Versatile and highly efficient oxidative C(sp3)–H bond functionalization of tetrahydroisoquinoline promoted by bifunctional diethyl azodicarboxylate (DEAD): scope and mechanistic insights†‡
Takuya
Suga
,
Sunao
Iizuka
and
Takahiko
Akiyama
 *
*
Department of Chemistry, Gakushuin University, 1-5-1 Mejiro, Toshima-ku, Tokyo 171-8588, Japan. E-mail: takahiko.akiyama@gakushuin.ac.jp
First published on 1st August 2016
Abstract
Oxidative C(sp3)–H bond functionalization of the 1-position of tetrahydroisoquinoline (THIQ) derivatives was performed by using diethyl azodicarboxylate (DEAD) as the oxidant. A wide range of nucleophiles, including ketene silyl acetal, silyl enol ether, nitroalkane, dimethyl malonate, terminal alkyne, ketone, phosphonate, trimethylsilyl cyanide, and allylstannane gave very high yields without their excessive use. A mechanistic study revealed that the oxidative and basic nature of DEAD is responsible for its high efficiency.
The oxidative C(sp3)–H bond functionalization of the α-positions of heteroatoms is a topic of keen interest in organic chemistry.1 It is regarded as a promising strategy for the straightforward generation of oxocarbenium ions or iminium ions from simple ethers and amines. The pioneering work of Murahashi2 and Li3 on oxidative iminium formation from tetrahydroisoquinoline (THIQ) derivatives by using a ruthenium or copper catalyst has inspired the exploration of numerous non-metal (iodine,4a hypervalent iodine,4b DDQ4c,d and CX4,4eetc.4f–i) and transition-metal (Cu,5a,b Fe,5c Ru,5d,e Ir,5f,g Au,5h and so on.5i) oxidants. However, neither the product yield nor the substrate scope and loading of nucleophiles have necessarily been taken into consideration despite the many studies indicated above, probably because those studies focused on the efficiency and novelty of the oxidation step. This has prompted us to develop a simpler and more efficient general reaction as a practical synthetic technique.
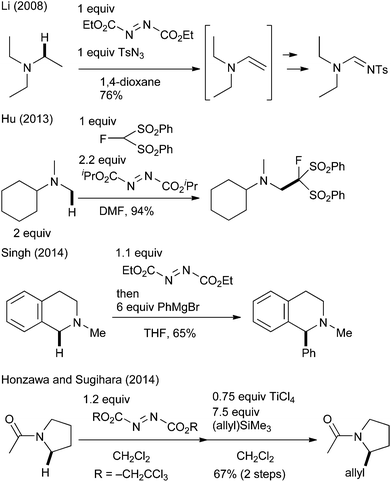
To realize efficient transformation, we focused on the oxidative character of azodicarboxylate derivatives. Although they are mostly known as reagents for the Mitsunobu reaction6 and as strong electrophiles,7 their utility as simple oxidants has also been investigated over the last 50 years.8 Notably, several researchers recently reported their applicability for the oxidation of amines.9 Williams9a and Li9b–d independently reported that diethyl azodicarboxylate (DEAD) oxidized aliphatic amines to the corresponding enamines or iminium ions. Later, Hu applied this azodicarboxylate-promoted oxidation to the oxidative Mannich-type reaction of N-methylamine and fluorine-containing nucleophiles.9e It was extended to the oxidative coupling reactions of THIQ and organometallic reagents by Singh (Scheme 1).9f,g Sugihara reported that the reaction of tertiary amides with di(2,2,2-trichloroethyl)azodicarboxylate gave stable aminal adducts that underwent a TiCl4-promoted Hosomi–Sakurai-type reaction to furnish allylated products.9h We wish to report herein a mild, highly efficient, and general C(sp3)–H bond functionalization of THIQ by using commercially available DEAD as the oxidant. Several mechanistic studies have revealed the mechanism underlying the distinctive reactivity of DEAD.3e
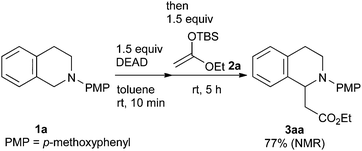
Our study was initiated by the observation of the rapid consumption of THIQ 1a when it was treated with DEAD in toluene at room temperature, which indicated that the oxidation of 1a took place (Scheme 2). The subsequent addition of 1.5 equiv. of ketene silyl acetal 2a gave coupling adduct 3aa in moderate yield, as expected. It is noteworthy that the application of ketene silyl acetal derivatives is quite limited in the oxidative functionalization of THIQ,10 probably because of the instability of the ketene silyl acetals under many oxidative conditions.
Encouraged by this result, we further optimized the reaction conditions (Table 1). In general, this reaction could be carried out in various solvents. Small excesses of DEAD (1.5 equiv.) and nucleophile (1.5 equiv.) sufficed for the completion of the reactions. The amount of DEAD could be further reduced to 1.1 equivalents (entries 7 and 8). Although less polar solvents, such as toluene, THF, and dichloromethane, were moderately suitable for the reaction (entries 1–3, 75–83% yield), highly polar solvents, such as N,N-dimethylformamide, acetonitrile, and dimethyl sulfoxide, were more effective (entries 4–6, 88% to quantitative yield). One of the features of our reaction is the separate addition of DEAD and nucleophiles. Indeed, their simultaneous addition gave an inferior result (entry 7). The longer reaction time for the oxidation step did not affect the yield (entry 6 vs. 8). Thus, the mild oxidation conditions allowed the in situ-formed oxidized species to remain intact for a long time.
| Entry | Solvent | Yield (%) |
|---|---|---|
| All the reactions were carried out under the conditions described in Scheme 2 except for the solvent used (0.1 M, 0.1 mmol scale). Yields were estimated by 1H NMR measurement.a 1.1 equiv. of DEAD was added.b DEAD and 2a were added at the same time. The reaction time was 1.5 h.c 1a was treated with DEAD for 30 min, and subsequently treated with 2a for 30 min.d Isolated yield. | ||
| 1 | Toluene | 77 |
| 2 | THF | 75 |
| 3 | CH2Cl2 | 83 |
| 4 | DMF | 88 |
| 5 | MeCN | 89 |
| 6 | DMSO | Quant. |
| 7a,b | DMSO | 81 |
| 8a,c | DMSO | 97d |
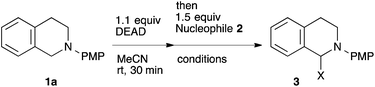
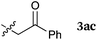
To our delight, a range of nucleophiles and pro-nucleophiles were applicable for this reaction (Table 2). Not only α-di-substituted ketene silyl acetal 2b (entry 2, 98%) and silyl enol ether 2c (entry 3, 85%), but also nitromethane (2d) (entry 4, 92%), dimethyl malonate (2e) (entry 5, 97%), allyltributylstannane (2f) (entry 6, 95%), dimethyl phosphonate (2g) (entry 7, 91%), and trimethylsilyl cyanide (2h) (entry 8, 94%) reacted successfully to afford the corresponding coupling adducts in excellent yields. Moreover, acetophenone (2i) was also applicable. Although it was less reactive in acetonitrile, the reaction proceeded efficiently when the solvent was switched from acetonitrile to methanol after the oxidation step (entries 9 and 10, 10% and 92%, respectively).11 Although the nucleophilic addition of phenylacetylene (2j) required the addition of 2 mol% CuI because copper acetylide is the true nucleophile for this substrate, the yield was very good (entry 11). The present reaction has several advantages as a practical method. First, it requires only a small excess of nucleophile (1.5 equiv.) to realize an excellent yield regardless of nucleophiles. Most of the known THIQ oxidation reactions require excessive amounts of nucleophiles.12 Second, a variety of nucleophiles and pro-nucleophiles are applicable.13 The reaction with acetophenone 2i is particularly remarkable because catalysts had been necessary to activate this species (e.g., proline14). Our reaction does not require such catalysts, and exchanging the solvent after the oxidation step would suffice.
| Entry | Nucleophile | Temp., time | X | Yield (%) |
|---|---|---|---|---|
| All the reactions were carried out in 0.1 mmol scale (0.1 M). Yields were calculated after isolation.a DMSO was used as solvent.b NMR yield.c Solvent was changed from acetonitrile to methanol after the oxidation step. See the ESI for details.d 2 mol% CuI was added. | ||||
| 1a |
|
rt, 0.5 h |
|
97 |
| 2 |
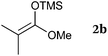
|
rt, 2 h |
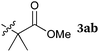
|
98 |
| 3a |

|
60 °C, 6 h |
|
85 |
| 4 |
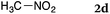
|
60 °C, 4 h |
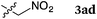
|
92 |
| 5 |
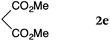
|
rt, 11 h |
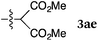
|
97 |
| 6 |
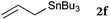
|
90 °C, 10 h |

|
95 |
| 7 |
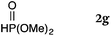
|
rt, 20 min |
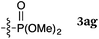
|
91 |
| 8 |
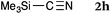
|
rt, 20 min |
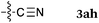
|
94 |
| 9 |
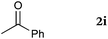
|
50 °C, 48 h |
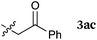
|
10b |
| 10c | 92 | |||
| 11d |

|
90 °C, 24 h |
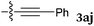
|
90 |
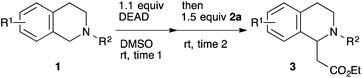
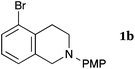
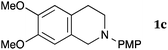
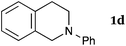
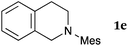
This method was effective for various kinds of THIQ. Then, the scope of THIQ was examined by using ketene silyl acetal 2a as the nucleophile (Table 3). The introduction of substituents on the aromatic ring of THIQ did not interrupt the reaction. For instance, 5-bromo-substituted and 6,7-dimethoxy-substituted THIQs 1b and 1c gave the desired products in 81% and 99% yields, respectively (entries 2 and 3), under almost identical conditions to those shown in Table 2. N-Phenyl-substituted THIQ 1d was also successfully oxidized to give the desired product quantitatively (entry 4, 97%). Sterically highly-demanding N-mesityl-substituted THIQ 1e also furnished the addition product in a high yield (entry 5, 85%). N-Methyl-substituted THIQ 1f also participated in the reaction with very good yield (entry 6, 93%).15 It should be noted that the reaction time for the first oxidation step was strongly dependent on the substituent on the nitrogen of THIQ, according to TLC analysis. THIQs 1a–1c, 1e and 1f were smoothly oxidized in almost 30 min after the addition of DEAD, whereas N-phenyl-substituted THIQ 1d required 3 h for complete oxidation.
| Entry | THIQ 1 | Time 1 (min) | Time 2 (min) | Yield (%) |
|---|---|---|---|---|
| All the reactions were carried out in the same manner as entry 1 in Table 2, except for the reaction times. Reaction times were determined by TLC analyses. Yields were calculated after isolation. PMP = p-methoxyphenyl. Mes = 2,4,6-Me3C6H2.a Prolonging the reaction time 2 to 23 h did not improve the yield. | ||||
| 1 |
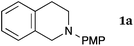
|
30 | 30 | 3aa |
| 97 | ||||
| 2a |
|
35 | 240 | 3ba |
| 81 | ||||
| 3 |
|
20 | 90 | 3ca |
| 99 | ||||
| 4 |
|
180 | 120 | 3da |
| 97 | ||||
| 5 |
|
20 | 30 | 3ea |
| 85 | ||||
| 6 |
|
15 | 30 | 3fa |
| 93 | ||||
Next, a mechanistic study was carried out to clarify why this reaction is distinctively effective for various nucleophiles and pro-nucleophiles. The electrophiles in the oxidative functionalization of THIQs would be the corresponding iminium ions as proposed in the literature, and their reactivities would likely be similar regardless of the oxidation method. Nevertheless, our reaction has wider generality than most of the other procedures, and even acetophenone was applicable in the absence of basic additives. Further studies of this difference would improve our understanding of the oxidative functionalization of THIQs.
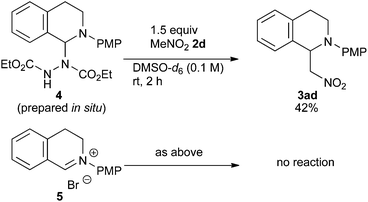
In order to determine the species generated in the reaction mixture, direct NMR analysis was conducted. THIQ 1a was treated with 1.1 equiv. of DEAD for 1 h at room temperature. 1H NMR measurement of the resulting mixture indicated the formation of one major species (Scheme 3, green). Although its signals were broad, the chemical shifts were clearly consistent with those of the neutral aminal structure 416 rather than an iminium salt. For instance, no signal was observed around 9.5 ppm, which is representative of the iminium moiety. On the other hand, the broad signal at 6.7 ppm could be assigned to the hydrogen atom of the benzylic aminal moiety.17 The 1H NMR spectrum of the isolated corresponding iminium ion 5 is also provided for comparison (brown).
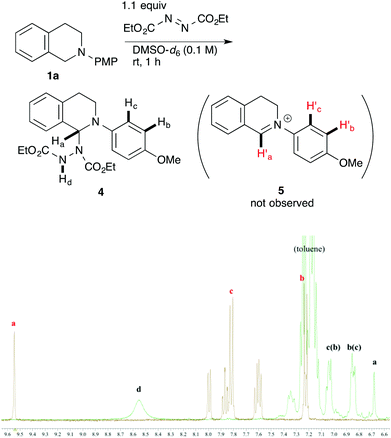 | ||
| Scheme 3 1H NMR spectral comparison between aminal 4 (green) and the corresponding iminium ion 5 (brown) in DMSO-d6. | ||
Then, the reactivities of aminal 4 and iminium ion 5 were compared by adding 1.5 equiv. of nitromethane 2d as the pro-nucleophile. To our surprise, aminal 4 was apparently more reactive to nitromethane 2d than iminium ion 5. After 2 h, 42% of 4 was converted into product 3ad. In contrast, no conversion of 5 into 3ad was observed under the same conditions. A reasonable explanation is that the high reactivity of aminal 4 originates from the basicity of the counter anion. Thus, the partially generated dicarboxyhydrazide anion would effectively deprotonate the pro-nucleophile as in the Mitsunobu reaction, so that the following nucleophilic addition would proceed swiftly. DEAD works not only as an oxidant, but also as a good precursor for a base.18 Klussmann pointed out that the acidity of the reaction system could alter the character of the C–H bond functionalization reaction of THIQs.3e The acidic CuCl2/O2 system (pH 5.3–6.13e) was suitable for “nucleophiles” (e.g. silyl enol ether) due to the high concentration of iminium ions, and the basic CuBr/tBuOOH system (pH 6.3–7.23e) was suitable for “pro-nucleophiles” (e.g. 2-naphthol) due to the efficient deprotonation. In contrast, DEAD is applicable to a wide range of nucleophiles and pro-nucleophiles. We consider that this is because aminal 4 easily liberates the dicarboxyhydrazide anion, which is basic enough to deprotonate pro-nucleophiles. Our reaction is unique in this regard as well.
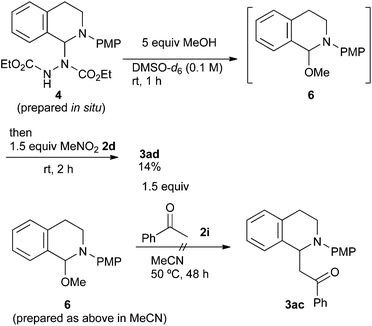
As has been already noted, the reaction with acetophenone 2i proceeded successfully only in methanol. In order to explain this result, we carried out further mechanistic studies (Scheme 5). The NMR experiment revealed that the treatment of aminal 4 with 5 equiv. of methanol gave N,O-acetal 6 in DMSO-d6 at room temperature after 1 h. However, the formation of 6 was unlikely to be due to the advantage brought about by methanol. The following reaction of N,O-acetal 6 and 1.5 equiv. of nitromethane 2d gave 3ad in only 14% yield under the conditions described in Scheme 4, while it was 42% in the absence of the added methanol. In addition, when the reaction of N,O-acetal 6 with acetophenone 2i (Table 2, entry 9) was examined in acetonitrile, 3ac was not obtained at all even though N,O-acetal 6 should be fully generated under such conditions. Therefore, we postulate that the advantage brought about by methanol is largely based on its solvent effect. Its high polarity and hydrogen bonding might promote the formation of iminium ions or the deprotonation of acetophenone 2i.
Conclusions
In summary, we have developed a versatile and highly efficient oxidative coupling of THIQ derivatives using DEAD as the oxidant. The reaction gave the corresponding aminal at ambient temperature. Subsequent addition of various nucleophiles furnished coupling products in generally excellent yields. The mechanistic study revealed that the reactivity of the iminium ion was strongly dependent on its counter anion. Such an effect has not been focused on, although it is clearly as decisive as the efficiency of the oxidation step. DEAD was found to be an ideal bifunctional reagent that worked as both an oxidant and a base. In the reaction with acetophenone 2i in methanol, the acetal exchange afforded the less reactive N,O-acetal 6. However, the solvent effect of methanol promoted the reaction. This study will provide a simple and useful method for the transformation of tetrahydroisoquinolines. Further investigation for the substrate scope and on the reaction mechanism is now ongoing.Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (B) (26288053) from JSPS.Notes and references
- (a) R. Narayan, K. Matcha and A. P. Antonchick, Chem. – Eur. J., 2015, 21, 14678 CrossRef CAS PubMed; (b) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed; (c) R. Rohlmann and O. G. Mancheño, Synlett, 2013, 6 CAS; (d) K. M. Jones and M. Klussmann, Synlett, 2012, 159 CAS; (e) M. Klussman and D. Sureshkumar, Synthesis, 2011, 353 CrossRef; (f) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed.
- (a) S. Murahashi, N. Komiya, H. Terai and T. Nakae, J. Am. Chem. Soc., 2003, 125, 15312 CrossRef CAS PubMed; (b) S. Murahashi, N. Komiya and H. Terai, Angew. Chem., Int. Ed., 2005, 44, 6931 CrossRef CAS PubMed.
- (a) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2004, 126, 11810 CrossRef CAS PubMed; (b) Z. Li and C.-Z. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef CAS PubMed; (c) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed; (d) Z. Li, D. S. Bohle and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS PubMed. For the mechanistic study on this subject, see: (e) E. Boess, C. Schmitz and M. Klussmann, J. Am. Chem. Soc., 2012, 134, 5317 CrossRef CAS PubMed.
- For selected examples, see: (a) R. A. Kumar, G. Saidulu, K. R. Prasad, G. S. Kumar, B. Sridhar and K. R. Reddya, Adv. Synth. Catal., 2012, 354, 2985 CrossRef CAS; (b) X.-Z. Shu, X.-F. Xia, Y.-F. Yang, K.-G. Ji, X.-Y. Liu and Y.-M. Liang, J. Org. Chem., 2009, 74, 7464 CrossRef CAS PubMed; (c) A. S. K. Tsang and M. H. Todd, Tetrahedron Lett., 2009, 50, 1199 CrossRef CAS; (d) Q. Chen, J. Zhou, Y. Wang, C. Wang, X. Liu, Z. Xu, L. Lin and R. Wang, Org. Lett., 2015, 17, 4212 CrossRef CAS PubMed; (e) J.-F. Franz, W.-B. Kraus and K. Zeitler, Chem. Commun., 2015, 51, 8280 RSC; (f) G. Jiang, J. Chen, J.-S. Huang and C.-M. Che, Org. Lett., 2009, 11, 4568 CrossRef CAS PubMed; (g) Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052 CrossRef CAS PubMed; (h) A. Tanoue, W.-J. Yoo and S. Kobayashi, Org. Lett., 2014, 16, 2346 CrossRef CAS PubMed; (i) Z. Xie, L. Liu, W. Chen, H. Zheng, Q. Xu, H. Yuan and H. Lou, Angew. Chem., Int. Ed., 2014, 53, 3904 CrossRef CAS PubMed.
- For the selected examples, see: (a) G. Zhang, Y. Ma, S. Wang, Y. Zhang and R. Wang, J. Am. Chem. Soc., 2012, 134, 12334 CrossRef CAS PubMed; (b) C. Min, A. Sanchawala and D. Seidel, Org. Lett., 2014, 16, 2756 CrossRef CAS PubMed; (c) M. O. Ratnikov, X. Xu and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 9475 CrossRef CAS PubMed; (d) M. Rueping, C. Vila, R. M. Koenigs, K. Poscharny and D. C. Fabry, Chem. Commun., 2011, 47, 2360 RSC; (e) D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094 CrossRef CAS PubMed; (f) A. C. Condie, J. C. González-Gómez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464 CrossRef CAS PubMed; (g) J. Xuan, T.-T. Zeng, Z.-J. Feng, Q.-H. Deng, Q. J.-R. Chen, L.-Q. Lu, W.-J. Xiao and H. Alper, Angew. Chem., Int. Ed., 2015, 54, 1625 CrossRef CAS PubMed; (h) J. Xie, H. Li, J. Zhou, Y. Cheng and C. Zhu, Angew. Chem., Int. Ed., 2012, 51, 1252 CrossRef CAS PubMed; (i) X.-Z. Shu, Y.-F. Yang, X.-F. Xia, K.-G. Ji, X.-Y. Liu and Y.-M. Liang, Org. Biomol. Chem., 2010, 8, 4077 RSC.
- (a) S. Fletcher, Org. Chem. Front., 2015, 2, 739 RSC; (b) K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551 CrossRef CAS PubMed.
- For recent examples, see: (a) J. Yan, L. Tay, C. Neo, B. R. Lee and P. W. H. Chan, Org. Lett., 2015, 17, 4176 CrossRef CAS PubMed; (b) Y. Wang, B. Frett, N. McConnell and H.-Y. Li, Org. Biomol. Chem., 2015, 13, 2958 RSC; (c) K. Fourmy and A. Voituriez, Org. Lett., 2015, 17, 1537 CrossRef CAS PubMed; (d) W.-Y. Yu, W.-N. Sit, K.-M. Lai, Z. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2008, 130, 3304 CrossRef CAS PubMed.
- (a) F. Yoneda, K. Suzuki and Y. Nitta, J. Am. Chem. Soc., 1966, 88, 2328 CrossRef CAS; (b) R. Axen, M. Chaykovsky and B. Witkop, J. Org. Chem., 1967, 32, 4117 CrossRef CAS PubMed; (c) K. Kato and O. Mitsunobu, J. Org. Chem., 1970, 35, 4227 CrossRef; (d) I. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044 CrossRef; (e) I. E. Markó, M. Tsukazaki, P. R. Giles, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed. Engl., 1997, 36, 2208 CrossRef; (f) H. T. Cao and R. Grée, Tetrahedron Lett., 2009, 50, 1493 CrossRef CAS; (g) N. Iranpoor, H. Firouzabadi, D. Khaii and R. Shahin, Tetrahedron Lett., 2010, 51, 3508 CrossRef CAS; (h) M. Hayashi, M. Shibuya and Y. Iwabuchi, J. Org. Chem., 2012, 77, 3005 CrossRef CAS PubMed; (i) Z. Yang, B. Wang, X. Xu, H. Wang and X. Li, Chin. J. Org. Chem., 2015, 35, 207 CrossRef CAS.
- (a) G. Vincent, Y. Chen, J. W. Lane and R. M. Williams, Heterocycles, 2007, 72, 385 CrossRef CAS; (b) X. Xu, X. Li, L. Ma, N. Ye and B. Weng, J. Am. Chem. Soc., 2008, 130, 14048 CrossRef CAS PubMed; (c) X. Xu and X. Li, Org. Lett., 2009, 11, 1027 CrossRef CAS PubMed; (d) X. Xu, P. Du, D. Cheng, H. Wang and X. Li, Chem. Commun., 2012, 48, 1811 RSC; (e) W. Huang, C. Ni, Y. Zhao and J. Hu, New J. Chem., 2013, 37, 1684 RSC; (f) K. N. Singh, P. Singh, A. Kaur and P. Singh, Synlett, 2012, 760 CrossRef CAS; (g) K. N. Singh, S. V. Kessar, P. Singh, P. Singh, M. Kaur and A. Batra, Synthesis, 2014, 2644 CrossRef CAS; (h) S. Honzawa, M. Uchida and T. Sugihara, Heterocycles, 2014, 88, 975 CrossRef CAS.
- (a) D. Sureshkumar, A. Sud and M. Klussmann, Synlett, 2009, 1558 CAS; (b) G. Bergonzini, C. S. Schindler, C.-J. Wallentin, E. N. Jacobsen and C. R. J. Stephenson, Chem. Sci., 2014, 5, 112 RSC; (c) L. Chu, X. Zhang and F.-L. Qing, Org. Lett., 2009, 11, 2197 CrossRef CAS PubMed.
- Methanol cannot be used in the oxidation step because of the undesired reaction between DEAD and excessive methanol. See ref. 8a.
- For representative examples using less than 2 equiv. nucleophiles, see: ref. 4d, g, 5b and 14a.
- Examples of relatively broad scope on the kinds of nucleophiles: (a) J. Dhineshkumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 15, 1092 CrossRef CAS PubMed; (b) W.-P. To, Y. Liu, T.-C. Lau and C.-M. Che, Chem. – Eur. J., 2013, 19, 5654 CrossRef CAS PubMed; (c) A. Tanoue, W.-J. Yoo and S. Kobayashi, Adv. Synth. Catal., 2013, 355, 269 CAS; (d) H. E. Ho, Y. Ishikawa, N. Asao, Y. Yamamoto and T. Jin, Chem. Commun., 2015, 51, 12764 RSC . See also ref. 3d, e and 4d.
- (a) A. Sud, D. Sureshkumar and M. Klussmann, Chem. Commun., 2009, 45, 3169 RSC; (b) Y. Shen, M. Li, S. Wang, T. Zhan, Z. Tan and C.-C. Guo, Chem. Commun., 2009, 953 RSC.
- The reaction of N-(4-nitrophenyl)-substituted THIQ gave the desired product only in 7% yield. The electron-withdrawing group on the nitrogen atom was not amenable to this transformation.
- The formation of the relevant N,O-acetals has been reported; (a) S. Murahashi, T. Naota and K. Yonemura, J. Am. Chem. Soc., 1988, 110, 8256 CrossRef CAS; (b) Z. Li, P. D. MacLeod and C.-J. Li, Tetrahedron: Asymmetry, 2006, 17, 590 CrossRef CAS . See also ref. 3a, c and e.
- See the ESI‡ for the detailed assignment.
- Todd reported that the oxidation reaction of THIQ by 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) gave the corresponding ion pair, but it scarcely reacted with the nitromethane solvent unless aqueous NaHCO3 was added (ref. 4c). This would also support our proposal that the basicity of the anion is very important. We confirmed that the reaction by using DEAD proceeded quantitatively under the same conditions without additional base. A. S.-K. Tsang, P. Jensen, J. M. Hook, A. S. K. Hashmi and M. H. Todd, Pure Appl. Chem., 2011, 83, 655 CrossRef CAS.
Footnotes |
| † Dedicated to Professor Barry M. Trost for his 75th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedure, analytical data for the compounds and spectra for the NMR experiments. See DOI: 10.1039/c6qo00249h |
| This journal is © the Partner Organisations 2016 |