 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fundamental, technical and environmental overviews of plastic chemical recycling†
Hui Luo
 *a,
Helen Tyrrell
b,
Jingyang Bai
b,
Rukayya Ibrahim Muazu
b and
Xiangyi Long
*a,
Helen Tyrrell
b,
Jingyang Bai
b,
Rukayya Ibrahim Muazu
b and
Xiangyi Long
 *b
*b
aSchool of Mechanical Engineering Sciences, University of Surrey, Guildford, GU2 7XH, UK. E-mail: hui_luo@surrey.ac.uk
bDepartment of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK
First published on 5th October 2024
Abstract
The accumulation of plastic waste is a severe environmental challenge worldwide. Although mechanical recycling methods are in place for plastics such as polyethylene terephthalate (PET), the physical and chemical properties are significantly compromised after a number of cycles, and they eventually reach end-of-life and end up in landfill. Chemical recycling is a collection of emerging innovative technologies that transform plastic waste into base chemicals, monomers and feedstocks. This approach complements mechanical recycling, bridging the gap between waste management and the petrochemical industry. However, with regard to the seven types of recyclable plastic, there is currently no clear overview of the suitable techniques. Therefore, we aim to provide a critical perspective on the suitability of different chemical processes towards recycling different types of plastic, by combining fundamental knowledge and research advancements in recent years, with an emphasis on assessing their environmental and economic impacts. Finally, based on the development status, we will highlight the current challenges and future opportunities in implementing chemical recycling technologies to meet the sustainability requirement of a climate-neutral circular economy.
Introduction
As of 2021, the global production of plastic amounted to nearly 391 million metric tons, with over 90% derived from virgin fossil-based resources, making the plastic industry heavily reliant on fossil fuels.3 However, a mere 10% of the total plastic volume collected is recycled, with just 2% processed through closed-loop recycling, while the majority (79%) is left to accumulate in landfills or other natural environments.5 The rapid growth of plastics production and the accumulated plastic waste exacerbate the triple planetary crisis of habitat loss, plastic pollution and greenhouse gas emissions.7 Thus, the effective management of plastic waste and their end-of-life treatment stands as a major challenge. Specifically, there is a growing interest in transitioning to a circular economy in which plastics will be efficiently and sustainably recycled back into the economy. Yet according to the Circularity Gap Report 2023 by the Circle Economy think tank, the current global circularity rate stands at only 7.2%.8 It means that we are still primarily operating within a linear economy, resulting in the loss of valuable raw materials. This urges us to work on global solutions, from preventing waste, to extracting more value out of this inevitable waste.Recent attempts to mitigate plastic waste, such as plastic prohibitions and the Extended Producer Responsibility (EPR) programme, have made substantial progress. Although reducing demand is a valuable strategy to decrease waste, completely eschewing plastics remains unfeasible due to their ubiquity and essentiality.9 On this note, the Circular Plastics Alliance aims to boost the EU market for recycled plastics, which covers the full plastics value chains and includes over 330 organisations representing industry, academia and public authorities.
Today, mechanical recycling is the most widely used process to recycle plastics. The mechanical recycling industry has the potential to reach 250 million tonnes by 2060, presenting a revenue opportunity of $300–400 billion in today's terms.3 Via mechanical recycling, plastics waste is ground, washed, extruded and pelletised to make recycled plastics. This process allows recycling of plastics waste several times, but with a progressive loss of properties. In addition, mechanical recyclability is restrained by their types and impurities introduced during usage and the post-consumer phase. According to a 2015 report from the Environment Protection Authority (EPA), the plastics with the highest recovery rates are polyethylene terephthalate (PET, SPI code 1, 19.5%), high-density polyethylene (HDPE, SPI code 2, 10%) and low-density polyethylene (LDPE, SPI code 4, 5%). All other plastics, including polypropylene (PP, SPI code 5) and polystyrene (PS, SPI code 6), were recovered in less than 1% of cases. The recovery rates for polyvinyl chloride (PVC, SPI code 3) and other plastics (SPI code 7) were effectively zero.10 However, more recent data show the recycling rate has grown significantly in the past few years. For example, VinylPlus reported that 813![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 tonnes of PVC waste were recycled within their framework in 2022, representing around 27% of the total PVC waste generated in the EU-27, Norway, Switzerland and the UK.11
266 tonnes of PVC waste were recycled within their framework in 2022, representing around 27% of the total PVC waste generated in the EU-27, Norway, Switzerland and the UK.11
Chemical recycling, on the other hand, is a process that, through the application of heat, chemicals, or catalytic agents, converts the plastic polymer chains into oligomers, monomers or other basic chemicals (such as carbon monoxide, carbon dioxide, methane, and hydrogen) prior to further reprocessing into monomers/polymers.14 Unlike mechanical recycling, which requires highly segregated feedstocks through intensive sorting and reprocessing, some of the chemical recycling technologies are largely nonselective and have a higher tolerance to feedstock contaminates, thereby saving the time and additional costs associated with plastic pre-treatment.15,16 Complementary to mechanical recycling, this process offers the possibility to transform hard-to-recycle or end-of-life plastic waste into petrochemical equivalent feedstocks for virgin plastic production. This prevents this type of waste from being sent to incineration or landfill, which requires extensive downstream gas cleaning due to the production of toxic dioxins during operations,16 bridging the gap between waste management and the petrochemical industry. It is generally understood that the chemical recycling of plastics like PET may be less environmentally favourable than mechanical recycling. However, recent studies suggest that directly comparing chemical recycling methods with mechanical recycling is now considered obsolete.17 Hence, the combined utilisation of multiple end-of-life (EoL) management approaches for specific plastic waste streams is currently being employed across various plastic waste management systems.18,19
However, compared with mechanical recycling, the development of chemical recycling is still at the early stages. According to Plastics Europe Circularity, out of 29.5 Mt post-consumer waste collected in the EU27 + 3, <0.1 Mt was chemically recycled, compared with 9.1 Mt sent to mechanical recycling plants.3 This is mainly due to the more complex chemical reaction process and sizable energy input. Despite this, according to a graph from Holland Circular Hotspot, based on data from Nexant/Technip Energies (NexantECA, 2021),20 chemical recycling is projected to grow significantly in the next few decades (Fig. 1). Therefore, research and innovation efforts in both academia and industry are looking to develop sustainable and energy-efficient chemical recycling processes.
 | ||
| Fig. 1 Plastic recycling outlook based on data from Nexant and Technip Energies. The percentage of plastics fall out of mechanical recycling, chemical recycling and energy recovery representing the stream that is sent for landfill (with leakage to nature). Reproduced from ref. 14 with permission from Holland Circular Hotspot, copyright 2023. | ||
Chemical recycling can be broadly categorised into three main technologies including depolymerisation (solvolysis), pyrolysis and gasification. Solvolysis turns waste plastic into monomers that can be re-polymerised to produce virgin plastics, while pyrolysis and gasification create recycled intermediate substances such as pyrolysis oil or syngas to be used as precursor feedstock for monomer chemical synthesis. Depending on the chemical structure of the plastic polymer chain and the desired reaction products, different chemical recycling techniques, reaction conditions, catalysts and reagents are being developed to maximize the resource efficiency and minimize energy consumption. Therefore, in this tutorial review, we aim to provide a recommendation of the suitability of chemical processes towards recycling different types of plastic, with an emphasis on assessing their economic and environmental impact. Finally, based on the development status, we will highlight the current challenges and future opportunities in implementing chemical recycling technologies.
Polyethylene terephthalate – SPI code 1
Polyethylene terephthalate (PET) is a low-cost, light-weight thermoplastic polyester that displays good durability towards heat and chemicals, has high moisture and gas barrier properties and has excellent optical clarity.21 Its ubiquitous use in consumer food and drinks packaging, in the form of bottles, trays and films, as well as fibres for textiles, results in vast amounts of plastic waste globally. Whilst 60% of PET bottles are currently collected for recycling in Europe, a standardised method for collecting other streams of PET waste has not been established. This contributes to the disposal of 5781 kt of PET waste to landfill and the environment in Europe alone.22 The durable, non-biodegradable nature of PET plastic means that recycling is necessary to tackle the mass accumulation of waste generated.The production of PET is a multi-step process involving an esterification reaction between terephthalic acid and ethylene glycol to form bis(hydroxyethyl terephthalate) (BHET), followed by polycondensation of BHET in the presence of antimony-base catalysts such as Sb2O3 and Sb(OAc)3. Until terephthalic acid became a readily available starting material, dimethyl terephthalate was used in a transesterification reaction with ethylene glycol.21 Typically, the starting molecules used to make PET are obtained from petrochemical sources. Companies such as Coca-Cola are increasing their efforts to incorporate bio-derived materials into the production of PET drinks bottles. In 2009, Coca-Cola released the 30% bio-based PlantBottle™ where petrochemical-derived ethylene glycol was substituted by sugarcane-derived ethylene glycol.23,24 Coca-Cola have since produced a 100% bio-based prototype and aims to replace all oil-derived PET with recycled and renewable PET by 2030 in Western Europe and Japan. Whilst the switch to biomass-derived monomers offers a pathway to more sustainable packaging and fibres, it will not solve the problem of tackling the masses of non-biodegradable PET waste accumulated globally. Therefore, it is important that research efforts continue to focus on both sustainable production and recycling of PET.
Mechanical recycling is a common industrial method to convert PET waste into new products due to its simplicity and low-cost. Unfortunately, the desirable properties of virgin-PET are not retained during mechanical recycling. Consequently, only limited amounts of recycled PET can be re-introduced into the plastic bottle production process.25 This results in the majority of PET being downcycled to lower value products, such as carpet fibres, which cannot enter the recycling loop again.26
Alternatively, a chemical recycling approach could address the limitations of current mechanical methods. Using solvolysis, polyester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds can undergo nucleophilic attack to enable the complete breakdown of the polymer chain to reform the initial monomers, oligomers or other useful small molecules. In a closed-loop recycling scenario, the monomers or oligomers would be re-introduced into the production process of virgin-grade PET. Various chemical recycling reactions can be applied depending on the desired products (Fig. 2). Various solvolysis approaches including hydrolysis, glycolysis and methanolysis will enable the recovery of feedstock molecules that are suitable for direct addition into the PET production process.
O bonds can undergo nucleophilic attack to enable the complete breakdown of the polymer chain to reform the initial monomers, oligomers or other useful small molecules. In a closed-loop recycling scenario, the monomers or oligomers would be re-introduced into the production process of virgin-grade PET. Various chemical recycling reactions can be applied depending on the desired products (Fig. 2). Various solvolysis approaches including hydrolysis, glycolysis and methanolysis will enable the recovery of feedstock molecules that are suitable for direct addition into the PET production process.
The hydrolysis of PET can be performed under neutral, acidic or alkaline conditions. Generally, neutral hydrolysis occurs in water between temperatures of 200–300 °C and 1–4 MPa to yield TPA and EG with small amounts of the TPA and EG monoester. A large excess of water is required for the hydrolysis; often mass ratios of PET/water up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 are used. Conducting hydrolysis at temperatures above 245 °C, where PET is in the molten state, has been found to accelerate the depolymerisation.27 This is done in the presence of metal salt catalysts based on Zn, Sb and Mn. Whilst the poor solubility of TPA in water facilitates precipitation and easier separation, impurities from the process also precipitate alongside it. As a result, additional costly purification steps are required to ensure high purities of the monomers.26
12 are used. Conducting hydrolysis at temperatures above 245 °C, where PET is in the molten state, has been found to accelerate the depolymerisation.27 This is done in the presence of metal salt catalysts based on Zn, Sb and Mn. Whilst the poor solubility of TPA in water facilitates precipitation and easier separation, impurities from the process also precipitate alongside it. As a result, additional costly purification steps are required to ensure high purities of the monomers.26
Acid hydrolysis is commonly performed in concentrated H2SO4 (>87%) at lower temperatures compared with neutral hydrolysis (<150 °C) and yields EG and TPA.28 Whilst this system does not require a catalyst, it is highly corrosive and the recovery of high purity EG from the reaction mixture is challenging. Additionally, the use of highly concentrated acid poses safety concerns and a high cost of recovering the acid.29 On the other hand, alkaline hydrolysis requires less-concentrated NaOH or KOH (<20%) to give yields of up to 100% EG and disodium or dipotassium terephthalate. This route is often associated with longer reaction times (3–5 h) and higher reaction temperatures (>200 °C).29 After the depolymerisation, acidification of the reaction mixture enables the precipitation and separation of TPA.26
Alternatively, methanolysis involves the depolymerisation in the presence of methanol to form EG and dimethyl terephthalate (DMT), an alternative to TPA for PET production. Common reaction conditions require 180–280 °C with pressures of 2–4 MPa in the presence of Zn(OAc)2.26 This method is being used industrially by Eastman, where production began at their first operational plant in Tennessee, US earlier this year and is estimated to reach 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year processing capacity. Additionally, Loop Industries recently announced that €35 million of funding will go towards the global rollout of their Infinite Loop™ technology based on methanolysis depolymerisation.28,30,31
000 tonnes per year processing capacity. Additionally, Loop Industries recently announced that €35 million of funding will go towards the global rollout of their Infinite Loop™ technology based on methanolysis depolymerisation.28,30,31
Glycolysis has gained popularity for chemical PET recycling due to the ease of recovering BHET using milder reaction conditions than hydrolysis processes. Obtaining BHET is advantageous as it can be re-introduced into the PET production process at the second step; this enables the first esterification reaction between TPA and EG to be bypassed.28,32 The glycolysis process involves combining PET with an excess of a glycol. In ethylene glycol, the depolymerisation proceeds around the boiling point (∼200 °C) and atmospheric pressure. Despite the requirement of an additional distillation step to separate the excess glycol, the separation of the glycol-based reaction mixture is much easier compared with the separation of acidic or basic reagents used in hydrolysis.33 Glycolysis can occur without the presence of a catalyst but at the detriment of longer reaction times (<5 h) with lower BHET yields.34 Therefore, a great research effort is being made to develop efficient catalysts to increase the efficiency of this process on an industrial scale. Earlier studies identified that metal salt catalysts, such as Zn(OAc)2,35,36 facilitate the glycolysis to BHET, with more recent studies demonstrating BHET yields up to 80% after a reduced time of 1 h on a lab scale.37 Concerns over the separation and environmental impacts of metal salt catalysts have led researchers to explore more “green” catalysts consisting of ionic liquids or deep eutectic solvents. These systems offer benefits such as low volatility, high stability, tunable properties and easier separation but are required in larger amounts and are more costly in comparison.38
Whilst most chemical recycling methods focus on the recovery of monomers or oligomers for re-processing into PET, other methods such as ammonolysis or aminolysis have been explored for the production of TPA and EG fine chemical derivatives. Ammonolysis and aminolysis involve the depolymerisation of PET using liquid ammonia and primary amines respectively. Ammonolysis results in the formation of terephthalamide and EG, whereas aminolysis forms di-amines of TPA and EG. These reactions occur in milder conditions (<100 °C) due to the increased nucleophilicity of amines compared with alcohol reagents in alcoholysis. However, ammonolysis and aminolysis methods use expensive, toxic reagents and have only been demonstrated at lab-scale so far.25 Alternatively, high-temperature hydrolysis using steam pyrolysis can also allow the recovery of TPA. EG cannot be recovered via this route as it decomposes into gases such as CO and CH4. As a result, this process is not commonly used on an industrial scale due to the variety of products formed and the associated separation costs. However, this option could be particularly suitable for chemical recycling of highly contaminated PET waste streams.29,39
Overall, the costs of the various chemical recycling reactions are estimated to be much higher compared with mechanical recycling. This is because chemical recycling processes require significant energy and/or chemical inputs plus any additional PET waste processing and purification steps to achieve high yields. The knowledge surrounding the tolerance of these processes towards coloured PET and contamination found in waste streams is still fairly limited. Studies by Aguado et al. demonstrated that alkaline hydrolysis is more tolerant towards industrial PET waste compared with glycolysis treatment.40 Alkaline hydrolysis and glycolysis methods were applied to both virgin-PET samples and PET-rich (>90%) industrial waste samples containing highly coloured PET and small quantities of other plastics, such as polyolefins. Whilst the yields of depolymerisation products decreased when applying real PET waste in both processes, the alkaline hydrolysis enabled the recovery of TPA with >90% yields in all cases, compared with >77% BHET yields for glycolysis. Similar studies by Barredo and López-Fonseca on industrial PET waste recycling via alkaline hydrolysis and glycolysis respectively yielded similar findings.32,41 Importantly, Barredo et al. demonstrated that TPA recovered from alkaline hydrolysis does not retain the colourants and pigments found in coloured PET waste streams.41
Despite requirements for highly pure PET waste streams, glycolysis is the preferred commercial route for PET chemical recycling. A number of global chemical companies, such as DuPont and Eastman Kodak, currently operate glycolysis of PET on an industrial scale.42 Unfortunately, complex mixed plastic waste streams are not compatible with the processes mentioned in this section as the breakdown occurs at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O polyester bonds that are absent in other types of plastic. Therefore, pre-sorting of waste to create PET-rich waste streams with minimal contamination is crucial for widespread application of these chemical recycling approaches.25 Despite these uncertainties, it is estimated that chemical recycling via depolymerisation could reach a capacity of 350 kt per year by 2025.22
O polyester bonds that are absent in other types of plastic. Therefore, pre-sorting of waste to create PET-rich waste streams with minimal contamination is crucial for widespread application of these chemical recycling approaches.25 Despite these uncertainties, it is estimated that chemical recycling via depolymerisation could reach a capacity of 350 kt per year by 2025.22
Polyolefins (HDPE, LDPE, PP and PS) – SPI code 2, 4, 5, 6
Polyolefins are a group of thermoplastic polymers composed of long, linear heat-responsive hydrocarbon chains. They include HDPE, LDPE, PP and PS, which are the most popular and widely used synthetic plastics, representing about two-thirds of all post-consumer plastic waste. The application of PE and PP comprises around 80% of all synthetic plastic.13 Due to their moldability, durability and low price, they have been widely used in various applications (Table 1). Polyolefins only consist of saturated C–C and C–H bonds, which are highly resistant to chemical functionalisation or degradation due to their strong bond strength. Although the aromatic rings in PS are easily reacted electrophilically, its polymer backbone is identical to that of polyolefins. Therefore, unlike the more reactive carbonyl-containing linkages in polyesters, polyamides, and polyurethanes, they are not easily degraded by chemical methods (such as solvolysis) or enzymatic processes.43,44Pyrolysis is one of the main processes for chemically recycling polyolefins, through thermal decomposition of the long polymer chains with less or absence of oxygen at elevated temperatures (300–900 °C) to produce smaller and less complex molecules in an inert atmosphere.45,46 Fig. 3 shows the pyrolysis of polyolefins and the main products, which includes alkanes, alkenes, aromatics such as benzene, toluene and xylenes (collectively known as BTX) and styrene. This has been discussed in more detail in the section on Liquid products. The liquid product can be used as fuel but can also be the source of valuable chemicals. Styrene is in demand to make new polystyrene. Alkanes and alkenes can be used to make benzene, which is a key building block in many chemical syntheses.
Pyrolysis has been extensively studied in a variety of reactor geometries and experimental setups, ranging from micro-pyrolysers47 and thermogravimetric analysers (TGA)48 for kinetics studies, to laboratory-scale batch reactors,49 semi-batch reactors50,51 and fixed bed reactors.52,53 Additionally, medium and large-scale equipment, especially fluidised bed reactors,54 have been used primarily for industrial applications. Batch and semi-batch reactors are often employed in laboratory settings because of their simple design and operation. Moreover, these types of reactor offer flexibility in the amount of plastic and particle sizes, making them more comparable to industrial conditions.51 Semi-batch reactors, with a carrier gas, typically utilise zeolites to study the catalytic pyrolysis of PP55,56 and PE,56 as well as the thermal cracking of PS.57,58 Batch reactors have been used to investigate the impact of different atmospheres (N2 vs. H2) on the pyrolysis of various plastics, including PE and PP,59,60 and the conversion of PS.61,62 Analysing these different studies indicates that the configuration of the reactor greatly affects the pyrolysis of plastics.
Depending on whether a catalyst has been involved in the process, pyrolysis can be divided into thermal pyrolysis and catalytic pyrolysis.
Thermal pyrolysis
Thermal pyrolysis is a cost-effective and simple technique for processing polyolefins.47,48 Apart from the reactor configuration which has been discussed above, temperature is one of the most crucial parameters in pyrolysis and is also one of the most extensively studied operational variables.49 This parameter has the greatest impact on the thermal cracking of plastics, thus significantly influencing the distribution of pyrolysis products. A common observation in studies on plastic pyrolysis is the advantageous oil yield at lower temperatures.50–53 Conversely, with rising temperatures, gas production increases, predominantly arising from the further breakdown of liquid products at elevated temperatures. However, it has been noted that at sufficiently low temperatures, the liquid yield increases as the temperature rises (from 250 °C to 300 °C for pyrolysis of PE and PP), thus restraining the cracking liquid products.54 This is due to the fact that the decomposition reactions of plastics are not thermodynamically favourable, resulting in lower conversion rates at lower temperatures. Hence, when energy efficiency is a priority, low temperatures with reasonable liquid yields are preferable in order to reduce energy costs.Catalytic pyrolysis
In the catalytic pyrolysis of plastics, a variety of catalysts have been employed to enhance the target reactions, reduce the reaction temperature and time, improve the product quality, and increase the process efficiency.55 Fig. 4 shows the catalytic pyrolysis mechanism over a typical zeolite catalyst, HZSM-5. Apart from the random scission mechanism of C–C bonds in the thermal pyrolysis, the chain scission also initiates on the acidic sites of the zeolite, which involves random and end-chain scission and carbocation reactions. The carbonium ion is created in the long-chain radical because the hydrogen is extracted by the HZSM-5 catalyst. The secondary reactions happen afterwards, including cyclization and aromatization, The Diels–Alder reaction, and isomerization, and are promoted because of the presence of HZSM-5. The choice of catalysts is influenced by factors such as the type of plastic feedstock, desired product distribution, and economic feasibility. Some widely used catalysts have been summarised in Table 2 and discussed further below.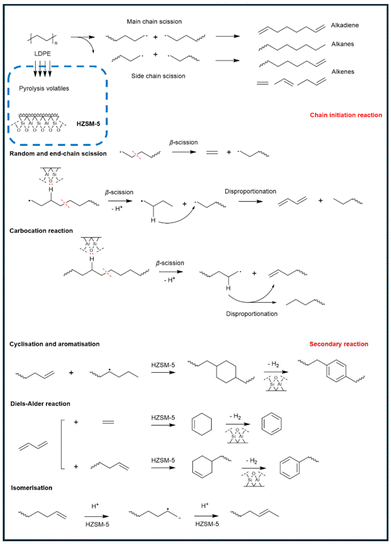 | ||
| Fig. 4 Mechanistic illustration of the catalytic pyrolysis process of LDPE over zeolites. Reproduced from ref. 78 with permission from Elsevier, copyright 2022. | ||
| Catalyst type | Examples | General characteristics |
|---|---|---|
| Zeolite | HZSM-5, HY, HUST, SBA-15, and MCM-41 | Producing higher aromatics and gasoline-range hydrocarbons |
| FCC | — | Negligible cost |
| Producing gasoline-range hydrocarbons and light olefins | ||
| Metal oxides | ZnO, MgO, CaO, and Fe2O3 | Some are effective, cheap, and sustainable |
| Promoting the cleavage of C–C bonds and suppressing undesirable side reactions | ||
| Metal-modified catalysts | Metal loaded Al2O3 and ZSM-5 | Suitable loading can improve catalytic efficacy in the pyrolysis process |
| Other novel catalysts | Carbon materials, and clays | Low cost, and special structure |
| Having excellent potential to achieve different target products |
Zeolite and fluid catalytic cracking (FCC) catalysts
Zeolites, particularly those with a high surface area and pore volume, have been extensively investigated due to their acidic sites and shape-selective properties, which promote the cracking of large hydrocarbon molecules into smaller, more desirable compounds.56–59 Compared with other catalysts for plastic pyrolysis, zeolites have the advantage of producing a higher content of aromatics.60 There are two groups of zeolites for plastic pyrolysis: microporous zeolites, including HZSM-5, HY, HUST, etc., and mesoporous zeolites, including SBA-15, MCM-41, etc.61 The acid sites are the key to pyrolysis. There are two types of acid site in the zeolites, namely Lewis and Brønsted acid sites, as the proton donors. The number of Brønsted acid sites is decided by the ratio of Si/Al in the framework.56 The Lewis acid sites can determine the content of aromatic and alkene chemicals in the pyrolysis product.62,63HZSM-5 is a typical zeolite for pyrolyzing plastic waste and producing aromatics.64 Gasoline-range hydrocarbons and monocyclic aromatic hydrocarbons can be produced with HZSM-5.65 HZSM-5 can participate in the cracking reaction at the end of polymers.66 HY has a larger micropore and is conducive to producing gasoline-range hydrocarbons.67 The selectivity of gasoline-range hydrocarbons in the liquid products can reach 97% in the HDPE pyrolysis reaction.68 One disadvantage of HY is the deactivation, which is faster than HZSM-5. The cavities in HY inhibit the diffusion of polycyclic aromatic hydrocarbons, which can finally form the coke blocking the active acid sites.69 Similarly, HUSY, which can produce considerable gasoline-range hydrocarbons, also suffers from deactivation. In comparison, HZSM-5 has the properties of high connectivity and no cavity, which has a longer lifetime.70
SBA-15 and MCM-41 are mesoporous zeolites with uniform channels and high specific surface area.71 The selectivity of oil is similar to that of microporous catalysts, but these mesoporous zeolites suffer from low stability and acidity.72 The metal-loaded mesoporous zeolites can be prepared to achieve an improved catalysis performance due to the introduction of Brønsted acid sites.72,73
The FCC catalysts are composited of silica-alumina and the binder, which have the zeolite crystal and non-zeolite matrix.74 The FCC unit is used in the petroleum industry to convert crude oils to gasoline, olefinic gases, and base chemicals.75 It has an interconnected network containing micro-, meso- and macropores, and the heavy compounds can enter the internal surface and be pre-cracked. The spent FCC catalyst is a promising option for plastic pyrolysis, considering the similar feedstock structure and the negligible cost. The petroleum-based plastic waste can be cracked and pyrolyzed in the meso- and macropores, while the gasoline-range hydrocarbons and light olefins can be produced in the micropores.76,77
Metal oxides and metal-modified catalysts
Metal oxides such as ZnO, MgO, CaO, and Fe2O3 have demonstrated promising catalytic activity by promoting the cleavage of C–C bonds and suppressing undesirable side reactions, thus improving the yield of desired products. MgO, which is effective, cheap, and sustainable for plastic upgrading, can achieve the goal of cracking diesel into gasoline and hydrogenating the alkenes to alkanes.79 The yield and quality of products can be increased by combining the base and solid acid catalysts.80–82 For example, combining the MgO and HZSM-5 catalysts and making a dual-stage catalytic bed can provide both basic and acidic sites. The quality of products can be improved because of the synergistic effect, and the yield of aromatic hydrocarbons can also be increased.83Moreover, supported metal catalysts, including noble metals such as Pt, Pd, and Ru, as well as non-noble metals like Ni and Co, have shown catalytic efficacy in enhancing the pyrolysis process. Al2O3 is a typical support for different metals. Fe, Ce, Co, Ni, Cu, and Pt have been loaded onto Al2O3 to prepare plastic pyrolysis catalysts.84–86 Meanwhile, metal modifying the zeolites is a crucial way to prepare more efficient catalysts. With a unique microporous structure and uniform acidic and basic sites, metal nanoparticle-modified zeolites have been used in many reactions, including catalytic isomerisation, cracking, and hydrogenation reactions.87–89 Iliopoulou et al. modified ZSM-5 with Ni and Co and found that Ni can promote dehydrogenation and facilitate the formation of aromatics.90 Nishino et al. prepared Ga-ZSM-5 for the PE pyrolysis reaction.91 The yield of liquid products was more than 50%, and the value-added aromatics accounted for more than 80% of the liquid.
Other novel catalysts
Carbon-based catalysts have the advantages of low cost, acidity, numerous pore structures, flexible surface modification, etc., which can convert plastic waste into fuels and value-added chemicals.92 González et al. tested activated carbon as a catalyst in polyethylene pyrolysis, and the activated carbon had a good selectivity to aromatics.93 Research shows that the pore sizes of activated carbons relate to the molecular weight of the products.94,95 Specifically, Sun et al. used H3PO4-actived carbon catalysts to convert waste polyethylene into aromatics, alkenes and alkanes, and studied the catalytic effect on the enrichment of the aromatics.96 The target products were affected by the phosphorus functional groups. Zhang et al. tested the activated carbons in the LDPE plastic pyrolysis and found the yield of aviation fuel could reach up to 100area.%.97 The selectivity of aromatics and alkanes was around 30area.% and 70area.%, respectively. Furthermore, Tsang et al. used the Pt/C catalyst to upcycle biomass-based polyisoprene rubbers into jet-fuel. Hydropyrolysis and vapor-phase hydrogenation were conducted in a two-stage fixed-bed reactor. The C10 cycloalkane yield reached 642.7 mg g−1 with a selectivity of 83.6% at 200 °C.98 Therefore, activated carbons have excellent potential to be applied in plastic pyrolysis. Recently, clays have been chosen for pyrolysis reactions owing to their low cost and high reserves. Their activation could be similar or even superior to zeolites at high temperatures,99 and the layer structure they have can form a 2D network with interconnected micropores.100 Clays tend to produce heavy olefin hydrocarbons, rather than aromatics, because of their mild acidity.84After introducing the different thermal and catalytic pyrolysis processes for polyolefin, the following sections will focus on reviewing the different product compositions.
Liquid products
The liquid products, include alkanes, alkenes, and aromatics such as BTEX and styrene, are central components in polyolefin pyrolysis. For thermal pyrolysis, the aromatic structures such as styrene and benzene mostly originate from PS. PP tends to form alkenes and some aromatics while polyethylene produces alkanes and alkenes. Moreover, the liquid and wax-like hydrocarbons from plastic waste have high calorific values. After upgrading and purification, they can be used as petroleum-like diesel for transportation.46,101–103In catalytic pyrolysis, the use of catalysts improved the yield of oil with high quality.104,105 Moreover, liquid products with a high concentration of aromatics can also be achieved. Aromatic hydrocarbons such as BTEX are key building blocks in many chemical syntheses. Currently, BTEX are mostly produced from fossil fuels via the thermal fractionation of coal and catalytic reforming and aromatising of petroleum. These traditional routes can be altered by using the pyrolysis of waste polyolefins to reduce fossil fuel consumption and carbon emissions.
Nishino et al. prepared Ga-ZSM-5 and transformed the plastic waste into aromatic liquid products at 520–550 °C.91 The yield was over 80%. Valle et al. prepared Ni-modified HZSM-5 and converted the heavy oil into total aromatic hydrocarbons, and the yield increased to 65%.106 Related research showed that the acid sites of zeolite are significant for the aromatic yield. Metal-modified zeolite can adjust the acid sites.107 The pyrolysis of plastic waste can be a promising way to produce BTEX.103 The liquid products (pyrolysis oil) can be used as fuel without upgrading and can also be the source of valuable chemicals with further separation such as distillation and membrane separation that are available in the traditional oil refinery industry. It is foreseen that employing the separation infrastructure in oil industry in the waste recycling chain does not require any modification in the production strategy or in the implementation of new units within the refinery complex.108
Gaseous products
Gaseous products from polyolefin pyrolysis include light alkanes and alkenes from C1 to C5 and H2. The formation of gaseous products is influenced by the type of catalyst in the catalytic pyrolysis.109 Ratnasari et al. found that ethene (C2), propene (C3), and butane (C4) were the major gases in HDPE pyrolysis over MCM-41 and ZSM-5 catalysts.110 Moreover, the amount of gas relates to the amount of zeolite catalysts.111 The composition can be tailored by the size of the zeolite catalysts. More than 50% of gaseous products were C1 to C4 hydrocarbons over the nano-sized HZSM-5 catalyst.112 Many studies also showed the relationship between the yield of gaseous products and the acidity of the catalysts.113 Furthermore, H2 is a promising energy carrier due to its efficiency, sustainability, and development potential. It can be produced from plastic waste, which is an efficient way to recycle plastic waste and have high-value products.Solid products
The solid product from waste polyolefins pyrolysis is a carbon-rich material. The reactor is usually in a two-stage style. The first stage (500–700 °C) is for the waste polyolefin pyrolysis, and the second stage (600–800 °C) is for the synthesis of carbon materials over catalysts.113 Various high-valued carbon materials, including carbon nanotubes (CNTs),114 carbon fibres,115 carbon nanospheres,116 and carbon nanosheets,117 can be produced.CNTs have potential applications because of their high mechanical resilience, interconnected pore structure, and high electrical conductivity.118 More and more research focuses on producing CNTs from plastic waste over specific catalysts.119 Acomb et al. produced CNTs from LDPE over Fe/Al2O3 catalyst, and the yield was 26 wt%.120 Yao et al. used Fe/α-Al2O3 and Fe/γ-Al2O3 catalysts to produce CNTs from real plastic waste, having a yield of 35 wt% and 33 wt%, respectively.121 Wu et al. produced CNTs from PP plastic and achieved a carbon yield of 29 wt% over the Fe/SiO2 catalyst.122 Nahil et al. added different metals, including Zn, Mg, Ca, Ce, Mo, and Mn, into Ni-based catalysts and produced CNTs from PP.123 Yao et al. used post-consumer mixed waste plastics to prepare CNTs over Ni–Fe bimetallic catalysts.124 Different supports, including MCM-41, ZSM-5, H-Beta, and NKF5, were used. The highest yield of carbon materials was over 55 wt%, over the Ni–Fe/MCM-41 catalyst. Wang et al. used cordierite as the support and prepared Fe/cordierite, Ni/cordierite, and Ni–Mg/cordierite catalysts.125 A high yield of filamentous carbon (93 wt%) was achieved. The economic viability of this process was verified by Cai et al.126 The CNTs prepared from plastic pyrolysis have been applied in many fields, such as electrocatalysts,127 solid oxide fuel cells,128 phase change material,129 and pollutant adsorption.130
Overall, pyrolysis is a promising chemical recycling method for polyolefins. It has been extensively studied across various reactor types, with temperature being a key parameter influencing product distribution. Lower temperatures favour oil production, while higher temperatures increase gas yields. Catalytic pyrolysis, employing various catalysts, enhances target reactions, reduces reaction times, and improves product quality and process efficiency. Zeolites are extensively studied for their shape-selective properties, promoting the cracking of large hydrocarbon molecules into smaller, desirable compounds. Metal oxides and metal-modified catalysts, such as supported metal catalysts and FCC catalysts, also show promise in enhancing pyrolysis efficiency. Carbon-based catalysts, including activated carbons and clays, offer low-cost alternatives for plastic waste conversion. Pyrolysis yields liquid, gaseous, and solid products. Liquid products, rich in alkanes, alkenes, and aromatics, find applications as fuels and chemical feedstocks. Gaseous products primarily comprise light hydrocarbons and hydrogen, influenced by catalyst type and acidity. Solid products, carbon-rich materials, can be further processed into high-value carbon materials like CNTs and carbon fibres, with various applications including in electrocatalysts and solid oxide fuel cells.
PVC (polyvinyl chloride) SPI code – 3
PVC is the world's third-largest thermoplastic by volume, after PE and PP. Due to its versatile properties such as light weight, durability, good insulation, high chemical and fire resistance, low cost and easy processability, it finds applications in a variety of fields, such as water pipes, building materials, electronic components and medical devices. PVC is produced from the polymerisation of ethylene dichloride. In the EU-27 + UK, Norway and Switzerland, about 6.5 million tonnes of PVC products are manufactured every year. The annual European consumption of PVC resin totals 5.1 million tonnes – 10% of all plastics used in Europe.131Chemical recycling is more suitable for an unsorted PVC waste stream for which mechanical recycling is not achievable or is uneconomical. However, there are also many challenges in chemical recycling, mostly caused by: (i) plasticiser additives, which are added to PVC at concentrations ranging from 30% to as high as 50% by weight and containing chemicals such as phthalates—which are carcinogenic and mutagenic; and (ii) thermal stabilisers that contains heavy metals such as lead, tin and barium. The solution to these problems is discussed in more detail elsewhere.132–134 In this review, we focus more on the chemical recycling of PVC polymer itself.
The chemical recycling of PVC waste essentially consists of two steps: dechlorination to remove Cl from the PVC macromolecule and the use of the remaining hydrocarbons (Fig. 5a). The first step is essential as the formed highly corrosive HCl gaseous product can significantly damage the reactor, cause potential environmental hazards, poison metal-based pyrolysis catalysts and contaminate the upcycling products. There are two routines, namely dehydrochlorination and dechlorination, for chlorine removal. In dehydrochlorination, chlorine is removed as gaseous HCl when heating up PVC to 400 °C, combined with Cl trapping at the exhaust. Major disadvantages of such treatment include that a certain amount of chlorine is locked in the cyclized and crosslinked structure when conjugate polyene is formed even in the presence of steam, and the service life of the equipment is short due to the corrosive nature of HCl.135,136 Dechlorination presents a more effective approach, where chlorine is removed by absorbents during the thermal treatment.
 | ||
| Fig. 5 (a) Illustration of the traditional (top arrows) and two-step (bottom arrows) methods for PVC-containing mixed polyolefin wastes, and schematic process of cyclization occurring in PVC pyrolysis. Reproduced from ref. 2 with permission from Springer Nature, copyright 2023. (b) Proposed redox-mediated paired-electrolysis mechanism for di(2-ethylhexyl) phthalate (DEHP) plasticizer containing PVC. Key steps are numbered: (1) reduction of plasticizer; (2) electron transfer to polymer; (3) dechlorination of polymer; (4) chloride oxidation; and (5) oxidative chlorination of the arene. Major products observed from the reaction are highlighted in the yellow boxes. Reproduced from ref. 4 with permission from Springer Nature, copyright 2023. (c) The proposed reaction route of the simultaneous upcycling of PVC and PET in ionic liquids (ILs). Reproduced from ref. 6 with permission from Springer Nature, copyright 2023. | ||
Absorbents, usually alkaline compounds such as NaOH,137,138 Na2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139,140 etc., or basic metal oxides such as Fe3O4,141–143 ZnO,144 CaO,145,146 mixed magnesia-alumina oxide2 etc., can significantly increase the chlorine removal effectiveness through catalytic acceleration of the process as well as mitigate hazardous gas emissions by forming water-soluble metal chloride. The dechlorination rate is determined by
139,140 etc., or basic metal oxides such as Fe3O4,141–143 ZnO,144 CaO,145,146 mixed magnesia-alumina oxide2 etc., can significantly increase the chlorine removal effectiveness through catalytic acceleration of the process as well as mitigate hazardous gas emissions by forming water-soluble metal chloride. The dechlorination rate is determined by
A two-stage process is clearly beneficial, as the Cl removal requires a lower temperature (usually below 400 °C even without any catalyst) than polymer chain cracking (>500 °C).141,148,149 Besides, performing the dechlorination and pyrolysis at the same time, especially when the second step involves a metal-based catalyst, the residue chlorine can severely poison the metal catalyst, resulting in a low pyrolysis liquid yield.150 For example, Kots and colleagues reported a two-stage strategy that is capable of upcycling polyolefins mixed with PVC by nearly completely trapping the Cl released from the PVC with Mg3AlO4.5 at 30 bar H2 and 250 °C.2 Subsequent hydrogenolysis over a Ru/TiO2 catalyst results in ∼70% yield for the liquid product. However, without the Cl trapping, the Ru/TiO2 showed almost no activity, with the residual solid yield reaching 99%.
As Cl is also an important reagent, research has also been pushing to recover the Cl from the absorbents, while simultaneously regenerating the absorbent materials. Calcination in steaming at 550 °C can lead to the recovery of HCl with negligible Cl content in the absorbent, following the metal chloride hydrolysis reaction:
| MCl2 + H2O → MO + HCl (M = metal) |
The HCl removed with steam can be separated from the water vapour in the downstream scrubber following standard industrial practices.2 For Cl trapped with alkaline compounds, which generated results in NaCl salt, electrodialysis using an ion exchange membrane coupled with NaCl electrolysis to form NaOH, Cl2 and H2 also demonstrates effective Cl recovery.151
Besides removing Cl with absorbents, recently researchers have also been looking into utilising Cl directly in other reactions as a reagent. A new approach reported by Fagnani et al. utilises the chloride anions generated from PVC under electro-reductive conditions directly in a tandem electro-oxidative chlorination reaction, as shown in Fig. 5b.4 One advantage of this approach is that the plasticizer, DEHP—a problem when it comes to recycling—could serve as a redox mediator. Another advantage is the direct use of electrons in an electrified process instead of the addition of hazardous reagents, resulting in a small ecological footprint. Similarly, Ma's group reported the co-upcycling of PVC and PET.6 Given the similar densities of the two plastics (1.33–1.45 g cm−3 for PET and 1.16–1.55 g cm−3 for PVC), posing extra challenges in separating the two, they developed an upcycling route to transform these two polymers simultaneously. As illustrated in Fig. 5c, by using a chlorine-containing ionic liquid as the catalyst/solvent and ZnCl2 as Lewis acid catalyst, the in situ generated HCl constituents from PVC dehydrochlorination are used to attack the Calkyl–O bonds on the main chain of PET step by step, resulting in the formation of terephthalic acid (TPA, yieldaromatics > 98%) and ethylene dichloride (EDC, yieldC2 > 94% and yieldCl > 97%) in the end. These innovative strategies offer a new perspective on treating mixed plastic wastes.
After the first step of Cl removal from the PVC chains, the remaining material is often treated via high-temperature pyrolysis (500–900 °C)152 or gasification (800–1500 °C).153 During high-temperature treatment, the removal of Cl is thought to initiate cyclisation of the remaining unsaturated polyene chains and subsequent thermal cracking to form benzene and other small hydrocarbons (Fig. 5a).152
As detailed in the previous section on polyolefins, pyrolysis yields valuable products including syngas, liquid fuels and carbon-rich solids. On the other hand, gasification at high temperatures in the presence of oxygen or steam transforms PVC into syngas that serves as a valuable chemical feedstock. In this case, Cl is recovered as HCl via a scrubbing process. Examples of industrial scale PVC recycling processes are documented by Ait-Touchente et al. including two gasification processes operating in Japan – Sumitomo Metals and the Ebara process.132
The advances in Cl removal methods detailed in this section will ensure that high-quality products are formed with minimal Cl content. It is clear that multi-step pyrolysis and gasification approaches are suitable for treating mixed-plastic waste containing PVC. Despite the complexity of the product mixture formed with pyrolysis, it is preferred over gasification due to the high-value and readily applicable liquid oil produced. As both pyrolysis and gasification approaches require high temperatures, further improvements should focus on reducing energy consumption.
Other plastics – SPI code – 7
The SPI code #7 category was designed as a catch-all for “other” plastics, such as bio-based polylactic acid (PLA, polyester), nylon (polyamide), polymethyl methacrylate (PMMA), polycarbonate (PC), acrylonitrile butadiene styrene (ABS), etc. Therefore, the reuse and recycling protocols are not standardised within this category. However, based on their polymer chain structures, similar chemical recycling methods introduced above can still be applied to them.PLA
As a flagship bio-based plastic, PLA is used in many applications such as food packaging materials. The main constituent, lactic acid, is produced through fermentation from biomass feedstocks such as food crops (corns) and waste sugars (cellulose, glucose). Industrially, the polymerisation to PLA is preferentially achieved by ring-opening polymerisation of lactide (a cyclic diester of lactic acid), rather than the polycondensation of lactic acid, which is limited by the need to remove water and difficulty in achieving a high molecular weight.154Compared with petroleum-based plastics, PLA is currently more expensive due to the increased production costs associated with the fermentation and purification of LA, which account for approximately 50% of the total cost. Besides, despite the dynamic growth of biopolymers, their waste streams are still small and scattered, and no separate recycling stream yet exists for PLA. A study has shown that it is more energetically favourable to attain lactic acid from chemical depolymerisation of PLA rather than to produce virgin feedstock from the costly fermentation route.156 Besides, as the formation of lactide from lactic acid can contribute up to 30% of the total cost of the polymerisation of PLA,157 there is also a tendency in research to obtain the dimer directly when chemically recycling PLA. This process is typically done via thermal depolymerisation in the presence of suitable catalysts (such as organic compounds of Zn, Sn, Al, Ti, Zr), at high temperature (200–250 °C) and under vacuum conditions.156,158–161 This process is also rather complex and expensive, both for the severe operating conditions and for the need for several purification steps of the final product.161 Therefore, recent research efforts have been leaning towards advancing base-catalysed hydrolysis which can also lead to lactide formation. Another high-value product that can be recovered from PLA is alkyl lactates, which are green solvents with good biodegradability and low toxicity. They can also be used to produce lactide with a similar transformation process to lactic acid, thus “closing the loop” on the PLA life-cycle. Fig. 6 presents the main chemical recycling methods for PLA.155
 | ||
| Fig. 6 Illustration of different chemical recycling reactions for PLA and the corresponding products: pyrolysis (lactide); acid hydrolysis (lactic acid); base hydrolysis (lactide); and alcoholysis (alkyl lactate). Reproduced from ref. 155 with permission from MDPI, copyright 2020. | ||
pH plays an important role in PLA hydrolysis. The use of acid and base provides alternative mechanisms for the depolymerisation. Under acidic conditions, the dominating process is a chain-end scission, whereby the terminal hydroxyl group is activated by protonation and is hydrolysed directly to lactic acid. The rate of degradation is independent of chain length due to the hydrophobicity of the polymer chain compared with the increased hydrophilicity of the chain end.162,163 In contrast, the use of basic conditions leads to random chain scission via a back-biting reaction to generate lactide, which is subsequently hydrolysed. Accordingly, the process kinetics is dictated by the nature of the hydroxyl terminal groups. When the terminating OH was blocked, no significant PLA degradation was observed by de Jong et al.164 By analysing the fraction of total initial lactide present as one of the chain-ends, random scission and chain-end scission can be determined.165
Due to the insolubility of PLA in aqueous media, hydrolysis can require high temperatures in the range of 180–350 °C.155 Various methods have been proposed to reduce the temperature requirement, including microwave heating (170 °C), high-pressure steam (100–130 °C) and combination with other solvents, such as 50% ethanol (90 °C) and ionic liquid (130 °C). Recently, Friscic and Auclair's research group demonstrated the use of a mechano-enzymatic depolymerisation method with moist-solid reaction mixtures with Humicola insolens cutinase (HiC) enzyme, to avoid the need to dissolve PLA in aqueous solution. After 5 days of milling + aging conditions, the percentage yield of depolymerisation reached over 90%, marking the effectiveness of this process.166
Alcoholysis of PLA to alkyl lactates follows a simple reaction pathway as illustrated in Fig. 6. A range of commercial reagents including alkali/alkaline metals (Li–K/Mg–Ba) and selected alkoxides Na(OEt), K(OEt), Ca(OMe)2, as well as organometallic/chloride Zn, Sn, and Al reagents are commonly used to catalyse the reaction.155 These catalysts, effective as Lewis acid sites, arise via binding to the carboxyl of the ester to facilitate the attack of the chemical bonds, thus driving the transesterification process.167
In addition to research advancements, an overview of the chemical recycling of PLA technologies disclosed in the published patent documents is provided by Niaounakis, where the pros and cons in an industrially relevant context are discussed.168
Nylon (polyamide)
Other commonly used plastics falling in this category are engineering plastics such as polyamides, which are designed to withstand harsh mechanical and environmental conditions, the same property that dictates the challenge in recycling. For example, Nylon-6 is a thermoplastic polyamide produced industrially by water-assisted ring-opening polymerization of ε-caprolactam on a 6.21 million ton annual scale in 2021,169 with the market size estimated at USD 34.39 billion in 2023.170 According to the World Wildlife Federation, up to 1 million tons of Nylon-based fishing gear are abandoned in the ocean each year, with fishing nets composed of Nylon-6 making up at least 46% of the Great Pacific Garbage Patch.Currently, less than 1% of Nylon is recycled worldwide due to the various technical challenges. Mechanical recycling is rarely used since the high manufacturing temperature leads to partial degradation of the polyamide chain, leaving recycled Nylon products with inferior properties.171 The idea of burning plastics for energy recovery does not apply to Nylon either, as a limitation for Nylons is that toxic HCN, CO, CO2, and NH3 are produced in combustion,172 and the economic value of fossil fuel-derived ε-caprolactam will be lost.
The current chemical recycling strategies for polyamides focus on pyrolysis, hydrolysis/solvolysis, hydrogenolysis, ammonolysis or aminolysis, as well as deploymerisation in ionic liquids. Fig. 7 presents the reaction temperature range among different methods. It can be seen that with the aid of metal complex catalyst, the hydrogenation of polyamide can take place under relatively low temperatures (150–200 °C), yet the high H2 pressure, usage of solvent as well as the low product yield still pose safety and efficiency concerns. The reaction is typically catalysed by ruthenium pincer complexes using DMSO as a solvent, which plays a critical role in the process by disrupting the hydrogen bonding of the polyamide and at the same time remaining uncoordinated to the metal center, thus allowing the catalysis to occur.173 Improvements are yet to be made to increase the product yield and find alternative proton sources to H2 for the hydrogenation of amide bonds. In comparison, hydrolysis with sub- and supercritical water and acid catalyst (e.g. HCl) can effectively decrease the reaction temperature requirement, while achieving a high product yield. Bockhorn and co-workers compared the apparent activation energy Ea of the thermal degradation of Nylon-6 by means of TG/MS.174 The Ea is calculated to be 211.3 kJ mol−1 without any catalyst. The value decreases to 163.9 kJ mol−1 in the presence of H3PO4, and further to 113 kJ mol−1 with the eutectic mixture of NaOH/KOH as catalyst. However, a disadvantage of this process is the high yield of salts and traces of acids in the recovered ε-caprolactam, which is a drawback for the production of virgin Nylon materials. Without the aid of an acid catalyst, hydrolysis takes place in the temperature range of 300–370 °C, with a varied product yield depending on the solvent type (water, toluene, methanol, etc.) and reaction time.
 | ||
| Fig. 7 (a) Examples of hydrolysis, pyrolysis and hydrogenolysis reactions of Nylon polymers. (b) A summary of Nylon depolymerisation product yield as a function of reaction temperature. | ||
A pyrolysis approach with reduced pressure and oxygen-free conditions normally delivers a high product yield. The temperature ranges from 240 °C to 400 °C, and varies depending on the catalyst being used. For example, Wursthorn et al. reported the use of Ln(N(TMS)2)3 (Ln = lanthanide), a commercially available lanthanide trisamide catalyst, to treat post-consumer Nylon-6.175 Under solvent-free conditions, 10–3 Torr and 240 °C, the ε-caprolactam yield can reach 93%. As shown in Fig. 8, experimental and theoretical mechanistic analysis argues that the reaction proceeds via a novel mechanism involving an initial deprotonation step of the Nylon amide N–H bond which binds the catalyst covalently to the polymer. This step is followed by predominant chain-end backbiting steps in which ε-caprolactam units are sequentially excised from the chain ends. There are rare reports on ammonolysis of Nylon in the literature, with a handful of examples documented in the patent domain.176–180 Therefore, we will not review this technique here in great detail.
 | ||
| Fig. 8 Mechanistic illustration of Nylon-6 depolymerisation with commercially available lanthanide trisamido catalysts Ln(N(TMS)2)3 (Ln = lanthanide). (A) Computed Nylon-6 model reaction. (B) Nylon coordination. (C) Proposed depolymerisation mechanism. Reproduced from ref. 175 with permission from Wiley, copyright 2023. | ||
To summarise, depending on the polymer structure, the reactions for chemical recycling of different plastics need to be carefully chosen. Table 3 provides a list of reaction types mentioned in this review, along with the operation temperatures. When energy efficiency is a priority, low temperatures may help reduce energy costs. Yet this needs to be reviewed alongside other parameters, such as catalyst type, product yield, post-separation, etc., in order to find the suitable process.
| Plastic type | Reaction | Temperature/°C |
|---|---|---|
| PET | Acid hydrolysis | <150 |
| Neutral hydrolysis | 200–300 | |
| Alkaline hydrolysis | >200 | |
| Methanolysis | 180–280 | |
| Glycolysis | ∼200 | |
| Ammonolysis | <100 | |
| Polyolefins | Thermal pyrolysis | 300–900 |
| Catalytic pyrolysis | <500 | |
| PVC | Dichlorination + pyrolysis | 400–500 |
| PLA | Hydrolysis | 180–350 |
| Nylon (polyamide) | Hydrogenolysis | 150–200 |
| Hydrolysis | 300–370 | |
| Pyrolysis | 240–400 | |
| Ammonolysis | 300–400 | |
Mixed plastics
Although upcycling single-component plastic waste is feasible, upcycling real-life plastic waste, that is, plastic mixtures comprising diverse plastic types (including polyolefins, polyesters, polyurethane, polyvinyl chloride and so on), is more challenging. It is claimed that PLA bottles can contaminate the PET waste stream, making its recycling process less efficient and increasing its cost by necessitating investment in new sorting equipment. It is noteworthy that these plastics cannot be easily or cheaply sorted by sight and the separation methods based on density are not efficient enough due to their similar densities. Only near infrared sensors can ensure good separation, but this technology requires an expensive initial investment, and it is not common place in current recycling facilities.Yet, we should aim to transform these mixed plastics. There are two strategies that warrant special consideration: (1) transformation of mixed plastics into a product with a simple composition, and (2) stepwise transformation of mixed plastics. In both cases, the rational design of the underlying carbon cycle is very crucial.9 In the former case, the pyrolysis method has been widely adapted by industry (e.g. ChemCycling® from BASF), to recycle low-quality mixed waste streams, such as municipal solid waste. Such a method has high tolerance to contaminants and impurities, and is more suitable for streams consisting of mostly polyolefins that have high carbon contents. However, polyolefins are difficult to selectively depolymerise due to the uniformity of chemical structure across the polymer chain, which lacks differentially cleavable bonds. Therefore, under reaction conditions, the C–C and C–H bonds break in a statistical fashion, resulting in a mixture of short, medium and long-chain alkanes (C1–C35), alkenes and aromatics. This phenomenon poses additional challenges in post-reaction product separation.15
The latter case is more adaptable to mixed streams containing other elements, such as PVC (Cl), polyester (O) and polyamides (N). For example, Sánchez and Collinson reported that the use of Zn(Ac)2 in PLA/PET mixer solvolysis can effectively depolymerise PLA, while PET remains as unconverted solid.167 Similarly, Yang et al. discovered, by adjusting the reaction conditions, that a zinc bis[bis(trimethylsilyl)amide] (Zn(HMDS)2) catalyst is capable of sequentially depolymerising mixed BPA-PC/PET, PLA/PBS and PLA/PBAT.181 These solvolysis-based catalytic depolymerisation processes are more sensitive to impurities, and therefore very often pre-treatment steps are still required.
Another type of more complex mixed plastic stream is multilayer flexible plastic film packaging, which typically includes PE, PP, PET, PS, PVC, polyamides, ethylene vinyl alcohol (EVOH) and other materials like papers, foils, inks and additives. Traditional mechanical recycling cannot be used for their separation, resulting in the current recycling rates of 1–2% in the United States, while the market is still growing annually at a rate of 3.4% to 37.5 million tons in 2026. The dissolution strategy, in which the mixed polymers are dissolved in suitable solvents and recovered selected by anti-solvents, has a clear advantage. For example, a method called solvent-targeted recovery and precipitation (STRAP) is currently being studied by Huber's group, where the multilayer film is dissolved in different solvents to selectively solubilise different polymers, such as PE/EVOH/PET,182 PP183 and PE.184 The main advantage of this process is that the recovered polymers exhibit high purity, which is comparable to virgin polymer resins. Yet since the distillation columns and the heat required to separate the solvent and antisolvents are the major cost drivers for this process, the process needs to be deployed at scale to be competitive against virgin polymer manufacture.182 Other solvent-based separation and recycling techniques for mixed waste plastics have also been summarised previously.185 In recent years, there has also been an increasing focus on developing bio-based, renewable solvents such as α-pinene,186 D-limonene187 and ionic liquids188 in order to improve the overall sustainability of the process.
Sustainability assessment of plastic waste chemical recycling
While chemical recycling is currently considered a promising route for plastic waste recycling to chemical feedstocks, there are concerns over the energy intensity of processing related to repolymerisation and catalyst use, environmental implications of solvent use, and associated costs. To fully understand the intricacies in chemical recycling and to make progress in this domain, it is imperative to evaluate the potential environmental, cost, safety and social consequences of chemically recycling plastic waste.Sustainability assessment is the most common and reliable way to measure the viability of potential or existing technologies.189 Life cycle assessment (LCA), techno-economic assessment (TEA) and social LCA (S-LCA) are standard methodologies that allow for the characterisation of environmental and economic and social aspects of technologies respectively. LCA examines the potential environmental impact of a product, process, or service over its entire life cycle from raw material source (extraction) through to product manufacture, product use, and end of life (EoL). LCA is conducted according to procedures set by ISO 14040–14044,190 and accounts for all the inputs and outputs within a defined system boundary.191 TEA combines technical and economic data to evaluate the feasibility and profitability of the system,192 while social LCA (S-LCA) focuses on identifying and managing impacts, both positive and negative, on different stakeholders across the value chains.193
Generally, sustainability assessments related to the chemical recycling of plastic waste are limited, and thus, information regarding the environmental performance is not common.194 In recent years, studies have attempted to capture the impacts of some of the plastics waste chemical recycling techniques18 including gasification, pyrolysis, feedstock recycling, solvolysis, and hydrocracking. Fig. 9 and Table S1† show the results of environmental and economic assessments from a review of 14 existing studies and 27 case studies. These studies show pyrolysis as the main route for chemical recycling being modelled using LCA.16,18,195–197 Over 90% of the 27 case studies focused on only LCA,18,197–200 while only two studies reported the economic potential of the assessed systems.195,201 Most of the studies employed a mass basis with a system function as 1t of treated plastic waste. A cradle-to-gate system boundary is common among the reviewed studies mainly due to limited or absence of EoL data of the products derived from the plastics, which is not uncommon among LCAs of emerging technologies.202 For comparative purposes, some of the studies omitted the cradle stage, i.e. the pre-conversion stages listed above, and thus, only the gate-to-gate impact was reported.197,201 Some of the plastics evaluated as mixed or individual plastics among the reviewed studies include PET, HDPE, low LDPE, PP, PVC, PS and others that were not specified in the reported studies.
 | ||
| Fig. 9 GWP impact of plastic waste management via chemical recycling from 27 case studies (Gasific. = gasification; MR: mechanical recycling; F = fast; LTP = low-temperature pyrolysis; Rcy = recycling; FR = feedstock recycling), MPW = mixed plastic waste (see Table S1† for specific composition of each mix);*cradle-to-gate plastic polymer production; **cradle-to-grave (incineration of plastic waste). | ||
Since greenhouse gas (GHG) emissions are viewed as the most important environmental aspect for plastic waste management or recycling, the most reported environmental impact indicator is the global warming potential (GWP). Thus, all the reported GWP impact values from the 27 case studies were normalised to a reference function of 1 kg treated plastic waste, to allow a fairer comparison of the assessment outcomes. Apart from the climate change impacts, a range of environmental impacts, particularly acidification potential (AP-kgSO2-eq), photochemical ozone creation potential (POCP-kg C2H4-eq), and abiotic depletion potential (ADP-(kgSb-eq.)), were also reported by some of the studies.16,197,199,203
The GWP impact of chemically recycling various plastic waste is in the range of 0.37 to 12 kgCO2-eq per kg of plastic waste across all the 27 case studies, employing different chemical recycling methods (Fig. 9). As with many LCA studies of other products, variations exist in the LCA outcomes of the reviewed studies due to differences in the technologies used for recycling, type of polymer and LCA methodological choices; nevertheless, some level of consistency across most of the studies can be observed.202,204
Rickert et al. proposed a new approach of evaluating the performance of pyrolysing mixed plastic wastes by using the environmental budget, which was defined as the margin of environmental impact by two systems supplying the same function, with one system supplying energy and the other focused on monomer recovery from the processed plastic waste (PET trays).16 Four scenarios as a function of monomer recovery and energy generation were analysed, of which the percentage monomer recovery and efficiency of the recovery influence the total environmental budget. In terms of GWP, 100% monomer recovery offered the highest environmental budget across all scenarios assessed in the study. Meanwhile, recovery without ethylene glycol offered a higher environmental budget than 50% recovery but lower environmental budget than 100% and 80% monomer recovery scenarios. Out of all the impact categories reported, acidification potential performed worst for the specific chemical recycling technology, which was also influenced by the recovery efficiency. A specific monomer recovery efficiency may be required for a given recycling system to operate within an environmental budget, consequently providing environmental benefits. A breakeven recovery efficiency was estimated at 40.95% for the modelled system.16 This is useful particularly with the complexity of the various chemical recycling options. For the recovery of pyrolysis fractions such as ethylene, propylene, -n-butane, 1-butene, isobutene, light naphtha, and aromatics, GWP impact values of 3.66 kgCO2-eq and 4.22 kgCO2-eq were associated with the pyrolysis route adopting consequential and attributional LCAs respectively.195
Low-temperature pyrolysis had a higher GWP impact compared with hydrocracking in the case of PET/PE treatment.198 Likewise, chemical recycling of PLA into dilactide via cyclic depolymerization followed by melt crystallization and ring-opening was 1.5 times greater than solvent-based treatment of PLA using selective dissolution to remove impurities such as adhesives, paints, and paper.196 Technical difficulties were reported in the case of the chemical route, but even so, a 100% lactic acid replacement was assumed. While solvent was recycled within the system, the loss of it contributed mainly to ozone formation, acidification and eutrophication potential.196 In terms of benefits to the system, the two main sources come from credit allocation and product substitution in the market. Selective dissolution and precipitation, which uses a solvent and anti-solvent to recover polymers of interest, was also reported to have a relatively low GWP of 1.9 kgCO2-eq compared with other assessed technologies including one solvolysis, one pyrolysis, and four mechanical recycling technologies and ten different incineration scenarios.44 The main setback for this system in terms of environmental consideration is the use of hazardous organic solvents, which can be reduced via high-rate recycling.
Comparing with mechanical recycling, Vollmer et al. reported CO2-eq values of ∼5.5, 4.3, 2.9, and 3.1 kg CO2-eq per kg of plastic waste for mechanical recycling of four types of polymer, namely acrylonitrile butadiene styrene, high-impact polystyrene, PET and PET-PE respectively.44 Khoo et al. obtained a GWP of 0.37 and 0.65 kgCO2-eq for gasification and pyrolysis of mixed plastic respectively (Table S1†); the same study obtained a GWP of 0.40 kgCO2-eq per kg of plastic waste via mechanical recycling, showing a slightly higher impact of mechanical recycling compared with pyrolysis and gasification for this specific case study.18 While mechanical recycling is known to have lower EoL processing impact, the lower product quality reduces its overall benefit against chemical technologies (Fig. 9). Their study combined gasification and pyrolysis routes alongside mechanical recycling and waste to energy across 8 different scenarios.
Most of the reviewed studies showed a significant reduction in GWP impact associated with the recovered products by credit allocation for avoided virgin material production, which contributed up to 59% GWP savings.199 This emphasises the need to maximise product quality and focus on high-value commodities. In terms of other environmental impact indicators, acidification potential, photochemical ozone creation and particular matter formation were also reported by some of the reviewed studies, with acidification potential as the most widely reported next to GWP. For 1 kg of plastic waste, a range of acidification potential values of chemical recycling by gasification were 0.00018 kgSO2-eq (ref. 18) and 0.0007 kgSO2-eq,197 while the value for pyrolysis was obtained as 0.00014 kgSO2-eq.18 Other chemical recycling had values of 0.0015 kgSO2-eq while solvent-based extraction was 0.00083 kgSO2-eq.196 For all the reviewed recycling pathways, recycling back to monomer (dimethyl terephthalate (DMT)) showed a higher value of 0.021 kgSO2-eq per kg PET fibre compared with the values reported in this study, and for mechanical recycling, with values in the range of 0.00064 kgSO2-eq (ref. 196) and 0.003 kgSO2-eq.200 The remaining indicators were sparsely reported, which presents a limitation for comparative analysis.
Considering the economics of plastic chemical recycling, a baseline scenario for the consequential LCA approach had a net present value (NPV) of $220.3 per t of HDPE plastic waste.195 Products with high yields such as propylene and butene had strong control of the market dynamics. These market dynamics pose significant environmental consequences with a specific influence on GWP (kgCO2-eq). It is worth noting that the literature on the economic analysis of plastic chemical recycling is relatively limited; however, emerging interest in low-cost sustainable pathways for plastic waste valorisation has stimulated increased research in this domain.205 For example, a study by Singh et al. reported a base-case minimum selling price (MSP) of $1.93 per kg of TPA recovered from waste PET via enzymatic recycling.206 Likewise, MSP values of $ 0.87 per kg, $ 0.96 per kg and $1.05 per kg were achieved for PET dissolution, glycolysis and methanolysis respectively.207 The same study reported an MSP in the range of $0.73 per kg to $1.10 per kg for the dissolution of polyolefins. The cost of feedstock (plastic waste) and its pre-treatment had a significant influence on the overall cost of recycling the plastic waste across all the reported studies, with up to 58% contribution to the total MSP.208 As with the environmental impact, the cost of the treatment pathways and product of interest are also key players in achieving viability of plastic chemical recycling processes. An MSP of $0.70 was achieved for 1 kg of methanol via gasification of mixed plastics. From both economic and environmental perspectives, plastic waste pre-processing including collection, transportation, sorting, cleaning, and flake production indicates significance for the overall performance of the recycling pathway. A 30% burden resulted from primary treatment preceding collection and sorting of PLA waste for solvent-based treatment.196 A large contribution of transportation to the total environmental impact of chemical recycling is further highlighted. In this case, the optimum location of the pre-processing and main recycling should be prioritised. More so, reducing the mass of plastic feedstock by pre-processing at source will lower the transportation burden.193,197 To maximise the benefits of plastic waste chemical recycling, the cost and environmental impact of feed and pre-treatment must be lowered through the adoption of improved logistics and advanced sorting techniques, and a reduction in the consumption of reagents and energy in the pre-treatment stages. More so, the optimisation of other parameters such as the main processing conditions, reagents and/or catalysts, feed quality and reference products should also be prioritised. Future sustainability assessments should also capture the influence of these parameters to provide a more holistic comparative analysis of emerging chemical recycling pathways.
Implementation of chemical recycling technologies
Today, most plastics are still produced from fossil-based feedstock. A lack of appropriate waste management infrastructure, policy incentives and business models is currently preventing the full value of plastics waste being captured. Transitioning to a circular, climate-neutral economy demands special investment and innovation to develop new feedstocks from recycled plastics to reduce dependence on fossil-based oil and gas, contributing to the goals of the Paris and Glasgow Agreements. Despite the technology development reviewed above, chemical recycling processes are more complex and thus more expensive, especially in the implementation phase, and therefore need more financial incentives and value chain considerations.• Over the last decade, the chemical recycling industry has grown significantly. As of 2022, the global chemical recycling input capacity has reached close to 1.2 million tonnes, with Europe at the forefront of technological developments.14 A significant number of demo and commercial plants are planned to launch in the upcoming years, as shown in Fig. 10. The size of installations matters and is expected to grow with TRL (Technology Readiness Level) and market maturity. For pyrolysis plants, it is expected that the size will grow from 30–50 kilotonnes per year in 2021 to 100 kilotonnes per year in 2030. In parallel, a development of improved and more efficient technologies needs to take place. These include technologies that are more tolerant for feedstock quality, have a higher efficiency and lower environmental impact, and are more scalable with much larger unit operations via improved process and reactor design. In recent years, emerging research that focuses on combining mechanical processing and biological treatment with chemical methods has gain momentum. For example, mechanochemical processes utilise mechanical energy and stoichiometry to drive plastic depolymerisation with minimal solvent usage;209–211 enzymolysis, such as the technique used by Carbios, utilises enzymes to effectively break down plastics such as PET.212,213
 | ||
| Fig. 10 Planned investments in chemical recycling in Europe. Data are based on the announcement made by members and non-members of Plastics Europe by May 2023. Adapted from ref. 1 with permission from Plastics Europe, copyright 2023. | ||
• In addition to the technical aspects reviewed above about catalyst and reaction conditions, there are two other key factors that influence the efficiency and output quality of these chemical recycling processes, namely the feedstock purity and size reduction. Among different recycling technologies, solvolysis depolymerisation typically requires high feedstock purity with minimum contaminants such as other plastic types, dyes, and adhesives. Smaller size particles (e.g. 5–20 mm) are preferred to improve surface area and reaction efficiency. Pyrolysis is more tolerable for this aspect as it can handle mixed plastic waste, but excessive contamination by non-plastic materials (metal, glass, paper) can negatively affect the process and damage equipment. A moderate size reduction can ensure consistent and uniform heating. Gasification has the lowest purity requirement amongst all three technologies. Not only can it handle mixed plastic waste, but some non-plastic wastes like biomass and municipal solid wastes are also tolerable. Taking these factors into account, one opportunity lies in having chemical recycling as an added step to the mechanical recycling process, to treat the stream that has been rejected for mechanical re-processing that otherwise goes to incineration or landfill.214
• Optimising waste management processes is essential to increase the level of resource efficiency, and thus the level of recycling. Encouraging separate waste collection is key as it leads to a much higher level of recycling rates. According to an EU report,3 plastics waste recycling rates are 13 times higher when collected separately compared with mixed collection schemes. In a recent social science study led by Walzberg et al., modelling results have also illustrated the importance of changing the habits of disposal behaviours. It is shown that in the US context, while behavioural interventions would require about 300–900 GJ of additional energy at end-of-life due to improved collection rates, they would avoid about 500–700 thousand metric tons of GHG emissions related to sorting and reprocessing.215
• Combining high recycling rates with renewable feedstocks improves the absolute sustainability of plastics. A recent study shows that if the plastics industry achieves a 75% recycling rate with advanced recycling technologies in 2030, plastics can comply with their assigned share of the safe operating space and be considered absolute environmentally sustainable regarding the considered eight planetary boundaries.7 The remaining virgin plastic production would predominantly rely on CO2 and renewable electricity from wind, hydro or nuclear power. A smaller portion of plastics would come from biomass.216
• Any attempt to upcycle plastics should be accompanied by a comprehensive LCA – from raw material extraction to recycling and transformation – to evaluate the overall process environmental impact. An aggressive implementation of multilayered strategies is required to curb the GHG emissions from plastics. Currently GHG mitigation strategies are often implemented within energy, materials, waste-reduction and management policies in isolation, yet the absolute reduction in GHG emissions of plastics’ life cycle requires a combination of energy infrastructure decarbonisation, recycling capability improvement, bio-based plastics adaption and demand management.217
• Although being the key to sustainability, recycling alone cannot cope with the growth in plastics demand predicted until 2050. Therefore, achieving absolute sustainability of plastics requires a fundamental change in both producing and using plastics.7 By now, many stakeholders have put a focus on either upstream solutions (so called pre-consumer) such as material design, substitution and plastic reduction, or downstream solutions (so called post-consumer) such as mechanical and chemical recycling. However, Systemiq's analysis pointed out the solutions need to be balanced out.218 Overall, reducing plastic consumption while treating plastic waste as a valuable resource will be essential for reducing the planetary footprint of plastics. Accordingly, society needs to decide whether or not to stop considering plastics as cheap and disposable and to start placing a higher value on this versatile and durable product.
Conclusion
This tutorial review highlights the considerations in developing, implementing and assessing chemical recycling technologies for different plastic wastes. The major conclusions are as follows:• Chemical recycling converts the plastic polymer chains into oligomers, monomers or other basic chemicals (such as carbon monoxide, carbon dioxide, methane, and hydrogen) prior to further reprocessing into monomers/polymers. Complementary to mechanical recycling, this process offers the possibility to transform hard-to-recycle or end-of-life plastic waste into petrochemical equivalent feedstocks for virgin plastic production.
• PET – Solvolysis processes, in particular glycolysis and alkaline hydrolysis, are recommended for the chemical recycling of PET back into the starting monomers or intermediates. As solvolysis targets the degradation of polyester bonds, mixed-plastic waste streams are not suitable for these processes. Future research in this area should focus on process optimisation using real industrial waste and investigating tolerances towards coloured PET streams and contamination.
• PP, PE, PS – Pyrolysis is a promising chemical recycling method for polyolefins. It thermally decomposes the long polymer chains to produce smaller and less complex molecules under an inert atmosphere. Liquid products, rich in alkanes, alkenes, and aromatics, can be utilised as fuels and chemical feedstocks. Gaseous products primarily comprise light hydrocarbons and hydrogen. Solid products, carbon-rich materials, can be further processed into high-value carbon materials with various applications including electrocatalysts and solid oxide fuel cells.
• PVC – Two-step process: dechlorination (remove Cl from the PVC macromolecule) + pyrolysis/gasification (use of the remaining hydrocarbons) has been developed in recent years to recover the Cl element and improve the quality of the final hydrocarbon products. However, due to the high additive content in PVC compared with other plastic types, novel methods are still required to maximise the recycling rate.
• Others and mixed plastics – the SPI-7 category was designed as a catch-all for “other” plastics, with the reuse and recycling protocols currently not standardised. These “others” waste is often mixed with the main plastic streams, adding complexity for mechanical and chemical recycling. However, based on their polymer chain structures, chemical recycling methods introduced similar to those above can still be applied on them, via “one-pot” transformation into products with simple composition, or sequential treatment processes.
• The existing literature indicates the importance of recovering high-value products via chemical recycling technologies as it underpins the sustainability of recycling technologies since up to ∼60% of the total burden could be avoided compared with 25% presented by some mechanical recycling technologies. Furthermore, the utilisation of renewables or alternative low-impact energy sources could further reduce the environmental intensity of processing plastic waste via chemical recycling. Economic benefits could also be obtained from the sale of high-value products as well as avoided CO2 costs.
• The sustainability profile of a recycling system is a function of several factors including the combination of polymer and the recycling process, as well as the assessment methodology. The suitability of polymers for a recycling technology should be assessed and prioritised as a combination that yields highest product of interest, to maximise the environmental and economic benefits of the recycling system.
• The implementation of chemical recycling technologies requires support from waste management infrastructure, policy and financial incentives as well as tailored business models. Alongside the deployment of higher TRL pyrolysis plants, the development of other technologies with better feedback quality tolerance and scalability is key. Stakeholders need to look at both the upstream of plastic production and downstream plastic recycling as a whole to achieve overall sustainability, where coupling with other renewable carbons (CO2, biomass) may come into play.
Author contributions
HL conceived the idea for the review. The manuscript was written and reviewed through contributions of all the authors.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
HL thanks the support from the Surrey Future Fellowship Scheme funded by the University of Surrey. HT would like to acknowledge the EPSRC Centre for Doctoral training in Next Generation Synthesis and Reaction Technology (Imperial College London, EP/S023232/1). RIM would like to acknowledge support from the VALUED project funded by the Engineering and Physical Sciences Research Council (EPSRC) UK, project grant: EP/W031019/1. XL gratefully acknowledges funding from the European Union Horizon 2020 Programme (iCAREPLAST Project, Grant agreement No. 820770).References
- Plastics Europe Chemical Recycling, https://plasticseurope.org/sustainability/circularity/recycling/chemical-recycling/, (accessed 10/08, 2024).
- P. A. Kots, B. C. Vance, C. M. Quinn, C. Wang and D. G. Vlachos, Nat. Sustainability, 2023, 6, 1258–1267 CrossRef.
- Plastic Europe, The Circular Economy for Plastics – A European Analysis, 2024 Search PubMed.
- D. E. Fagnani, D. Kim, S. I. Camarero, J. F. Alfaro and A. J. McNeil, Nat. Chem., 2023, 15, 222–229 CrossRef.
- E. Anglou, A. Ganesan, Y. Chang, K. M. Gołąbek, Q. Fu, W. Bradley, C. W. Jones, C. Sievers, S. Nair and F. Boukouvala, Chem. Eng. J., 2024, 481, 148278 CrossRef.
- R. Cao, M. Q. Zhang, Y. Jiao, Y. Li, B. Sun, D. Xiao, M. Wang and D. Ma, Nat. Sustainability, 2023, 6, 1685–1692 CrossRef.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Nat. Sustainability, 2023, 6, 599–610 CrossRef.
- Circle Economy, The circularity gap report, 2023, pp. 1–64 Search PubMed.
- D. Ma, Nat. Sustainability, 2023, 6, 1142–1143 CrossRef.
- A. Rahimi and J. M. García, Nat. Rev. Chem., 2017, 1, 1–11 CrossRef.
- VinylPlus, Progress Report, https://www.vinylplus.eu/our-achievements/progress-report-2023/.
- UNEP, Single-use plastics: A roadmap for sustainability, 2018 Search PubMed.
- A. Lee and M. S. Liew, J. Mater. Cycles Waste Manage., 2021, 23, 32–43 CrossRef.
- Holland Circular Hotspot, Chemical Recycling in circular perspective, 2023 Search PubMed.
- R. A. Clark and M. P. Shaver, Chem. Rev., 2024, 124, 2617–2650 CrossRef PubMed.
- J. Rickert, F. Cerdas and C. Herrmann, Proc. CIRP, 2020, 90, 426–431 CrossRef.
- R. D. Allen and M. I. James, in Circular Economy of Polymers: Topics in Recycling Technologies, American Chemical Society, 2021, vol. 1391, ch. 4, pp. 61–80 Search PubMed.
- H. H. Khoo, Resour., Conserv. Recycl., 2019, 145, 67–77 CrossRef.
- M. G. Davidson, R. A. Furlong and M. C. McManus, J. Cleaner Prod., 2021, 293, 126163 CrossRef.
- NexantECA, Advances in depolymerization technologies for recycling. Retrieved from, https://www.nexanteca.com/news-and-media/advances-depolymerization-technologies-recycling.
- R. Nisticò, Polym. Test., 2020, 90, 106707 CrossRef.
- Zero Waste Europe, How circular is PET?, 2022 Search PubMed.
- Coca-Cola Collaborates with Tech Partners to Create Bottle Prototype Made from 100% Plant-Based Sources, https://www.coca-colacompany.com/media-center/100-percent-plant-based-plastic-bottle, (accessed 09/08, 2024).
- R. Mori, RSC Sustainability, 2023, 1, 179–212 RSC.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3765 RSC.
- J. Payne and M. D. Jones, Journal, 2021, 14, 4041–4070 Search PubMed.
- P. Pereira, P. E. Savage and C. W. Pester, ACS Sustainable Chem. Eng., 2023, 11, 7203–7209 CrossRef.
- V. Sinha, M. R. Patel and J. V. Patel, J. Polym. Environ., 2010, 18, 8–25 CrossRef.
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1347 CrossRef.
- Loop Industries And Reed Management Sign Agreement for €35 Million Financing For Global Commercialization of The INFINITE LOOP™ Technology and to Form French Joint Venture, https://www.loopindustries.com/cms/loop-industries-and-reed-management-sign-agreement-for-e35-million-financing-for-global-commercialization-of-the-infinite-loop-technology-and-to-form-french-joint-venture/, (accessed 09/08, 2024).
- Eastman's depolymerisation plant starts production, generates revenue, https://www.sustainableplastics.com/news/eastmans-depolymerisation-plant-starts-production-generates-revenue, (accessed 09/08, 2024).
- R. López-Fonseca, I. Duque-Ingunza, B. de Rivas, S. Arnaiz and J. I. Gutiérrez-Ortiz, Polym. Degrad. Stab., 2010, 95, 1022–1028 CrossRef.
- A. Bohre, P. R. Jadhao, K. Tripathi, K. K. Pant, B. Likozar and B. Saha, ChemSusChem, 2023, 16, e202300142 CrossRef.
- M. P. Ekart, W. S. Murdock Jr. and T. M. Pell Jr., USPat., US6410607B1, 2002 Search PubMed.
- U. R. Vaidya and V. M. Nadkarni, Ind. Eng. Chem. Res., 1987, 26, 194–198 CrossRef.
- S. Baliga and W. T. Wong, J. Polym. Sci., Part A: Polym. Chem., 1989, 27, 2071–2082 CrossRef.
- R. López-Fonseca, I. Duque-Ingunza, B. de Rivas, L. Flores-Giraldo and J. I. Gutiérrez-Ortiz, Chem. Eng. J., 2011, 168, 312–320 CrossRef.
- J. Xin, Q. Zhang, J. Huang, R. Huang, Q. Z. Jaffery, D. Yan, Q. Zhou, J. Xu and X. Lu, J. Environ. Manage., 2021, 296, 113267 CrossRef PubMed.
- W. Kaminsky, Fuel Commun., 2021, 8, 100023 CrossRef.
- A. Aguado, L. Martínez, L. Becerra, M. Arieta-araunabeña, S. Arnaiz, A. Asueta and I. Robertson, J. Mater. Cycles Waste Manage., 2014, 16, 201–210 CrossRef.
- A. Barredo, A. Asueta, I. Amundarain, J. Leivar, R. Miguel-Fernández, S. Arnaiz, E. Epelde, R. López-Fonseca and J. I. Gutiérrez-Ortiz, J. Environ. Chem. Eng., 2023, 11, 109823 CrossRef.
- N. George and T. Kurian, Ind. Eng. Chem. Res., 2014, 53, 14185–14198 CrossRef CAS.
- C. W. S. Yeung, J. Y. Q. Teo, X. J. Loh and J. Y. C. Lim, ACS Mater. Lett., 2021, 3, 1660–1676 CrossRef CAS.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Energy Convers. Manage., 2016, 115, 308–326 CrossRef CAS.
- R. Miandad, M. A. Barakat, A. S. Aburiazaiza, M. Rehan and A. S. Nizami, Process Saf. Environ. Prot., 2016, 102, 822–822 CrossRef CAS.
- Y. Zhang, G. Ji, D. Ma, C. Chen, Y. Wang, W. Wang and A. Li, Process Saf. Environ. Prot., 2020, 142, 203–211 CrossRef CAS.
- J. Jiang, K. Shi, X. Zhang, K. Yu, H. Zhang, J. He, Y. Ju and J. Liu, J. Environ. Chem. Eng., 2022, 10, 106867–106867 CrossRef CAS.
- O. Dogu, M. Pelucchi, R. Van de Vijver, P. H. M. Van Steenberge, D. R. D'Hooge, A. Cuoci, M. Mehl, A. Frassoldati, T. Faravelli and K. M. Van Geem, Prog. Energy Combust. Sci., 2021, 84, 100901–100901 CrossRef.
- A. López, I. de Marco, B. M. Caballero, M. F. Laresgoiti and A. Adrados, Chem. Eng. J., 2011, 173, 62–71 CrossRef.
- E. A. Williams and P. T. Williams, J. Anal. Appl. Pyrolysis, 1997, 40–41, 347–363 CrossRef.
- Y. Liu, J. Qian and J. Wang, Fuel Process. Technol., 2000, 63, 45–55 CrossRef CAS.
- R. Bagri and P. T. Williams, J. Anal. Appl. Pyrolysis, 2002, 63, 29–41 CrossRef CAS.
- I. Ahmad, M. I. Khan, H. Khan, M. Ishaq, R. Tariq, K. Gul and W. Ahmad, Int. J. Green Energy, 2015, 12, 663–671 CrossRef CAS.
- D. P. Serrano, J. Aguado and J. M. Escola, ACS Catal., 2012, 2, 1924–1941 CrossRef CAS.
- R. Thahir, M. Irwan, A. Alwathan and R. Ramli, Results Eng., 2021, 11, 100231–100231 CrossRef CAS.
- K. Sivagami, K. V. Kumar, P. Tamizhdurai, D. Govindarajan, M. Kumar and I. Nambi, RSC Adv., 2022, 12, 7612–7620 RSC.
- S. L. Wong, S. Armenise, B. B. Nyakuma, A. Bogush, S. Towers, C. H. Lee, K. Y. Wong, T. H. Lee, E. Rebrov and M. Muñoz, J. Anal. Appl. Pyrolysis, 2023, 169, 105793–105793 CrossRef CAS.
- Y. Chai, W. Dai, G. Wu, N. Guan and L. Li, Acc. Chem. Res., 2021, 54, 2894–2904 CrossRef CAS.
- L. Dai, N. Zhou, Y. Lv, Y. Cheng, Y. Wang, Y. Liu, K. Cobb, P. Chen, H. Lei and R. Ruan, Prog. Energy Combust. Sci., 2022, 93, 101021 CrossRef.
- W. Vermeiren and J. P. Gilson, Top. Catal., 2009, 52, 1131–1161 CrossRef CAS.
- R. K. Singh, B. Ruj, A. K. Sadhukhan and P. Gupta, J. Environ. Manage., 2019, 239, 395–406 CrossRef CAS PubMed.
- R. K. Singh, B. Ruj, A. K. Sadhukhan and P. Gupta, J. Environ. Manage., 2020, 261, 110112–110112 CrossRef CAS.
- J. Jae, G. A. Tompsett, A. J. Foster, K. D. Hammond, S. M. Auerbach, R. F. Lobo and G. W. Huber, J. Catal., 2011, 279, 257–268 CrossRef CAS.
- X. Zhang, H. Lei, G. Yadavalli, L. Zhu, Y. Wei and Y. Liu, Fuel, 2015, 144, 33–42 CrossRef CAS.
- M. Artetxe, G. Lopez, M. Amutio, G. Elordi, J. Bilbao and M. Olazar, Ind. Eng. Chem. Res., 2013, 52, 10637–10645 CrossRef CAS.
- P. Castano, G. Elordi, M. Olazar, A. T. Aguayo, B. Pawelec and J. Bilbao, Appl. Catal., B, 2011, 104, 91–100 CrossRef CAS.
- Y.-H. Seo, K.-H. Lee and D.-H. Shin, J. Anal. Appl. Pyrolysis, 2003, 70, 383–398 CrossRef CAS.
- A. Marcilla, M. I. Beltrán and R. Navarro, Appl. Catal., B, 2009, 86, 78–86 CrossRef CAS.
- E. Epelde, M. Ibañez, A. T. Aguayo, A. G. Gayubo, J. Bilbao and P. Castaño, Microporous Mesoporous Mater., 2014, 195, 284–293 CrossRef CAS.
- G. A. Eimer, L. B. Pierella, G. A. Monti and O. A. Anunziata, Catal. Commun., 2003, 4, 118–123 CrossRef CAS.
- S. J. Choi, Y.-K. Park, K.-E. Jeong, T.-W. Kim, H.-J. Chae, S. H. Park, J.-K. Jeon and S.-S. Kim, Korean J. Chem. Eng., 2010, 27, 1446–1451 CrossRef CAS.
- A. Marcilla, A. Gómez-Siurana and D. Berenguer, Appl. Catal., A, 2006, 301, 222–231 CrossRef CAS.
- T. F. Degnan Jr, Top. Catal., 2000, 13, 349–356 CrossRef.
- E. Rodríguez, A. Gutiérrez, R. Palos, F. J. Vela, M. J. Azkoiti, J. M. Arandes and J. Bilbao, Chem. Eng. J., 2020, 382, 122602–122602 CrossRef.
- E. Rodríguez, A. Gutiérrez, R. Palos, F. J. Vela, J. M. Arandes and J. Bilbao, Waste Manage., 2019, 93, 162–172 CrossRef.
- G. Elordi, M. Olazar, P. Castaño, M. Artetxe and J. Bilbao, Ind. Eng. Chem. Res., 2012, 51, 14008–14017 CrossRef CAS.
- Y. Wu, K. Wang, B. Wei, H. Yang, L. Jin and H. Hu, Sci. Total Environ., 2022, 806, 151287 CrossRef CAS PubMed.
- L. Fan, Y. Zhang, S. Liu, N. Zhou, P. Chen, Y. Liu, Y. Wang, P. Peng, Y. Cheng and M. Addy, Energy Convers. Manage., 2017, 149, 432–441 CrossRef CAS.
- H. Zhang, J. Zheng, R. Xiao, Y. Jia, D. Shen, B. Jin and G. Xiao, Energy Fuels, 2014, 28, 4294–4299 CrossRef CAS.
- J. Wang, B. Zhang, Z. Zhong, K. Ding, A. Deng, M. Min, P. Chen and R. Ruan, Energy Convers. Manage., 2017, 139, 222–231 CrossRef CAS.
- H. Iftikhar, M. Zeeshan, S. Iqbal, B. Muneer and M. Razzaq, Bioresour. Technol., 2019, 289, 121647–121647 CrossRef CAS.
- G. Kabir and B. H. Hameed, Renewable Sustainable Energy Rev., 2017, 70, 945–967 CrossRef CAS.
- J. Wang, J. Jiang, X. Meng, M. Li, X. Wang, S. Pang, K. Wang, Y. Sun, Z. Zhong, R. Ruan and A. J. Ragauskas, Environ. Sci. Technol., 2020, 54, 8390–8400 CrossRef CAS PubMed.
- F. Zhang, M. Zeng, R. D. Yappert, J. Sun, Y. H. Lee, A. M. LaPointe, B. Peters, M. M. Abu-Omar and S. L. Scott, Science, 2020, 370, 437–437 CrossRef CAS PubMed.
- Ç. Çelikgöğüs and A. Karaduman, Energy Sources, Part A, 2015, 37, 2507–2513 CrossRef.
- J. Kang, K. Cheng, L. Zhang, Q. Zhang, J. Ding, W. Hua, Y. Lou, Q. Zhai and Y. Wang, Angew. Chem., Int. Ed., 2011, 50, 5200–5203 CrossRef CAS.
- X. Peng, K. Cheng, J. Kang, B. Gu, X. Yu, Q. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2015, 54, 4553–4556 CrossRef CAS.
- C. Zhao and J. A. Lercher, Angew. Chem., Int. Ed., 2012, 51, 5935–5940 CrossRef CAS PubMed.
- E. F. Iliopoulou, S. D. Stefanidis, K. G. Kalogiannis, A. Delimitis, A. A. Lappas and K. S. Triantafyllidis, Appl. Catal., B, 2012, 127, 281–290 CrossRef CAS.
- J. Nishino, M. Itoh, H. Fujiyoshi and Y. Uemichi, Fuel, 2008, 87, 3681–3686 CrossRef CAS.
- Y. Chen, Y. Zhu, Z. Wang, Y. Li, L. Wang, L. Ding, X. Gao, Y. Ma and Y. Guo, Adv. Colloid Interface Sci., 2011, 163, 39–52 CrossRef CAS PubMed.
- Y. S. González, C. Costa, M. C. Márquez and P. Ramos, J. Hazard. Mater., 2011, 187, 101–112 CrossRef.
- A. do Couto Fraga, C. P. B. Quitete, V. L. Ximenes, E. F. Sousa-Aguiar, I. M. Fonseca and A. M. B. Rego, J. Mol. Catal. A: Chem., 2016, 422, 248–257 CrossRef.
- Z. Yang, H. Lei, Y. Zhang, K. Qian, E. Villota, M. Qian, G. Yadavalli and H. Sun, Appl. Energy, 2018, 220, 426–436 CrossRef CAS.
- K. Sun, Q. Huang, X. Meng, Y. Chi and J. Yan, Energy Fuels, 2018, 32, 9772–9781 CrossRef CAS.
- Y. Zhang, D. Duan, H. Lei, E. Villota and R. Ruan, Appl. Energy, 2019, 251, 113337 CrossRef CAS.
- J. Wang, J. Jiang, Z. Zhang, X. Meng, Y. Sun, A. J. Ragauskas, Q. Zhang and D. C. W. Tsang, Energy Convers. Manage., 2022, 270, 116250 CrossRef CAS.
- K. M. Rajendran, V. Chintala, A. Sharma, S. Pal, J. K. Pandey and P. Ghodke, Mater. Today Commun., 2020, 24, 100982 CrossRef.
- K. Li, J. Lei, G. Yuan, P. Weerachanchai, J.-Y. Wang, J. Zhao and Y. Yang, Chem. Eng. J., 2017, 317, 800–809 CrossRef CAS.
- S. Lee, K. Yoshida and K. Yoshikawa, Energy Environ. Res., 2015, 5, 18–18 Search PubMed.
- S. Frigo, M. Seggiani, M. Puccini and S. Vitolo, Fuel, 2014, 116, 399–408 CrossRef CAS.
- M. B. Al Rayaan, Clean. Eng. Technol., 2021, 2, 100062 CrossRef.
- J. Chattopadhyay, T. S. Pathak, R. Srivastava and A. C. Singh, Energy, 2016, 103, 513–521 CrossRef CAS.
- C. Li, C. Zhang, M. Gholizadeh and X. Hu, J. Hazard. Mater., 2020, 399, 123075 CrossRef CAS PubMed.
- B. Valle, A. G. Gayubo, A. Alonso, A. T. Aguayo and J. Bilbao, Appl. Catal., B, 2010, 100, 318–327 CrossRef CAS.
- E.-M. El-Malki, R. A. Van Santen and W. M. H. Sachtler, J. Phys. Chem. B, 1999, 103, 4611–4622 CrossRef CAS.
- R. Palos, A. Gutiérrez, F. J. Vela, M. Olazar, J. M. Arandes and J. Bilbao, Energy Fuels, 2021, 35, 3529–3557 CrossRef CAS PubMed.
- F. J. Christopher, P. S. Kumar, L. Jayaraman and G. Rangasamy, Fuel, 2023, 332, 126168 CrossRef CAS.
- D. K. Ratnasari, M. A. Nahil and P. T. Williams, J. Anal. Appl. Pyrolysis, 2017, 124, 631–637 CrossRef CAS.
- B. Kunwar, B. R. Moser, S. R. Chandrasekaran, N. Rajagopalan and B. K. Sharma, Energy, 2016, 111, 884–892 CrossRef CAS.
- A. L. Figueiredo, A. S. Araujo, M. Linares, Á. Peral, R. A. García, D. P. Serrano and V. J. Fernandes, J. Anal. Appl. Pyrolysis, 2016, 117, 132–140 CrossRef CAS.
- H. Qiao, X. Hou, H. Zhou, C. Song, L. Yin, J. Huang, E. Yuan and T. Cui, Fuel, 2023, 351, 128821 CrossRef CAS.
- D. Yao and C.-H. Wang, Appl. Energy, 2020, 265, 114819 CrossRef CAS.
- J. M. Younker, T. Saito, M. A. Hunt, A. K. Naskar and A. Beste, J. Am. Chem. Soc., 2013, 135, 6130–6141 CrossRef CAS.
- H. Zhang, X.-L. Zhou, L.-M. Shao, F. Lü and P.-J. He, ACS Sustainable Chem. Eng., 2019, 7, 3801–3810 CrossRef CAS.
- Y. Wen, K. Kierzek, X. Chen, J. Gong, J. Liu, R. Niu, E. Mijowska and T. Tang, Waste Manage., 2019, 87, 691–700 CrossRef CAS.
- R. Vicentini, W. Nunes, B. G. A. Freitas, L. M. Da Silva, D. M. Soares, R. Cesar, C. B. Rodella and H. Zanin, Energy Storage Mater., 2019, 22, 311–322 CrossRef.
- S. He, Y. Xu, Y. Zhang, S. Bell and C. Wu, J. Hazard. Mater., 2021, 402, 123726 CrossRef CAS PubMed.
- J. C. Acomb, C. Wu and P. T. Williams, Appl. Catal., B, 2016, 180, 497–510 CrossRef CAS.
- D. Yao, Y. Zhang, P. T. Williams, H. Yang and H. Chen, Appl. Catal., B, 2018, 221, 584–597 CrossRef CAS.
- X. Liu, Y. Zhang, M. A. Nahil, P. T. Williams and C. Wu, J. Anal. Appl. Pyrolysis, 2017, 125, 32–39 CrossRef CAS.
- M. A. Nahil, C. Wu and P. T. Williams, Fuel Process. Technol., 2015, 130, 46–53 CrossRef CAS.
- D. Yao, H. Yang, Q. Hu, Y. Chen, H. Chen and P. T. Williams, Appl. Catal., B, 2021, 280, 119413 CrossRef CAS.
- J. Wang, B. Shen, M. Lan, D. Kang and C. Wu, Catal. Today, 2020, 351, 50–57 CrossRef CAS.
- N. Cai, S. Xia, X. Li, L. Sun, P. Bartocci, F. Fantozzi, H. Zhang, H. Chen, P. T. Williams and H. Yang, J. Cleaner Prod., 2021, 315, 128240 CrossRef CAS.
- N. Cai, S. Xia, X. Zhang, Z. Meng, P. Bartocci, F. Fantozzi, Y. Chen, H. Chen, P. T. Williams and H. Yang, ChemSusChem, 2020, 13, 938–944 CrossRef CAS.
- Q. Liu, F. Wang, E. Hu, R. Hong, T. Li, X. Yuan, X. B. Cheng, N. Cai, R. Xiao and H. Zhang, iScience, 2022, 25, 104855 CrossRef CAS.
- Y. Wang, J. Bailey, Y. Zhu, Y. Zhang, S. K. S. Boetcher, Y. Li and C. Wu, Waste Manage., 2022, 154, 96–104 CrossRef CAS.
- D. Yao, H. Li, B. C. Mohan, A. K. Prabhakar, Y. Dai and C.-H. Wang, ACS Sustainable Chem. Eng., 2022, 10, 1125–1136 CrossRef CAS.
- The European Council of Vinyl Manufacturers, PVC applications, https://pvc.org/pvc-applications/, (accessed 10/08, 2024).
- Z. Ait-Touchente, M. Khellaf, G. Raffin, N. Lebaz and A. Elaissari, Polym. Adv. Technol., 2024, 35, e6228 CrossRef CAS.
- K. Lewandowski and K. Skórczewska, Polymers, 2022, 14, 3035 CrossRef CAS.
- S. Xu, Z. Han, K. Yuan, P. Qin, W. Zhao, T. Lin, T. Zhou and F. Huang, Nat. Rev. Methods Primers, 2023, 3, 44–44 CrossRef CAS.
- G. Wang, S. Liu, H. Zhang, J. Wang and Q. Xue, Polymers, 2022, 14, 1689 CrossRef CAS.
- Z. Yuan, J. Zhang, P. Zhao, Z. Wang, X. Cui, L. Gao, Q. Guo and H. Tian, ACS Omega, 2020, 5, 11291–11298 CrossRef CAS PubMed.
- J. Lu, S. Borjigin, S. Kumagai, T. Kameda, Y. Saito and T. Yoshioka, Waste Manage., 2019, 99, 31–41 CrossRef CAS PubMed.
- T. Kameda, M. Ono, G. Grause, T. Mizoguchi and T. Yoshioka, Ind. Eng. Chem. Res., 2008, 47, 8619–8624 CrossRef CAS.
- D. Torres, Y. Jiang, D. A. Sanchez-Monsalve and G. A. Leeke, J. Anal. Appl. Pyrolysis, 2020, 149, 104831 CrossRef CAS.
- G. Jiang, D. A. Sanchez Monsalve, P. Clough, Y. Jiang and G. A. Leeke, ACS Sustainable Chem. Eng., 2021, 9, 1576–1589 CrossRef CAS.
- J. Hubáček, J. Lederer, P. Kuráň, P. Koutník, Z. Gholami, M. Zbuzek and M. Bačiak, Fuel Process. Technol., 2022, 231, 107226 CrossRef.
- L. Ye, T. Li and L. Hong, Waste Manage., 2021, 126, 832–842 CrossRef CAS PubMed.
- L. Hong, L.-H. Ye and T.-L. Li, De-chlorination effectiveness and efficiency of poly(vinyl) chloride (PVC) co-pyrolyzing with iron oxide, SSRN, preprint, DOI:10.2139/ssrn.4402944.
- A. M. Hapipi, H. Suda, M. A. Uddin and Y. Kato, Energy Fuels, 2018, 32, 7792–7799 CrossRef CAS.
- H. Nishibata, M. A. Uddin and Y. Kato, Polym. Degrad. Stab., 2020, 179, 109225 CrossRef CAS.
- H. Mio, S. Saeki, J. Kano and F. Saito, Environ. Sci. Technol., 2002, 36, 1344–1348 CrossRef CAS PubMed.
- M. H. Cho, S. H. Jung and J. S. Kim, Energy Fuels, 2010, 24, 1389–1395 CrossRef CAS.
- K. B. Park, S. J. Oh, G. Begum and J. S. Kim, Energy, 2018, 157, 402–411 CrossRef CAS.
- T. Bhaskar, R. Negoro, A. Muto and Y. Sakata, Green Chem., 2006, 8, 697–700 RSC.
- L. Chen, Y. Zhu, L. C. Meyer, L. V. Hale, T. T. Le, A. Karkamkar, J. A. Lercher, O. Y. Gutiérrez and J. Szanyi, React. Chem. Eng., 2022, 7, 844–854 RSC.
- S. Kumagai, J. Lu, Y. Fukushima, H. Ohno, T. Kameda and T. Yoshioka, Resour., Conserv. Recycl., 2018, 133, 354–361 CrossRef.
- M. Sadat-Shojai and G. R. Bakhshandeh, Journal, 2011, 96, 404–415 CAS.
- J. Huang, A. Veksha, W. P. Chan, A. Giannis and G. Lisak, Renewable Sustainable Energy Rev., 2022, 154, 111866 CrossRef CAS.
- M. L. Di Lorenzo and R. Androsch, Synthesis, Structure and Properties of Poly(lactic acid), Springer, 2018 Search PubMed.
- P. McKeown and M. D. Jones, Sustainable Chem., 2020, 1, 1–22 CrossRef.
- V. Piemonte, S. Sabatini and F. Gironi, J. Polym. Environ., 2013, 21, 640–647 CrossRef CAS.
- L. Shen, J. I. Haufe and M. K. Patel, Product overview and market projection of emerging bio-based plastics, PRO-BIP 2009: final report, Copernic Institute for Sustainable Development and Innovation - Utrecht University, the Netherlands, 2009 Search PubMed.
- F. d. Kopinke, M. Remmler, K. Mackenzie and M. Milder, Polym. Degrad. Stab., 1996, 53, 329–342 CrossRef CAS.
- D. Cam and M. Marucci, Polymer, 1997, 38, 1879–1884 CrossRef CAS.
- H. Nishida, T. Mori, S. Hoshihara, Y. Fan, Y. Shirai and T. Endo, Polym. Degrad. Stab., 2003, 81, 515–523 CrossRef CAS.
- H. Abe, N. Takahashi, K. J. Kim, M. Mochizuki and Y. Doi, Biomacromolecules, 2004, 5, 1606–1614 CrossRef CAS PubMed.
- S. Lazzari, F. Codari, G. Storti, M. Morbidelli and D. Moscatelli, Polym. Degrad. Stab., 2014, 110, 80–90 CrossRef CAS.
- C. F. Van Nostrum, T. F. J. Veldhuis, G. W. Bos and W. E. Hennink, Polymer, 2004, 45, 6779–6787 CrossRef CAS.
- S. J. De Jong, E. R. Arias, D. T. S. Rijkers, C. F. Van Nostrum, J. J. Kettenes-Van Den Bosch and W. E. Hennink, Polymer, 2001, 42, 2795–2802 CrossRef CAS.
- C. Shih, J. Controlled Release, 1995, 34, 9–15 CrossRef CAS.
- M. Pérez-Venegas, T. Friščić and K. Auclair, ACS Sustainable Chem. Eng., 2023, 11, 9924–9931 CrossRef.
- A. C. Sánchez and S. R. Collinson, Eur. Polym. J., 2011, 47, 1970–1976 CrossRef.
- M. Niaounakis, Eur. Polym. J., 2019, 114, 464–475 CrossRef CAS.
- Statista, Production of polyamide fibers worldwide from 1975 to 2022, https://www.statista.com/statistics/649908/polyamide-fiber-production-worldwide/#:~:text=In%202022%2C%20global%20polyamide%20production,of%20polyamide%20fibers%20produced%20worldwide, (accessed 12/08, 2024).
- Nylon Market Size, Share & Trends Analysis Report, https://www.grandviewresearch.com/industry-analysis/nylon-6-6-market, (accessed 12/08, 2024).
- M. J. Lozano-González, M. T. Rodriguez-Hernandez, E. A. Gonzalez-De Los Santos and J. Villalpando-Olmos, J. Appl. Polym. Sci., 2000, 76, 851–858 CrossRef.
- M. Nielsen, P. Jurasek, J. Hayashi and E. Furimsky, J. Anal. Appl. Pyrolysis, 1995, 35, 43–51 CrossRef CAS.
- A. Kumar, N. Von Wolff, M. Rauch, Y. Q. Zou, G. Shmul, Y. Ben-David, G. Leitus, L. Avram and D. Milstein, J. Am. Chem. Soc., 2020, 142, 14267–14275 CrossRef CAS PubMed.
- H. Bockhorn, A. Hornung, U. Hornung and J. Weichmann, Thermochim. Acta, 1999, 337, 97–110 CrossRef CAS.
- L. Wursthorn, K. Beckett, J. O. Rothbaum, R. M. Cywar, C. Lincoln, Y. Kratish and T. J. Marks, Angew. Chem., Int. Ed., 2023, 62, e202212543 CrossRef CAS PubMed.
- R. J. Mckinney, USPat., US005302756A, 1994 Search PubMed.
- E. Canivenc and J.-F. Thierry, WO1997003040A1, 1996.
- S. B. Fergusson and Y. Yan, WO2001070665A2, 2001.
- R. J. Mckinney, US Pat., US005395974A, 1995 Search PubMed.
- J. A. J. Hendrix, M. Booij and Y. H. Frentzen, US Pat., US005668277A, 1997 Search PubMed.
- R. Yang, G. Xu, B. Dong, X. Guo and Q. Wang, ACS Sustainable Chem. Eng., 2022, 10, 9860–9871 CrossRef CAS.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed.
- J. Yu, A. d. C. Munguía-López, V. S. Cecon, K. L. Sánchez-Rivera, K. Nelson, J. Wu, S. Kolapkar, V. M. Zavala, G. W. Curtzwiler, K. L. Vorst, E. Bar-Ziv and G. W. Huber, Green Chem., 2023, 25, 4723–4734 RSC.
- K. L. Sánchez-Rivera, C. Granger, H. Appiah, K. Nelson, S. Grey, D. J. Sun, J. E. Estela-García, E. Chen, Z. Xu, T. A. Osswald, L.-S. Turng, A. G. McDonald, R. C. Van Lehn, E. Bar-Ziv and G. W. Huber, ACS Mater. Lett., 2024, 4042–4050, DOI:10.1021/acsmaterialslett.4c01048.
- Y. B. Zhao, X. D. Lv and H. G. Ni, Chemosphere, 2018, 209, 707–720 CrossRef CAS PubMed.
- S. C. Aparício, P. M. Castro, B. D. Ribeiro and I. M. Marrucho, Green Chem., 2024, 26, 6799–6811 RSC.
- M. Ramírez-Martínez, S. L. Aristizábal, G. Szekely and S. P. Nunes, Green Chem., 2022, 25, 966–977 RSC.
- M. Mohan, J. D. Keasling, B. A. Simmons and S. Singh, Green Chem., 2022, 24, 4140–4152 RSC.
- A. Lindfors, Environ. Sustainability Indicators, 2021, 12, 100149 CrossRef.
- ISO 14044:2006 Environmental management—Life cycle assessment—Requirements and guidelines, https://www.iso.org/standard/38498.html, (accessed on 17/10/2024).
- R. I. Muazu, R. Rothman and L. Maltby, J. Cleaner Prod., 2021, 293, 126120 CrossRef.
- I. G. Hakeem, A. Sharma, T. Sharma, A. Sharma, J. B. Joshi, K. Shah, A. S. Ball and A. Surapaneni, Biofuels, Bioprod. Biorefin., 2023, 17, 718–750 CrossRef CAS.
- UNEP, Guidelines for Social Life Cycle Assessment of Products and Organizations, 2020 Search PubMed.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–24 CrossRef CAS PubMed.
- X. Zhao and F. You, ACS Sustainable Chem. Eng., 2021, 9, 12167–12184 CrossRef CAS.
- D. Maga, M. Hiebel and N. Thonemann, Resour., Conserv. Recycl., 2019, 149, 86–96 CrossRef.
- A. Demetrious and E. Crossin, J. Mater. Cycles Waste Manage., 2019, 21, 850–860 CrossRef CAS.
- F. Perugini, M. L. Mastellone and U. Arena, Environ. Prog., 2005, 24, 137–154 CrossRef CAS.
- S. M. Al-Salem, S. Evangelisti and P. Lettieri, Chem. Eng. J., 2014, 244, 391–402 CrossRef CAS.
- L. Shen, E. Worrell and M. K. Patel, Resour., Conserv. Recycl., 2010, 55, 34–52 CrossRef.
- G. Faraca, V. Martinez-Sanchez and T. F. Astrup, Resour., Conserv. Recycl., 2019, 143, 299–309 CrossRef.
- R. I. Muazu, P. Yaseneva, N. Shah and M.-M. Titirici, unpublished work.
- Bank of England, LCA of Management Options for Polymer Waste from Bank Notes - Final Study Report, 2015.
- N. Ryan and P. Yaseneva, Philos. Trans. R. Soc., A, 2021, 379, 20200335 CrossRef CAS PubMed.
- BOTTLE™ research analysis, https://www.bottle.org/research/analysis, (accessed 13/08, 2024).
- A. Singh, N. A. Rorrer, S. R. Nicholson, E. Erickson, J. S. DesVeaux, A. F. T. Avelino, P. Lamers, A. Bhatt, Y. Zhang, G. Avery, L. Tao, A. R. Pickford, A. C. Carpenter, J. E. McGeehan and G. T. Beckham, Joule, 2021, 5, 2479–2503 CrossRef CAS.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- G. Yadav, A. Singh, A. Dutta, T. Uekert, J. S. DesVeaux, S. R. Nicholson, E. C. D. Tan, C. Mukarakate, J. A. Schaidle, C. J. Wrasman, A. C. Carpenter, R. M. Baldwin, Y. Román-Leshkov and G. T. Beckham, Energy Environ. Sci., 2023, 16, 3638–3653 RSC.
- A. H. Hergesell, R. J. Baarslag, C. L. Seitzinger, R. Meena, P. Schara, Ž. Tomović, G. Li, B. M. Weckhuysen and I. Vollmer, J. Am. Chem. Soc., 2024, 146, 26139–26147 CrossRef CAS PubMed.
- V. Štrukil, ChemSusChem, 2021, 14, 330–338 CrossRef PubMed.
- S. Kaabel, J. Arciszewski, T. H. Borchers, J. P. D. Therien, T. Friščić and K. Auclair, ChemSusChem, 2023, 16, e202201613 CrossRef CAS PubMed.
- V. Tournier, C. M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M. L. Desrousseaux, H. Texier, S. Gavalda, M. Cot, E. Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne and A. Marty, Nature, 2020, 580, 216–219 CrossRef CAS PubMed.
- C.-C. Chen, L. Dai, L. Ma and R.-T. Guo, Nat. Rev. Chem., 2020, 4, 114–126 CrossRef PubMed.
- P. U. Vasileios Rizos, E. Righetti and A. Kassab, Chemical Recycling of Plastics: Technologies, trends and policy implications, CEPS, 2023 Search PubMed.
- J. Walzberg, S. Sethuraman, T. Ghosh, T. Uekert and A. Carpenter, Energy Res. Soc. Sci., 2023, 100, 103116 CrossRef.
- E. T. C. Vogt and B. M. Weckhuysen, Nature, 2024, 629, 295–306 CrossRef CAS PubMed.
- J. Zheng and S. Suh, Nat. Clim. Change, 2019, 9, 374–378 CrossRef.
- SYSTEMIQ, ReShaping Plastics: Pathways to a Circular, Climate Neutral Plastics System in Europe, 2022 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available.Life-cycle assessment studies included in this review. See DOI: https://doi.org/10.1039/d4gc03127j |
| This journal is © The Royal Society of Chemistry 2024 |















