Solvent-free synthesis of stable heterostructured-CsPbBr3/Cs2PbBr5 assisted by SiO2 for white light-emitting diodes†
Jisong
Yao
*,
Tianliang
Yao
,
Kaishuai
Zhang
,
Wenxuan
Fan
,
Zhi
Yang
,
Leimeng
Xu
,
Shalong
Wang
and
Jizhong
Song
 *
*
Key Laboratory of Materials Physics of Ministry of Education, School of Physics and Microelectronics, Zhengzhou University, Daxue Road 75, Zhengzhou 450052, China. E-mail: yaojisong@zzu.edu.cn; songjizhong@zzu.edu.cn
First published on 10th January 2024
Abstract
Continuously improving the stability of lead halide perovskite nanocrystals (NCs) to meet the requirement of industrialization has received tremendous attention. Phase structure modulation or matrix protection has been proven to be a feasible strategy, but these preparations are still hindered by the usage of organic solvents, complex operations, and unsatisfactory batch reproducibility, conflicting with scaled-up production. Here, we have successfully prepared highly stable cesium lead bromide perovskites with superior luminescence properties (photoluminescence quantum yield, PLQY > 80%) by solid-state synthesis using SiO2 nanospheres as a reaction medium, which present heterostructured-CsPb2Br5/CsPbBr3 (H-CPB). Profiting from the dual-phase structure and SiO2 matrix protection, the as-obtained H-CPB shows high stability with a retained PL of above 90% under continuous UV light irradiation, which is significantly higher than the 42% for the control sample. Importantly, the proposed method features solvent-free, rapid (within 5 mins), and room-temperature synthesis, which could also be used to scale up production evidenced by a batch of 760 g H-CPB powders without compromising their performance. The resulting H-CPB phosphors and commercial red-emitting phosphor coated on blue GaN chips exhibit tunable color temperatures from warm white to cool white, which indicates that the H-CPB perovskites have huge potential for lighting applications.
Introduction
Lead halide perovskite nanocrystals (LHP NCs) have emerged as promising materials for various optoelectronic devices, such as light-emitting diodes (LEDs), solar cells, photodetectors, and lasers, due to their excellent photophysical properties (high photoluminescence quantum yields (PLQYs), narrow emission width, tunable emission spectra, etc.).1–6 Moreover, LHP NCs can easily be prepared quickly even at room temperature, reflecting their unique advantages in the synthesis and processing.7 However, LHP NCs are characterized by very poor stability due to their low formation energy and soft ionic crystal structure.7–10 Many studies have shown that LHP NCs could quickly lose photoactivity and even decompose when they are exposed to photoirradiation, heat, and humidity, which has limited their further development and practical applications.11Studies have demonstrated that embedding LHP NCs into inorganic matrixes is one of the most effective strategies to improve their stability.12–15 Particularly, low-dimensional inorganic perovskites, such as two-dimensional (2D) CsPb2Br5 (referred to as the 2D phase) and zero-dimensional (0D) Cs4PbBr6, are effective matrixes for enhancing the stability and luminescent properties of three-dimensional (3D) CsPbBr3 due to their superior stability and high similarity in lattice constants.16–18 Many research examples have been realized by solution-based chemistry for the synthesis of LHP heterostructures, enabling improved performance of LEDs.16,19–22 Despite these great progress, a large amount of organic solvents is usually used in the synthetic and purification processes, which is not only harmful but also increases the production cost.23 In contrast, solid-state reactions can effectively avoid the use of hazardous solvents and tend to form LHP heterostructures, which have recently received increasing attention in the past few years.24–27 However, solid-state synthesis of LHP heterostructures still suffers from some drawbacks including high temperatures (above 300 °C), complicated preparation processes, and low PL efficiency of perovskite products, which hinder their practical applications.24,25,28 Although the heterostructured-Cs4PbBr6/CsPbBr3 have been recently synthesized at room temperature by mechanochemical synthesis, it is still necessary to introduce organic solvents into the reaction system when ligand passivation is required to enhance the PL efficiency as well as the synthesis requires rather long reaction times (tens of hours).24 Therefore, it is imperative to develop a solvent-free, room-temperature, and rapid synthesis method for efficient and stable LHP heterostructures.29
Herein, we report a facile all-solid synthesis of stable heterostructured-CsPb2Br5/CsPbBr3 (H-CPB) by embedding CsPbBr3 NCs into CsPb2Br5 matrixes at room temperature. In our synthesis system, SiO2 was used as a reaction medium to control the nucleation and growth of H-CPB perovskites, which can effectively avoid the use of organic solvents. Moreover, the organic ligands can be easily introduced into the reaction system for passivating the H-CPB perovskites. More importantly, the final products are highly stable in some harsh conditions, e.g., stored in humid air and under UV light irradiation. Finally, we demonstrate that the H-CPB powders can be used as down-converting material for a white LED with tunable color temperature from warm white to cool white. The WLEDs also showed good stability in the air (relative humidity ∼ 50%) with minimal change in the emission color during the operation for more than 12 h under the 10-mA driving current.
Results and discussion
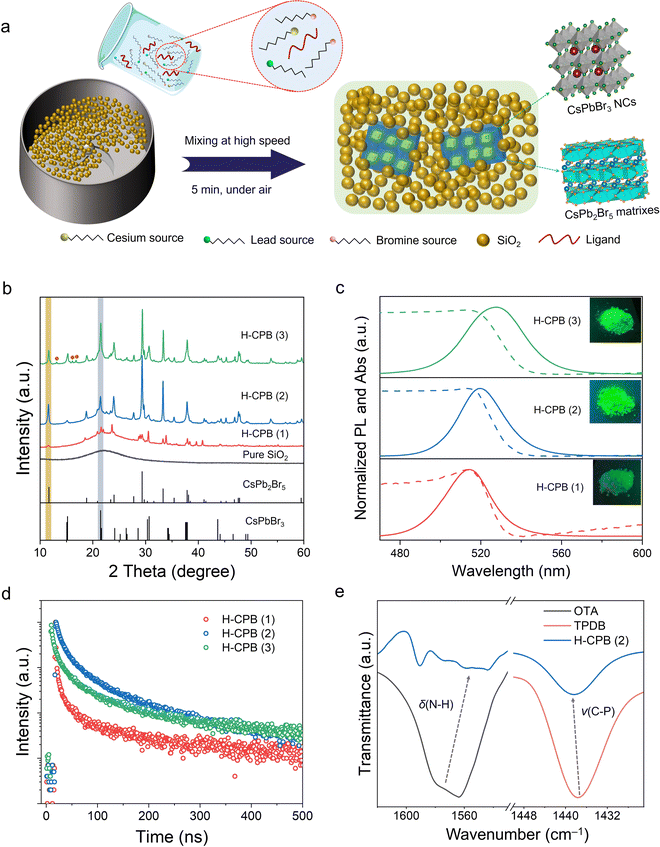
H-CPB composites were obtained through a solid-state synthesis, which was quite simple and only required rapid mixing of the raw materials (refers to the materials used to synthesize H-CPB) with SiO2 powder at room temperature (Fig. 1a). In a typical experiment, guanidine carbonate (Gu2CO3) cesium stearate, lead stearate, nickel stearate, octylamine (OTA), and SiO2 were firstly mixed in a mixer at high speed for 2 min. Then, a certain amount of triphenylphosphine dibromide (TPDB) was added into the above reaction system (molar ratio of Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb
Pb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br
Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 is 3
SiO2 is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) and stirred rapidly for 3 min to obtain H-CPB composites. It is worth mentioning that the whole process of the above reaction was carried out in the air without any heating equipment. In order to optimize the performance of the final composites, we prepared three different ratios of LHPs to SiO2. Hereafter, the products synthesized by inadequate, moderate, and excess LHPs are denoted as H-CPB (1), H-CPB (2), and H-CPB (3), respectively (See ESI† for details). As shown in Fig. 1b, the powder X-ray diffraction (XRD) confirmed the coexistence of monoclinic CsPbBr3 (PDF# 18-0364) and tetragonal CsPb2Br5 (PDF# 25-0211) in the material system. It can be seen that the diffraction peaks of CsPbBr3 in the CPB/SiO2 composites were exhibited more significantly as the proportion of SiO2 decreased. When the proportion of LHPs is in excess (H-CPB (3)), we also observed the diffractions from impurities (marked by brown balls), which may have originated from the raw materials without the reaction. The broad diffraction band ranging from 10° to 30° should be attributed to the existence of amorphous silica, which was consistent with the XRD pattern of pure SiO2.
70) and stirred rapidly for 3 min to obtain H-CPB composites. It is worth mentioning that the whole process of the above reaction was carried out in the air without any heating equipment. In order to optimize the performance of the final composites, we prepared three different ratios of LHPs to SiO2. Hereafter, the products synthesized by inadequate, moderate, and excess LHPs are denoted as H-CPB (1), H-CPB (2), and H-CPB (3), respectively (See ESI† for details). As shown in Fig. 1b, the powder X-ray diffraction (XRD) confirmed the coexistence of monoclinic CsPbBr3 (PDF# 18-0364) and tetragonal CsPb2Br5 (PDF# 25-0211) in the material system. It can be seen that the diffraction peaks of CsPbBr3 in the CPB/SiO2 composites were exhibited more significantly as the proportion of SiO2 decreased. When the proportion of LHPs is in excess (H-CPB (3)), we also observed the diffractions from impurities (marked by brown balls), which may have originated from the raw materials without the reaction. The broad diffraction band ranging from 10° to 30° should be attributed to the existence of amorphous silica, which was consistent with the XRD pattern of pure SiO2.
The optical properties and surface chemistry of H-CPB composites were further investigated. As shown in Fig. 1c, the H-CPB composites featured green emission peaks at ∼520 nm with a narrow full width at half maximum (FWHM) of ∼26 nm. A clear red-shift of PL spectra (from 514 to 526 nm) was also seen, which was caused by the larger size of LHP particles according to SEM image results (Fig. 2a and Fig. S1, ESI†). The PLQY values of H-CPB (1), H-CPB (2), and H-CPB (3) were determined to be 15%, 83%, and 54%, respectively, indicating that the ratio of SiO2 nanospheres plays a key role in regulating the crystallization process of the H-CPB composites. Meanwhile, it can be clearly seen that the H-CPB (2) exhibits brighter luminescence under UV light. The time-resolved PL spectra of the H-CPB powders were also measured (Fig. 1d), and they can be well-fitted by a double-exponential decay function. The corresponding fitted parameters and relevant discussion are shown in Table S1 (ESI†). The obtained average lifetime of the three samples was measured to be 7.9 ns for H-CPB (1), 41.5 ns for H-CPB (2), and 32.0 ns for H-CPB (3), which was consistent with the PLQY results. To further explore the effect of SiO2 on H-CPB composites, SiO2 with different specific surface areas was also studied systematically (Fig. S2, ESI†). Hereafter, the products produced by 100 m2 g−1, 170 m2 g−1, and 230 m2 g−1 of SiO2 nanospheres were denoted by H-CPB (2), H-CPB (4), and H-CPB (5), respectively (details in the Experimental Section). The results from XRD and SEM showed that the SiO2 with different specific surface areas could act as a reaction medium to promote the formation of H-CPB (Fig. S3 and S4, ESI†). However, when the specific surface area of SiO2 gradually increased, the optical performance of H-CPB composites gradually decreased, and even the PL spectrum of H-CPB (5) was obviously blue-shifted and broadened (Fig. S5, ESI†). We further measured Fourier-transform infrared spectroscopy (FTIR) on the as-prepared samples, as depicted in Fig. 1e. Comparing with the raw material ligands, FTIR reveals that the amino group scissoring vibration δ(NH2) of OTA and the stretching vibration ν(C–P) of TPDB are broadened and shift significantly in H-CPB composites, indicating that the unsaturated Pb dangling bonds due to halide vacancy can be passivated by OTA and TPDB ligands. In addition, the characteristic vibrational band of N–H bending (1630 cm−1) was detected on the surface of pure SiO2 nanospheres (Fig. S6, ESI†), which also favors the formation of highly luminescent H-CPB composites.30
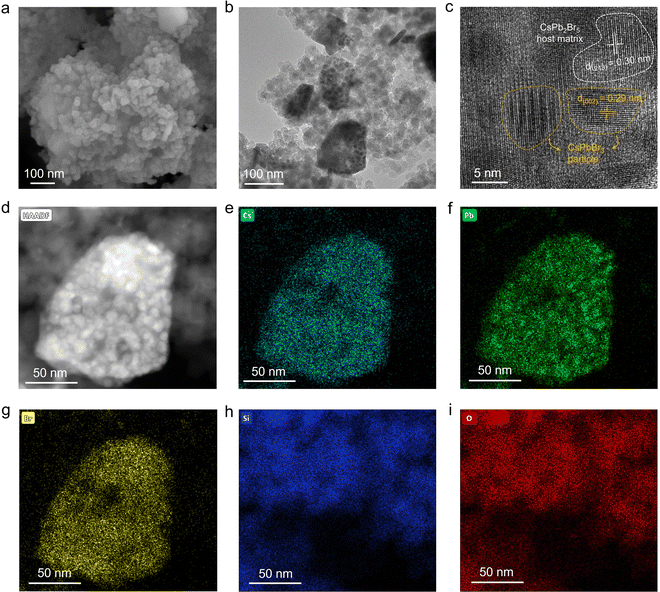
To reveal the morphology and the structure of H-CPB composites, we performed scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. Hereafter, we will focus on analyzing H-CPB (2) composites as a representative to show the advantages of our proposed method. As shown in Fig. S2 (ESI†), the pure SiO2 nanospheres have a size of about tens of nanometers. In contrast, the H-CPB composites showed complexes of SiO2 nanospheres and larger crystalline particles (Fig. 2a), indicating that the large particles belong to H-CPB combined with the XRD analysis. Moreover, the H-CPB particles were wrapped by SiO2 nanospheres and the number of H-CPB particles also increased with the proportion of introducing raw materials (Fig. S1, ESI†). The TEM image also showed that the H-CPB particles were present between the SiO2 nanospheres (Fig. 2b). Furthermore, we found that the smaller nanoparticles of about ten nanometers were distributed inside the H-CPB particles. Consistent with this result, the high-resolution TEM (HRTEM) image indicated a mixture of tetragonal and monoclinic lattice fringes corresponding to 2D CsPb2Br5 and 3D CsPbBr3 phases, respectively, indicating the presence of H-CPB (Fig. 2c). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) (Fig. 2d) and energy-dispersive X-ray spectroscopy (EDS) (Fig. 2e–i) were used to reveal the elemental distribution. The elements Cs, Pb, and Br elements were homogeneously distributed in the H-CPB part, and Si and O elements were deposited around it. Furthermore, the quantification of the EDS spectrum revealed a Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb
Pb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br atomic ratio of 1
Br atomic ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.92
1.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.77 (this ratio is between the corresponding CsPbBr3 of 1
3.77 (this ratio is between the corresponding CsPbBr3 of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:3 and CsPb2Br5 of 1
1:3 and CsPb2Br5 of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:5) in the as-synthesized H-CPB (Fig. S7, ESI†), further indicating the formation of CsPb2Br5/CsPbBr3 heterostructures.
2:5) in the as-synthesized H-CPB (Fig. S7, ESI†), further indicating the formation of CsPb2Br5/CsPbBr3 heterostructures.
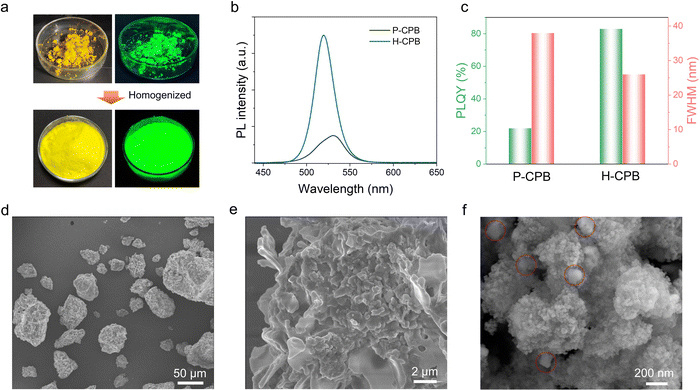
To demonstrate the role of SiO2 as a reaction medium, we further investigated the direct synthesis of LHPs without SiO2 (denoted as pure CPB (P-CPB)). As can also be seen from the photographs of P-CPB (Fig. 3a), the dark yellow powders are agglomerated. On a relative basis, the H-CPB powders showed more homogeneity and emitted a brighter green light. Moreover, as shown in Fig. 3b and c, the P-CPB features an asymmetric peak at ∼530 nm with a broader FWHM of ∼36 nm and lower PLQY of 22% compared to the H-CPB composites (FWHM = 26 nm, PLQY = 83%), which showed that the SiO2 as a reaction medium can sufficiently homogenize CPB particles and improve their PL efficiency. The crystal structure of the P-CPB was evaluated using XRD analysis (Fig. S8, ESI†). Diffraction peaks proved that the P-CPB contains some impurities (marked by purple crosses) that may have originated from unreacted salts in addition to the coexistence of monoclinic CsPbBr3 and tetragonal CsPb2Br5, which further demonstrates the role of SiO2 as a mediator in promoting LHP generation in the solid-state reactions. Furthermore, the low-magnification SEM image showed that the P-CPB is composed of irregular micron-sized particles (Fig. 3d). Moreover, the high-magnification SEM image shows noticeable wrinkles on the surface of the P-CPB, which is different from that of the H-CPB composites (Fig. 3e and f). In addition, the TEM analysis further confirmed that CsPbBr3 NCs were embedded in the CsPb2Br5 matrix in the pure CPB (Fig. S9, ESI†), which is consistent with the XRD results. As a result, the SiO2 nanospheres not only play a role in promoting the formation of the CsPb2Br5/CsPbBr3 heterostructures but also effectively avoids the agglomeration of products, enabling high-quality LHP heterostructures.
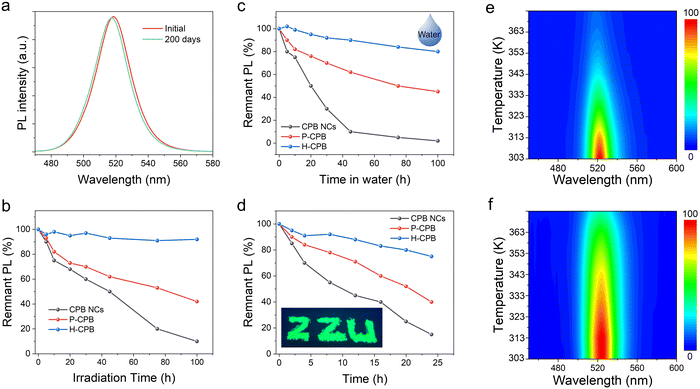
In order to meet the needs of commercial applications, LHP NCs are supposed to be stable under practical application situations. Therefore, we studied the stability of H-CPB composites upon storage and light irradiation under ambient conditions. The PL spectra of H-CPB hardly change at all even after storage for 100 days and could maintain 95% of the original PLQY (Fig. 4a). Furthermore, the photostability test of H-CPB, P-CPB, and colloidal CsPbBr3 NCs was implemented under continuous UV light irradiation (365 nm, 106 mW cm−2). As shown in Fig. 4b, the relative PL intensity of P-CPB and colloidal CsPbBr3 NCs was decreased to 42% and 10% of the initial intensity after the light irradiation for 100 h, respectively. On the contrary, the relative PL intensity of H-CPB composites was still maintained above 90%, and no obvious PL decrease was observed under irradiation for 100 h (Fig. S10, ESI†). To assess the environmental stability of H-CPB composites, water tolerance tests were carried out by immersing CsPbBr3 NCs, C-CPB, and H-CPB composites in water under ambient conditions (Fig. 4c). It should be pointed out that the CsPbBr3 NCs, C-CPB, and H-CPB composites are encapsulated in UV-curing resin. It was observed that the relative PL intensity of CsPbBr3 NCs was decreased to 15% and the green-light emission completely disappeared after being immersed in water for 100 hours. In striking contrast, the H-CPB composites could still maintain 80% of the initial PL intensity value. In addition, the thermal stability tests were carried out by monitoring the variation of the PL intensity of the sample before and after annealing at 85 °C for 24 h under ambient conditions. As depicted in Fig. 4d, the H-CPB composites still maintained 75% of the initial PL intensity value after annealing for 24 h. However, the relative PL intensity of CsPbBr3 NCs decreased to 15% and was almost completely quenched after 24 h. Meanwhile, the H-CPB composites exhibited higher stability with increasing temperatures from 30 to 100 °C (Fig. 4e and f). The above results clearly show that the H-CPB composites could significantly improve the stability of LHP NCs due to the synergistic effect of dual-phase structure and SiO2 matrix protection, which could also promote their practical applications.
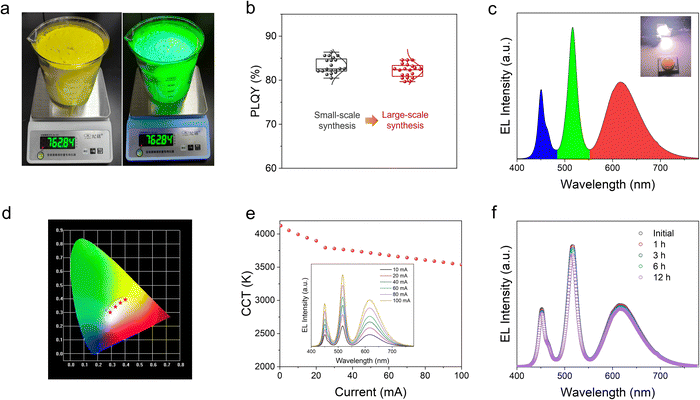
The high stability and efficiency of H-CPB composites make them promising candidates for white LEDs (WLEDs). More remarkably, by simply increasing the batch size, this proposed solid-state approach can be scaled up to an industrial scale. To support this conclusion, we have successfully prepared a batch size of 760 g for H-CPB composites (Fig. 5a). Moreover, the H-CPB composites demonstrate good performance reproducibility evidenced by the statistical PLQY distribution even when prepared in large batches, as shown in Fig. 5b. Therefore, WLEDs were prepared by pumping the phosphor blend of a commercial red-emitting CaAlSiN3:Eu2+ (CASN) phosphor and H-CPB composites on a 455 nm blue-LED chip. As seen in Fig. 5c, the electroluminescence (EL) spectrum obviously consists of three emission bands of blue, green, and red, which belong to the blue LED chip, H-CPB composites, and CASN phosphor, respectively. The Commission Internationale de L’Eclairage (CIE) chromaticity coordinate of (0.379, 0.386) and a correlated color temperature (CCT) value of 3994 K were obtained for the resulting WLED, which corresponds to an excellent warm white light emission. Meanwhile, the white light ranging from “warm” to “cold” was achieved by simply varying the ratio of green and red phosphors (Fig. 5d). Fig. 5e illustrates the detailed CCT variation and EL spectra under different driving currents. Small changes of CCT increase from 4125 K at 0.5 mA to 3538 K at 100 mA suggest high stability of the obtained WLED device against current variations. WLEDs also showed good stability in the air (relative humidity ∼50%) with minimal change in the emission color during the operation for more than 12 h under the 5-mA driving current, as shown in Fig. 5f. These results indicate that the stability of H-CPB composites has been significantly improved.
Conclusion
In summary, we synthesized H-CPB composites in large batches by a quick solid-state reaction under ambient conditions. We demonstrated that the SiO2 nanospheres can be used as a reaction medium to control the growth of LHPs, realizing the preparation of H-CPB composites, which exhibit high chemical and optical stability without encapsulation. Benefitting from these excellent properties and high stability, we have combined green H-CPB composites and commercial red phosphors with blue LED chips to obtain WLEDs with excellent stability and adjustable CCT from warm to cool white light. We believe that the H-CPB composites with improved stability and their retaining of excellent optical properties will energetically facilitate their practical applications.Data availability
All of the necessary data had been included in the ESI.†Author contributions
J. Y. and J. S. devised the method and conceived the project. J. Y., T. Y., and K. Z. designed and performed the experiments. W. F. carried out the optical measurements. Z.Y., L. X., and S. W. conducted the analytical characterization. J. Y. and J. S. co-wrote the manuscript. All authors contributed to discussions and finalizing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by National Natural Science Foundation of China (Grant No. 52272166, 22205214, and 12204427), China Postdoctoral Science Foundation (Grant No. 2022M712910), Natural Science Foundation of Henan Province of China (Grant No. 222300420299 and 232300421214), Zhengzhou University Youth Talent Start-up Grant (Grant No. 32340213).Notes and references
- T. H. Han, K. Y. Jang, Y. T. Dong, R. H. Friend, E. H. Sargent and T. W. Lee, A roadmap for the commercialization of perovskite light emitters, Nat. Rev. Mater., 2022, 7, 757–777 CrossRef.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Properties and potential optoelectronic applications of lead halide perovskite nanocrystals, Science, 2017, 358, 745–750 CrossRef CAS PubMed.
- X. K. Liu, W. Xu, S. Bai, Y. Jin, J. Wang, R. H. Friend and F. Gao, Metal halide perovskites for light-emitting diodes, Nat. Mater., 2021, 20, 10–21 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- L. N. Quan, F. P. G. de Arquer, R. P. Sabatini and E. H. Sargent, Perovskites for Light Emission, Adv. Mater., 2018, 30, 1801996 CrossRef PubMed.
- J. S. Yao, L. Wang, K. H. Wang, Y. C. Yin, J. N. Yang, Q. Zhang and H. B. Yao, Calcium-tributylphosphine oxide passivation enables the efficiency of pure-blue perovskite light-emitting diode up to 3.3%, Sci. Bull., 2020, 65, 1150–1153 CrossRef CAS PubMed.
- Q. A. Akkerman, G. Raino, M. V. Kovalenko and L. Manna, Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals, Nat. Mater., 2018, 17, 394–405 CrossRef CAS PubMed.
- J. W. Hou, P. Chen, A. Shukla, A. Krajnc, T. S. Wang, X. M. Li, R. Doasa, L. H. G. Tizei, B. Chan, D. N. Johnstone, R. J. Lin, T. U. Schulli, I. Martens, D. Appadoo, M. S. Ari, Z. L. Wang, T. Wei, S. C. Lo, M. Y. Lu, S. C. Li, E. B. Namdas, G. Mali, A. K. Cheetham, S. M. Collins, V. Chen, L. Z. Wang and T. D. Bennett, Liquid-phase sintering of lead halide perovskites and metal-organic framework glasses, Science, 2021, 374, 621–625 CrossRef CAS PubMed.
- K. Sun, D. Z. Tan, X. Y. Fang, X. T. Xia, D. J. Lin, J. Song, Y. H. Lin, Z. J. Liu, M. Gu, Y. Z. Yue and J. R. Qiu, Three-dimensional direct lithography of stable perovskite nanocrystals in glass, Science, 2022, 375, 307–310 CrossRef CAS PubMed.
- J. S. Yao, J. J. Wang, J. N. Yang and H. B. Yao, Modulation of metal halide structural units for light emission, Acc. Chem. Res., 2021, 54, 441–451 CrossRef CAS PubMed.
- H. Cho, Y. H. Kim, C. Wolf, H. D. Lee and T. W. Lee, Improving the stability of metal halide perovskite materials and light-emitting diodes, Adv. Mater., 2018, 30, 1704587 CrossRef PubMed.
- Y. Duan, C. Ezquerro, E. Serrano, E. Lalinde, J. García-Martínez, J. R. Berenguer and R. D. Costa, Meeting high stability and efficiency in hybrid light-emitting diodes based on SiO2/ZrO2 coated CsPbBr3 perovskite nanocrystals, Adv. Funct. Mater., 2020, 30, 2005401 CrossRef CAS.
- H. Hu, L. Wu, Y. Tan, Q. Zhong, M. Chen, Y. Qiu, D. Yang, B. Sun, Q. Zhang and Y. Yin, Interfacial synthesis of highly stable CsPbX3/Oxide janus nanoparticles, J. Am. Chem. Soc., 2018, 140, 406–412 CrossRef CAS PubMed.
- Z. J. Li, E. Hofman, J. Li, A. H. Davis, C. H. Tung, L. Z. Wu and W. Zheng, Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals, Adv. Funct. Mater., 2017, 28, 1704288 CrossRef.
- A. Loiudice, S. Saris, E. Oveisi, D. T. L. Alexander and R. Buonsanti, CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat, Angew. Chem., Int. Ed., 2017, 56, 10696–10701 CrossRef CAS PubMed.
- S. Bera and N. Pradhan, Perovskite nanocrystal heterostructures: Synthesis, optical properties, and applications, ACS Energy Lett., 2020, 5, 2858–2872 CrossRef CAS.
- K. Du, L. He, S. Song, J. Feng, Y. Li, M. Zhang, H. Li, C. Li and H. Zhang, In situ embedding synthesis of highly stable CsPbBr3/CsPb2Br5@PbBr(OH) nano/microspheres through water assisted strategy, Adv. Funct. Mater., 2021, 31, 2103275 CrossRef CAS.
- L. N. Quan, R. Quintero-Bermudez, O. Voznyy, G. Walters, A. Jain, J. Z. Fan, X. Zheng, Z. Yang and E. H. Sargent, Highly emissive green perovskite nanocrystals in a solid state crystalline matrix, Adv. Mater., 2017, 29, 1605945 CrossRef PubMed.
- G. K. Grandhi, N. S. M. Viswanath, J. H. In, H. B. Cho and W. B. Im, Robust, brighter red emission from CsPbI3 perovskite nanocrystals via endotaxial protection, J. Phys. Chem. Lett., 2020, 11, 3699–3704 CrossRef CAS PubMed.
- B. Wang, C. Y. Zhang, S. L. Huang, Z. C. Li, L. Kong, L. Jin, J. H. Wang, K. F. Wu and L. Li, Postsynthesis phase transformation for CsPbBr3/Rb4PbBr6 core/shell nanocrystals with exceptional photostability, ACS Appl. Mater. Interfaces, 2018, 10, 23303–23310 CrossRef CAS PubMed.
- J. W. Xu, W. X. Huang, P. Y. Li, D. R. Onken, C. C. Dun, Y. Guo, K. B. Ucer, C. Lu, H. Z. Wang, S. M. Geyer, R. T. Williams and D. L. Carroll, Imbedded nanocrystals of CsPbBr3 in Cs4PbBr6: Kinetics, enhanced oscillator strength, and application in light-emitting, Adv. Mater., 2017, 29, 1703703 CrossRef PubMed.
- X. L. Zhang, B. Xu, J. B. Zhang, Y. Gao, Y. J. Zheng, K. Wang and X. W. Sun, All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: Dual-phase CsPbBr3-CsPb2Br5 composites, Adv. Funct. Mater., 2016, 26, 4595–4600 CrossRef CAS.
- B. Wang, C. Zhang, W. Zheng, Q. Zhang, Z. Bao, L. Kong and L. Li, Large-scale synthesis of highly luminescent perovskite nanocrystals by template-assisted solid-state reaction at 800 °C, Chem. Mater., 2019, 32, 308–314 CrossRef.
- K. Y. Baek, W. Lee, J. Lee, J. Kim, H. Ahn, J. I. Kim, J. Kim, H. Lim, J. Shin, Y. J. Ko, H. D. Lee, R. H. Friend, T. W. Lee, J. Lee, K. Kang and T. Lee, Mechanochemistry-driven engineering of 0D/3D heterostructure for designing highly luminescent Cs-Pb-Br perovskites, Nat. Commun., 2022, 13, 4263 CrossRef CAS PubMed.
- C. Fan, H. P. Liu, J. W. Zhou, X. L. Dai, H. P. He and Z. Z. Ye, Ultrastable and highly efficient CsPbBr3 composites achieved by dual-matrix encapsulation for display devices, InfoMat, 2023, 5, e12417 CrossRef CAS.
- A. Karmakar, M. S. Dodd, X. Y. Zhang, M. S. Oakley, M. Klobukowski and V. K. Michaelis, Mechanochemical synthesis of 0D and 3D cesium lead mixed halide perovskites, Chem. Commun., 2019, 55, 5079–5082 RSC.
- F. Palazon, Y. El Ajjouri, P. Sebastia-Luna, S. Lauciello, L. Manna and H. J. Bolink, Mechanochemical synthesis of inorganic halide perovskites: evolution of phase-purity, morphology, and photoluminescence, J. Mater. Chem. C, 2019, 7, 11406–11410 RSC.
- Z. Y. Zhu, Q. Q. Yang, L. F. Gao, L. Zhang, A. Y. Shi, C. L. Sun, Q. Wang and H. L. Zhang, Solvent-free mechanosynthesis of composition-tunable cesium lead halide perovskite quantum dots, J. Phys. Chem. Lett., 2017, 8, 1610–1614 CrossRef CAS PubMed.
- K. Zhang, W. Fan, T. Yao, S. Wang, Z. Yang, J. Yao, L. Xu and J. Song, Polymer-surface-mediated mechanochemical reaction for rapid and scalable manufacture of perovskite QD phosphors, Adv. Mater., 2024, 2310521 CrossRef PubMed.
- X. M. Li, Y. Wang, H. D. Sun and H. B. Zeng, Amino-mediated anchoring perovskite quantum dots for stable and low-threshold random lasing, Adv. Mater., 2017, 29, 1701185 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization, and spectroscopic data. See DOI: https://doi.org/10.1039/d3qm01233f |
| This journal is © the Partner Organisations 2024 |