Silver-mediated oxidative annulation of N-arylthio succinimides with alkynes: direct access to benzo[b]thiophenes†
E.
Ramesh
,
Majji
Shankar
,
Suman
Dana
and
Akhila K.
Sahoo
*
School of Chemistry, University of Hyderabad, Hyderabad-500046, India. E-mail: akhilchemistry12@gmail.com; akssc@uohyd.ac.in
First published on 25th July 2016
Abstract
A convenient synthetic route to 2,3-diarylbenzo[b]thiophene derivatives via Ag-catalyzed intermolecular oxidative cyclization between bench-stable N-arylthio succinimides and unactivated internal alkynes is demonstrated herein. The reaction indicates a broad scope, facilitating the construction of diverse arrays of π-conjugated 2,3-diaryl substituted benzothiophenes. The method involves oxidative cleavage of the S–N bond and annulation of alkynes with the concurrent 1,2-S-migration to yield benzo[b]thiophenes.
Introduction
Benzothiophenes are privileged structural motifs found largely in numerous natural products,1 biologically active molecules,2 and organic materials3 (Fig. 1A). Consequently, there is continuing interest in the development of concise and efficient methods for the fabrication of benzothiophene and its derivatives.3 The construction of heterocyclic motifs via functionalization of C–H bonds of the molecules is considered effective, as these methods directly use non-pre-functionalized precursors,4 offering ready accessibility, broad scope, and the production of fewer by-products.4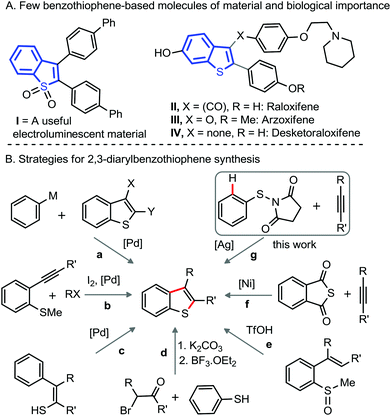 | ||
| Fig. 1 (A) Importance of benzothiophene derivatives. (B) Synthetic methods for 2,3-diarylbenzothiophene. | ||
Although indole and benzofuran derivatives are readily fabricated via direct annulations of aniline/phenol derivatives with unactivated alkynes under transition metal (TM) catalysis,5 a complementary method for the construction of 2,3-diarylbenzo[b]thiophenes from thiols and diaryl-alkynes remains underdeveloped. Perhaps the rapid dimerization of thiol motifs under oxidative conditions, hydrothiolation of the alkyne-moiety, and the possibility of catalyst poisoning by the thiol-group intimately obstruct the reaction outcome. Activated alkynes (Michael acceptor) successfully undergo annulation with thiols to afford benzothiophene derivatives,6 which involves an electrophilic/radical mode of cyclization; while the reaction with unactivated alkynes under the identical system is unsuccessful.6
The construction of 2,3-diarylbenzo[b]thiophenes mostly involves TM-catalyzed cross-couplings of 2,3-dihalo benzothiophenes and arene nucleophiles (path a, Fig. 1B),7 iodine triggered oxidative cyclization of 2-alkynyl-thioanisoles (path b),8 Pd-catalyzed intramolecular cyclization of 2-arylthioenols (path c),9 nucleophilic displacement and dehydrative cyclization between thiophenols and desyl halides (path d),10 and triflic acid mediated cyclization of 2-alkenyl-sulfoxides (path e);11 most of these methods obviously require preformed precursors, obtained through multiple synthetic manipulations. An elegant demonstration of 2,3-diarylbenzo[b]thiophene involves the Ni-catalyzed decarbonylative annulation of thiophthalic anhydride with unactivated alkynes (path f, Fig. 1B).12 Arenesulfonyl chlorides or arenedisulfides are successfully employed for the synthesis of 2,3-diarylbenzo[b]thiophene derivatives.13
Facile cleavage of oxidizable N–N, N–O, and O–N bonds under TM-catalysis has been employed for the synthesis of indole and benzofuran skeletons,14 the construction of benzothiophene motifs through oxidative cleavage of the S–N bond has not yet been developed. Despite significant challenges and shortcomings, we herein discuss the realization of a synthetic route to 2,3-disubstituted benzo[b]thiophenes via Ag-catalyzed oxidative annulation of easily accessible bench-stable N-arylthio succinimides with unactivated alkynes (path g, Fig. 1B).
Results and discussion
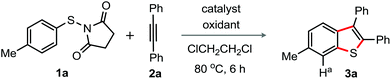
To realize the feasibility of the envisioned process sketched in path g, Fig. 1B, our investigation was initiated by reacting N-(p-tolyl-thio)-succinimide (1a) with 1,2-diphenyl acetylene (2a) in the presence of oxidative catalytic conditions comprising various silver-salts and oxidizing agents (additives).15 To our surprise, the formation of 6-methyl-2,3-diphenyl benzothiophene (3a; 18%) was observed, when the reaction was conducted in the presence of AgSbF6 and PhI(OAc)2 in 1,2-dichloroethane (DCE) at 80 °C (entry 1, Table 1). 1H NMR studies established the structure of 3a; comparison of the representative proton chemical shift among 3a and 5-methyl-2,3-diphenyl-benzothiophene clearly suggested for 3a.15 The appearance of a Ha (singlet at δ = 7.67) solely indicated for 3a.15 Arguably, the formation of 3a was possible through 1,2-migration of the S-moiety. The use of other oxidants Na2S2O8 or (NH4)2S2O8 resulted in the enhanced yield of the product (entries 2 and 3); while Cu(OAc)2·H2O turned out poor (entry 4). Gratifyingly, addition of K2S2O8 improved the production of 3a (73%) with complete consumption of 1a (entry 5); under this condition, 3a was isolated in 64% yield (0.5 mmol scale, entry 5). In none of the cases, the benzylic C–H oxidation product was observed. In the absence of K2S2O8, the reaction was poor (entry 6). Disappointingly, the reaction failed in the absence of AgSbF6 (entry 7). Other Ag-catalysts AgBF4 and AgOTf were found moderate, while AgNO3 and AgOAc were ineffective (entries 8–11). Surprisingly, the reaction in the presence of TEMPO (1.0 equiv.) did not provide the product 3a (entry 12), while di-p-tolyl disulfide was exclusively formed when BHT (1.0 equiv.) was used (entry 13); these results indicate the participation of a radical intermediate in this reaction.16| Entry | Catalyst (30 mol%) | Oxidant (50 mol%) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol). b Conversion based on crude 1H NMR of the starting material. c 1a (0.5 mmol), ClCH2CH2Cl (2.0 mL), isolated yields. d TEMPO (0.1 mmol). e BHT (0.1 mmol). NR = no reaction; DP = di-p-tolyl disulfide formed. | |||
| 1 | AgSbF6 | PhI(OAc)2 | 18 |
| 2 | AgSbF6 | Na2S2O8 | 58c |
| 3 | AgSbF6 | (NH4)2S2O8 | 59c |
| 4 | AgSbF6 | Cu(OAc)2·H2O | 16 |
| 5 | AgSbF 6 | K 2 S 2 O 8 | 73 (64 ) |
| 6 | AgSbF6 | — | 23 |
| 7 | — | K2S2O8 | NR |
| 8 | AgBF4 | K2S2O8 | 18 |
| 9 | AgOTf | K2S2O8 | 58 |
| 10 | AgNO3 | K2S2O8 | NR |
| 11 | AgOAc | K2S2O8 | NR |
| 12 | AgSbF6 | K2S2O8 | NRd |
| 13 | AgSbF6 | K2S2O8 | DPe |
To explore the generality for the preparation of 2,3-diaryl benzothiophenes, the reaction of N-(p-substituted aryl-thio)-succinimide derivatives 1 with 2a was at first conducted under the optimized conditions shown in entry 5, Table 1 (Scheme 1).
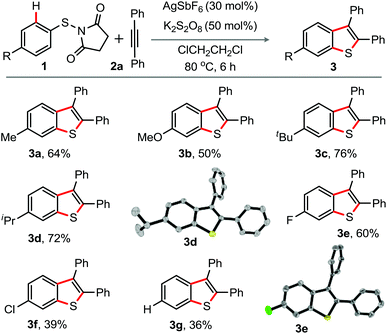 | ||
| Scheme 1 Substrate scope I. Reaction conditions: 1 (0.5 mmol), 2a (0.5 mmol), AgSbF6 (30 mol%), K2S2O8 (50 mol%), ClCH2CH2Cl (2.0 mL) at 80 °C, 6 h. Isolated yields. | ||
As observed previously in Table 1, the desired products 6-substituted-2,3-diphenyl-benzothiophenes 3a–f, attained through 1,2-S-migration, were solely isolated in moderate to good yields. The electron-rich (Me, OMe, t-Bu, i-Pr) or the halo groups (F, Cl) in the aryl moiety in 1 did not affect the reaction outcome. Structures of 3d and 3e were once again established by X-ray crystallographic analysis (Scheme 1),15 while the reaction of the electron-neutral N-phenylthio derivative provided 36% of 3g.
The facile reactivity of 1 with 2a (Scheme 1) inspired us to investigate the annulation of 1 with various diaryl alkynes (Fig. 2). The reaction of 1a/1c (p-Me/p-t-Bu containing aryl moieties) with p-F (2b), p-Cl (2c), or p-Br (2d) substituted diaryl alkynes under the optimized conditions delivered the corresponding 6-Me/6-t-Bu possessing 2,3-diarylbenzothiophenes 4a–f in 69–83% isolated yields. The 1,2-di-p-tolyl acetylene (2e) was not an exception, as the electron-rich alkynes were poor in undergoing annulations, to yield 4g (48%) and 4h (52%). Likewise, annulation of N-p-F-phenyl-thio compound 1e with p-F/Br containing diaryl alkynes 2b/2d successfully produced 4i (62%) and 4j (64%), respectively. The products 4k–m were reliably constructed through the annulation of m-CF3 (2f) and m,p-di-chloro (2g) substituted 1,2-diaryl acetylenes with 1a/1c (Fig. 2). Disappointingly, annulations of 1 with 1,2-dialkyl acetylenes failed.
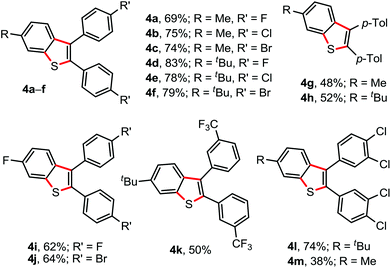 | ||
| Fig. 2 Substrate scope II. Reaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), AgSbF6 (30 mol%), K2S2O8 (50 mol%), ClCH2CH2Cl (2.0 mL) at 80 °C, 6 h. Isolated yields. | ||
We next examined the annulation of 1 with the unactivated unsymmetrical alkynes. In general, the reaction of unsymmetrical alkynes would provide a mixture of two regioisomers. Interestingly, the reaction of N-arylthio derivatives 1d/1a (4-i-Pr/4-Me containing aryl moieties) with 1,2-phenylalkyl acetylene exclusively delivered 2-alkyl-3-phenyl benzothiophenes 5a−c along with a minor amount of uncharacterized side product, albeit in moderate yield (Fig. 3).15 The participation of a phenyl-stabilized alkenyl radical is possibly responsible for the high regioselectivity (Scheme 4).16 Annulation between 1a and unsymmetrical 1,2-diaryl acetylenes (phenyl and 4-F/Cl/Br bearing aryl) readily produced an inseparable mixture of two regioisomers 5d−f (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in appreciable yields (Fig. 3), whereas the reaction of 1a with 1-o-Br-phenyl and 2-phenyl bearing acetylene provided a mixture of products 5g/5g′ (5/1; 63%).
1) in appreciable yields (Fig. 3), whereas the reaction of 1a with 1-o-Br-phenyl and 2-phenyl bearing acetylene provided a mixture of products 5g/5g′ (5/1; 63%).
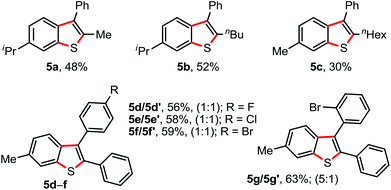 | ||
| Fig. 3 Annulation with unsymmetrical alkynes. Reaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), AgSbF6 (30 mol%), K2S2O8 (50 mol%), ClCH2CH2Cl (2.0 mL) at 80 °C, 6 h. Isolated yields. | ||
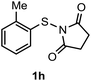
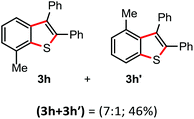
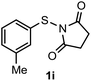
Surprisingly, the annulation of N-(o-tolyl-thio)-succinimide (1h) with 2a under the optimized conditions led to a mixture of 7-Me and 4-Me containing 2,3-diphenyl benzothiophenes 3h (with retention of S-moiety) and 3h′ (with 1,2-migration of S-moiety), respectively (entry 1, Table 2). Likewise, exposure of N-(m-tolyl-thio)-succinimide (1i) with 2a provided a complex reaction profile, forming three benzothiophene products with the retention and 1,2-migration of the S-moiety (entry 2, Table 2).
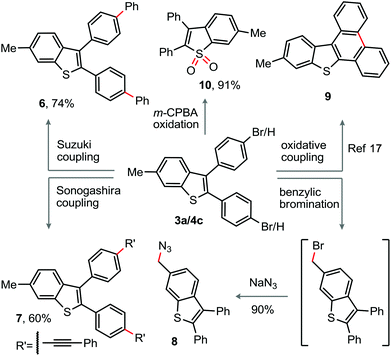
To demonstrate the utility of the present protocol, peripheral modification of the parent 2,3-diaryl-benzothiophene skeleton was envisaged. The bromo-group on the periphery of the benzothiophene 4c was successfully employed for the arylation and alkynylation through Suzuki and Sonogashira reactions to construct the π-conjugated benzothiophenes 6 (74%) and 7 (60%), respectively (Scheme 2). Next, m-CPBA oxidation of 3a delivered the corresponding sulfone derivative 10 (91%) (Scheme 2). The oxidative dehydrogenative coupling among the C–H bonds of the diaryl moieties may provide 9.17 A sequential benzylic bromination followed by nucleophilic azide replacement of 3a produced the azide bearing benzothiophene derivative 8 in 90% yield (Scheme 2), which is amenable to [3 + 2]-cycloaddition.
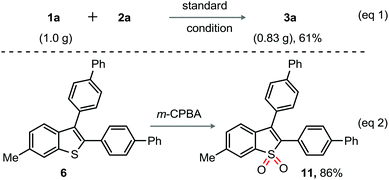
A gram scale annulation experiment of 1a (1.0 g) with 2a was successfully conducted under the optimized conditions to furnish 3a (0.83 g) (eqn (1), Scheme 3); this result reflects the reaction scalability and the efficacy of the catalytic system. The V-shaped thiophene based oligomer,3c for example 11 is used as an electroluminescent device. Interestingly, the m-CPBA mediated oxidation of 6 directly led to 11 in 86% yield (eqn (2), Scheme 3). We thus believe that the current method is useful for the design, development, and fabrication of novel π-extended benzothiophene derivatives as electroluminescent materials.
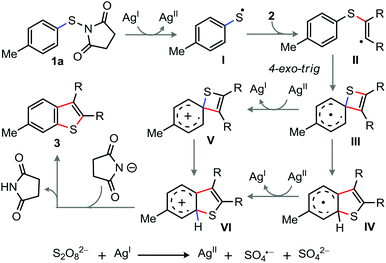
On the basis of control experiments and precedents, the plausible reaction pathway is sketched in Scheme 4. The reaction begins with the single electron transfer from Ag(I) to 1a to produce thiyl radical I and a succinimide-anion.16 Next, the concomitant attack of I to alkyne followed by the ipso-carbon attack of arene to the vinyl-radical in II leads to a highly strained spirocyclohexadienyl radical intermediate III.16 Oxidation of III then provides spirocyclohexadienyl arenium species V, which rapidly undergoes ring expansion involving 1,2-S-migration to give VI. Finally, deprotonation–aromatization of VI affords 3. Alternatively, 1,2-S-migration of III followed by oxidation of IV provides VI, which then transforms to 3.
Conclusions
In summary, we have shown a Ag-catalyzed oxidative annulation of readily accessible and bench-stable S–N bearing thiol derivatives with unactivated alkynes for the direct construction of 2,3-diaryl benzothiophene derivatives, which is scalable. Peripheral modifications of halo groups build π-extended and V-shaped benzothiophene analogues, which are useful for electroluminescent devices. Modifications that would address the shortcomings of the current method are being actively pursued in our laboratory.Acknowledgements
We thank CSIR (02/0155/13), India for financial support. ER, MS and SD thank CSIR, India for the fellowship.Notes and references
- For benzothiophene containing natural products, see: D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed.
- (a) J. W. Ellingboe, T. R. Alessi, T. M. Dolak, T. T. Nguyen, J. D. Tomar, F. Guzzo, J. F. Bagli and M. L. Mccaleb, J. Med. Chem., 1992, 35, 1176 CrossRef CAS; (b) S. M. Malamas, J. Sredy, C. Moxham, A. Katz, W. Xu, R. Mcdevitt, F. O. Adebayo, D. R. Sawicki, L. Seestaller, D. Sullivan and J. R. Taylor, J. Med. Chem., 2000, 43, 1293 CrossRef PubMed; (c) B. L. Flynn, E. Hamel and M. K. Jung, J. Med. Chem., 2002, 47, 2670 CrossRef; (d) U. Schopfer, P. Schoeffter, S. F. Bischoff, J. Nozulak, D. Feuerbach and P. Floershim, J. Med. Chem., 2002, 45, 1399 CrossRef CAS PubMed; (e) Z. Qin, I. Kastrati, R. E. P. Chandrasena, H. Liu, P. Yao, P. A. Petukhov, J. L. Bolton and G. R. J. Thatcher, J. Med. Chem., 2007, 50, 2682 CrossRef CAS PubMed.
- (a) A. J. Seed, K. J. Toyne, J. W. Goodby and M. Hird, J. Mater. Chem., 2000, 10, 2069 RSC; (b) I. Fouad, Z. Mecbal, K. I. Chane-Ching, A. Adenier, F. Maurel, Z. J. –J. Aaron, P. Vodicka, K. Cernovska, V. Kozmik and J. Svoboda, J. Mater. Chem., 2004, 14, 1711 RSC; (c) G. Barbarella, A. Favaretto, A. Zanelli, G. Gigli, M. Mazzeo, M. Anni and A. Bongini, Adv. Funct. Mater., 2005, 15, 664 CrossRef CAS.
- (a) L. Ackermann, Acc. Chem. Res., 2014, 47, 281 CrossRef CAS PubMed; (b) G. Naresh, R. Kant and T. Narender, Org. Lett., 2015, 17, 3446 CrossRef CAS PubMed.
- (a) D. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 16474 CrossRef CAS PubMed; (b) T. Satoh and M. Miura, Chem. – Eur. J., 2010, 16, 11212 CrossRef CAS PubMed; (c) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (d) M. R. Kuram, M. Bahanuchandra and A. K. Sahoo, Angew. Chem., Int. Ed., 2013, 52, 4607 CrossRef CAS PubMed; (e) W. Zeng, W. Wu, H. Jiang, L. Huang, Y. Sun, Z. Chen and X. Li, Chem. Commun., 2013, 49, 6611 RSC; (f) R. Zhu, J. Wei and Z. Shi, Chem. Sci., 2013, 4, 3706 RSC.
- (a) K. Liu, F. jia, H. Xi, Y. Li, X. Zheng, Q. Guo, B. Shen and Z. Li, Org. Lett., 2013, 15, 2026 CrossRef CAS PubMed; (b) D. Yang, K. Yan, W. Wei, L. Tian, Q. Li, J. You and H. Wang, RSC Adv., 2014, 4, 48547 RSC; (c) K. Yan, D. Yang, M. Zhang, W. Wei, Y. Liu, L. Tian and H. Wang, Synlett, 2015, 26, 1890 CrossRef CAS.
- (a) K. Yamamura, Y. Houda, M. Hashimoto, T. Kimura, M. Kamezawa and T. Otani, Org. Biomol. Chem., 2004, 2, 1413 RSC; (b) M. Miyasaka, K. Hirano, T. Satoh and M. Miura, Adv. Synth. Catal., 2009, 351, 2683 CrossRef CAS; (c) L. Zhao, C. Bruneau and H. Doucet, Tetrahedron, 2013, 69, 7082 CrossRef CAS.
- B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937 CrossRef CAS PubMed.
- K. Inamoto, Y. Arai, K. Hiroya and T. Doi, Chem. Commun., 2008, 5529 RSC.
- G. V. P. Chandramouli, B. Prasanna, P. N. Kumar and P. V. Reddy, Phosphorus Sulfur Silicon Relat. Elem., 2002, 177, 511 CrossRef CAS.
- (a) K. Nobushige, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 1188 CrossRef CAS PubMed; (b) K. Padala and M. Jeganmohan, Chem. Commun., 2014, 50, 14573 RSC.
- T. Inami, Y. Baba, T. Kurahashi and S. Matsubara, Org. Lett., 2011, 13, 1912 CrossRef CAS PubMed.
- (a) L. Benati, P. C. Montevecchi and P. Spagnolo, J. Chem. Soc., Perkin Trans. 1, 1991, 2103 RSC; (b) D. Wan, Y. Yang, X. Liu, M. Li, S. Zhoo and J. You, Eur. J. Org. Chem., 2016, 55 CrossRef CAS.
- (a) D. Zhao, Z. Shi and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 12426 CrossRef CAS PubMed; (b) Z. Zhou, G. Liu, Y. Chen and X. Lu, Adv. Synth. Catal., 2015, 357, 2944 CrossRef CAS; (c) G. Liu, Y. Shen, Z. Zhou and X. Lu, Angew. Chem., Int. Ed., 2013, 52, 6033 CrossRef CAS PubMed.
- See the ESI.†.
- (a) Y. Unoh, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2013, 52, 12975 CrossRef CAS PubMed; (b) Y.-R. Chen and W.-L. Duan, J. Am. Chem. Soc., 2013, 135, 16754 CrossRef CAS PubMed; (c) W. Ma and L. Ackermann, Synthesis, 2014, 46, 2297 CrossRef CAS.
- A. Buquet, A. Couture, A.-L. Combier and A. Pollet, Tetrahedron, 1981, 37, 75 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of the 1H NMR, 13C NMR and HRMS data for all products. CCDC 1479463 and 1479468. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00259e |
| This journal is © the Partner Organisations 2016 |