Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A focus on applying 63/65Cu solid-state NMR spectroscopy to characterize Cu MOFs
Zhenfeng
Pang
 and
Kong Ooi
Tan
and
Kong Ooi
Tan
 *
*
Laboratoire des Biomolécules, LBM, Département de Chimie, École Normale Supérieure, PSL University, Sorbonne Université, CNRS, 75005 Paris, France. E-mail: kong-ooi.tan@ens.psl.eu
First published on 16th April 2024
Abstract
Metal–organic frameworks (MOFs) are a class of hybrid organic and inorganic porous materials that have shown prospects in applications ranging from gas storage, separation, catalysis, etc. Although they can be studied using various characterization techniques, these methods often do not provide local structural details that help explain their functionality. Zhang et al. (W. Zhang, B. E. G. Lucier, V. V. Terskikh, S. Chen and Y. Huang, Chem. Sci., 2024, https://doi.org/10.1039/D4SC00782D) have recently exploited 63/65Cu solid-state NMR spectroscopy (for the first time) and DFT calculations to elucidate the structures of Cu(I) centers in MOFs. While there are still many challenges in overcoming issues in resolution and sensitivity, this work lays the foundation for further development of solid-state NMR technology in characterizing copper in MOFs or other amorphous solids.
Metal–organic frameworks (MOFs) are a novel class of materials that have gained popularity in recent decades due to their highly porous structures and exceptional tunability, making them attractive candidates for diverse applications such as gas storage, separation, and catalysis.1 A critical factor influencing their functional properties is the incorporation of metal centers within the MOF structure. Because of the unique properties of Cu+ or Cu2+, copper-based MOFs have shown uniquely high photocatalytic activity,2 enhanced luminescence properties,3 and promising biomedical applications.4 Copper can adopt various coordination environments within an MOF, such as two-coordinate linear, three-coordinate trigonal planar, or four-coordinate tetrahedral. The coordination number plays a crucial role in determining the electrical conductivity, structural stability, and reactivity of the MOF. Characterization of these copper centers is challenging, especially for Cu(I), because, unlike Cu(II), Cu(I) is ‘invisible’ to EPR or UV-vis spectroscopy. Moreover, other spectroscopic techniques, such as powder X-ray diffraction, energy dispersive spectroscopy (EDS), etc., often do not yield high-resolution information on local structural details of the metal centers. Hence, Zhang et al. have exploited 63/65Cu solid-state nuclear magnetic resonance (NMR) spectroscopy, a powerful method of choice to extract site-specific information of these copper environments in MOFs [(https://doi.org/10.1039/D4SC00782D)5 and ref. 6].
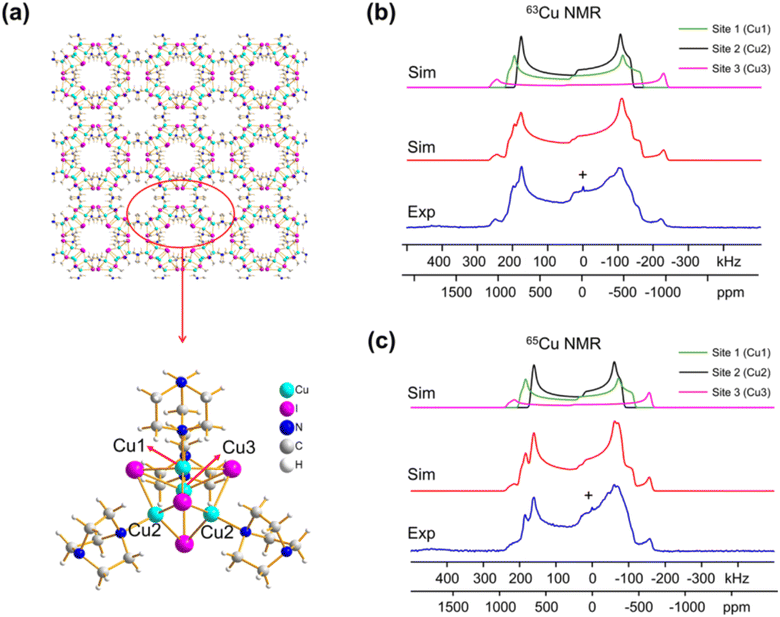
Despite the promising aspects, 63/65Cu NMR is not commonly employed, mainly because 63Cu and 65Cu are both spin-3/2 particles that possess quadrupolar interactions that are often too large to be averaged by the magic-angle spinning (MAS) technique.7 Hence, their NMR spectra are very broad (>50 kHz) and the poor resolution usually limits the application of 63/65Cu NMR to materials with a single site. Although it can be applied to materials with multiple well-defined sites, the results are highly dependent on the quality of spectra fitting, and the conclusions are sometimes subjective or debatable. Moreover, the broad NMR spectra also inherently result in poor NMR sensitivity, which also limits its use to mostly simple 1D NMR experiments. Nevertheless, the linewidths of the 63/65Cu NMR spectra performed under static (non-spinning) conditions are primarily determined by chemical-shift anisotropy (CSA) and electric-field gradient (EFG) tensors, which contain rich structural information. Zhang et al. have meticulously performed high-field (21.1 T) NMR experiments and density functional theory (DFT) calculations to extract the CSA and EFG tensors of 13 different Cu MOFs. For instance, they showed that the experimental 63/65Cu NMR spectra of [Cu4I4(DABCO)2] (Fig. 1) can be very well simulated using the fitted NMR interactions. Moreover, it was shown that the three different Cu sites in the MOF can be remarkably well distinguished, which is a non-trivial task. The experimental NMR data were effectively combined with DFT calculations, so that specific NMR parameters could be confidently assigned to specific copper sites within the MOF structure. This synergy between experiment and theory provides a powerful approach for a comprehensive understanding of the copper environment.
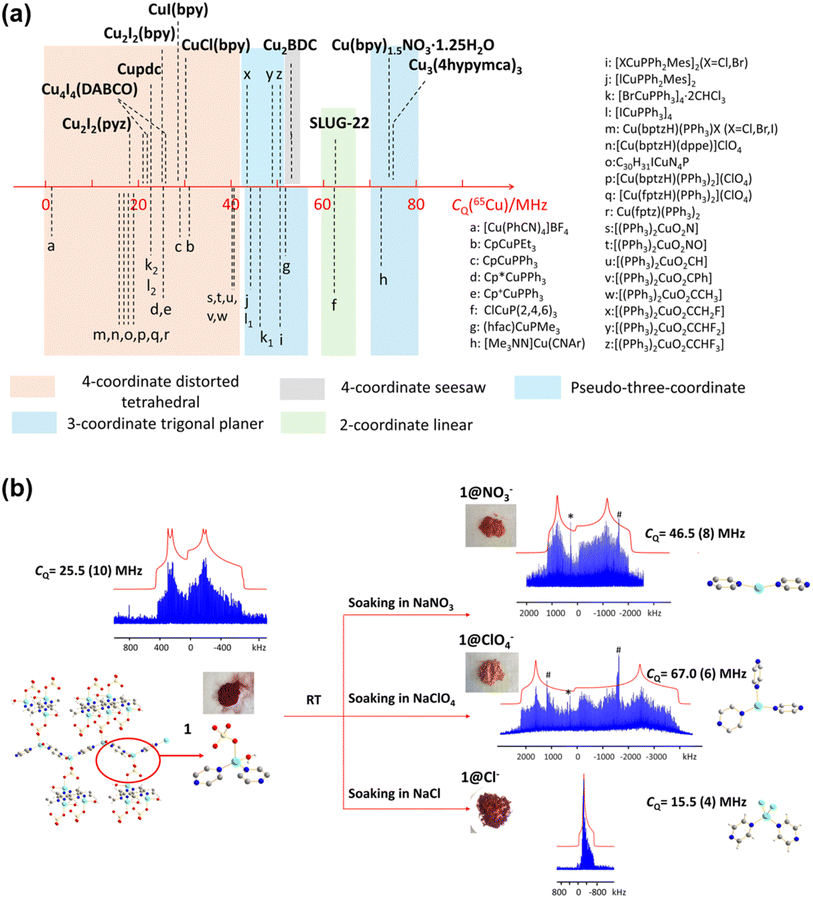
Moreover, Zhang et al. also provided a general tool (Fig. 2a) for estimating the chemical environments of Cu(I) via their quadrupolar coupling constants (CQ). This allows them to elucidate the structural change in a Cu MOF participating in an anion exchange reaction. Fig. 2b shows that the CQ in the Cu4I4(DABCO)2 MOF has increased significantly when the MOF is soaked in NaNO3 or NaClO4 solutions. Using the results obtained earlier (Fig. 2a), it was inferred that the Cu(I) center has transitioned from a distorted tetrahedral configuration to either a two- or three-coordinate structure. The results were then compared with PXRD measurements performed on independently synthesized samples, and it was concluded that the connectivities are similar but not identical.
Although the application of solid-state NMR in characterizing ultra-wideline (UW) nuclei still faces challenges due to poor NMR sensitivity and resolution, we are optimistic that new NMR technologies, i.e., ultra-high-field NMR and hyperpolarization, will help circumvent these issues. It is known that ultra-high-field NMR is exceptionally beneficial in characterizing half-integer quadrupolar nuclei (e.g., 63/65Cu, 47/49Ti, 95Mo, 91Zr, 33S, 67Zn, etc.) because it grants higher-resolution spectra, in addition to higher sensitivity due to the larger Boltzmann population. The latter feature is due to the fact that the linewidth of the NMR central transitions is inversely proportional to the B0 magnetic field.7 These advantages have recently been exploited to study 95Mo, 33S, and 67Zn using either a commercially available 28.2 T magnet or the world’s highest-field 35.2 T magnet available in the US national facility (MagLab).8–10 On the other hand, dynamic nuclear polarization (DNP) is an NMR sensitivity enhancement technique that can usually boost the NMR signals by several orders of magnitude.11–13
In conclusion, Zhang et al. have shown that 63/65Cu NMR spectroscopy is a powerful tool for probing the copper environments within MOFs. By offering site-specific information about the coordination number and geometry of copper centers, it provides crucial insights into the factors governing the properties of MOFs. While challenges remain in overcoming signal broadening, sensitivity limitations, and the need for strategic isotopic enrichment, ongoing advancements in NMR technology, data-processing methods, and integration with other techniques hold immense promise for pushing the boundaries of 63/65Cu NMR spectroscopy and further enhancing our understanding of copper-based MOFs. This comprehensive understanding will ultimately pave the way for the rational design of MOFs with tailored properties for specific applications. Moreover, this NMR method could be extended to many different fields involving Cu(I) species, such as catalysis, surface chemistry, solar cells, and biochemistry.
Author contributions
Z. P. and K. O. T. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by funding obtained from the French National Research Agency (HFPulsedDNP, ANR-21-CE29-0019), RESPORE (no. 339299), and Junior Fellows PSL 2022 (no. 2022-306).References
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- D. Shi, R. Zheng, M. Sun, X. Cao, C. Sun, C. Cui, C. Liu, J. Zhao and M. Du, Angew. Chem., 2017, 129, 14829 CrossRef.
- L. Chen, X. Chen, R. Ma, K. Lin, Q. Li, J.-P. Lang, C. Liu, K. Kato, L. Huang and X. Xing, J. Am. Chem. Soc., 2022, 144, 13688 CrossRef CAS PubMed.
- M. Al Sharabati, R. Sabouni and G. A. Husseini, Nanomaterials, 2022, 12, 277 CrossRef CAS PubMed.
- W. Zhang, B. E. G. Lucier, V. V. Terskikh, S. Chen and Y. Huang, Chem. Sci., 2024 10.1039/D4SC00782D.
- B. E. G. Lucier, W. Zhang, A. Sutrisno and Y. Huang, A Review of Exotic Quadrupolar Metal NMR in MOFs A Review of Exotic Quadrupolar Metal NMR in MOFs, Elsevier Inc., 3rd edn, 2022 Search PubMed.
- R. W. Schurko, Acc. Chem. Res., 2013, 46, 1985 CrossRef CAS PubMed.
- Z. J. Berkson, R. Zhu, C. Ehinger, L. Lätsch, S. P. Schmid, D. Nater, S. Pollitt, O. V. Safonova, S. Björgvinsdóttir, A. B. Barnes, Y. Román-Leshkov, G. A. Price, G. J. Sunley and C. Copéret, J. Am. Chem. Soc., 2023, 145, 12651 CrossRef CAS PubMed.
- A. J. Stirk, S. T. Holmes, F. E. S. Souza, I. Hung, Z. Gan, J. F. Britten, A. W. Rey and R. W. Schurko, CrystEngComm, 2024, 26, 1219 RSC.
- E. Bellan, F. Maleki, M. Jakoobi, P. Fau, K. Fajerwerg, D. Lagarde, A. Balocchi, P. Lecante, J. Trébosc, Y. Xu, Z. Gan, L. Pautrot-D'Alençon, T. Le Mercier, H. Nagashima, G. Pacchioni, O. Lafon, Y. Coppel and M. L. Kahn, J. Phys. Chem. C, 2023, 127, 17809 CrossRef CAS.
- A. J. Rossini, A. Zagdoun, M. Lelli, A. Lesage, C. Copéret and L. Emsley, Acc. Chem. Res., 2013, 46, 1942 CrossRef CAS PubMed.
- P. Berruyer, L. Emsley and A. Lesage, eMagRes, 2018, 7, 93 Search PubMed.
- K. O. Tan, L. Yang, M. Mardini, C. Boon Cheong, B. Driesschaert, M. Dincă and R. G. Griffin, Chem.–Eur. J., 2022, 28, e202202556 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |