Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
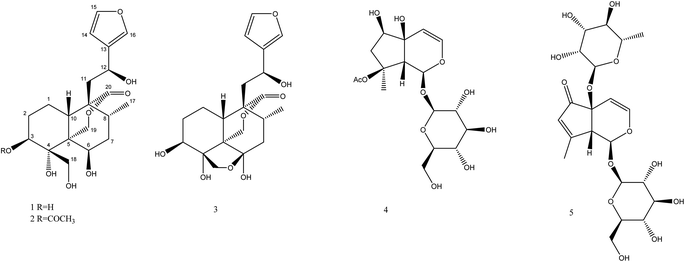
Fatimanols Y and Z: two neo-clerodane diterpenoids from Teucrium yemense†
Ahmed Elbermawi *ab,
Fazila Zulfiqarb,
Ikhlas A. Khanb and
Zulfiqar Alib
*ab,
Fazila Zulfiqarb,
Ikhlas A. Khanb and
Zulfiqar Alib
aDepartment of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura, 35516, Egypt. E-mail: asbeder@mans.edu.eg; Tel: +20 10-0481-1533
bNational Center for Natural Products Research, School of Pharmacy, University of Mississippi, University, MS 38677, USA
First published on 20th October 2023
Abstract
Teucrium yemense (Defl.), a medicinal plant, grows in Yemen and Saudi Arabia and is also referred to as Reehal Fatima. The plant has a long history of use in these regions for the treatment of diabetes, rheumatism, and renal conditions. Phytochemical investigation of the aerial parts of T. yemense yielded two previously undescribed neo-clerodane diterpenoids, namely fatimanols Y and Z (1 and 2) along with the known teulepicephin (3), 8-acetylharpagide (4) and teucardosid (5). Structure elucidation was accomplished from their 1D and 2D NMR, ECD, and MS characteristics as well as by comparing them to related reported compounds. The new molecules expand understanding of secondary metabolites of this genus. Compounds 1–5 did not show antimicrobial activity against various bacterial and fungal strains.
1. Introduction
Teucrium L. (Lamiaceae), commonly known as germanders, is a cosmopolitan genus of about 300 species mainly distributed in South and Central America, Southern Asia, and the Middle East but predominantly prevalent in the Mediterranean basin. Plants in this genus are generally perennial, herbs or shrubs, and the corollas are mostly white to cream-colored with characteristic reduced upper lips.1,2 Teucrium species have been used traditionally as diuretic, diaphoretic, antipyretic, and antiseptic agents for centuries in many parts of the world.3 Several biological activities such as anthelmintic, insecticide, antiulcer, antispasmodic, analgesic, antioxidant, anti-inflammatory, antifeedant, and antimicrobial have been related to Teucrium.4–7 In Egypt, Teucrium is used as an appetizer, expectorant, and hypoglycemic.8 About 300 compounds including flavonoids, terpenoids, iridoids, steroids, phenylethanoids and mainly diterpenoids have been reported from different species of Teucrium. The Teucrium genus is a rich source of diterpenoids, particularly neo-clerodanes which are used as chemotaxonomic markers for Teucrium species. More than 220 diterpenes have been described so far from Teucrium.9Teucrium yemense (Defl.), commonly known as Reehal Fatima, is a therapeutic plant that is frequently grown in Yemen and Saudi Arabia. The plant has a long history of use in these areas for the treatment of diabetes, rheumatism, and renal ailments.10–12 Over thirty neo-clerodane diterpene derivatives from this species have been identified and four of them showed potential antidiabetic activity.2,13,14 Based on the aforementioned facts, the aerial parts of T. yemense were selected to explore further chemical investigation.
2. Result and discussion
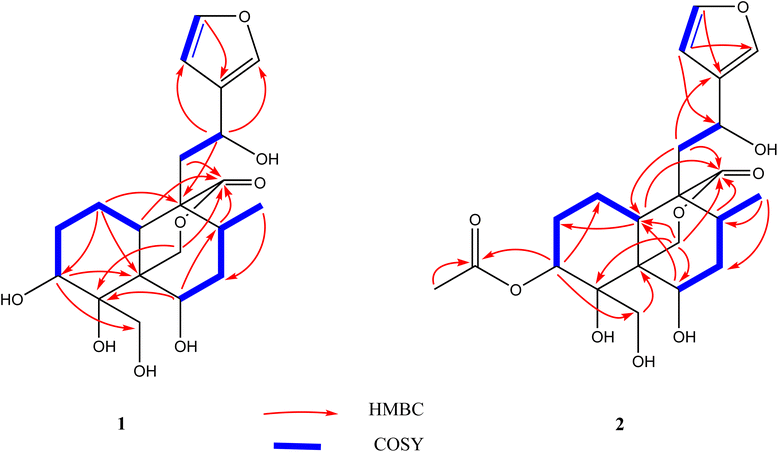
Using a combination of chromatographic techniques, five compounds (1–5) (Fig. 1) were isolated from the methanolic extract of the aerial parts of T. yemense. Compounds 1 and 2 (fatimanols Y and Z) were previously undescribed and were identified as (12S)-15,16-epoxy-3β,4α,6β,12-tetrahydroxy-18-hydroxy-neo-cleroda-13(16),14-dien-20,19-olide and (12S)-15,16-epoxy-3β-acetyl-4α,6β,12-tetrahydroxy-18-hydroxy-neo-cleroda-13(16),14-dien-20,19-olide, respectively, based on 1D and 2D NMR spectroscopic and mass spectral data.Compound 1 was obtained as colorless gum with a molecular formula C20H28O8 deduced on the basis an [M–H]− ion peak in the HRESIMS at m/z 395.1702 (calcd m/z 395.1711) and the number of carbon resonances in the DEPTQ-135 spectrum. The NMR data showed resonances, typical for a furanyl moiety [δH/C 6.49 (d, 1.9 Hz)/109.7 (CH-14), 7.47 (t, 1.7 Hz)/144.6 (CH-15), and 7.49 (d, 1.6 Hz)/139.9 (CH-16) and δC 132.1 (C-13)], a methyl [δH/C 0.80 (d, 6.8 Hz)/16.8 (CH3-17)], three oxy-methines [δH/C 4.06/72.9 (CH-3), 4.15/69.4 (CH-6), and 4.86/63.3 (CH-12)], two isolated oxy-methylenes [δH/C 3.96, 4.05/59.9 (CH2-18), and 3.97, 4.74/74.5 (CH2-19)], an ester carbonyl [δC 175.8 (C-20)], and an oxy-nonprotonated carbon [δC 77.3 (C-4)]. Besides, resonances in the aliphatic region for four methylenes, two methines, and two quaternary carbons were also observed. Based on the NMR data (Table 1), a neo-clerodane diterpenoid skeleton was assumed for 1. The presence of two isolated oxy-methylenes (CH2-18 and CH2-19)] and a carbonyl (C-20) indicated that three methyl groups of neo-clerodane were oxidized. The locations of oxygenated methines were supported by the HMBC correlations of H-12 (δH 4.86) with C-14 (δC 109.7), C-16 (δC 139.9), and C-9 (δC 51.3); H-3 (δH 4.06) with C-4 (δC 77.3), C-5 (δC 46.1), and C-18 (δC 59.9); and H-6 (δH 4.15) with C-4 (δC 77.3), C-5 (δC 46.1), and C-8 (δC 30.2) (Fig. 2). The C-18 and C-19 oxy-methylenes as well as non-protonated oxycarbon C-4 were confirmed by the HMBC correlations of H2-18 with C-3, C-4, and C-5 and H2-19 with C-4 and C-5. The HMBC correlations of H2-19 (oxy-methylene), H2-11, and H-10 with carbonyl (δC 175.8) supported five-membered lactone (C-5–C-19–O–C-20–C-9–C-10).
| Compound 1 | Compound 2 | |||
|---|---|---|---|---|
| Position | δC | δHa mult. (J in Hz) | δC | δHa mult. (J in Hz) |
| a Multiplicity is not clear for some signals due to overlapping. | ||||
| 1 | 25.3 | 1.12 m | 24.9 | 1.19 m |
| 2.44 dq (13.1, 3.8) | 2.48 dq (13.5, 2.5) | |||
| 2 | 29.9 | 1.46 qd (13.1, 3.9) | 27.6 | 1.71 |
| 1.82 | 1.87 | |||
| 3 | 72.9 | 4.06 dd (12.6, 4.6) | 75.9 | 5.28 dd (12.7, 4.8) |
| 4 | 77.3 | 76.3 | ||
| 5 | 46.1 | 46.7 | ||
| 6 | 69.4 | 4.15 br. t (2.9) | 69.2 | 4.20 br. t (2.8) |
| 7 | 37.5 | 1.63 ddd (14.9, 12.8, 2.5) | 37.6 | 1.63 |
| 1.83 | 1.82 | |||
| 8 | 30.2 | 2.59 m | 30.2 | 2.64 m |
| 9 | 51.3 | 51.2 | ||
| 10 | 35.8 | 2.93 dd (13.1, 4.5) | 35.8 | 3.04 dd (13.0, 4.4) |
| 11 | 37.2 | 2.03 dd (15.8, 7.3) | 37.2 | 2.06 |
| 2.37 dd (15.8, 3.4) | 2.38 dd (15.8, 3.3) | |||
| 12 | 63.3 | 4.86 | 63.3 | 4.86 |
| 13 | 132.1 | 132.1 | ||
| 14 | 109.7 | 6.49 d (1.9) | 109.7 | 6.50 dd (2.0, 0.9) |
| 15 | 144.6 | 7.47 br. t (1.7) | 144.6 | 7.48 br. t (1.7) |
| 16 | 139.9 | 7.49 br. s | 139.9 | 7.50 d (1.6) |
| 17 | 16.8 | 0.80 d (6.8) | 16.8 | 0.82 d (6.8) |
| 18 | 59.9 | 3.96 d (11.5) | 61.4 | 3.91 d (11.7) |
| 4.05 d (11.5) | 4.10 d (11.7) | |||
| 19 | 74.5 | 3.97 d (13.8) | 74.3 | 4.03 d (13.9) |
| 4.74 d (13.8) | 4.77 d (13.9) | |||
| 20 | 175.8 | 175.4 | ||
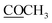 |
172.5 | |||
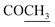 |
21.2 | 2.11 s | ||
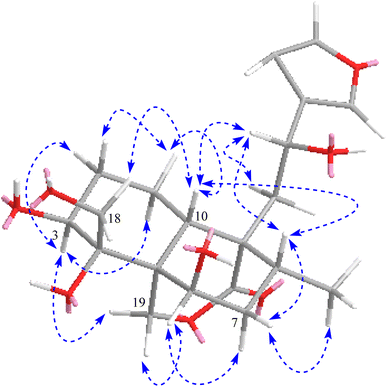
The absolute configuration at C-12 was determined to be S due to the negative cotton effect at 242–260 nm in the experimental ECD spectrum of 1 (Fig. S34†) as it had been reported so by Aydoğan et al. based on the experimental and calculated ECD data of teusandrin H.15 The relative stereochemistry of the other chiral points was determined based on the NOESY correlations (Fig. 3) and characteristic coupling constant values. The characteristic larger coupling constant (13.1 Hz) exhibited by biogenetically β-faced H-10 with H-1ax supported its axial orientation 10S configuration. Similarly, the larger coupling constant value of H-3 (12.6 Hz) with H-2ax and smaller coupling constant value of H-6 (br. t, 2.9 Hz) with H-7ax/eq supported the axial and equatorial orientations of H-3 and H-6, respectively, which ultimately revealed equatorially oriented OH-3 and axially orientated OH-6 with 3S and 6R configurations. The NOESY correlations of H-10ax with H-8ax/H-12/H-18 revealed their co-faced orientations with 8R, 12S, 4R configurations. Similarly, the NOESY correlation of H-3ax with H-1ax/H-19, and H-6 with H-19/H-7ax confirmed their co-faced assimilation on the other side of the plane and eventually supported 5R and 9R configurations (Fig. 3). The NMR data of 1 were comparable to those of teuluteumin A except for the missing resonances of methoxy group.16 Ultimately, compound 1 was elucidated as (3S,4R,5R,6R,8R,9R,10S,12S)-15,16-epoxy-3,4,6,12-tetrahydroxy-18-hydroxy-neo-cleroda-13(16),14-dien-20,19-olide and named fatimanol Y.
Compound 2 exhibited an [M–H]− ion peak in the HRESIMS at m/z 437.1816 (calcd for C22H29O9, 437.1817) corresponding to the molecular formula of C22H30O9. The NMR data of 2 was comparable to 1 except for the additional resonances [δH/C 2.11/21.2 (CH3) and δC 172.5 (carbonyl)] of an acetyl group in 2. The acetyl group was located as an acetoxy group at C-3 based on the HMBC correlations of H-3 (δH 5.28) and methyl group (δH 2.11) with carbonyl (δC 172.5). The complete assignment of 1H and 13C NMR resonances was accomplished by HSQC, COSY, and HMBC spectroscopic data. Based on the ECD spectrum (Fig. S35†), the NOESY correlations (Fig. 3), and characteristic coupling constant values, the stereogenic centers of 2 were defined similarly as described for compound 1. Thus compound 2 was elucidated as (3S,4R,5R,6R,8R,9R,10S,12S)-15,16-epoxy-3 acetyl-4,6,12-tetrahydroxy-18-hydroxy-neo-cleroda-13(16),14-dien-20,19-olide and named fatimanol Z.
Based on the NMR and HRESIMS data analysis as well as by comparing with the literature data, the known compounds were identified as teulepicephin (3),2 8-acetylharpagide (4),17 teucardosid (5).18
All isolates were screened for in vitro antimicrobial activities. None of the isolated metabolites showed significant antimicrobial activity (up to 20 μg mL−1) against Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, methicillin-resistant Staphylococcus aureus (MRS), Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia and Enterococcus faecium (VRE).
3. Material and methods
3.1. General experimental procedure
Optical rotations were measured in MeOH using AUTOPOL II Automatic Polarimeter (Rudolph, Hackettstown, NJ, USA). ECD spectra were collected using Olis DSM 20 CD digital spectropolarimeter (Bogart, GA, USA). IR spectra were determined on an Agilent Technologies Cary 630 FTIR. UV spectra were measured on a Thermo Scientific Evolution 201 UV-visible spectrophotometer. NMR experiments were carried out on a Bruker Avance III 400 MHz spectrometer using CD3OD as a solvent and methanol residue signals were used as the internal standard. An Agilent Technologies 6200 series mass spectrometer was employed to acquire mass data. Column chromatography (CC) was performed over flash silica gel (SiliaFlashV®P60, SiliCycle Inc., USA). Analytical TLC was carried out on silica gel F254 aluminum sheet (20 cm × 20 cm, SiliCycle, Canada) or reversed phase C-18 aluminum sheet (20 cm × 20 cm, Sorbent Tech., USA). The detection of the spots was made possible by visualization under UV-254 nm and by spraying with 1% vanillin in H2SO4–EtOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90), followed by heating. Analytical grade solvents (Fischer chemicals) were used for the isolation and purification procedures.
90), followed by heating. Analytical grade solvents (Fischer chemicals) were used for the isolation and purification procedures.
3.2. Plant material
Teucrium yemense (Defl.) aerial parts were collected from Abha, Saudi Arabia in March 2013. The plant identity was confirmed by a taxonomist at the College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The plant material was air-dried in shade at room temperature. A voucher specimen, coded Ty/018, was kept in the Pharmacognosy Department, Faculty of Pharmacy, Mansoura University.3.3. Extraction and isolation
The powdered air-dried aerial parts (280 g) were extracted by maceration with aqueous MeOH (90%) at room temperature. The dried extract (30 g), obtained on the removal of the solvent by rotary evaporator, was subjected to vacuum liquid chromatography (VLC) over reversed phase C-18 silica gel (30 cm × 5 cm), eluted firstly with 100% H2O, then H2O–MeOH 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, followed by increasing the MeOH proportions by 10% till 100% MeOH. Fraction eluted with H2O–MeOH 90
10, followed by increasing the MeOH proportions by 10% till 100% MeOH. Fraction eluted with H2O–MeOH 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 & 80
10 & 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 were mixed (2.7 g) and chromatographed over silica gel column (4 cm × 120 cm), eluted with DCM–MeOH 95
20 were mixed (2.7 g) and chromatographed over silica gel column (4 cm × 120 cm), eluted with DCM–MeOH 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (4 L) and 90
5 (4 L) and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (4 L) to purify compounds 3 (5.6 mg), 4 (124 mg), and 5 (80 mg). Fraction eluted with H2O–MeOH 70
10 (4 L) to purify compounds 3 (5.6 mg), 4 (124 mg), and 5 (80 mg). Fraction eluted with H2O–MeOH 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (1.9 g) was subjected to repeated column chromatography [silica gel (3 cm × 120 cm), eluted with EtOAc–DCM–MeOH–H2O 15
30 (1.9 g) was subjected to repeated column chromatography [silica gel (3 cm × 120 cm), eluted with EtOAc–DCM–MeOH–H2O 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (4 L) and [silica gel (2 cm × 120 cm), eluted with DCM–MeOH 9
0.5 (4 L) and [silica gel (2 cm × 120 cm), eluted with DCM–MeOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 L) to obtain compound 1 (29 mg). Fraction eluted with H2O–MeOH 60
1 (2 L) to obtain compound 1 (29 mg). Fraction eluted with H2O–MeOH 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (1.3 g) was subjected to repeated column chromatography [silica gel (3 cm × 120 cm), eluted with EtOAc–DCM–MeOH 15
40 (1.3 g) was subjected to repeated column chromatography [silica gel (3 cm × 120 cm), eluted with EtOAc–DCM–MeOH 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (3 L) and [silica gel (2 cm × 120 cm), eluted with DCM–MeOH 9
0.5 (3 L) and [silica gel (2 cm × 120 cm), eluted with DCM–MeOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 L) to obtain compound 2 (22 mg).
1 (2 L) to obtain compound 2 (22 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 210 (6.5) nm; IR υ 3378, 2935, 1701, 1202, 875 cm−1, 1H and 13C NMR data, see Table 1; HRESIMS m/z 395.1702 [M–H]− (calcd for C20H27O8, 395.1711).
ε) 210 (6.5) nm; IR υ 3378, 2935, 1701, 1202, 875 cm−1, 1H and 13C NMR data, see Table 1; HRESIMS m/z 395.1702 [M–H]− (calcd for C20H27O8, 395.1711).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 211 (3.7) nm IR υ 3403, 2965, 1720, 1239, 1049 cm−1, 1H and 13C NMR data see Table 1; HRESIMS m/z 437.1816 [M–H]− (calcd for C22H29O9, 437.1817).
ε) 211 (3.7) nm IR υ 3403, 2965, 1720, 1239, 1049 cm−1, 1H and 13C NMR data see Table 1; HRESIMS m/z 437.1816 [M–H]− (calcd for C22H29O9, 437.1817).3.4. In vitro antimicrobial activity
The antimicrobial activity of the isolated compounds was evaluated against Candida albicans, ATCC 90028, Aspergillus fumigatus ATCC 204305, Cryptococcus neoformans ATCC 90113, methicillin-resistant Staphylococcus aureus ATCC 1708 (MRS), Escherichia coli ATCC 2452, Pseudomonas aeruginosa ATCCBAA-2018, Klebsiella pneumonia ATCC 2146 and Enterococcus faecium (VRE) ATCC 700221. From the American Type Culture Collection, the strains were purchased (ATCC, Manassas, VA). An altered version of the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) procedures was used for the susceptibility testing.19 A final DMSO concentration of 1% was maintained in the assay while serially diluting all samples in 20% DMSO/saline and transferring them in duplicate to 384 well flat-bottom microplates. Following the McFarland standard, inocula were created by adjusting the OD630 of microbe suspensions in incubation broth.20 For C. albicans RPMI 1640 (2% dextrose/0.03% glutamine/MOPS at pH 6.0) was used, for C. neoformans, Sabouraud dextrose was used, while, cation-adjusted Mueller–Hinton pH 7.0 for MRS, VRE, E. coli, K. pneumonia, and P. aeruginosa, and RPMI 1640 broth (2% dextrose, 0.03% glutamine, buffered with 0.165 M MOPS at pH 7.0) for A. fumigatus in accordance with the CLSI procedure, to afford recommended inocula as per CLSI protocol. Each assay contained drug controls for bacteria and fungi. MRS, VRE, E. coli, K. pneumoniae, P. aeruginosa, C. albicans, and A. fumigatus were incubated at 35 °C for 48 hours, while C. neoformans was incubated at 35 °C for 68–72 hours. A Bio-Tek plate reader was used to record the optical density (530 nm) or fluorescence (544ex/590em) of A. fumigatus, VRE, and MRS before and after incubation.4. Conclusion
Teucrium yemense (Defl.), known as Reehal Fatima, has lately been identified as a potential source for new neo-clerodane diterpenoids. Consequently, this study's goal was to further explore the chemistry of this plant. The present investigation revealed two undescribed neo-clerodane diterpenoids, namely fatimanol Y and fatimanol Z, together with the known teulepicephin, 8-acetylharpagide and teucardosid from the aerial parts of T. yemense.Author contributions
Ahmed Elbermawi: conceptualization, investigation, methodology, writing – original draft. Fazila Zulfiqar: investigation, validation. Ikhlas A. Khan: resources, supervision. Zulfiqar Ali: supervision, review & editing.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The first author is grateful to the Egyptian Government for financial support.References
- F. Menichini, F. Conforti, D. Rigano, C. Formisano, F. Piozzi and F. Senatore, Food Chem., 2009, 115, 679–686 CrossRef CAS.
- M. Nur-E-Alam, M. Yousaf, S. Ahmed, E. S. Al-Sheddi, I. Parveen, D. M. Fazakerley, A. Bari, H. A. Ghabbour, M. D. Threadgill, K. C. L. Whatley, K. F. Hoffmann and A. J. Al-Rehaily, J. Nat. Prod., 2017, 80, 1900–1908 CrossRef CAS PubMed.
- M. Bakhtiari and J. Asgarpanah, J. Essent.Oil Bear. Plants, 2015, 18, 1174–1179 CrossRef CAS.
- M. C. Carreiras, B. Rodríguez, F. Piozzi, G. Savona, M. R. Torres and A. Perales, Phytochemistry, 1989, 28, 1453–1461 CrossRef CAS.
- M. C. de La Torre, B. Rodríguez, M. Bruno, G. Savona, F. Piozzi and O. Servettaz, Phytochemistry, 1988, 27, 213–216 CrossRef CAS.
- A. Gonzalez-Coloma, C. Gutierrez, J. M. Miguel del Corral, M. Gordaliza, M. L. de la Puente and A. San Feliciano, J. Agric. Food Chem., 2000, 48, 3677–3681 CrossRef CAS PubMed.
- N. Fatima, Sci. Int., 2016, 28, 1229–1231 CAS.
- A. Kamel, J. Nat. Prod., 1995, 58, 428–431 CrossRef CAS.
- F. Aydoğan, Z. Ali, F. Zulfiqar, C. Karaalp, I. A. Khan and E. Bedir, Phytochem. Lett., 2023, 54, 45–49 CrossRef.
- E. Abdei-Sattar, Arch. Pharmacal Res., 1998, 21, 785–786 CrossRef PubMed.
- N. M. Al-Musayeib, R. A. Mothana, A. Matheeussen, P. Cos and L. Maes, BMC Complementary Altern. Med., 2012, 12, 49 CrossRef PubMed.
- M. A. Rahman, J. S. Mossa, M. S. Al-Said and M. A. Al-Yahya, Fitoterapia, 2004, 75, 149–161 CrossRef PubMed.
- M. Nur-E-Alam, I. Parveen, B. Wilkinson, S. Ahmed, R. M. Hafizur, A. Bari, T. J. Woodman, M. D. Threadgill and A. J. Al-Rehaily, Sci. Rep., 2021, 11, 8074 CrossRef CAS PubMed.
- E. A. Sattar, J. S. Mossa, I. Muhammad and F. S. El-Feraly, Phytochemistry, 1995, 40, 1737–1741 CrossRef CAS.
- F. Aydoğan, E. H. Anouar, M. Aygün, H. Yusufoglu, C. Karaalp and E. Bedir, J. Mol. Struct., 2021, 1231, 129919 CrossRef.
- A. Castro, S. Moco, J. Coll and J. Vervoort, J. Nat. Prod., 2010, 73, 962–965 CrossRef CAS PubMed.
- Y. Takeda, S. Tsuchida and T. Fujita, Phytochemistry, 1987, 26, 2303–2306 CrossRef CAS.
- J. Ruhdorfer and H. Rimpler, Z. Naturforsch., C: J. Biosci., 1981, 36, 697–707 CrossRef.
- J. Meletiadis, J. F. G. M. Meis, J. W. Mouton, J. P. Donnelly and P. E. Verweij, J. Clin. Microbiol., 2000, 38, 2949 CrossRef CAS PubMed.
- S. G. Franzblau, R. S. Witzig, J. C. Mclaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson and R. H. Gilman, J. Clin. Microbiol., 1998, 36, 362 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of compounds 1–5 are available as supplementary materials. See DOI: https://doi.org/10.1039/d3ra06083g |
| This journal is © The Royal Society of Chemistry 2023 |