Anion-dependent spin crossover in solution for an iron(II) complex of a 1H-pyrazolyl ligand†
Simon A. Barrett and
Malcolm A. Halcrow*
School of Chemistry, University of Leeds, Woodhouse Lane, Leeds, LS2 9JT, UK. E-mail: m.a.halcrow@leeds.ac.uk; Fax: +44 (0)113 343 6565; Tel: +44 (0)113 343 6506
First published on 12th February 2014
Abstract
The spin-crossover equilibrium midpoint temperature (T1/2) in [Fe(3-bpp)2]X2 (3-bpp = 2,6-di{pyrazol-3-yl}pyridine) varies from 259 K when X− = BPh4− to 277 K when X− = Br−, at 10 mM concentrations in an acetone–water solvent mixture.
Metal-organic spin-crossover (SCO) materials continue to be heavily studied in the solid state,1 with particular current interest in their applications in nanoscience.2 However, while ultrafast spectroscopy in solution has elucidated the atomistic mechanism of the spin-transition event,3 interest in solution-phase SCO has otherwise developed more slowly.4,5 Individual examples of cooperative SCO switching in micelles,6 a spin-state dependent MRI response from an iron complex,7 an SCO complex that binds barbiturate in solution8 and designs of anion-responsive SCO centre,9,10 have all been demonstrated. These results imply the UV/vis and paramagnetic NMR changes induced by SCO could be of use for sensor applications.5
The anion-dependent complexes [Fe(H2bip)2L]2+ (H2bip = 2,2′-bi{1,4,5,6-tetrahydropyrimidine}; L = H2bip, bipy etc.) are the best characterised system where SCO is triggered by supramolecular host–guest binding.9 The low-spin state of these complexes is favoured in the presence of strongly associating halide anions, which interact with the chelating N–H groups at the periphery of the H2bip ligands. Earlier investigations of anion-dependent SCO in other compounds had shown negative results,11,12 possibly because those studies were performed in aqueous solution or water-containing solvent mixtures. Water tends to disrupt host–guest interactions to anions, all other things being equal, because of its polarity and strong hydrogen-bonding character.13 A contributing factor to the successful observation of anion binding by [Fe(H2bip)2L]2+ may be that those studies were performed in the less competitive solvent dichloromethane.9
The complex [Fe(3-bpp)2]2+ (12+; 3-bpp = 2,6-di{pyrazol-3-yl}pyridine) has been important to the development of several aspects of SCO research.14 Its chemistry was originally developed by Goodwin et al.,12,15–17 but it has since been employed by others in a variety of supramolecular and multi-functional spin-crossover materials.18,19 These studies have been facilitated by the unusual stability of 12+ in water,20 which has allowed a large number of salts of this complex to be precipitated and crystallised. Twenty years ago Goodwin et al. reported solution-phase SCO data for the I−, BF4− and PF6− salts of 12+ in an unspecified acetone–water mixture, concluding that “…all three salts show essentially the same behaviour”.12 However, reexamination of their data implies that the SCO midpoint temperature (T1/2) for [Fe(3-bpp)2]I2 (1I2) lies 10 K higher than for the other two salts (ESI†). That follows the trend expected from the [Fe(H2bip)2L]2+ system,9 and would be another rare observation of anion-dependent spin-crossover. This result required clarification, however, since SCO in 12+ in acetone–water is sensitive to the composition of the solvent mixture.20 We report here a re-examination of this system which confirms that guest-responsive SCO can be observed in 12+, even in a competitive solvent.
The salts 1X2 (X− = BPh4−, BF4−, CF3SO3−, NO3− and Br−) were prepared by the literature procedures.‡12,15,19 The BPh4−, BF4− and CF3SO3− salts were recrystallised from MeNO2/Et2O, while the other less soluble salts were recrystallised from MeOH/Et2O. While 1[BF4]2 was isolated as a solvent-free powder after drying in vacuo, all the other salts contained water or methanol of crystallisation in their purified forms by microanalysis (ESI†; hydrate formation is a common feature of the chemistry of 1X2 salts14). Preliminary screening by 1H NMR in CD3CN, (CD3)2CO and a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CD3)2CO–D2O mixture at 293 K established a small, but consistent dependence of the paramagnetic isotropic shifts from 1X2 on the anion X− (ESI†). In both solvents, the contact shifts (and hence the magnetic moment21) of the sample followed the order in X−
1 v/v (CD3)2CO–D2O mixture at 293 K established a small, but consistent dependence of the paramagnetic isotropic shifts from 1X2 on the anion X− (ESI†). In both solvents, the contact shifts (and hence the magnetic moment21) of the sample followed the order in X−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BPh4− ≈ BF4− > CF3SO3− > NO3− ≈ Br− (1[NO3]2 and 1Br2 were only soluble in the mixed solvent system). This is the trend expected if the high–low-spin state population of the complex in solution is perturbed by more coordinating anions.22 Consistent with that suggestion, all five salts gave identical isotropic shifts within experimental error in the more polar solvent CD3OD, where hydrogen bonding between 12+ and X− should be weaker.
BPh4− ≈ BF4− > CF3SO3− > NO3− ≈ Br− (1[NO3]2 and 1Br2 were only soluble in the mixed solvent system). This is the trend expected if the high–low-spin state population of the complex in solution is perturbed by more coordinating anions.22 Consistent with that suggestion, all five salts gave identical isotropic shifts within experimental error in the more polar solvent CD3OD, where hydrogen bonding between 12+ and X− should be weaker.
These initial observations were quantified by variable temperature Evans method measurements (Fig. 1, Table 1). These were performed in the 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CD3)2CO–D2O solvent mixture, corresponding to 31.2 mol % D2O. Addition of water to the solvent was necessary to afford a medium in which all five salts were sufficiently soluble. The data for 1[BF4]2 under these conditions are consistent with those we have reported for that salt in other (CD3)2CO–D2O solvent compositions.20
1 v/v (CD3)2CO–D2O solvent mixture, corresponding to 31.2 mol % D2O. Addition of water to the solvent was necessary to afford a medium in which all five salts were sufficiently soluble. The data for 1[BF4]2 under these conditions are consistent with those we have reported for that salt in other (CD3)2CO–D2O solvent compositions.20
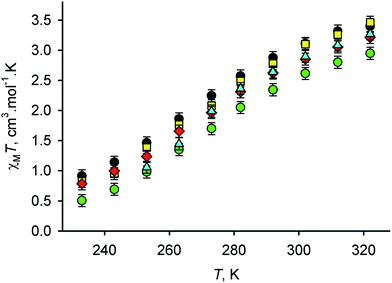 | ||
Fig. 1 Variable temperature magnetic susceptibility data for 1X2 in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CD3)2CO–D2O, with X− = BPh4− (black circles), BF4− (yellow squares), CF3SO3− (red diamonds), NO3− (cyan triangles) and Br− (green circles).§ 1 v/v (CD3)2CO–D2O, with X− = BPh4− (black circles), BF4− (yellow squares), CF3SO3− (red diamonds), NO3− (cyan triangles) and Br− (green circles).§ | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CD3)2CO–D2O and pure (CD3)2CO, measured by Evans method (Fig. 1 and 2).§ See ref. 22 for the definition of βN
1 v/v (CD3)2CO–D2O and pure (CD3)2CO, measured by Evans method (Fig. 1 and 2).§ See ref. 22 for the definition of βN
| X− | βN | T1/2a, K | ΔH, kJ mol−1 | ΔS, J mol−1 K−1 |
|---|---|---|---|---|
| a From ref. 12. The stoichiometry of the (CD3)2CO–D2O solvent mixture used in ref. 12 was not specified, but is probably similar to that in this work.20b From ref. 20. | ||||
1X2, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 v/v (CD3)2CO–D2O 1 v/v (CD3)2CO–D2O |
||||
| BPh4− | 0 | 259(1) | 31.5(4) | 121(2) |
| PF6−a | 0.64 | 258 | — | — |
| BF4− | 0.69 | 261(1) | 30.6(4) | 117(2) |
| CF3SO3− | 0.74 | 264(1) | 29.9(4) | 113(2) |
| NO3− | 0.86 | 268(1) | 33.2(4) | 124(2) |
| I−a | 0.88 | 268 | — | — |
| Br− | 0.93 | 274(1) | 25.9(4) | 95(2) |
| 1X2, (CD3)2CO | ||||
| BPh4− | 0 | 243(1) | 20.3(2) | 83(1) |
| BF4−b | 0.69 | 247(1) | 24.8(2) | 100(1) |
| CF3SO3− | 0.74 | 252(1) | 22.0(2) | 87(1) |
1[BPh4]2 + y[NBu4]Br, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 v/v (CD3)2CO–D2O 1 v/v (CD3)2CO–D2O |
||||
| y = 0 | — | 259(1) | 31.5(4) | 121(2) |
| y = 0.78 | — | 264(1) | 29.7(4) | 111(2) |
| y = 1.71 | — | 269(1) | 25.0(4) | 93(2) |
All five salts exhibit an SCO equilibrium under these conditions, centred just below room temperature (Fig. 1). The T1/2 values obtained show the clear trend in X−:
| BPh4− ≈ BF4− > CF3SO3− > NO3− > Br− |
This correlates perfectly with the hydrogen-bonding capability of those anions, as expressed by Lungwitz and Spange's βN parameter (Fig. 2).22 Importantly, Goodwin's original data for 1[PF6]2 and 1I2 in an unspecified (CD3)2CO–D2O solvent composition also agree well with these new results (Table 1, Fig. 2).12 The enthalpy and entropy of SCO for four of the salts in Table 1 (from van'T Hoff isochore plots) are similar, and are consistent with previously reported values for 1[BF4]2 in (CD3)2CO–D2O mixtures.20 The exception is 1Br2, whose ΔH and ΔS values are unexpectedly lower, and closer to those shown by salts of 12+ in pure organic solvents including (CD3)2CO (Table 1). A reduction in ΔH and ΔS was also observed when Br− was titrated into 1[BPh4]2 (see below, Table 1). We suggest that it may reflect a weaker solvation shell about the 12+ cations induced by the strongly associated Br− anions, which would reduce the rearrangement of the solvent accompanying SCO. That remains to be confirmed, however. Notably nucleophilic displacement of 3-bpp from the iron centre by Br−, which is a potential side-reaction in the high-spin form of the complex, would have the opposite effect of raising ΔH and ΔS.4
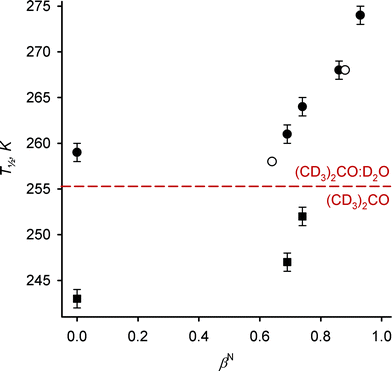 | ||
Fig. 2 Plot of the spin-crossover mid-point temperature T1/2 of the salts 1X2 vs. the hydrogen-bonding power of the X− anion (βN (ref. 21)) in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CD3)2CO–D2O (circles) and pure (CD3)2CO (squares).§ The black data points are from this work, while the white circles are the I− and PF6− salts measured by Goodwin et al.12 The solvent dependence of these data is discussed in ref. 20. 1 v/v (CD3)2CO–D2O (circles) and pure (CD3)2CO (squares).§ The black data points are from this work, while the white circles are the I− and PF6− salts measured by Goodwin et al.12 The solvent dependence of these data is discussed in ref. 20. | ||
For comparison, the three 1X2 salts that are soluble in pure (CD3)2CO were also measured in that solvent (Table 1, Fig. 2 and ESI†). The results are consistent with those above in showing a 9 K increase in T1/2 for 1[CF3SO3]2 compared to 1[BPh4]2, a slightly larger difference than in the more polar solvent mixture. Lastly, titration of [NBu4]Br into 1[BPh4]2 in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CD3)2CO–D2O yielded an increase in T1/2 with increasing bromide concentration, that is consistent with the behaviour of the pure 1[BPh4]2 and 1Br2 salts (Table 1 and ESI†).
1 (CD3)2CO–D2O yielded an increase in T1/2 with increasing bromide concentration, that is consistent with the behaviour of the pure 1[BPh4]2 and 1Br2 salts (Table 1 and ESI†).
The salts 1[BPh4]2, 1[BF4]2, 1[CF3SO3]2, 1[NO3]2 and 1Br2 all show the same UV/vis metal-to-ligand charge-transfer (MLCT) maximum, at λmax = 456 nm (εmax = 3.6 ± 0.1 × 103 dm3 mol−1 cm−1) in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v (CH3)2CO–H2O at 293 K (ESI†). The invariance of these spectra with the anion present is inconsistent with the Evans method data, since εmax of this MLCT band should increase with T1/2 which raises the low-spin fraction of the complex at room temperature.20 That might reflect the sample concentrations in the UV/vis measurements (0.2 mM), which were ca. 50× lower than for the Evans method experiments (10 mM). Low concentrations promote host–guest dissociation in solution, which would explain the discrepancy between the techniques.
1 v/v (CH3)2CO–H2O at 293 K (ESI†). The invariance of these spectra with the anion present is inconsistent with the Evans method data, since εmax of this MLCT band should increase with T1/2 which raises the low-spin fraction of the complex at room temperature.20 That might reflect the sample concentrations in the UV/vis measurements (0.2 mM), which were ca. 50× lower than for the Evans method experiments (10 mM). Low concentrations promote host–guest dissociation in solution, which would explain the discrepancy between the techniques.
In conclusion, we have demonstrated a dependence between spin-crossover in [Fe(3-bpp)2]2+ (12+) and the presence of hydrogen bonding anions, in a polar solvent mixture at NMR concentrations (ca. 10 mM). As with the [Fe(H2bip)2L]2+ system,9 more strongly associating anions favour the low-spin state of the complex and increase T1/2. That is noteworthy, because evidence for the influence of hydrogen-bonding anions on T1/2 in solid SCO materials has been contradictory up to now.23 The sensitivity of 12+ to hydrogen bonding to anions (and to solvent20) arises because the hydrogen bond-donor N–H groups in 3-bpp are directly covalently bonded to the metal-donor N atoms. Hence small perturbations in the electronic character of the ligand, caused by changes in hydrogen bonding, are transmitted efficiently to the coordinated iron atom.
Although the response of T1/2 to different anions in 12+ is smaller than in [Fe(H2bip)2L]2+ derivatives, this work was performed in more competitive solvents (including an acetone–water mixture) where hydrogen bonding between 12+ and X− is expected to be weaker.13 The fact that any correlation between T1/2 and X− is observed under our conditions is noteworthy for a monodentate hydrogen bond-donor like 12+, and confirms that SCO in 12+ is sensitive to host–guest interactions. Therefore, the [Fe(3-bpp)2]2+ motif is a promising platform for the development of SCO-based sensor applications. Our current work aims to modify the 3-bpp ligand design, to maximise its host–guest binding capabilities.
Acknowledgements
This work was funded by the University of Leeds.Notes and references
- Spin-crossover materials – properties and applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, UK, 2013, p. 568 Search PubMed.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313 RSC.
- A. Cannizzo, C. J. Milne, C. Consani, W. Gawelda, C. Bressler, F. van Mourik and M. Chergui, Coord. Chem. Rev., 2010, 254, 2677 CrossRef CAS PubMed.
- N. Hassan, A. B. Koudriavtsev and W. Linert, Pure Appl. Chem., 2008, 80, 1281 CrossRef CAS.
- M. P. Shores, C. M. Klug and S. R. Fiedler, in Spin-crossover materials – properties and applications, ed. M. A. Halcrow, John Wiley & Sons, Chichester, UK, 2013, ch. 10, pp. 281–301 Search PubMed.
- C. Gandolfi, C. Moitzi, P. Schurtenberger, G. G. Morgan and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 14434 CrossRef CAS PubMed; P. N. Martinho, Y. Ortin, B. Gildea, C. Gandolfi, G. McKerr, B. O'Hagan, M. Albrecht and G. G. Morgan, Dalton Trans., 2012, 41, 7461 RSC.
- J. Hasserodt, New J. Chem., 2012, 36, 1707 RSC.
- M. C. Young, E. Liew, J. Ashby, K. E. McCoy and R. J. Hooley, Chem. Commun., 2013, 49, 6331 RSC.
- Z. Ni and M. P. Shores, J. Am. Chem. Soc., 2009, 131, 32 CrossRef CAS PubMed; Z. Ni and M. P. Shores, Inorg. Chem., 2010, 49, 10727 CrossRef PubMed; Z. Ni, A. M. McDaniel and M. P. Shores, Chem. Sci., 2010, 1, 615 RSC; Z. Ni, S. R. Fiedler and M. P. Shores, Dalton Trans., 2011, 40, 944 RSC.
- A. M. McDaniel, C. M. Klug and M. P. Shores, Eur. J. Inorg. Chem., 2013, 943 CrossRef CAS.
- H. L. Chum, J. A. Vanin and M. I. D. Holanda, Inorg. Chem., 1982, 21, 1146 CrossRef CAS; J. W. Turner and F. A. Schultz, Inorg. Chem., 2001, 40, 5296 CrossRef.
- K. H. Sugiyarto, D. C. Craig, A. D. Rae and H. A. Goodwin, Aust. J. Chem., 1994, 47, 869 CrossRef CAS.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648 RSC.
- M. A. Halcrow, Coord. Chem. Rev., 2005, 249, 2880 CrossRef CAS PubMed.
- K. H. Sugiyarto and H. A. Goodwin, Aust. J. Chem., 1988, 41, 1645 CrossRef CAS; K. H. Sugiyarto, K. Weitzner, D. C. Craig and H. A. Goodwin, Aust. J. Chem., 1997, 50, 869 CrossRef.
- K. H. Sugiyarto, M. L. Scudder, D. C. Craig and H. A. Goodwin, Aust. J. Chem., 2000, 53, 755 CrossRef CAS.
- T. Buchen, P. Gütlich and H. A. Goodwin, Inorg. Chem., 1994, 33, 4573 CrossRef CAS; T. Buchen, P. Gütlich, K. H. Sugiyarto and H. A. Goodwin, Chem.–Eur. J., 1996, 2, 1134 CrossRef; S. Marcén, L. Lecren, L. Capes, H. A. Goodwin and J.-F. Létard, Chem. Phys. Lett., 2002, 358, 87 CrossRef; K. H. Sugiyarto, W.-A. McHale, D. C. Craig, A. D. Rae, M. L. Scudder and H. A. Goodwin, Dalton Trans., 2003, 2443 RSC.
- E. Coronado, M. C. Giménez-López, C. Giménez-Saiz and F. M. Romero, CrystEngComm, 2009, 11, 2198 RSC; M. Clemente-León, E. Coronado, M. C. Giménez-López, F. M. Romero, S. Asthana, C. Desplanches and J.-F. Létard, Dalton Trans., 2009, 8087 RSC; I. A. Gass, S. R. Batten, C. M. Forsyth, B. Moubaraki, C. J. Schneider and K. S. Murray, Coord. Chem. Rev., 2011, 255, 2058 CrossRef CAS PubMed.
- P. King, J. J. Henkelis, C. A. Kilner and M. A. Halcrow, Polyhedron, 2013, 52, 1449 CrossRef CAS PubMed.
- S. A. Barrett, C. A. Kilner and M. A. Halcrow, Dalton Trans., 2011, 40, 12021 RSC.
- B. Weber and F. A. Walker, Inorg. Chem., 2007, 46, 6794 CrossRef CAS PubMed.
- R. Lungwitz and S. Spange, New J. Chem., 2008, 32, 392 RSC.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119 RSC and references therein.
- D. Fedaoui, Y. Bouhadja, A. Kaiba, P. Guionneau, J.-F. Létard and P. Rosa, Eur. J. Inorg. Chem., 2008, 1022 CrossRef CAS; F. El Hallak, P. Rosa, P. Vidal, I. Sheikin, M. Dressel and J. van Slageren, EPL, 2011, 95, 57002 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, elemental microanalysis, NMR, Evans method and UV/vis data. See DOI: 10.1039/c4ra00230j |
| ‡ The salt 1[NCS]2 (ref. 16) was also investigated in this work. However, solutions of this compound substantial amounts of uncoordinated 3-bpp by 1H NMR (ESI†), which probably reflects competitive displacement of 3-bpp from the metal centre by the nucleophilic NCS− ion.24 For this reason, 1[NCS]2 was not investigated further during this study. Smaller amounts (<10%) of free 3-bpp are also present in solutions of 1[NO3]2 and 1Br2 by NMR, and ligand displacement equilibria may make a small contribution to ΔH and ΔS of SCO in those salts.4,20 |
| § The differing values of χMT at the high- and low-temperature ends of these plots reflect the temperature window of the measurements, which was limited by the liquid range of the solvent. Hence the data do not cover the full the spin-state equilibria, which will span a temperature range of ca. 150 K from start to finish.4,5 The T1/2 values in Table 1 and Fig. 2, and the van'T Hoff plots, were calculated assuming that the fully high-spin complex exhibits χMT = 3.5 ± 0.1 cm3 mol−1 K under all the conditions used. That approximation is supported by our earlier work.20 |
| This journal is © The Royal Society of Chemistry 2014 |