fast response/recovery (125 ms/140 ms), and stable sensing performance in harsh environments such as acids (pH = 1), alkali (pH = 14), salt solutions (1 M NaCl), and alcohols (Fig. 15c).
5. Summary and outlook
Hydrogels with a unique three-dimensional conductive network have been widely used in the field of flexible strain sensors. However, the applications of hydrogel-based flexible strain sensors under extreme environmental conditions are still in the early stages, with numerous challenges remaining unresolved. In this review, we summarize the main challenges associated with the performance of hydrogel-based flexible strain sensors under various extreme environmental conditions, including extreme temperature, extreme humidity, high mechanical stretching, and high corrosion. Correspondingly, we discuss the improvement strategies proposed to address these challenges in different extreme environments and evaluate their current applications, which remain unsatisfactory. Based on this analysis, we propose the following potential future research directions (Fig. 16): | ||
| Fig. 16 Proposed future research directions for hydrogel-based flexible strain sensors operating under harsh environmental conditions. | ||
(1) The depth and prevalence of hydrogel-based strain sensor applications have not yet been thoroughly investigated. Current hydrogel flexible strain sensors are still at the laboratory level and have not been commercialized. The challenges faced in practical applications in various extreme environments are complex. The physical and chemical changes that occur within hydrogel networks in such extreme environments are not well understood and require further exploration, while most research has focused on extreme conditions such as temperature, humidity, and high mechanical stretching; other extreme environments, such as high radiation, also impact hydrogel networks and sensor performance, which needs further investigation. In the future, the application of hydrogel-based flexible strain sensors in various harsh environments can be achieved through material selection and optimization (utilizing high radiation-resistant materials and polymer-based composites with high pressure tolerance), advanced packaging technologies (incorporating metallic materials and composite shielding to ensure sensor integrity), sensor structural design (integrating smart algorithms, self-calibration functions, and compensation mechanisms), and the integration of multiple sensors.
(2) Enhancing the adaptability of strain sensors in extreme environments requires the integration of advanced technologies. The performance of hydrogel flexible strain sensors can be precisely tuned by combining frontier technologies such as 3D printing and lithography. Additionally, combining these sensors with machine learning and human–computer interaction can enable more efficient and versatile sensing capabilities.
(3) The current form factor of hydrogel-based flexible sensors limits their application in smart wearables. Most existing hydrogel-based flexible sensors are in the form of 2D films or 3D foams, limiting the application of smart wearables. Future research should focus on fabricating fibrous sensors through spinning techniques and integrating them into textiles using weaving technologies such as plain weave and twill weave. This approach would enable sensors to be worn on the body for all-weather, real-time monitoring of physiological conditions.
(4) Exploration of extreme environments not only increases the demand for hydrogel flexible strain sensors but also poses challenges to their reuse and recycling. To enhance the performance of hydrogels in extreme environments, toxic solvents (dimethyl sulfoxide, etc.) are often used, which can be hazardous to the human body when used for long periods. In addition, the degrading and recycling of some existing hydrogel materials for flexible strain sensors present significant challenges.
(5) Hydrogels for flexible sensors offer excellent conductivity, enabling integrated applications with other devices. Hydrogels with ionic conductivity not only endow flexible strain sensors with excellent flexibility and sensing properties but also serve as electric and energy storage devices (batteries, nanofriction generators, ionic skin, solar cells, etc.). They also have applications in the medical field (drug delivery, tissue engineering scaffolds, etc.), etc. Therefore, the self-powering of flexible sensors can be realized by integrating sensors and solar cells through conductive hydrogels.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
M. L. and J. P. engaged in discussions regarding the content and structure. J. P. and Q. C. provided guidance and revisions on the manuscript content. W. Z., Y. G., T. M., and J. C. contributed significant insights. All authors contributed to writing the manuscript. C. G. supervised the whole project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National Key Research and Development Program (2022YFE0121000), Natural Science Foundation of Shaanxi (2022ZY2-JCYJ-01-10), Natural Science Foundation of Xi'an (24ZDCYJSGG0039), and Fundamental Research Funds for the Central Universities. The authors also acknowledge the support from Xi'an Polytechnic University.Notes and references
- S. J. Zheng, X. W. Wang, W. Z. Li, Z. Y. Liu, Q. N. Li and F. Yan, Nat. Electron., 2024, 7, 576–585 CrossRef CAS.
- Z. Wang, Y. Liu, Z. Zhou, P. Chen and H. Peng, Nat. Rev. Electr. Eng., 2024, 1, 466–477 CrossRef.
- Y. Sun, Y. Su, Z. Chai, L. Jiang and L. Heng, Nat. Commun., 2024, 15, 7290 CrossRef CAS.
- L. Zhang, L. Chen, S. Wang, S. Wang, D. Wang, L. Yu, X. Xu, H. Liu and C. Chen, Nat. Commun., 2024, 15, 3859 CrossRef CAS PubMed.
- A. M. Moran, V. T. Vo, K. J. McDonald, P. Sultania, E. Langenbrunner, J. H. V. Chong, A. Naik, L. Kinnicutt, J. Li and T. Ranzani, Communications Engineering, 2024, 3, 117 CrossRef PubMed.
- Z. Li, Z. Li, W. Tang, J. Yao, Z. Dou, J. Gong, Y. Li, B. Zhang, Y. Dong, J. Xia, L. Sun, P. Jiang, X. Cao, R. Yang, X. Miao and R. Yang, Nat. Commun., 2024, 15, 7275 CrossRef CAS.
- B.-H. Xiao, K. Xiao, J.-X. Li, C.-F. Xiao, S. Cao and Z.-Q. Liu, Chem. Sci., 2024, 15, 11229–11266 RSC.
- S. H. Kim, A. Basir, R. Avila, J. Lim, S. W. Hong, G. Choe, J. H. Shin, J. H. Hwang, S. Y. Park and J. Joo, Nature, 2024, 629, 1047–1054 CrossRef CAS.
- Z. Ma, W. Shi, K. Yan, L. Pan and G. Yu, Chem. Sci., 2019, 10, 6232–6244 RSC.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1880 CrossRef CAS PubMed.
- Y. Sun, J. Huang, Y. Cheng, J. Zhang, Y. Shi and L. Pan, SmartMat, 2024, e1269 CrossRef.
- Z. Jiang, N. Chen, Z. Yi, J. Zhong, F. Zhang, S. Ji, R. Liao, Y. Wang, H. Li and Z. Liu, Nat. Electron., 2022, 5, 784–793 CrossRef CAS.
- Q. Zhuang, K. Yao, C. Zhang, X. Song, J. Zhou, Y. Zhang, Q. Huang, Y. Zhou, X. Yu and Z. Zheng, Nat. Electron., 2024, 7, 598–609 CrossRef CAS.
- Y. Zhang, N. Li, Y. Xiang, D. Wang, P. Zhang, Y. Wang, S. Lu, R. Xu and J. Zhao, Carbon, 2020, 156, 506–513 CrossRef CAS.
- M. Gwiazda, A. Kaushik, A. Chlanda, E. Kijeńska-Gawrońska, J. Jagiełło, K. Kowiorski, L. Lipińska, W. Święszkowski and S. K. Bhardwaj, Appl. Surf. Sci. Adv., 2022, 9, 100258 CrossRef.
- Z. Zhai, X. Zhang, J. Wang, H. Li, Y. Sun, X. Hao, Y. Qin, B. Niu and C. Li, Chem. Eng. J., 2022, 428, 131720 CrossRef CAS.
- D. Maurya, S. Khaleghian, R. Sriramdas, P. Kumar, R. A. Kishore, M. G. Kang, V. Kumar, H.-C. Song, S.-Y. Lee and Y. Yan, Nat. Commun., 2020, 11, 5392 CrossRef CAS PubMed.
- C. Li, S. Cong, Z. Tian, Y. Song, L. Yu, C. Lu, Y. Shao, J. Li, G. Zou and M. H. Rümmeli, Nano Energy, 2019, 60, 247–256 CrossRef CAS.
- Y. Liu, Z. Xu, X. Ji, X. Xu, F. Chen, X. Pan, Z. Fu, Y. Chen, Z. Zhang and H. Liu, Nat. Commun., 2024, 15, 5354 CrossRef CAS.
- S. Seyedin, P. Zhang, M. Naebe, S. Qin, J. Chen, X. Wang and J. M. Razal, Mater. Horiz., 2019, 6, 219–249 RSC.
- Z. Wang, Y. Cong and J. Fu, J. Mater. Chem. B, 2020, 8, 3437–3459 RSC.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- D. Caccavo, S. Cascone, G. Lamberti and A. Barba, Chem. Soc. Rev., 2018, 47, 2357–2373 RSC.
- J. Zhao, Z. Wang, L. Mo, X. Meng, L. Li and Z. Peng, Prog. Chem., 2022, 34, 2202 CAS.
- X. Xuan, H. S. Yoon and J. Y. Park, Biosens. Bioelectron., 2018, 109, 75–82 CrossRef CAS.
- A. Mehmood, N. Mubarak, M. Khalid, R. Walvekar, E. Abdullah, M. T. H. Siddiqui, H. A. Baloch, S. Nizamuddin and S. Mazari, J. Environ. Chem. Eng., 2020, 8, 103743 CrossRef CAS.
- X. Qi, X. Li, H. Jo, K. S. Bhat, S. Kim, J. An, J.-W. Kang and S. Lim, Sens. Actuators, A, 2020, 301, 111697 CrossRef CAS.
- J. H. Yoon, S.-M. Kim, H. J. Park, Y. K. Kim, D. X. Oh, H.-W. Cho, K. G. Lee, S. Y. Hwang, J. Park and B. G. Choi, Biosens. Bioelectron., 2020, 150, 111946 CrossRef CAS PubMed.
- J. Pu, Y. Gao, Z. Geng, Y. Zhang, Q. Cao, J. Yang, X. Zhao, Y. Wang, J. Wang and C. Guan, Adv. Funct. Mater., 2024, 34, 2304453 CrossRef CAS.
- T. Yan, Y. Wu, J. Tang and Z. Pan, Mater. Res. Bull., 2021, 143, 111452 CrossRef CAS.
- Z. Zhao, Q. Li, Y. Dong, J. Gong, Z. Li, X. Qiao and J. Zhang, Energy Technol., 2021, 9, 2100166 CrossRef.
- B. Zhang, L. Zhang, W. Deng, L. Jin, F. Chun, H. Pan, B. Gu, H. Zhang, Z. Lv and W. Yang, ACS Nano, 2017, 11, 7440–7446 CrossRef CAS.
- J. Pu, Y. Gao, Q. Cao, G. Fu, X. Chen, Z. Pan and C. Guan, SmartMat, 2022, 3, 608–618 CrossRef CAS.
- F. Bu, Y. Gao, W. Zhao, Q. Cao, Y. Deng, J. Chen, J. Pu, J. Yang, Y. Wang and N. Yang, Angew. Chem., 2024, 136, e202318496 CrossRef.
- M. Hou, M. Yu, W. Liu, H. Zhang, Z. Wang, J. Du, L. Xu, N. Li and J. Xu, Chem. Eng. J., 2024, 483, 149299 CrossRef CAS.
- Q. Zhang, H. Lu, G. Yun, L. Gong, Z. Chen, S. Jin, H. Du, Z. Jiang and W. Li, Adv. Funct. Mater., 2024, 34, 2308113 CrossRef CAS.
- Z. Han, P. Wang, Y. Lu, Z. Jia, S. Qu and W. Yang, Sci. Adv., 2022, 8, eabl5066 CrossRef CAS.
- Y. Wang, P. Chen, X. Zhou, Y. Liu, N. Wang and C. Gao, ACS Appl. Mater. Interfaces, 2022, 14, 47100–47112 CrossRef CAS.
- S. Zhang, R. Sun, J. Wang, Z. Jiang, M. Liu, H. Chen, Z. Hu, X. Zhan, F. Gao and Q. Zhang, Mater. Horiz., 2024 10.1039/d4mh00970c.
- Y. Ni, X. Zang, Y. Yang, Z. Gong, H. Li, J. Chen, C. Wu, J. Huang and Y. Lai, Adv. Funct. Mater., 2024, 34, 2402853 CrossRef CAS.
- S. Zhang, F. Guo, X. Gao, M. Yang, X. Huang, D. Zhang, X. Li, Y. Zhang, Y. Shang and A. Cao, Advanced Science, 2024, 2405880 CrossRef.
- S.-N. Li, Z.-R. Yu, B.-F. Guo, K.-Y. Guo, Y. Li, L.-X. Gong, L. Zhao, J. Bae and L.-C. Tang, Nano Energy, 2021, 90, 106502 CrossRef CAS.
- F. Wang, J. Chen, X. Cui, X. Liu, X. Chang and Y. Zhu, ACS Appl. Mater. Interfaces, 2022, 14, 30268–30278 CrossRef CAS.
- Q. Feng, K. Wan, C. Zhang and T. Liu, J. Polym. Sci., 2022, 60, 2710–2719 CrossRef CAS.
- M. Zhu, X. Wang, H. Tang, J. Wang, Q. Hao, L. Liu, Y. Li, K. Zhang and O. G. Schmidt, Adv. Funct. Mater., 2020, 30, 1907218 CrossRef CAS.
- R. Hou, Y. Xie, R. Song, J. Bao, Z. Shi, C. Xiong and Q. Yang, Cellulose, 2024, 31, 4247–4262 CrossRef CAS.
- Q. Rong, W. Lei, L. Chen, Y. Yin, J. Zhou and M. Liu, Angew. Chem., Int. Ed., 2017, 56, 14159–14163 CrossRef CAS PubMed.
- L. Li, Y. Zheng, E. Liu, X. Zhao, S. Yu, J. Wang, X. Han, F. Xu, Y. Cao and C. Lu, Chem. Eng. J., 2022, 437, 135399 CrossRef CAS.
- S. Wu, P. Liu, W. Tong, J. Li, G. Xu, F. Teng, J. Liu, H. Feng, R. Hu and A. Yang, Compos. Sci. Technol., 2023, 231, 109816 CrossRef CAS.
- S. Niu, S. He, Y. Chen, Z. Zhu, X. Chang, C. Yang, J. Li, Y. Jiang, D. Wang and Y. Zhu, Adv. Mater. Technol., 2023, 8, 2300867 CrossRef CAS.
- L. Zhao, Q. Ling, X. Fan and H. Gu, ACS Appl. Mater. Interfaces, 2023, 15, 40975–40990 CrossRef CAS PubMed.
- J. S. Kim and A. Yethiraj, J. Chem. Phys., 2008, 129, 124504 CrossRef PubMed.
- B. Yao, S. Wu, R. Wang, Y. Yan, A. Cardenas, D. Wu, Y. Alsaid, W. Wu, X. Zhu and X. He, Adv. Funct. Mater., 2022, 32, 2109506 CrossRef CAS.
- Z. Liu, J. Zhang, J. Liu, Y. Long, L. Fang, Q. Wang and T. Liu, J. Mater. Chem. A, 2020, 8, 6219–6228 RSC.
- Y. Wang, L. Zhang and A. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 41710–41716 CrossRef CAS.
- H. Liu, X. Wang, Y. Cao, Y. Yang, Y. Yang, Y. Gao, Z. Ma, J. Wang, W. Wang and D. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 25334–25344 CrossRef CAS PubMed.
- C. Luo, X. Deng and S. Xie, J. Mater. Chem. C, 2021, 9, 17042–17049 RSC.
- O. Morgenstern, F. M. O'Connor, B. T. Johnson, G. Zeng, J. P. Mulcahy, J. Williams, J. Teixeira, M. Michou, P. Nabat, L. W. Horowitz, V. Naik, L. T. Sentman, M. Deushi, S. E. Bauer, K. Tsigaridis, D. T. Shindell and D. E. Kinnison, Geophys. Res. Lett., 2020, 47, e2020GL088295 CrossRef CAS.
- Z. Wu, G. Guo, B. K. Biswal, J. Dai and G. Chen, Bioresour. Technol., 2020, 313, 123574 CrossRef CAS.
- W. Wang, S. Xu, Q. Li and S. Dong, Constr. Build. Mater., 2022, 317, 126164 CrossRef CAS.
- C. Wang, B. Yang, R. Xiang, J. Ji, Y. Wu and S. Tan, ACS Nano, 2023, 17, 23194–23206 CrossRef CAS.
- S.-N. Li, X.-F. He, Z.-F. Zeng, B. Jiang, Q. Wu, L.-X. Gong, Y. Li, J. Bae, S. Wang and L.-C. Tang, Nano Energy, 2022, 103, 107789 CrossRef CAS.
- G. Chen, J. Huang, J. Gu, S. Peng, X. Xiang, K. Chen, X. Yang, L. Guan, X. Jiang and L. Hou, J. Mater. Chem. A, 2020, 8, 6776–6784 RSC.
- Z. He, Z. Zhou and W. Yuan, ACS Appl. Mater. Interfaces, 2022, 14, 38205–38215 CrossRef CAS PubMed.
- Z. Wu, W. Shi, H. Ding, B. Zhong, W. Huang, Y. Zhou, X. Gui, X. Xie and J. Wu, J. Mater. Chem. C, 2021, 9, 13668–13679 RSC.
- A. Chandra, Phys. Rev. Lett., 2000, 85, 768 CrossRef CAS PubMed.
- H. Liao, X. Guo, P. Wan and G. Yu, Adv. Funct. Mater., 2019, 29, 1904507 CrossRef.
- Y. Zhao, Z. Chen, F. Mo, D. Wang, Y. Guo, Z. Liu, X. Li, Q. Li, G. Liang and C. Zhi, Advanced Science, 2021, 8, 2002590 CrossRef CAS PubMed.
- L.-Y. Zeng, X.-C. Wang, Y. Wen, H.-M. Chen, H.-L. Ni, W.-H. Yu, Y.-F. Bai, K.-Q. Zhao and P. Hu, Carbohydr. Polym., 2023, 300, 120229 CrossRef CAS PubMed.
- Z. Qin, D. Dong, M. Yao, Q. Yu, X. Sun, Q. Guo, H. Zhang, F. Yao and J. Li, ACS Appl. Mater. Interfaces, 2019, 11, 21184–21193 CrossRef CAS.
- X. Pan, Q. Wang, R. Guo, Y. Ni, K. Liu, X. Ouyang, L. Chen, L. Huang, S. Cao and M. Xie, J. Mater. Chem. A, 2019, 7, 4525–4535 RSC.
- S. Bao, J. Gao, T. Xu, N. Li, W. Chen and W. Lu, Chem. Eng. J., 2021, 411, 128470 CrossRef CAS.
- Y. Jian, S. Handschuh-Wang, J. Zhang, W. Lu, X. Zhou and T. Chen, Mater. Horiz., 2021, 8, 351–369 RSC.
- D. Sun, N. Li, J. Rao, S. Jia, Z. Su, X. Hao and F. Peng, J. Mater. Chem. A, 2021, 9, 14381–14391 RSC.
- C. Shao, M. Wang, L. Meng, H. Chang, B. Wang, F. Xu, J. Yang and P. Wan, Chem. Mater., 2018, 30, 3110–3121 CrossRef CAS.
- J. Cai, X. Zhang, W. Liu, J. Huang and X. Qiu, Polymer, 2020, 202, 122643 CrossRef CAS.
- Y. Liu, G. Tian, Y. Du, P. Shi, N. Li, Y. Li, Z. Qin, T. Jiao and X. He, Adv. Funct. Mater., 2024, 34, 2315813 CrossRef CAS.
- J. Zhang, Y. Liang, Z. Deng, H. Xu, H. Zhang, B. Guo and J. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 29902–29913 CrossRef CAS PubMed.
- L. Xu, Z. Huang, Z. Deng, Z. Du, T. L. Sun, Z. H. Guo and K. Yue, Adv. Mater., 2021, 33, 2105306 CrossRef CAS PubMed.
- A. Agafonov, L. Ramenskaya, E. Grishina and N. Kudryakova, RSC Adv., 2021, 11, 38605–38615 RSC.
- Q. Xu, M. Hou, L. Wang, X. Zhang and L. Liu, Chem. Eng. J., 2023, 477, 147065 CrossRef CAS.
- W. Zhao, J. Jiang, W. Chen, Y. He, T. Lin and L. Zhao, Chem. Eng. J., 2023, 468, 143660 CrossRef CAS.
- D. M. Correia, L. C. Fernandes, M. M. Fernandes, B. Hermenegildo, R. M. Meira, C. Ribeiro, S. Ribeiro, J. Reguera and S. Lanceros-Mendez, Nanomaterials, 2021, 11, 2401 CrossRef CAS.
- J. Liu, X. Zhang, Y. Cui, Y. Liu, W. Wang, Y. Guo, Q. Wang and X. Dong, ACS Appl. Mater. Interfaces, 2024, 16, 5208–5216 CrossRef CAS PubMed.
- Y. Ye, H. Oguzlu, J. Zhu, P. Zhu, P. Yang, Y. Zhu, Z. Wan, O. J. Rojas and F. Jiang, Adv. Funct. Mater., 2023, 33, 2209787 CrossRef CAS.
- R. Liu, L. Cui, H. Wang, Q. Chen, Y. Guan and Y. Zhang, Results Phys., 2021, 13(35), 42052–42062 CAS.
- Y. Yang, Y. Yang, Y. Cao, X. Wang, Y. Chen, H. Liu, Y. Gao, J. Wang, C. Liu and W. Wang, Chem. Eng. J., 2021, 403, 126431 CrossRef CAS.
- Y. Wang, Y. Xia, P. Xiang, Y. Dai, Y. Gao, H. Xu, J. Yu, G. Gao and K. Chen, Chem. Eng. J., 2022, 428, 131171 CrossRef CAS.
- Z. He, C. Wu, M. Hua, S. Wu, D. Wu, X. Zhu, J. Wang and X. He, Matter, 2020, 2, 723–734 CrossRef.
- I. K. Voets, Soft Matter, 2017, 13, 4808–4823 RSC.
- R. Liu, Y. Liu, S. Fu, Y. Cheng, K. Jin, J. Ma, Y. Wan and Y. Tian, Small, 2024, 20, 2308092 CrossRef CAS PubMed.
- D. Sun, Y. Feng, S. Sun, J. Yu, S. Jia, C. Dang, X. Hao, J. Yang, W. Ren, R. Sun, C. Shao and F. Peng, Adv. Funct. Mater., 2022, 32, 2201335 CrossRef CAS.
- Y. K. Choi, T. Park, D. H. D. Lee, J. Ahn, Y. H. Kim, S. Jeon, M. J. Han and S. J. Oh, Nanoscale, 2022, 14, 8628–8639 RSC.
- T. Park, H. K. Woo, B. K. Jung, B. Park, J. Bang, W. Kim, S. Jeon, J. Ahn, Y. Lee and Y. M. Lee, ACS Nano, 2021, 15, 8120–8129 CrossRef CAS PubMed.
- W. Xu, T. Shen, Y. Ding, H. Ye, B. Wu and F. Chen, Small, 2024, 20, 2310072 CrossRef CAS.
- G. Su, Y. Zhang, X. Zhang, J. Feng, J. Cao, X. Zhang and T. Zhou, Chem. Mater., 2022, 34, 1392–1402 CrossRef CAS.
- H. Sun, Y. Zhao, S. Jiao, C. Wang, Y. Jia, K. Dai, G. Zheng, C. Liu, P. Wan and C. Shen, Adv. Funct. Mater., 2021, 31, 2101696 CrossRef CAS.
- M. Li, Y. Yang, C. Yue, Y. Song, M. Manzo, Z. Huang and L. Cai, Cellulose, 2022, 29, 1897–1909 CrossRef CAS.
- L. Zhou, B. Zhao, J. Liang, F. Lu, W. Yang, J. Xu, J. Zheng, Y. Liu, R. Wang and Z. Liu, Mater. Horiz., 2024, 11, 3856–3866 RSC.
- Y. Bai, B. Chen, F. Xiang, J. Zhou, H. Wang and Z. Suo, Appl. Phys. Lett., 2014, 105, 151903 CrossRef.
- C. Cai, C. Wen, W. Zhao, S. Tian, Y. Long, X. Zhang, X. Sui, L. Zhang and J. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 23692–23700 CrossRef CAS PubMed.
- H. Zhou, J. Lai, X. Jin, H. Liu, X. Li, W. Chen, A. Ma and X. Zhou, Chem. Eng. J., 2021, 413, 127544 CrossRef CAS.
- S. Shi, X. Peng, T. Liu, Y.-N. Chen, C. He and H. Wang, Polymer, 2017, 111, 168–176 CrossRef CAS.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang, H. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 CrossRef.
- Y. Lu, Y. Yue, Q. Ding, C. Mei, X. Xu, S. Jiang, S. He, Q. Wu, H. Xiao and J. Han, Infomat, 2023, 5, e12409 CrossRef CAS.
- T. Zhu, C. Jiang, M. Wang, C. Zhu, N. Zhao and J. Xu, Adv. Funct. Mater., 2021, 31, 2102433 CrossRef CAS.
- H. Chen, J. Huang, J. Liu, J. Gu, J. Zhu, B. Huang, J. Bai, J. Guo, X. Yang and L. Guan, J. Mater. Chem. A, 2021, 9, 23243–23255 RSC.
- B. Li, J. Luo, X. Huang, L. Lin, L. Wang, M. Hu, L. Tang, H. Xue, J. Gao and Y.-W. Mai, Composites, Part B, 2020, 181, 107580 CrossRef CAS.
- J. Wu, W. Huang, Z. Wu, X. Yang, A. G. P. Kottapalli, X. Xie, Y. Zhou and K. Tao, ACS Mater. Lett., 2022, 4, 1616–1629 CrossRef CAS.
- Q. Wang, X. Zhou, J. Zeng and J. Wang, Nucl. Instrum. Methods Phys. Res., Sect. B, 2016, 368, 90–95 CrossRef CAS.
- Z. Chen, Y. Li and Q. M. Li, Mater. Des., 2021, 207, 109819 CrossRef CAS.
- S. Mousazadeh and M. Kokabi, Polymer, 2020, 191, 122280 CrossRef CAS.
- Y. Zhan, W. Fu, Y. Xing, X. Ma and C. Chen, Mater. Sci. Eng., C, 2021, 127, 112208 CrossRef CAS PubMed.
- D. Yao, L. Wu, S. Peng, X. Gao, C. Lu, Z. Yu, X. Wang, C. Li and Y. He, ACS Appl. Mater. Interfaces, 2021, 13, 11284–11295 CrossRef CAS PubMed.
- H. Omidian and K. Park, Biomedical applications of hydrogels handbook, 2010, vol. 1, pp. 1–16 Search PubMed.
- Y. Cai, K. Wan, Q. Chen, M. Hong, Z.-X. Zhou and H. Fu, J. Mater. Chem. C, 2023, 11, 12981–12991 RSC.
- C. Qi, Z. Dong, Y. Huang, J. Xu and C. Lei, ACS Appl. Mater. Interfaces, 2022, 14, 30385–30397 CrossRef CAS.
- M. Antonietti, J. Conrad and A. Thuenemann, Macromolecules, 1994, 27, 6007–6011 CrossRef CAS.
- P. Du, J. Wang, Y.-I. Hsu and H. Uyama, Chem. Mater., 2024, 36, 1318–1332 CrossRef CAS.
- S. Wang, L. Wang, X. Qu, B. Lei, Y. Zhao, Q. Wang, W. Wang, J. Shao and X. Dong, ACS Appl. Mater. Interfaces, 2022, 14, 50256–50265 CrossRef CAS PubMed.
- J. Wei, Y. Zheng and T. Chen, Mater. Horiz., 2021, 8, 2761–2770 RSC.
- Z. Sun, C. Dong, B. Chen, W. Li, H. Hu, J. Zhou, C. Li and Z. Huang, Small, 2023, 19, 2303612 CrossRef CAS PubMed.
- J. Ren, Y. Liu, Z. Wang, S. Chen, Y. Ma, H. Wei and S. Lü, Adv. Funct. Mater., 2022, 32, 2107404 CrossRef CAS.
- Z. Wang, S. Lü, Y. Liu, T. Li, J. Yan, X. Bai, B. Ni, J. Yang and M. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 31393–31401 CrossRef CAS PubMed.
- X. Hu, S. Chen, H. Wang, Z.-X. Zhou, J. Min, Q. Chen, M. Hong and H. Fu, React. Funct. Polym., 2023, 190, 105624 CrossRef CAS.
- J. Wu, Z. Wu, X. Lu, S. Han, B.-R. Yang, X. Gui, K. Tao, J. Miao and C. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 9405–9414 CrossRef CAS PubMed.
- Y. Chen, Y. Xu, Q. Gao, H. Qian and R. Yang, IEEE Sens. J., 2020, 21, 6802–6810 Search PubMed.
- X. Li, D. Lou, H. Wang, X. Sun, J. Li and Y. N. Liu, Adv. Funct. Mater., 2020, 30, 2007291 CrossRef CAS.
- L. Zhao, H. Xu, L. Liu, Y. Zheng, W. Han and L. Wang, Advanced Science, 2023, 10, 2303922 CrossRef CAS PubMed.
- L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang, L. Fang, K. Liu, C. W. Chan and Y. Tang, Small, 2017, 13, 1601916 CrossRef PubMed.
- J. Huang, R. Zhou, Z. Chen, Y. Wang, Z. Li, X. Mo, N. Gao, J. He and C. Pan, Mater. Today Phys., 2023, 35, 101123 CrossRef.
- C. Wang, Y. Liu, X. Qu, B. Shi, Q. Zheng, X. Lin, S. Chao, C. Wang, J. Zhou and Y. Sun, Adv. Mater., 2022, 34, 2105416 CrossRef CAS PubMed.
- X. Sui, H. Guo, C. Cai, Q. Li, C. Wen, X. Zhang, X. Wang, J. Yang and L. Zhang, Chem. Eng. J., 2021, 419, 129478 CrossRef CAS.
- M. Guo, J. Yan, X. Yang, J. Lai, P. An, Y. Wu, Z. Li, W. Lei, A. T. Smith and L. Sun, J. Mater. Chem. A, 2021, 9, 7935–7945 RSC.
- J. Wen, J. Tang, H. Ning, N. Hu, Y. Zhu, Y. Gong, C. Xu, Q. Zhao, X. Jiang, X. Hu, L. Lei, D. Wu and T. Huang, Adv. Funct. Mater., 2021, 31, 2011176 CrossRef CAS.
- S. Zhao, Y. Zuo, T. Liu, S. Zhai, Y. Dai, Z. Guo, Y. Wang, Q. He, L. Xia, C. Zhi, J. Bae, K. Wang and M. Ni, Adv. Energy Mater., 2021, 11, 2101749 CrossRef CAS.
- W. Wang, X. Deng, J. Lu and C. Luo, J. Mater. Chem. C, 2023, 11, 13857–13864 RSC.
- M. T. I. Mredha, H. H. Le, J. Cui and I. Jeon, Advanced Science, 2020, 7, 1903145 CrossRef CAS PubMed.
- H. Zhu, J. Xu, X. Sun, Q. Guo, Q. Guo, M. Jiang, K. Wu, R. Cai and K. Qian, J. Mater. Chem. A, 2022, 10, 23366–23374 RSC.
- J. Yang, J. Cheng, G. Qi and B. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 17163–17174 CrossRef CAS PubMed.
- X. Meng, Y. Qiao, C. Do, W. Bras, C. He, Y. Ke, T. P. Russell and D. Qiu, Adv. Mater., 2022, 34, 2108243 CrossRef CAS PubMed.
- X. Meng, Y. Qiao, C. Do, W. Bras, C. He, Y. Ke, T. P. Russell and D. Qiu, Adv. Mater., 2022, 34, 2108243 CrossRef CAS PubMed.
- H. Yao, W. Yang, W. Cheng, Y. J. Tan, H. H. See, S. Li, H. P. A. Ali, B. Z. Lim, Z. Liu and B. C. Tee, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 25352–25359 CrossRef CAS.
- X. Liu, J. Miao, Q. Fan, W. Zhang, X. Zuo, M. Tian, S. Zhu, X. Zhang and L. Qu, Adv. Fiber Mater., 2022, 4, 361–389 CrossRef CAS.
- H. Liu, C. Liu, J. Luo, H. Tang, Y. Li, H. Liu, J. Wu, F. Han, Z. Liu and J. Guo, InfoMat, 2024, 6, e12511 CrossRef.
- H. Zhang, W. Niu and S. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 32640–32648 CrossRef CAS PubMed.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS.
- K. Lei, M. Chen, P. Guo, J. Fang, J. Zhang, X. Liu, W. Wang, Y. Li, Z. Hu, Y. Ma, H. Jiang, J. Cui and J. Li, Adv. Funct. Mater., 2023, 33, 2303511 CrossRef CAS.
- Y. Wang, Y. Zhang, P. Ren, S. Yu, P. Cui, C. B. Nielsen, I. Abrahams, J. Briscoe and Y. Lu, Nano Energy, 2024, 125, 109599 CrossRef CAS.
- J. Han, T. Lei and Q. Wu, Carbohydr. Polym., 2014, 102, 306–316 CrossRef CAS PubMed.
- J.-n. Liu, Q. He, M.-y. Pan, K. Du, C.-B. Gong and Q. Tang, J. Mater. Chem. A, 2022, 10, 25118–25128 RSC.
- M. Liao, P. Wan, J. Wen, M. Gong, X. Wu, Y. Wang, R. Shi and L. Zhang, Adv. Funct. Mater., 2017, 27, 1703852 CrossRef.
- Q. Chen, H. Chen, L. Zhu and J. Zheng, J. Mater. Chem. B, 2015, 3, 3654–3676 RSC.
- Y. Zhou, C. Wan, Y. Yang, H. Yang, S. Wang, Z. Dai, K. Ji, H. Jiang, X. Chen and Y. Long, Adv. Funct. Mater., 2019, 29, 1806220 CrossRef.
- H. Zheng, N. Lin, Y. He and B. Zuo, ACS Appl. Mater. Interfaces, 2021, 13, 40013–40031 CrossRef CAS PubMed.
- H. Luan, D. Zhang, Z. Xu, W. Zhao, C. Yang and X. Chen, J. Mater. Chem. C, 2022, 10, 7604–7613 RSC.
- S. Han, C. Liu, X. Lin, J. Zheng, J. Wu and C. Liu, ACS Appl. Polym. Mater., 2020, 2, 996–1005 CrossRef CAS.
- J. Zhou, T. Chen, Z. He, L. Sheng and X. Lu, J. Mater. Chem. C, 2023, 11, 13476–13487 RSC.
- W. Feng, H. Luo, Y. Wang, S. Zeng, L. Deng, X. Zhou, H. Zhang and S. Peng, RSC Adv., 2018, 8, 2398–2403 RSC.
- Y. Huang, L. Xiao, J. Zhou, T. Liu, Y. Yan, S. Long and X. Li, Adv. Funct. Mater., 2021, 31, 2103917 CrossRef CAS.
- T. Long, Y. Li, X. Fang and J. Sun, Adv. Funct. Mater., 2018, 28, 1804416 CrossRef.
- X. Zhang, H. Geng, X. Zhang, Y. Liu, J. Hao and J. Cui, J. Mater. Chem. A, 2023, 11, 2996–3007 RSC.
- Q. Yang, M. Li, R. Chen, D. Gao, Z. Wang, C. Qin, W. Yang, H. Liu and P. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 51774–51784 CrossRef CAS PubMed.
- H. Wu, H. Qi, X. Wang, Y. Qiu, K. Shi, H. Zhang, Z. Zhang, W. Zhang and Y. Tian, J. Mater. Chem. C, 2022, 10, 8206–8217 RSC.
- L. Xing, Y. Song, X. Zou, H. Tan, J. Yan and J. Wang, J. Mater. Chem. C, 2023, 11, 13376–13386 RSC.
- H. Wu, Y. Wu, J. Yan, W. Xiao, Y. Wang, H. Zhang, X. Huang, H. Xue, L. Wang, L. Tang, Y. Mai and J. Gao, Chem. Eng. J., 2024, 488, 150963 CrossRef CAS.
- J. Kim, G. Zhang, M. Shi and Z. Suo, Science, 2021, 374, 212–216 CrossRef CAS PubMed.
- A. Motealleh and N. S. Kehr, Adv. Healthcare Mater., 2017, 6, 1600938 CrossRef PubMed.
- D. Gan, T. Shuai, X. Wang, Z. Huang, F. Ren, L. Fang, K. Wang, C. Xie and X. Lu, Nano-Micro Lett., 2020, 12, 1–16 CrossRef PubMed.
- X. Yu, Y. Zheng, H. Zhang, Y. Wang, X. Fan and T. Liu, Chem. Mater., 2021, 33, 6146–6157 CrossRef CAS.
- X. Liu, Q. Zhang, L. Duan and G. Gao, Adv. Funct. Mater., 2019, 29, 1900450 CrossRef.
- P. Rao, T. L. Sun, L. Chen, R. Takahashi, G. Shinohara, H. Guo, D. R. King, T. Kurokawa and J. P. Gong, Adv. Mater., 2018, 30, 1801884 CrossRef PubMed.
- K. Haraguchi, Y. Xu and G. Li, Macromol. Rapid Commun., 2010, 31, 718–723 CrossRef CAS PubMed.
- J. Yin, W. Sun, X. Song, H. Ji, Y. Yang, S. Sun, W. Zhao and C. Zhao, Sep. Purif. Technol., 2020, 238, 116497 CrossRef CAS.
- J. Ma, Z. Li, H. Song, X. Xu, C. Long, Y. Li, Y. Qing and C. Liu, Chem. Eng. J., 2024, 488, 150797 CrossRef CAS.
- H. Gotoh, C. Liu, A. B. Imran, M. Hara, T. Seki, K. Mayumi, K. Ito and Y. Takeoka, Sci. Adv., 2018, 4, eaat7629 CrossRef CAS PubMed.
- J. Liu, J. Huang, Q. Cai, Y. Yang, W. Luo, B. Zeng, Y. Xu, C. Yuan and L. Dai, ACS Appl. Mater. Interfaces, 2020, 12, 20479–20489 CrossRef CAS PubMed.
- O. Galant, S. Bae, M. N. Silberstein and C. E. Diesendruck, Adv. Funct. Mater., 2020, 30, 1901806 CrossRef CAS.
- H. Zhang, M. Yue, T. Wang, J. Wang, X. Wu and S. Yang, New J. Chem., 2021, 45, 4647–4657 RSC.
- S. Dai, H. Hu, Y. Zhang, J. Xu, Y. Zhong, G. Cheng and J. Ding, J. Mater. Chem. C, 2023, 11, 2688–2694 RSC.
| This journal is © The Royal Society of Chemistry 2024 |







