Open Access Article
Open Access ArticleNew perspective crosslinking electrochemistry and other research fields: beyond electrochemical reactors†
Yu
Zhang
 and
Yuen
Wu
and
Yuen
Wu
 *
*
School of Chemistry and Materials Science, University of Science and Technology of China, Hefei 230026, China. E-mail: yuenwu@ustc.edu.cn
First published on 16th April 2024
Abstract
Over the years, electrochemical reactors have evolved significantly, with modern reactors now able to achieve a high current density and power output in compact sizes. This leap in performance has not only greatly accelerated the rate of electrochemical reactions but also had a broader impact on the environment. Traditional research perspectives, focused primarily on the internal working systems of reactors, possibly overlook the potential of electrochemical systems in regulating their surrounding environment. A novel research perspective considering the interaction between electrochemical processes and their environmental context as a unified subject of study has gradually emerged alongside the dramatic development of electrochemical techniques. This viewpoint introduces a paradigm shift: electrochemical reactors are not isolated entities but rather are integral parts that interact with their surroundings. Correspondingly, this calls for an innovative research methodology that goes beyond studying the electrochemical processes in isolation. Rather, it integrates the design of the electrochemical system with its specific application environment, ensuring seamless integration for optimal performance under various practical conditions. Therefore, performance metrics should include not only the basic parameters of the electrochemical reactions but also the adaptability of the electrochemical system in real-world scenarios beyond the laboratory. By focusing on environmental integration and application-driven design, the applications of electrochemical technology can be more effectively leveraged. This perspective is exemplified by an electrochemical system based on coupled cathodic oxygen reduction and anodic oxygen evolution reactions. By adopting this new research paradigm, the applications of this electrochemical system can be extended to fields like medical treatment, food science, and microbial fermentation, with an emphasis on tailored designs for these specific application fields. This comprehensive and systematic new research approach aims to fully explore the potential applications of electrochemical technology and foster interdisciplinary collaboration in the electrochemical field.
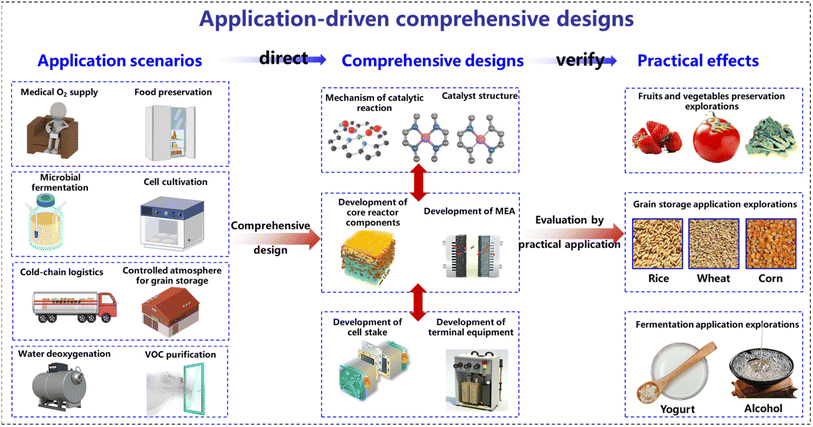
Introduction
Electrochemical technology, powered by electrical energy to drive chemical reactions, plays a pivotal role in scientific research and holds vast potential in practical applications.1,2 Historically, the focus of research in the electrochemistry field has been primarily confined to electrochemical reactors, and investigating electrode reactions, product types, and yields. Such a limited scope was initially shaped by the early structure of electrochemical reactors, which led to low current densities and limited electrochemical reaction efficiency.3,4 Consequently, the subtle impact of the electrochemical processes at such low current densities often led researchers to overlook their environmental interactions. Advancements in technology have since led to a considerable improvement in electrochemical reactors, now transitioning to flow-type electrolytic cells.3,5 This structure allows continuous reactant supply, especially supporting continuous gas-phase reactions with the introduction of gas diffusion electrodes (GDEs).6,7 Further progress includes the design of membrane electrode assembly (MEA) reactors with ion-exchange membranes between the anode and cathode.8–10 This near zero-gap structure minimizes internal polarization resistance, thus considerably boosting the electrochemical reaction efficiency.11,12 Modern electrochemical reactors have evolved into modular stacks based on MEA structures, maintaining a quite compact size while delivering high power and current density for prolonged, stable, and efficient electrochemical reactions.13–15 The ability to achieve high current densities reaching ampere levels for modern electrochemical reactors signifies the faster electrochemical reaction rates, but deepens their environmental footprints.16 It has become evident that such a narrow focus on electrode reactions within the reactors is insufficient because it neglects the wider environmental and systemic impacts. Therefore, an in-depth exploration of electrochemical technology should not be confined only to the reactions inside reactors, but also encompass their roles and applications in a broader environmental context. Adopting such a holistic research perspective is pivotal to fully tap into the potential value of electrochemical technology across various domains.In this new research paradigm, where electrochemical reactors and their surrounding environment are considered an interactive whole, an innovative research approach is required. Traditional strategies often follow a linear research process involving studies of the catalytic mechanism, catalyst, electrode reaction, and reaction product. However, such a research approach falls short in considering complex interactions between the reactor and its surrounding environment. The new research strategy emphasizes a practical application-oriented study, for designing everything from electrochemical mechanisms to reactor structures. As depicted in Fig. 1, this application-driven approach can ensure the seamless integration of electrochemical technology with its environment, achieving faster reactions and more effective environmental control. Furthermore, consideration must be given to the interplay between electrochemical reactions and external environments, as well as to the adaptability of reaction systems to the actual operating conditions. Performance assessments of designed electrochemical systems should extend beyond laboratory conditions towards real-world application environments, ensuring optimal overall performance in actual operations. In fact, even with the same electrochemical reaction mechanisms, different application scenarios and objectives may lead to entirely different design considerations. In the following sections, an electrochemical system coupling a cathodic oxygen reduction reaction (ORR) and anodic oxygen evolution reaction (OER) is considered as an example to illustrate the design of electrochemical systems tailored to specific application goals. By adopting this new research perspective, the potential applications of the coupled ORR–OER system can be extended to medical treatment, food preservation, and microbial fermentation fields. While the applications of electrochemical technology in these fields are still in the nascent stages of exploration, it is expected that this perspective will encourage researchers in the domain of electrochemistry to explore these promising areas further. In conclusion, considering the electrochemical system and its environment as an integrated entity as well as a purpose-oriented research approach has the potential to propel electrochemical technology to the forefront of interdisciplinary intersections, and prompt new collaborative efforts across diverse research fields.
Discussion
Electrochemical system: coupling cathodic ORR and anodic OER
Oxygen (O2), a crucial component of the earth's atmosphere, constituting approximately 21% of our breathable air, is omnipresent in our planet and plays a key role in numerous chemical and biological processes. In the field of electrochemistry, the ORR and the OER are two fundamental reactions. The ORR process involves the consumption of O2, reducing it to water or other oxygen-containing ions, while the OER is the reverse process, electrochemically generating O2 from water or oxygen-containing ions.10By coupling these two electrochemical reactions, O2 production, removal, or concentration modulation can be achieved. These are pivotal for various applications, such as O2 production in the medical field, O2 removal for food preservation, or manipulating the O2 concentration to regulate microbial metabolism in microbial fermentation.
In the coupled electrochemical system of the cathodic ORR and anodic OER with a 4-electron transfer (4ET) pathway, the reactions at the cathode and anode for an acid system are shown as follows:17,18
Cathode reaction:
| 4H+ + O2 + 4e− → 2H2O |
Anode reaction:
| 2H2O − 4e− → 4H+ + O2 |
In an alkaline system, the cathode and anode electrode equations are as follows:17,18
Cathode reaction:
| 2H2O + O2 + 4e− → 4OH− |
Anode reaction:
| 4OH− − 4e− → 2H2O + O2 |
Upon combining the above cathodic and anodic half-reactions, the total electrochemical reaction can be derived as follows:
| O2 (from air) → O2 |
The above electrode reactions encapsulate the depletion of atmospheric O2 at the cathode and the generation of an O2 stream at the anode, which can be considered as the effective concentration of O2 obtained from the air, as shown in the equation. This process, characterized by a reduction in entropy, requires energy input to the system for its activation. In electrochemistry, the energy necessary for initiating such an entropy reduction process is supplied in the form of electrical power. The relationship between the free energy change (ΔG) and potential in electrochemical reactions is defined by the equation:19
| ΔG = −nFE |
| ΔG = ΔH − TΔS |
| −nFE = ΔH − TΔS |
Assuming a constant temperature and driving a process where environmental entropy decreases (i.e., ΔS < 0) through electrical energy input, it is derived as follows:
| TΔS = −nFE − ΔH |
The above equation delineates the relationship between electrical energy input and environmental entropy change in the electrochemical reaction process, unveiling its immense potential in precise environmental parameter control. Subsequent sections will delve into the potential applications of this ORR–OER electrochemical system across various domains. Furthermore, the following sections will specifically elaborate on guiding the overall designs of the electrochemical systems based on application scenarios.
Medical treatment field: electrochemical in situ production of high-purity medical O2
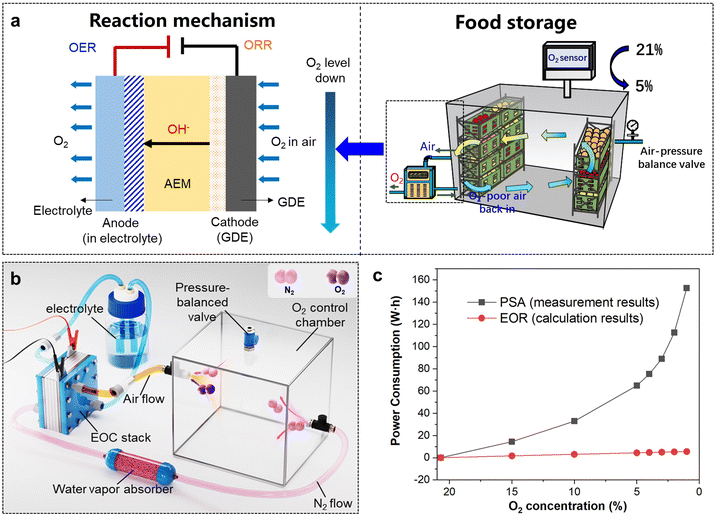
Medical O2, with a purity exceeding 99.5%, is extensively utilized in treating respiratory diseases, cardiac issues, emergency resuscitations, and as an adjunct in anaesthesia during surgeries.21 During the COVID-19 pandemic, medical O2 became a critical healthcare resource, with severe shortages reported in many countries.22,23 This crisis primarily stemmed from the COVID-19 virus's severe impact on the lungs of patients, necessitating critically ill patients to rely on high-purity medical O2 for assisted breathing.24 The surge in patient numbers led to a drastic shortage in the supply of medical O2. However, existing O2 supply devices, such as O2 cylinder and on-site pressure swing adsorption (PSA) equipment, exhibited inherent limitations.25 O2 cylinders are bulky, difficult to transport, and pose significant safety risks, while PSA equipment typically produces O2 with a purity of only 93%,26,27 which fails to meet medical O2 standards directly.28 In this context, the application of electrochemical technology for the on-site production of medical O2 presents itself as an innovative and viable solution, potentially alleviating the challenges of medical O2 supply in hospitals.Upon identifying hospital O2 supply as the application scenario, it is imperative to design and optimize the entire electrochemical reaction system with the specific demands of this application. The primary application requirements are as follows: (1) high O2 purity, as only O2 with a purity exceeding 99.5% meets the standards for medical O2; (2) high energy efficiency, to ensure low energy consumption of the O2 production equipment; (3) a high rate of O2 production, which needs to reach at least 1 L min−1 to satisfy practical application requirements.29,30 To meet the above criteria, it is essential to concurrently focus on optimizations and designs in terms of the catalytic mechanism, catalyst, and reactor structure. In a previous report,10 Zhang et al. designed a coupled ORR and OER electrocatalytic system to construct a high-performance electrochemical O2 generator (EOG) for in situ medical O2 production. As illustrated in Fig. 2, this EOG achieved a high O2 purity (>99.9%), high O2 production rate (>1.5 L min−1), and high energy efficiency (496 L kW−1 h−1), along with offering other advantages, such as high portability (4.7 kg), instant start-up (<1 s), and low noise (<50 dB), demonstrating superior performance and significant potential to replace traditional PSA O2 generators.
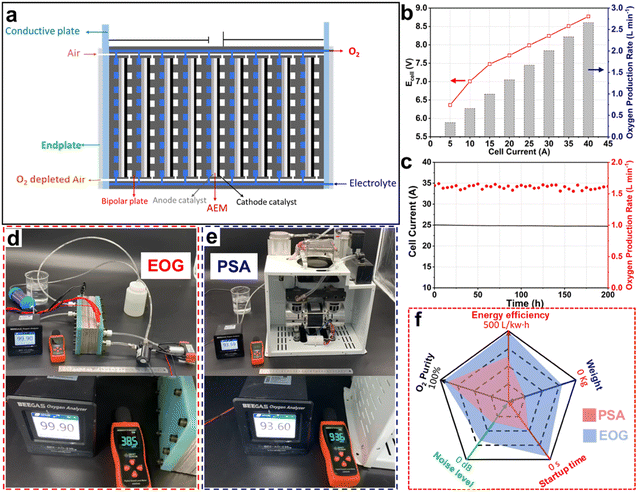 | ||
| Fig. 2 (a) Structural schematic of the electrochemical O2 generator (EOG) cell stack comprising 9-unit cells. (b) I–V curves and O2 production rates of the EOG cell stack with a 100 cm2 working area. (c) Stability test of the EOG cell stack. Digital photos showing comparisons of (d) EOG and (e) PSA devices for O2 purity (the values on the black apparatus) and noise (the values on the orange apparatus). (f) Five-star chart showing the comparison of the user experience for EOG and PSA devices.10 Reproduced with the permission from copyright 2024, Royal Society of Chemistry. | ||
In the work, Zhang et al. conducted a series of designs and optimizations to enhance the performance of the electrochemical O2 generator (EOG).10 In alkaline conditions, the ORR and OER can proceed through either a 4-electron transfer (4ET) mechanism adopting OH− ions as intermediates or a 2ET mechanism applying HO2− ions as intermediates. The 2ET pathway, by halving the energy requirement per unit of O2 produced, considerably enhances the O2-generation efficiency compared to the 4ET pathway. In terms of catalyst optimization, the development of ORR catalysts with high activity, stability, and 2ET selectivity was pursued to reduce the activation energy of the ORR, thereby decreasing the electrochemical polarization resistance and enhancing the reaction efficiency. In designing the electrocatalytic reactor structure, the cathodic ORR process is characterized as a three-phase-boundary reaction.31 This necessitates the incorporation of GDEs, which are instrumental in enhancing the mass-transfer efficiency of the O2 reactant within the ORR process, thereby effectively mitigating concentration polarization. The use of an alkaline AEM in the MEA structure minimizes the anode–cathode gap, reduces ohmic resistance, and ensures high O2 purity by allowing only ion transfer and preventing gas exchange between the anode and cathode. Through these optimizations and designs, Zhang et al. improved the O2 production of a single MEA cell to 180 mL min−1.10 However, this O2-production rate still fell short of the applicable standard of >1 L min−1. Their subsequent strategy involved assembling nine MEA units into an integrated stack and specifically improving the structure design of the polar plates. This highly integrated, series-connected stack structure enhanced the overall device's power output while minimizing the volume and weight, crucial for achieving the miniaturization and high portability of the electrochemical O2-generation device (Fig. 2a and d).
In electrochemical O2 production, the aim is for a high rate anodic OER, thus ensuring the ORR process has an excessive supply of air-sourced O2 over its consumption is crucial. An insufficient O2 supply for the cathodic ORR may limit the OER rate in turn, thereby lowering the overall O2 production. To address these issues, the design of the cathode flow channels should aim to maximize the air supply for the ORR side, reducing resistance to air mass transfer and accelerating the ORR reaction rates. Implementing multiple parallel straight channel-type flow fields and shortening the length of individual channels is an effective strategy to ensure sufficient gas flux for the ORR per unit time.32 Meanwhile, adjusting the land-to-channel ratio in the flow field is also crucial, as proper control of this ratio affects not only the flow rate and pressure drop of the gas reactants but also has great impacts on the water-removal efficiency, mechanical stability, temperature distribution, and overall conductivity of the cell stack.33 During the electrochemical reaction process, the effective contact area between gas reactants and the electrode, i.e., the land area, directly determines the distribution of the gas concentration within the electrode. This is vital for optimizing the entire electrochemical reaction process. For instance, Rajesh Boddu utilized computational fluid dynamics models to analyze the flow characteristics on bipolar plates with different serpentine flow field configurations.34 Their findings revealed that increasing the number of parallel flow channels and reducing their size could achieve a more efficient and uniform contact surface area and lower pressure drops, thereby enhancing the fuel-cell cathode ORR rate. Furthermore, research by Kahraman et al. suggested that wide flow channels could facilitate reactant transport and water removal, while wide land areas could improve the conductivity, thermal conductivity, and mechanical stability. Based on the data and experimental results in the research work, the flow channel width should be maintained below 1.5 mm to ensure mechanical stability, while the land-to-channel ratio should be as low as possible to minimize voltage loss.33 In summary, the importance of the flow channel design and land-to-channel ratio has been widely recognized in the fuel cells field.33–38 However, the design of novel, application-specific electrochemical reactors still requires tailored approaches. Variations in the viscosity and wettability of diverse reaction media necessitate unique land-to-channel ratios for each. Therefore, relying solely on existing design principles is insufficient for the design and optimization of future electrochemical reactors. This calls for researchers to not only deepen their understanding of existing principles but also to explore new design solutions.
On the anodic side, the rapid production of O2 on the anode under high current densities often triggers drastic bubble release, leading to issues such as bubble shielding effects and catalyst detachment.39 Numerous studies have demonstrated that self-supporting electrodes engineered through interfacial engineering techniques can effectively address these challenges.39–45 For instance, Hao et al. successfully deposited copper phosphide (Cu3P) microsheets on nickel foam, significantly enhancing the catalytic activity for the hydrogen evolution reaction (HER) and the oxygen evolution reaction.44 The binder-free, hierarchical Cu3P structure, with its superhydrophilic and superaerophobic traits, enhanced electrolyte–electrode interactions and bubble release, reducing bubble-induced dead zones and boosting the catalytic performance. Yan et al. developed a universally applicable and efficient cathodic electrodeposition strategy to successfully prepare different metal hydroxides on conductive substrates, creating electrodes that not only boasted high-quality loading but also exhibited outstanding hydrophilicity and oxygen-repelling properties.39 Zhang et al. elaborately constructed MOF-derived 2D nanosheet arrays loaded on a 3D porous Ni substrate.45 This design could significantly improve the surface roughness and hydrophilicity, enhancing water contact and active material interaction over flat surfaces. This structure accelerated gas bubble release from the electrode surface, preventing the obstruction of active sites and ensuring uninterrupted electrochemical processes. The above research examples demonstrate that OER catalysts developed through interfacial engineering techniques commonly possess rough surfaces and can eliminate the requirement for hydrophobic binders. These characteristics endow the anode with an excellent degassing property, hastening O2 bubble detachment, thus allowing achieving high activity and stability under high current density conditions.
Through these designs and improvements, the performance of the EOG finally met the preset objectives, achieving over 99.9% O2 purity, with the highest O2 production rate of over 2.5 L min−1 (Fig. 2f), and 496 L kW−1 h−1 energy efficiency.10 In their study, Zhang et al. then examined the practical stability of the stack, confirming its continuous 200 h operation at 1.5 L per min O2 production without degradation (Fig. 2c), demonstrating excellent prospects for practical application. Finally, to enhance the adaptability of the EOG to application environments, additional optimizations are necessary. For instance, give this EOG's reliance on alkaline electrolytes, the integration of an alkali vapor absorber at the anode outlet would be imperative for ensuring user safety. Moreover, to comply with hospital safety standards for electrical use, specific optimizations in the electrode area and cell unit number of the series-connected stack are required. These adjustments would allow for maintaining the overall current of the EOG device below the environmental maximum limitation without compromising its O2-production ability.
The aforementioned study realized the development of an alkaline electrolyte-based EOG device with a 2ET reaction pathway. The designs of EOGs tend to focus on attaining a high O2 purity, substantial O2 production, and high energy efficiency. These features are specifically tailored to meet the demands of professional operation settings, such as hospitals and medical institutions, where reaching high working performance is required. However, for patients with chronic cardiopulmonary diseases who rely on medical O2 in their daily lives, the design criteria for electrochemical O2 generators in household applications differ significantly. In addition to ensuring basic application standards, like a high O2 purity and production rate, safety also becomes a paramount consideration. The use of an alkaline electrolyte in the EOG system poses a risk of alkali leakage, which is unsuitable for non-professional, domestic environments. This calls for specific designs tailored to the requirements of home settings. Our current goal is to engineer a system that functions without any electrolytes, operating only by pure water. This necessitates reconceiving the reaction mechanism, catalyst, and electrode structure. The elimination of electrolytes necessitates the exploration of alternative ion-conduction materials. A prime candidate is the use of proton-exchange membrane (PEM) to facilitate the ion conductivity between the anode and cathode by its inner high proton concentration. This requires a modification in the MEA fabrication method, favoring a catalyst-coated membrane (CCM) approach.46 Here, the catalyst is directly adhered onto the PEM, ensuring robust bonding between the catalyst and the membrane. This greatly enhances the ion-transport efficiency, thereby enabling the electrochemical system to operate with pure water. However, the use of the PEM introduces a new challenge, where a harsh localized environment with a high proton concentration and strong acidity is formed on the catalyst interface. This leads to the need for a reconsideration of the catalyst selection, necessitating the development of stable, acid-resistant OER and ORR catalysts that can withstand acidic electro-corrosion. The above-discussed PEM–CCM technology has been extensively utilized in the fields of water electrolysis and fuel cells.47–56 In particular, such water electrolysis technology has been deeply developed for O2 generation on space stations. Although traditional water electrolysis technology has been challenging for home O2 generators due to the production of large amounts of explosive hydrogen at the cathode, adopting the ORR–OER coupled electrocatalytic system may effectively circumvents this issue. Given the similarities in the electrochemical environment of electrochemical systems based on PEM–CCM, one can draw on the advanced catalyst designs from the water electrolysis and fuel cell fields to optimize the ORR–OER system. Wu et al. introduced a nickel-stabilized ruthenium dioxide (Ni–RuO2) catalyst as an efficient iridium alternative, enhancing acidic OER activity and durability significantly. The Ni addition notably improved RuO2's stability, achieving over 1000 h of operation at 200 mA cm−2, indicating its practical application viability.51 For a PEM-based ORR catalyst design, Guo et al. utilized a gas-promoted dealloying technique to develop a Pt-skin Pt1.5Ni1−x alloy electrocatalyst with increased Ni site density. This hybrid catalyzed the ORR through a relay process, reducing Pt usage and boosting the ORR activity to 4.10 A mgPt−1, about 15 times higher than commercial Pt/C.56 Drawing on the latest advances in water electrolysis and fuel cell technologies is expected to enable overcoming the challenges associated with employing PEM–CCM structures in ORR–OER systems, thereby paving the way for safer, more efficient, and sustainable O2-production solutions. However, the electrochemical ORR and OER mechanisms by adopting a PEM–CCM approach typically follow 4ET pathways. This can result in reduced energy efficiency compared to the alkaline EOG system that adopts the 2ET reaction pathway. However, the prioritization of safety in the design of home appliances necessitates certain trade-offs, including some degree of compromise in energy efficiency.
The above-outlined research strategy underscores the complexity and comprehensiveness inherent in designing electrochemical reaction systems and device structures tailored to specific application environments. This endeavor necessitates a holistic approach, where the design of individual components is not viewed in isolation but as part of an interconnected whole. Establishing definitive and clear requirements is crucial for guiding the design and optimization of each component within the system. In addressing the potential conflicts that arise between varying target needs and component designs, it becomes essential to prioritize key criteria while making strategic adjustments in other areas. Such a balanced and coordinated approach is fundamental to ensuring the adaptability of electrochemical system across diverse application scenarios and their successful implementation. This research methodology not only deepens the understanding of the interplay among various components within an electrochemical device and their collective impact on system performance but also highlights how these interactions shape the overall functionality of the system. Adopting this integrated design strategy, with a focus on practical applications, is pivotal for harnessing the full potential of electrochemical technology and aligning it seamlessly with the requirements of its intended application environments.
Food preservation field: electrochemical in situ deoxygenation
Numerous studies have confirmed that creating a low-O2 environment can effectively extend the shelf life and improve the storage quality of fresh foods, including fruits, vegetables, and grains.57–59 Under low-O2 conditions, the growth of bacteria and moulds is suppressed, significantly reducing microbial-induced spoilage.60,61 More importantly, such an environment restrains the aerobic respiration of produce, crucial for maintaining food freshness. The consumption of sugars and other key nutrients during aerobic respiration affects the taste and nutritional value of these plant foods;62 and a low-O2 environment, by slowing down the respiration rate, helps reduce the loss of these nutrients, thereby extending the preservation time.63 Moreover, a low-O2 environment also reduces the production and accumulation of ethylene, a plant hormone that promotes fruit ripening and ageing, thus effectively helping delay the ageing process of produce.64Traditional methods of O2 removal, such as using nitrogen (N2) gas cylinders or in situ PSA N2 generators, involve injecting high-purity N2 into the preservation space to displace O2.65 However, these externally reliant N2 source methods face multiple challenges, including high N2 supply costs, large equipment size, and crucially, an inherently low O2 removal efficiency. Also, the N2-replacement O2-removal strategy becomes less efficient as O2 levels decrease, often resulting in huge N2 wastage and an incomplete deoxygenation due to issues like dead corners and an uneven airflow. Typically, achieving complete O2 displacement requires using N2 gas several times the volume of the deoxygenation space, leading to high energy and time consumption. This not only escalates costs but also limits their practicality in broad-space or energy-sensitive environments, highlighting the urgent need for a more cost-effective and efficient O2-removal solution.
In the context of food preservation, the electrochemical ORR process may provide an effective means for efficiently diminishing environmental O2 levels. In the above-discussed ORR–OER coupled electrochemical system, the focus was on utilizing electrical energy to drive the concentration process of O2 from air by the electrochemical reactor, aimed at producing high-purity O2 at the anode for medical applications. In contrast, for food preservation, the emphasis shifts to the ORR process at the cathode. This strategic focus is on enabling the rapid, energy-efficient, and effective in situ reduction of the O2 concentration, thereby fostering a low-O2 environment conducive to the extended preservation of fresh fruits, vegetables, and grains. As illustrated in Fig. 3a, for the design of an electrochemical O2-removal (EOR) system, an air-tight space is directly linked to the cathode of the EOR device, to efficiently remove the O2 in air by an ORR process under gaseous circulation. While the anode is exposed to the external environment, and discharges the OER-generated O2 towards the external atmosphere. Considering the EOR device and the sealed space as an integrated system, the coupled ORR–OER electrochemical system facilitates a direct, in situ, and unidirectional transfer of O2 from the preservation area to the external environment. This process, which reduces entropy, is powered by electrical energy, standing in contrast to the traditional N2-displacement strategy for O2 removal. EOR offers a fundamentally superior approach by directly consuming O2 on-site with the electrochemical device, thereby eliminating the need for external gas sources and reducing costs. Moreover, the advanced mechanism of EOR is characterized by its energy consumption being determined by the amount of O2 removed, irrespective of the O2 concentration. This characteristic makes the EOR more energy-efficient and potentially more cost-effective compared to traditional N2-displacement methods, especially in scenarios where low O2 levels need to be maintained consistently. To validate the above conclusions, a preliminary comparison was conducted on the energy consumption of different O2-removal strategies. Actual experiments were performed with a 5 L sealed chamber using a PSA N2 generator for O2 removal, yielding specific data on the energy consumption. For electrochemical O2 removal, a simulated electrochemical cyclic system was designed to estimate the energy consumption. As depicted in the structural configuration shown in Fig. 3b, O2 removal was achieved by continuously circulating the air within the chamber into the electrochemical reactor. The specific calculation parameters and equations are shown in the ESI.† The energy consumption for the two O2-removal strategies at various target O2 concentrations are summarized in Fig. 3c. Energy consumption from N2 displacement was observed to escalate quickly with decreasing the target O2 concentrations, experiencing an exponential increase when the target O2 level fell below 5%. This was attributed to the minimal gas concentration gradient at low O2 levels, therefore significantly reducing the displacement efficiency. In contrast, the energy consumption for O2 removal driven by the electrochemical ORR was markedly lower than that by N2 displacement, exhibiting a linear increasing trend. This was due to the fact that the energy consumption for the ORR process depends on the amount of O2 reacted, regardless of its concentration. In addition, employing a cyclic O2-removal approach enables the repeated use of N2, thereby further lowering the overall energy consumption. The above analysis preliminarily confirmed the energy efficiency advantages and technological advancements of electrochemical O2 removal, providing a theoretical foundation for further studies in this area.
Upon identifying the application scenario for fresh food preservation, the establishment of specific application requirements is crucial for guiding the design of EOR devices. Despite sharing the same ORR–OER coupling mechanism with the EOG system previously discussed, the distinct application scenarios necessitate unique design considerations for the electrochemical O2-removal apparatus. The primary application requirements include: (1) low energy consumption; (2) no pollution; and (3) a high O2-removal rate. These criteria not only guide the design parameters but also shape the operational strategies of EOR devices, ensuring the efficacy and safety in fresh food preservation.
Following the above-established application requirements, comprehensive designs are required for EOR devices. Considering these in order: (1) low energy consumption: the catalytic mechanism employs a 2ET pathway for the coupled ORR–OER electrochemical system with HO2− as an intermediate, consuming half the energy of the 4ET pathway, thereby significantly boosting the energy efficiency; (2) no pollution: given the direct connection between the cathode of the EOR and the sealed space with direct gas exchange taking place, it is necessary to ensure that the operation of the EOR does not result in any contamination of the fruits and vegetables, such as by electrolyte leakage, air outflow, or the dissolution of metal atoms from the catalyst. The choice and design of the catalyst play a crucial role in this process. An ideal catalyst should be non-toxic, exhibit high 2ET-ORR activity and selectivity, and possess excellent stability to avoid electro-corrosion. Single-atom catalysts represent a suitable choice, typically utilizing non-toxic carbon materials as substrates, offering excellent activity and selectivity, and high stability to effectively prevent metal atom dissolution under working potentials, thus avoiding contamination.65–68 Additionally, electrolytes should be eliminated to prevent potential leakage risk. The above-discussed CCM–MEA structure is well suited for this EOR device, as it can efficiently operate with only pure water; (3) a high O2 removal rate: to enhance the efficiency of O2 removal, optimization of the ORR process at the cathode is necessary to increase the current density and reaction rate. The optimizations can be divided into two aspects. First, since the ORR is a three-phase-boundary reaction involving gaseous O2, liquid water, and a solid catalyst, it is essential to ensure that the catalyst is in full contact with both the gaseous and liquid reactants, alongside sustaining efficient mass transfer of these reactants. In the MEA structure, introducing GDEs to the MEA may accelerate the rate of O2 mass transfer.3 Current applicable GDEs typically comprise a current collector layer, a diffusion layer, and a catalyst layer, where the current collector layer possesses high electrical conductivity, sufficient mechanical strength, and a larger pore size and porosity; the diffusion layer is a conductive microporous layer with high hydrophobicity and air permeability; and the catalyst layer employs appropriate binders to achieve uniform catalyst loading. To further improve catalyst utilization, enhancing the wettability of the catalyst layer is suggested, such as by fabricating GDEs with hydrophobic polymer concentration gradient diffusion layers to adjust the balance between the hydrophobicity and hydrophilicity.69 Through these methods, while accommodating the O2 mass transfer and wetting needs of the ORR catalyst, the issue of increased internal resistance due to water evaporation on the ion-exchange membrane can be significantly mitigated. Second, to improve the O2-removal efficiency, a shift of study emphasis is required, targeting ways to facilitate a complete depletion of O2 in air by the cathodic ORR process. Besides increasing the gas pressure to enhance the contact of O2 with the inner layer catalyst, redesign of the cathode flow channels to induce forced convection and turbulence is also required.70 This can be achieved by integrating obstruction structures and using multiple serpentine channels with increased bends, thus ensuring better O2 consumption. Such design alterations can not only enhance the O2-reduction rates but also reduce the operational demands on air pumps and the O2-removal time. In stark contrast to the above-discussed EOG that focused on O2 generation, EOR systems prioritize maximizing the O2 consumption, leading to fundamentally different design strategies for GDEs and for the flow channel of the polar plate to achieve better fruit and vegetable preservation in applications.
After finalizing the structural design of an EOR device, its practical application in fruit, vegetable, or grain storage is essential to validate the effectiveness of the EOR in real conditions. This step is crucial to evaluate the actual EOR performance and gather data for further improvements. Key considerations of the EOR device deployments include managing internal pressure changes of the storage space caused by O2 depletion, and adjusting for variations in O2-removal rates due to fluctuating O2 levels. Necessary adaptations thus include the introduction of pressure balance valves to maintain pressure balance in the conservation space, as well as increasing the gas flow rate to compensate for lower O2 concentrations. These modifications are crucial for optimizing the ultimate performance of EOR in real-world preservation scenarios.
The advantages of EOR devices in fresh food preservation merit extensive exploration, which may offer distinct advantages across the following three primary dimensions. (1) Energy-saving advantage: due to the intrinsic advancement of the electrochemical O2-removal mechanism, EOR devices are inherently more energy efficient compared to traditional N2-replacement equipment; (2) humidity maintenance advantage: the traditional O2-removal strategy often leads to significant humidity loss in the storage space due to the continuous injection of dry N2 airflows, which can adversely affect the freshness of the fruits and vegetables. In contrast, an EOR device can effectively remove O2 while maintaining humidity. It achieves this by removing electrolytes, enabling the device system with pure water loading to naturally humidify the space during gas circulation, thereby helping to preserve the freshness and taste flavour of the stored items; (3) further energy-saving advantages by combining with low-temperature storage: integrating EOR in low-temperature storage environments for fruits and vegetables could lessen the reliance on refrigeration. By creating a low-O2 environment, it would be feasible to maintain produce freshness at relatively higher ambient temperatures, potentially leading to a significant reduction in energy use for cooling purposes. Moreover, the utility of the EOR system could be broadened to encompass the removal of dissolved O2 from water, underscoring its potential to boost industrial efficiency. In critical industrial operations, like boiler water treatment and metal processing, eliminating dissolved O2 is essential for preventing metal corrosion and extending the life of equipment. The adoption of EOR systems in these expanded fields highlights the wide-ranging application prospects of EOR systems in diverse settings.
The development of an EOR system is a comprehensive research process that requires careful consideration of various aspects, including the electrochemical mechanism, catalyst materials, and reactor structure design, for optimal efficiency and safety. Assessing the system's functionality in real-world conditions is vital to ensure it meets specific environmental needs. By efficiently bridging theory and practice, this strategy opens new avenues for applying EOR in food preservation.
Microbial culture and fermentation field: electrochemical O2 concentration control
In modern biotechnology, the application of microorganisms has become a core technology, widely used in diverse fields including food production, pharmaceuticals, and energy generation.71–73 The growth and metabolic activities of microorganisms are critically influenced by the O2 level of the environment. Microorganisms are categorized as aerobic, facultative anaerobic, or strictly anaerobic, depending on their different O2 needs.74 Precise O2 control in microbial fermentation and culture is crucial for optimizing growth, enhancing the bioproduct yields, and directing metabolic pathways towards specific products.75 Building on the deeply discussed ORR–OER coupled electrochemical mechanism above, this section delves into approaches to adapt this mechanism for precise O2 regulation in the applications of microorganism cultivation and fermentation.Traditional methods for environmental O2 regulation typically use N2 and O2 supply systems, controlling O2 levels through gas flow and gas route adjustments, as illustrated in Fig. 4a. However, these methods require separate O2 and N2 gas sources and suffer from low efficiency and poor mobility, as well as being costly and space-consuming. The traditional principle for controlling the O2 concentration lies in injecting pure O2 or N2 into a sealed space to either increase or decrease the O2 level. Electrochemical technology may simplify the above processes by just switching electrode reactions between the OER and ORR, corresponding to the O2-generation process to increase its level, and the O2-consumption process to decease its level, respectively (Fig. 4b). The direct use of conventional ORR–OER systems is, however, limited by the inconvenience of frequent switching between the cathode and anode gas routes and the system connect configuration, reducing the system efficiency and practicality. Developing an effective electrochemical O2 control (EOC) device requires a design focused on precision, user-friendliness, portability, and adaptability to varied environments. This device should offer efficient O2-concentration control, easy operation, and flexible application across different scenarios. The following section details these design requirements and their implementation.
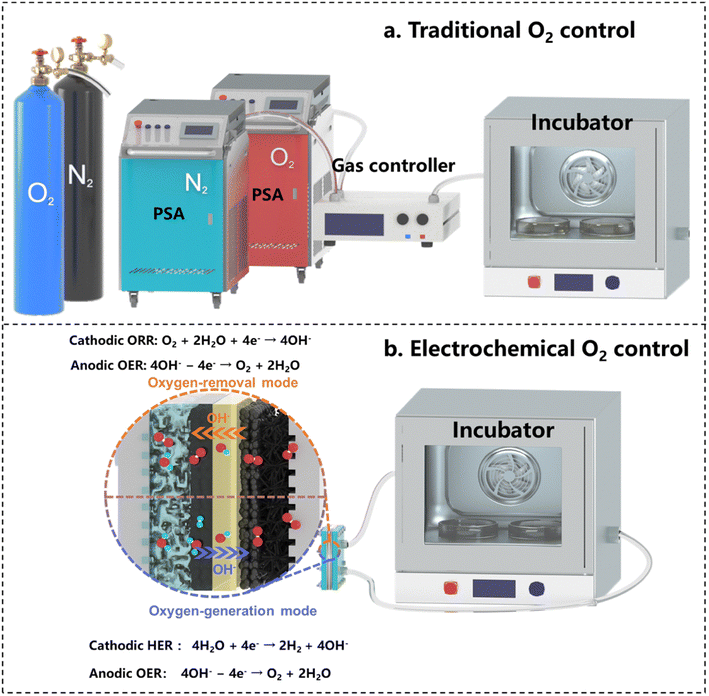 | ||
| Fig. 4 Working mechanisms of different O2-control methods: (a) traditional O2-control method, and (b) electrochemical O2-control method. | ||
For operational portability, the electrochemical system needs redesigning for continuous connection of one electrode to the O2-controlled environment. This would allow seamless switching processes between O2 removal and O2 generation by altering the electrode reactions of the ORR and OER through only altering the voltage directions, without changing any gas routing or device deployment. However, this poses structural challenges for MEA due to the different reaction environments of the two electrodes. Addressing these challenges necessitates a specific design of the reaction mechanism of the EOC to accommodate the MEA structure. A novel approach involves connecting the GDE side of the EOC to a sealed space. In this configuration, the O2-consumption process can be efficiently executed to continuously reduce the O2 concentration within this sealed space. This set-up facilitates the following cathode and anode reactions to promote the O2-removal function:
Cathodic ORR (GDE):
| O2 + 2H2O + 4e− → 4OH− |
Anodic OER (in liquid solution):
| 4OH− − 4e− → O2 + 2H2O |
To increase the O2 level of the sealed space, altering the voltage direction may exchange the roles of the cathode and anode. In this way, the electrode connected to the environment, initially a cathode, becomes an anode to enable the OER, thereby raising O2 levels. The opposite electrode, immersed in a liquid solution, now turns into a cathode. Although this cathode is unsuitable for the ORR due to the lack of a gas-transfer pathway, it may initiate the hydrogen evolution reaction (HER) to couple with the OER. In industrial settings for microbial cultivation and fermentation, exhaust gas-treatment systems allow the hydrogen produced by the HER to be properly managed, making the HER a viable choice in this application. The specific cathode and anode reactions for facilitating the O2-generation function are:
Cathodic HER (in liquid solution):
| 4H2O + 4e− → 2H2 + 4OH− |
Anodic OER (GDE):
| 4OH− − 4e− → O2 + 2H2O |
The design of the reaction mechanism outlined above enables the instantaneous switching functions of O2 generation and O2 removal, significantly optimizing the operational process with just a simple switch in the voltage direction (Fig. 4b). This electrochemical device achieves O2-concentration control through the integration of electrochemical O2 generation and removal functions. Therefore, based on the previous discussions about the energy consumption of electrochemical O2 generation and removal processes, it can be inferred that electrochemical O2 control also has a significant advantage over traditional strategies.
To ensure efficient O2 control performance, careful design of the catalyst is critical. Considering the O2-control mechanism involves the coupling of the ORR, OER, and HER, we need to develop a multifunctional catalyst. This catalyst should be effective in catalyzing these three reactions at different potentials and be suitable for the bipolar use of the electrochemical reactor. Suitable candidates can be selected from dual single-atom catalysts that are reported to have excellent stability, activity, and low toxicity.76,77 The new catalytic mechanism fits perfectly with the gaseous and liquid environments of the two sides in the MEA structure. Finally, by assembling multiple MEA units into a stack, the oxygen-control efficiency can be significantly enhanced.
In specific application scenarios, such as microbial culture and fermentation, detailed modifications to the design of electrochemical O2-regulation devices are indispensable. This entails the integration of pressure balance valves to mitigate pressure fluctuations in the culture chamber attributable to O2-concentration changes. To maintain a sterile environment, it is essential that all gas inlets and outlets be outfitted with bacterial filters, thereby preventing external microbial contamination. Considering the substantial CO2 production from bacterial metabolism, the gas discharged from the chamber should be directed through a CO2-absorption system prior to entering the EOC device, thus protecting the electrochemical device and prolonging its operational life. Moreover, if the EOC device employs an alkaline electrolyte, an alkali absorption unit must be installed to prevent any interference for the microbial culture environment. These customized designs for specific application scenario requirements are crucial for enhancing the compatibility of the EOC with the application environment and improving the overall functionality of the system.
Conclusions
Through an in-depth exploration of applications of the ORR–OER coupled electrochemical system in various research domains, it was demonstrated how the applications of the electrochemistry can be significantly broadened by shifting the research focus from being confined within the reactor to integrating with the surrounding environment. This perspective elaborately details how electrochemical reactors can be functionalized and customized for various application scenarios, encompassing overall design and detail adjustments. While these applications are all based on the same ORR–OER coupled electrochemical mechanism, they diverge into uniquely tailored designs responsive to different application needs. This research approach, centred around application objectives, places greater emphasis on feasibility and adaptability in real-world application scenarios. This shift in research perspective is not merely a technical evolution but also a progression in thinking methodology. It reflects a more comprehensive and systematic approach to problem-solving, particularly underscoring the importance of practical application and interdisciplinary integration. This trend will profoundly influence the field of electrochemistry, driving innovation in technology and diversifying applications. Furthermore, this comprehensive and systematic approach to research may bring new insights to other scientific fields, encouraging researchers to adopt a more integrated perspective, consider the interplay and impact across different disciplines, and thereby foster interdisciplinary collaboration, accelerating development and innovation in science and technology.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Y. W., and Y. Z. conceived and designed this perspective, and cowrite this article.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by National Key R&D Program of China (No. 2021YFA1501003), the Key Technologies R & D Program of Anhui Province (2022a05020053, 2023z04020010), Anhui Provincial Natural Science Foundation (2208085UD04, 2108085UD06), USTC Research Funds of the Double First-Class Initiative (YD2060006005).References
- T. Yan, X. Chen, L. Kumari, J. Lin, M. Li, Q. Fan, H. Chi, T. J. Meyer, S. Zhang and X. Ma, Multiscale CO2 Electrocatalysis to C2+ Products: Reaction Mechanisms, Catalyst Design, and Device Fabrication, Chem. Rev., 2023, 123, 10530–10583 CrossRef CAS PubMed.
- Y. Yang, P. Li, X. Zheng, W. Sun, S. X. Dou, T. Ma and H. Pan, Anion-exchange membrane water electrolyzers and fuel cells, Chem. Soc. Rev., 2022, 51, 9620–9693 RSC.
- C.-T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. García de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton and E. H. Sargent, CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface, Science, 2018, 360, 783–787 CrossRef CAS PubMed.
- Y. Wang, Y. Pang, H. Xu, A. Martinez and K. S. Chen, PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development – a review, Energy Environ. Sci., 2022, 15, 2288–2328 RSC.
- Z.-Z. Niu, L.-P. Chi, R. Liu, Z. Chen and M.-R. Gao, Rigorous assessment of CO2 electroreduction products in a flow cell, Energy Environ. Sci., 2021, 14, 4169–4176 RSC.
- E. W. Lees, B. A. W. Mowbray, F. G. L. Parlane and C. P. Berlinguette, Gas diffusion electrodes and membranes for CO2 reduction electrolysers, Nat. Rev. Mater., 2022, 7, 55–64 CrossRef CAS.
- D. Wakerley, S. Lamaison, J. Wicks, A. Clemens, J. Feaster, D. Corral, S. A. Jaffer, A. Sarkar, M. Fontecave, E. B. Duoss, S. Baker, E. H. Sargent, T. F. Jaramillo and C. Hahn, Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers, Nat. Energy, 2022, 7, 130–143 CrossRef CAS.
- C. Zhao, G. Luo, X. Liu, W. Zhang, Z. Li, Q. Xu, Q. Zhang, H. Wang, D. Li, F. Zhou, Y. Qu, X. Han, Z. Zhu, G. Wu, J. Wang, J. Zhu, T. Yao, Y. Li, H. J. M. Bouwmeester and Y. Wu, In Situ Topotactic Transformation of an Interstitial Alloy for CO Electroreduction, Adv. Mater., 2020, 32, 2002382 CrossRef CAS PubMed.
- A. Ozden, Y. Wang, F. Li, M. Luo, J. Sisler, A. Thevenon, A. Rosas-Hernández, T. Burdyny, Y. Lum, H. Yadegari, T. Agapie, J. C. Peters, E. H. Sargent and D. Sinton, Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene, Joule, 2021, 5, 706–719 CrossRef CAS.
- Y. Zhang, K. Xie, F. Zhou, F. Wang, Q. Xu, J. Hu, H. Ding, P. Li, Y. Tan, D. Li, J. Zhu, H. Zhou, C. Zhao, S. Lin and Y. Wu, Electrochemical Oxygen Generator With 99.9% Oxygen Purity and High Energy Efficiency, Adv. Energy Mater., 2022, 12, 2201027 CrossRef CAS.
- Z. Yin, H. Peng, X. Wei, H. Zhou, J. Gong, M. Huai, L. Xiao, G. Wang, J. Lu and L. Zhuang, An alkaline polymer electrolyte CO2 electrolyzer operated with pure water, Energy Environ. Sci., 2019, 12, 2455–2462 RSC.
- R. Phillips, A. Edwards, B. Rome, D. R. Jones and C. W. Dunnill, Minimising the ohmic resistance of an alkaline electrolysis cell through effective cell design, Int. J. Hydrogen Energy, 2017, 42, 23986–23994 CrossRef CAS.
- K. Jiao, J. Xuan, Q. Du, Z. Bao, B. Xie, B. Wang, Y. Zhao, L. Fan, H. Wang, Z. Hou, S. Huo, N. P. Brandon, Y. Yin and M. D. Guiver, Designing the next generation of proton-exchange membrane fuel cells, Nature, 2021, 595, 361–369 CrossRef CAS PubMed.
- D. Chen, P. Pei, Y. Li, P. Ren, Y. Meng, X. Song and Z. Wu, Proton exchange membrane fuel cell stack consistency: Evaluation methods, influencing factors, membrane electrode assembly parameters and improvement measures, Energy Convers. Manage., 2022, 261, 115651 CrossRef CAS.
- B. Endrődi, E. Kecsenovity, A. Samu, F. Darvas, R. V. Jones, V. Török, A. Danyi and C. Janáky, Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency, ACS Energy Lett., 2019, 4, 1770–1777 CrossRef PubMed.
- Y. Zhang, P. Li, C. Zhao, G. Zhou, F. Zhou, Q. Zhang, C. Su and Y. Wu, Multicarbons generation factory: CuO/Ni single atoms tandem catalyst for boosting the productivity of CO2 electrocatalysis, Sci. Bull., 2022, 67, 1679–1687 CrossRef CAS PubMed.
- F. Si, Y. Zhang, L. Yan, J. Zhu, M. Xiao, C. Liu, W. Xing and J. Zhang, in Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, ed. W. Xing, G. Yin and J. Zhang, Elsevier, Amsterdam, 2014, pp. 133–170, DOI:10.1016/B978-0-444-63278-4.00004-5.
- R. L. Doyle and M. E. G. Lyons, in Photoelectrochemical Solar Fuel Production: From Basic Principles to Advanced Devices, ed. S. Giménez and J. Bisquert, Springer International Publishing, Cham, 2016, pp. 41–104, DOI:10.1007/978-3-319-29641-8_2.
- J. Newman and N. P. Balsara, Electrochemical Systems, John Wiley & Sons, 2021 Search PubMed.
- A. Rao, J. Maclay and S. Samuelsen, Efficiency of electrochemical systems, J. Power Sources, 2004, 134, 181–184 CrossRef CAS.
- G. G. H. T. Force and P. P. E. Directive, Technical Specifications for Pressure Swing Adsorption (PSA) Oxygen Plants, 2020 Search PubMed.
- A. Chandra, U. Chakraborty, J. Pal and P. Karmakar, Silent hypoxia: a frequently overlooked clinical entity in patients with COVID-19, BMJ Case Rep., 2020, 13, e237207 CrossRef PubMed.
- D. M. Halpin, G. J. Criner, A. Papi, D. Singh, A. Anzueto, F. J. Martinez, A. A. Agusti and C. F. Vogelmeier, Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease, Am. J. Respir. Crit. Care Med., 2021, 203, 24–36 CrossRef CAS PubMed.
- A. D. Usher, Medical oxygen crisis: a belated COVID-19 response, Lancet, 2021, 397, 868–869 CrossRef CAS PubMed.
- J.-G. Jee, M.-B. Kim and C.-H. Lee, Pressure swing adsorption processes to purify oxygen using a carbon molecular sieve, Chem. Eng. Sci., 2005, 60, 869–882 CrossRef CAS.
- J. C. Santos, P. Cruz, T. Regala, F. D. Magalhães and A. Mendes, High-Purity Oxygen Production by Pressure Swing Adsorption, Ind. Eng. Chem. Res., 2007, 46, 591–599 CrossRef CAS.
- S. Hayashi, M. Kawai and T. Kaneko, Dynamics of high purity oxygen PSA, Gas Sep. Purif., 1996, 10, 19–23 CrossRef CAS.
- T. Tsuru and S. T. Hwang, Production of high-purity oxygen by continuous membrane column combined with PSA oxygen generator, Ind. Eng. Chem. Res., 1994, 33, 311–316 CrossRef CAS.
- B. O'driscoll, L. Howard, J. Earis and V. Mak, BTS guideline for oxygen use in adults in healthcare and emergency settings, Thorax, 2017, 72, ii1–ii90 CrossRef PubMed.
- C. F. McDonald, Home oxygen therapy, Aust. Prescr., 2022, 45, 21–24 CrossRef PubMed.
- H. Jiang, R. Luo, Y. Li and W. Chen, Recent advances in solid–liquid–gas three-phase interfaces in electrocatalysis for energy conversion and storage, EcoMat, 2022, 4, e12199 CrossRef CAS.
- X. Li and I. Sabir, Review of bipolar plates in PEM fuel cells: Flow-field designs, Int. J. Hydrogen Energy, 2005, 30, 359–371 CrossRef CAS.
- H. Kahraman and M. F. Orhan, Flow field bipolar plates in a proton exchange membrane fuel cell: Analysis & modeling, Energy Convers. Manage., 2017, 133, 363–384 CrossRef CAS.
- R. Boddu, U. K. Marupakula, B. Summers and P. Majumdar, Development of bipolar plates with different flow channel configurations for fuel cells, J. Power Sources, 2009, 189, 1083–1092 CrossRef CAS.
- A. Kumar and R. G. Reddy, Effect of channel dimensions and shape in the flow-field distributor on the performance of polymer electrolyte membrane fuel cells, J. Power Sources, 2003, 113, 11–18 CrossRef CAS.
- X. Chen, Z. Yu, C. Yang, Y. Chen, C. Jin, Y. Ding, W. Li and Z. Wan, Performance investigation on a novel 3D wave flow channel design for PEMFC, Int. J. Hydrogen Energy, 2021, 46, 11127–11139 CrossRef CAS.
- L. Rostami, M. Haghshenasfard, M. Sadeghi and M. Zhiani, A 3D CFD model of novel flow channel designs based on the serpentine and the parallel design for performance enhancement of PEMFC, Energy, 2022, 258, 124726 CrossRef CAS.
- Z. Qin, W. Huo, Z. Bao, C. Tongsh, B. Wang, Q. Du and K. Jiao, Alternating Flow Field Design Improves the Performance of Proton Exchange Membrane Fuel Cells, Advanced Science, 2023, 10, 2205305 CrossRef CAS PubMed.
- Z. Yan, H. Sun, X. Chen, H. Liu, Y. Zhao, H. Li, W. Xie, F. Cheng and J. Chen, Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution, Nat. Commun., 2018, 9, 2373 CrossRef PubMed.
- C. Zhao, Y. Wang, Z. Li, W. Chen, Q. Xu, D. He, D. Xi, Q. Zhang, T. Yuan, Y. Qu, J. Yang, F. Zhou, Z. Yang, X. Wang, J. Wang, J. Luo, Y. Li, H. Duan, Y. Wu and Y. Li, Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction, Joule, 2019, 3, 584–594 CrossRef CAS.
- Y. Li, S. Chen, D. Xi, Y. Bo, R. Long, C. Wang, L. Song and Y. Xiong, Scalable Fabrication of Highly Active and Durable Membrane Electrodes toward Water Oxidation, Small, 2018, 14, 1702109 CrossRef PubMed.
- F. Zhang, R. Zhao, Y. Wang, L. Han, J. Gu, Z. Niu, Y. Yuan, N. Qu, J. Meng and D. Wang, Superwettable Surface-Dependent efficiently electrocatalytic water splitting based on their excellent liquid adsorption and gas desorption, Chem. Eng. J., 2023, 452, 139513 CrossRef CAS.
- A. Xie, B. Yuan, X. Hu, B. Chen, L. Huang, S. Zhu, Y. Qian, D. Wu and S. Luo, NiCo-layered double hydroxides/Co9S8 with heterogeneous structure as ultra-high performance electrocatalyst for oxygen evolution reaction, Int. J. Hydrogen Energy, 2024, 51, 349–361 CrossRef CAS.
- J. Hao, W. Yang, Z. Huang and C. Zhang, Superhydrophilic and Superaerophobic Copper Phosphide Microsheets for Efficient Electrocatalytic Hydrogen and Oxygen Evolution, Adv. Mater. Interfaces, 2016, 3, 1600236 CrossRef.
- H. Zhang, H. Guo, Y. Zhang, J. Zhao, Y. Li, X. Li, J. Ren and R. Song, Metal–Organic Framework-Derived Multidimensional Hierarchical Assembling Body with a Superhydrophilic and Superaerophobic Surface Toward Efficient Electrochemical Overall Water Splitting, ACS Sustain. Chem. Eng., 2022, 10, 6402–6413 CrossRef CAS.
- K.-H. Kim, K.-Y. Lee, H.-J. Kim, E. Cho, S.-Y. Lee, T.-H. Lim, S. P. Yoon, I. C. Hwang and J. H. Jang, The effects of Nafion® ionomer content in PEMFC MEAs prepared by a catalyst-coated membrane (CCM) spraying method, Int. J. Hydrogen Energy, 2010, 35, 2119–2126 CrossRef CAS.
- Y. Zhao, D. P. Adiyeri Saseendran, C. Huang, C. A. Triana, W. R. Marks, H. Chen, H. Zhao and G. R. Patzke, Oxygen Evolution/Reduction Reaction Catalysts: From In Situ Monitoring and Reaction Mechanisms to Rational Design, Chem. Rev., 2023, 123, 6257–6358 CrossRef CAS PubMed.
- L. Chong, G. Gao, J. Wen, H. Li, H. Xu, Z. Green, J. D. Sugar, A. J. Kropf, W. Xu, X.-M. Lin, H. Xu, L.-W. Wang and D.-J. Liu, La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis, Science, 2023, 380, 609–616 CrossRef CAS PubMed.
- H. Wu, Y. Wang, Z. Shi, X. Wang, J. Yang, M. Xiao, J. Ge, W. Xing and C. Liu, Recent developments of iridium-based catalysts for the oxygen evolution reaction in acidic water electrolysis, J. Mater. Chem. A, 2022, 10, 13170–13189 RSC.
- J. Torrero, T. Morawietz, D. García Sanchez, D. Galyamin, M. Retuerto, V. Martin-Diaconescu, S. Rojas, J. A. Alonso, A. S. Gago and K. A. Friedrich, High Performance and Durable Anode with 10-Fold Reduction of Iridium Loading for Proton Exchange Membrane Water Electrolysis, Adv. Energy Mater., 2023, 13, 2204169 CrossRef CAS.
- Z.-Y. Wu, F.-Y. Chen, B. Li, S.-W. Yu, Y. Z. Finfrock, D. M. Meira, Q.-Q. Yan, P. Zhu, M.-X. Chen, T.-W. Song, Z. Yin, H.-W. Liang, S. Zhang, G. Wang and H. Wang, Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis, Nat. Mater., 2023, 22, 100–108 CrossRef CAS PubMed.
- L. Yan, P. Li, Q. Zhu, A. Kumar, K. Sun, S. Tian and X. Sun, Atomically precise electrocatalysts for oxygen reduction reaction, Chem, 2023, 9, 280–342 CAS.
- H. T. Chung, D. A. Cullen, D. Higgins, B. T. Sneed, E. F. Holby, K. L. More and P. Zelenay, Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst, Science, 2017, 357, 479–484 CrossRef CAS PubMed.
- R. Gui, H. Cheng, M. Wang, X. Tai, H. Zhang, C. Liu, X. Cao, C. Chen, M. Ge, H. Wang, X. Zheng, W. Chu, Y. Lin, Y. Xie and C. Wu, Symmetry-Induced Regulation of Pt Strain Derived from Pt3Ga Intermetallic for Boosting Oxygen Reduction Reaction, Adv. Mater., 2024, 36, 2307661 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhao, B. Danil, W. Xiao and X. Yang, Oxygen-Vacancy-Induced Formation of Pt-Based Intermetallics on MXene with Strong Metal-Support Interactions for Efficient Oxygen Reduction Reaction, Adv. Mater., 2024, 2400198 CrossRef PubMed.
- W. Guo, X. Gao, M. Zhu, C. Xu, X. Zhu, X. Zhao, R. Sun, Z. Xue, J. Song, L. Tian, J. Xu, W. Chen, Y. Lin, Y. Li, H. Zhou and Y. Wu, A closely packed Pt1.5Ni1−x/Ni–N–C hybrid for relay catalysis towards oxygen reduction, Energy Environ. Sci., 2023, 16, 148–156 RSC.
- M. Zhang, X. Meng, B. Bhandari, Z. Fang and H. Chen, Recent Application of Modified Atmosphere Packaging (MAP) in Fresh and Fresh-Cut Foods, Food Rev. Int., 2015, 31, 172–193 CrossRef CAS.
- S. Sandhya, Modified atmosphere packaging of fresh produce: Current status and future needs, LWT–Food Sci. Technol., 2010, 43, 381–392 CrossRef.
- A. A. Kader, D. Zagory, E. L. Kerbel and C. Y. Wang, Modified atmosphere packaging of fruits and vegetables, Crit. Rev. Food Sci. Nutr., 1989, 28, 1–30 CrossRef CAS PubMed.
- O. Couvert, L. Koullen, A. Lochardet, V. Huchet, J. Thevenot and Y. Le Marc, Effects of carbon dioxide and oxygen on the growth rate of various food spoilage bacteria, Food Microbiol., 2023, 114, 104289 CrossRef CAS PubMed.
- F. Liu and Y. Li, Storage characteristics and relationships between microbial growth parameters and shelf life of MAP sliced onions, Postharvest Biol. Technol., 2006, 40, 262–268 CrossRef CAS.
- S. Ali, A. S. Khan, A. U. Malik and M. Shahid, Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits, Food Chem., 2016, 206, 18–29 CrossRef CAS PubMed.
- R. Lakakul, R. M. Beaudry and R. J. Hernandez, Modeling Respiration of Apple Slices in Modified-Atmosphere Packages, J. Food Sci., 1999, 64, 105–110 CrossRef CAS.
- L. Torregrosa, G. Echeverria, J. Illa and J. Giné-Bordonaba, Emission of VOCs and quality evolution in response to repeated oxygen pull downs on ‘Conference’ pears during long-term cold storage, Postharvest Biol. Technol., 2020, 170, 111322 CrossRef CAS.
- P. V. Chinh, N. T. Hieu, V. D. Tien, T.-Y. Nguyen, H. N. Nguyen, N. T. Anh and D. V. Thom, Simulation and Experimental Study of a Single Fixed-Bed Model of Nitrogen Gas Generator Working by Pressure Swing Adsorption, Processes, 2019, 7, 654 CrossRef.
- Y. Wang, R. Shi, L. Shang, G. I. N. Waterhouse, J. Zhao, Q. Zhang, L. Gu and T. Zhang, High-Efficiency Oxygen Reduction to Hydrogen Peroxide Catalyzed by Nickel Single-Atom Catalysts with Tetradentate N2O2 Coordination in a Three-Phase Flow Cell, Angew. Chem., Int. Ed., 2020, 59, 13057–13062 CrossRef CAS PubMed.
- K. Jiang, S. Back, A. J. Akey, C. Xia, Y. Hu, W. Liang, D. Schaak, E. Stavitski, J. K. Nørskov, S. Siahrostami and H. Wang, Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination, Nat. Commun., 2019, 10, 3997 CrossRef PubMed.
- C. Tang, L. Chen, H. Li, L. Li, Y. Jiao, Y. Zheng, H. Xu, K. Davey and S.-Z. Qiao, Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres, J. Am. Chem. Soc., 2021, 143, 7819–7827 CrossRef CAS PubMed.
- Y. Wan, D. Qiu, P. Yi, L. Peng and X. Lai, Design and optimization of gradient wettability pore structure of adaptive PEM fuel cell cathode catalyst layer, Appl. Energy, 2022, 312, 118723 CrossRef CAS.
- D. Yu and S. Yu, Analysis of Flow Variation in a Straight Channel with Baffled Obstacles on a Bipolar Plate in a Proton-Exchange Membrane Fuel Cell, Int. J. Automot. Technol., 2023, 24, 759–771 CrossRef.
- P. Morales, V. Rojas, M. Quirós and R. Gonzalez, The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture, Appl. Microbiol. Biotechnol., 2015, 99, 3993–4003 CrossRef CAS PubMed.
- W. Sun, M. H. Shahrajabian and M. Lin, Research Progress of Fermented Functional Foods and Protein Factory-Microbial Fermentation Technology, Fermentation, 2022, 8, 688 CrossRef CAS.
- X. Liang, C. Li, W. Cao, W. Cao, F. Shen and Y. Wan, Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode, Molecules, 2021, 26, 3867 CrossRef CAS PubMed.
- B. Geinitz, A. Hüser, M. Mann and J. Büchs, Gas Fermentation Expands the Scope of a Process Network for Material Conversion, Chem. Ing. Tech., 2020, 92, 1665–1679 CrossRef CAS.
- H. Xu, W. Dou, H. Xu, X. Zhang, Z. Rao, Z. Shi and Z. Xu, A two-stage oxygen supply strategy for enhanced l-arginine production by Corynebacterium crenatum based on metabolic fluxes analysis, Biochem. Eng. J., 2009, 43, 41–51 CrossRef CAS.
- M. Zhao, R. Yang, Y. Wei, J. Su, X. Wang, N. Zhang, P. Sun, D. Chen and Y. Zhao, Dual isolated bimetal single-atom catalysts for tumor ROS cycle and parallel catalytic therapy, Nano Today, 2022, 44, 101493 CrossRef CAS.
- Y. Fan, S. Liu, Y. Yi, H. Rong and J. Zhang, Catalytic Nanomaterials toward Atomic Levels for Biomedical Applications: From Metal Clusters to Single-Atom Catalysts, ACS Nano, 2021, 15, 2005–2037 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc06983d |
| This journal is © The Royal Society of Chemistry 2024 |