Three-dimensional narrow-bandgap perovskite semiconductor ferroelectric methylphosphonium tin triiodide for potential photovoltaic application†
Han-Yue
Zhang
 * and
Ren-Gen
Xiong
* and
Ren-Gen
Xiong

State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, P. R. China. E-mail: zhanghanyue@seu.edu.cn
First published on 15th December 2022
Abstract
A novel A-site three-dimensional organic–inorganic halide perovskites (3D OIHP) ferroelectric, methylphosphonium tin triiodide (MPSnI3), featuring a narrow bandgap of 1.43 eV, was synthesized. The integration of ferroelectricity with initially moderate efficiency (2.23%) may afford a promising platform to investigate the ferroelectric photovoltaic effect in organic–inorganic halide perovskite solar cells.
The superior performance of methylammonium lead iodide (MAPbI3) in photovoltaic applications is a milestone achievement in perovskite chemistry. It has provoked booming research on organic–inorganic halide perovskites (OIHPs).1,2 OIHP structures containing organic cations and inorganic frameworks combine the merits of easy processability of solutions, lightweight, highly structural tunability, and additional functionalities.3–5 These features render OIHPs prominent performance in light-emitting diodes, solar cells, optoelectronic detectors, and ferroelectric devices.6,7 Three-dimensional (3D) ABX3 OIHPs (A = organic cations, B = metal, X = halogen), in which A-cations occupy the voids between BX6 octahedra (most notably (MA or FA)(Pb or Sn)(I or Br)3 where FA is formamidinium), are considered “superstar materials” in optoelectronics and photovoltaics.8 During the past decade, the power conversion efficiency (PCE) of MAPbI3-based perovskite solar cells (PSCs) has improved from 3.8% to >25%.9–11 More recently, Zhan and colleagues converted α-FAPbI3 films into PSCs with an efficiency of >23%,12 comparable with that of commercialized crystalline silicon and GaAs solar cells. Besides the appropriate bandgaps (∼1.5 eV) close to the optimal bandgap (∼1.34 eV),10,12 the high photovoltaic performance of MAPbI3 is deemed to be associated to its polar nature and ferroelectric polarization at room temperature.13–15
Ferroelectric materials with intrinsic spontaneous polarization in photovoltaic devices contribute significantly to formation of a strong local built-in field to facilitate effective separation of electron-hole pairs that are similar to the p–n junctions in conventional solar cells.16,17 Hence, a ferroelectric photovoltaic effect has long been expected to be a preferable choice for conversion of solar energy. However, the low absorption coefficient and wide bandgap in conventional ferroelectric materials (e.g., BTO, PZT, and LiNbO3) are unacceptable in photovoltaic research.18 Therefore, ferroelectric materials with a narrow bandgap are very important for high-efficiency photovoltaic devices. BiFeO3 has a small bandgap and can absorb many photons, but large leakage currents caused by ineluctable Fe2+ and oxygen vacancies seriously affect ferroelectric polarization.17,19,20 In OIHPs, Pb-based PSCs have toxic effects on the body and environment. In the past few years, the most promising Sn-based OIHPs have been investigated intensively for applications of solar cells, but they must be lead-free and realize comparable visible-light absorption. Unfortunately, their photovoltaic performances are far from those of lead-based devices.21–32 FA(Pb or Sn)(I or Br)3 and MAPbBr3 crystallize in centrosymmetric space groups at room temperature and fail to meet the polar symmetry for ferroelectrics.33–35 In recent years, extensive research has been conducted to extend the family of functional OIHPs,4,36 whereas the development of new photovoltaic OIHPs has stalled because of the difficulties in stabilizing the parent 3D structural motif. Subjected to the Goldschmidt tolerance factor t (0.8 < t < 1), 3D BX3− cubic frameworks can accommodate only a few small cations, MA, and FA to support a photoactive black perovskite phase.37,38 At this stage of their development, construction of 3D OIHP ferroelectrics with new A-site cations seems an incredible challenge.
Phosphonium-based molecules are promising as A-site cations because the P atom and N atom (which belong to the same group in the Periodic Table) have similar covalent characteristics. Thus, phosphonium cations and ammonium cations can enable materials to have similar structures and functions, whereas their differences in atomic radius, mass, electron structure, and bond length may elicit interesting variables in physical and chemical properties. Based on theoretical calculations of the tolerance factor rule, the methylphosphonium (MP+) cation has been predicted to be available to fill a 3D cage, thereby meeting the demand for the formation of a stable 3D OIHP structure.39 Very recently, we synthesized a 3D perovskite molecular ferroelectric MPSnBr3. At the experimental level we showed (for the first time) that MP+ can serve as a stable A-site cation in OIHPs.40 Moreover, in 1999, Mitzi et al. demonstrated the conducting property of tin iodide frameworks.41,42 In view of those observations, we synthesized a new 3D lead-free OIHP: methylphosphonium tin iodide (MPSnI3). It features a narrow bandgap (1.43 eV), which is close to the optimal bandgap (∼1.34 eV), thereby making it a competitive candidate for photovoltaic and optoelectronic applications. MPSnI3 is also a multiaxial ferroelectric with a Curie temperature (Tc) of 298 K. These attributes may boost the photoelectric performance of MPSnI3-based solar cells. Compared with MPSnBr3, substituting bromine for iodine: (i) heralded a new structure; (ii) resulted in a black perovskite phase with a narrower bandgap; (iii) broke through the long-term limitation of A-site 3D iodide perovskite species. We believe our discoveries afford a promising platform to investigate the ferroelectric photovoltaic effect in OIHP solar cells and other optoelectronic applications.
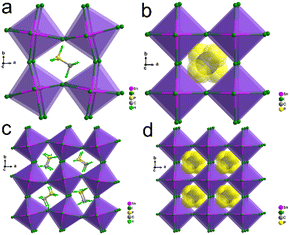
We prepared crystals of MPSnI3 in a hydroiodic acid solution of equimolar SnI2 and MPI (Fig. S1–S3, ESI†). Single-crystal structure studies revealed that MPSnI3 adopted a 3D ABX3 OIHP structure (A = MP+, B = Sn2+, and X = I−), in which organic MP+ cations were located in 3D-framework cavities surrounded by SnI6 octahedra, isostructural to the 3D MASnI3 (Fig. 1). The crystal structure of MPI adopted a monoclinic space group P21/m (Fig. S4 and Table S1, ESI†).
At 273 K, MPSnI3 crystallized in polar space group Pba2 (point group = mm2) (Table S1, ESI†). This corresponded to the low-temperature ferroelectric phase (LFP). In the LFP, the MP+ cations located on 2-fold symmetry axes in the lattice and thus showed 2-fold orientational disorder (Fig. 1a and c). The SnI6 octahedron was distorted, showing short (average of 3.057(5) Å) and long (average of 3.306(5) Å) Sn–I interatomic distances, and an angle of Sn–I–Sn between 158.9° and 178.0° (Fig. S5a and Table S2, ESI†). At 313 K (high-temperature paraelectric phase (HPP)), the crystal structure of MPSnI3 was refined to the non-centrosymmetric cubic space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (point group
3m (point group ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m) (Table S1, ESI†) based on the significant piezoelectric signal response by piezoresponse force microscopy (PFM) (Fig. S6, ESI†). Also, MP cations were modelled to be highly disordered spheres to satisfy the cubic symmetry (Fig. 1b and d). The SnI6 octahedron also changed to a regular octahedron with only one type of Sn–I interatomic distance of 3.1732(1) Å, and the Sn–I–Sn angle became 180.0° (Fig. S5b and Table S2, ESI†). Therefore, the ferroelectric phase transition from LFP to HPP was associated with the order–disorder transition of MP+ cations and deformation of the [SnI3]− framework. According to the Aizu rule, this phase transition in MPSnI3 should be ferroelectric with Aizu notion
3m) (Table S1, ESI†) based on the significant piezoelectric signal response by piezoresponse force microscopy (PFM) (Fig. S6, ESI†). Also, MP cations were modelled to be highly disordered spheres to satisfy the cubic symmetry (Fig. 1b and d). The SnI6 octahedron also changed to a regular octahedron with only one type of Sn–I interatomic distance of 3.1732(1) Å, and the Sn–I–Sn angle became 180.0° (Fig. S5b and Table S2, ESI†). Therefore, the ferroelectric phase transition from LFP to HPP was associated with the order–disorder transition of MP+ cations and deformation of the [SnI3]− framework. According to the Aizu rule, this phase transition in MPSnI3 should be ferroelectric with Aizu notion ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3mFmm2.43
3mFmm2.43
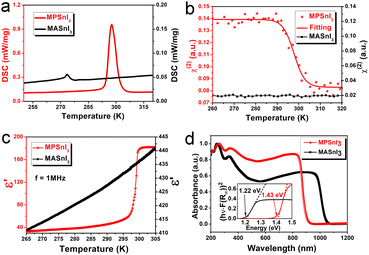
Differential scanning calorimetry (DSC) was undertaken to detect the phase transition. During heating, an endothermic peak around 298 K revealed a phase transition for MPSnI3, which was higher by 25 K than that for MASnI3 (Fig. 2a). We confirmed the phase transition by the temperature-dependent second-harmonic generation (SHG) response (Fig. 2b). MPSnI3 exhibited clear SHG signals in the ferroelectric phase. The SHG intensity of MPSnI3 had a marked change in Tc, indicating the appearance of significant symmetry breakage and in accordance with the transition of non-centrosymmetric mm2 and ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m crystallographic symmetry in ferroelectric and paraelectric phases. However, a change in SHG signal was not observed before or after the phase transition temperature (Tc = 273 K) for MASnI3. We also confirmed the phase transition of MPSnI3 by the dielectric anomaly around Tc in the temperature-dependent dielectric real part (ε′) (Fig. 2c). The huge variation of ε′ around Tc revealed the ferroelectric-to-paraelectric phase transition for MPSnI3. The Curie–Weiss law is not applicable in such an inappropriate ferroelectric compound.7 The behavior of dielectric permittivity around Tc can be explained by a fitted model based on the Landau–Ginzburg theory (Fig. S7, ESI†).
3m crystallographic symmetry in ferroelectric and paraelectric phases. However, a change in SHG signal was not observed before or after the phase transition temperature (Tc = 273 K) for MASnI3. We also confirmed the phase transition of MPSnI3 by the dielectric anomaly around Tc in the temperature-dependent dielectric real part (ε′) (Fig. 2c). The huge variation of ε′ around Tc revealed the ferroelectric-to-paraelectric phase transition for MPSnI3. The Curie–Weiss law is not applicable in such an inappropriate ferroelectric compound.7 The behavior of dielectric permittivity around Tc can be explained by a fitted model based on the Landau–Ginzburg theory (Fig. S7, ESI†).
The optical property of a MPSnI3 crystal was investigated by solid-state UV-vis diffuse reflectance spectroscopy at room temperature. The UV-vis absorbance spectrum displayed intense absorption at a band-edge onset of 900 nm (Fig. 2d). The feature of a direct bandgap semiconductor could be concluded (Fig. 2d, insert). From the Tauc plot, the measured optical bandgap for the direct transition was 1.43 eV, which is slightly higher than that for the 3D analogue: MASnI3 (1.22 eV).
To characterize the ferroelectricity of MPSnI3, we employed PFM to enable non-destructive visualization and control of ferroelectric domains on single crystals at 288 K. The clear-domain phase contrast and domain wall are shown in Fig. S3a and S3b (ESI†). The domain structure did not overlap with the topography (Fig. S8, ESI†). For MPSnI3, 180° and non-180° domains could coexist on a single crystal, thereby demonstrating the multiaxial nature (Fig. S9, ESI†).
These stable polarizations in the domains could also be switchable under an external electric field. Fig. 3c and d display the well-defined hysteresis loops and typical butterfly-shaped curves for the vertical phase and amplitude signals versus DC bias, respectively, which confirmed the switchable polarization of MPSnI3. We also undertook PFM tip poling experiments to observe domain switching directly. Switched domain patterns could be written on a single crystal of MPSnI3 under application of opposite voltages, which provided solid evidence for ferroelectric polarization switching (Fig. S10, ESI†).44,45
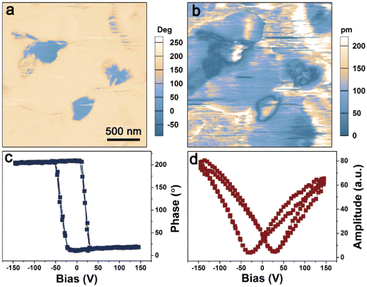 | ||
| Fig. 3 Vertical PFM phase (a) and amplitude (b) images on the crystal surface of MPSnI3 at 288 K. Vertical PFM phase (c) and amplitude (d) signals versus the tip bias at a selected location. | ||
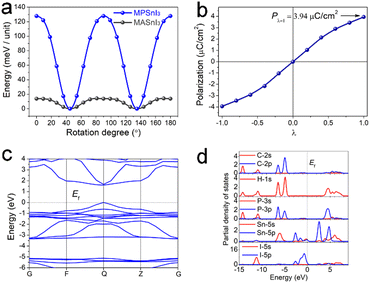
We calculated the hypothetical molecular rotation of MA and MP in the inorganic SnI3− framework, respectively (Fig. S11, ESI†). The state with the lowest energy emerged at 45° and 135° (Fig. 4a), so the molecule was most stable in the face-center direction, in accordance with the measured crystal structure. Compared with MA cations, the energy barrier of MP rotation was much higher, suggesting a higher ferroelectric phase-transition temperature. Such a high barrier for rotational energy of MPSnI3 may originate from a heavier molecular mass and larger molecular volume.
To gain deep insight into ferroelectric polarization reversal, density functional theory (DFT) calculations were carried out to evaluate the origin of polarization.46,47 Based on the modern theory of polarization,48,49 a dynamic path between two ferroelectric states is constructed according to the crystal structure acquired from single-crystal X-ray diffraction.
Considering the rotational and displacive motion of MP cations in the anionic SnI3− framework, the other states were obtained from the matrix transformation of the coordinates. The variation of polarization versus the dynamic path is displayed in Fig. 4b, from which the ferroelectric polarization with 3.94 μC cm−2 could be extracted from two equivalent ferroelectric configurations (λ = ±1). During ferroelectric switching (−1 < λ < 1), the polarization value changed smoothly, and turned to zero at λ = 0, suggesting a reference phase with zero polarization.
We also calculated the band structure to obtain deep insight into the electronic structure of MPSnI3 (Fig. 4c). The conduction band (CB) minimum and valence band (VB) maximum were localized at the same k-vector in the Brillouin zone, so MPSnI3 was a direct bandgap semiconductor, in good agreement with experimental phenomena. The calculated bandgap was 1.58 eV, close to the experimental value of 1.43 eV. Furthermore, bands can be assigned according to the partial density of states (PDOS), as plotted in Fig. 4d. From PDOS, the bands at the top of the VB originated from the nonbonding states of I-5p, and those at the CB bottom were mainly from the unoccupied Sn-5p orbitals. Clearly, the bandgap of the material was determined by the inorganic {SnI3}− framework.
We conducted preliminary device fabrication to test the photovoltaic performance of MPSnI3 with a device structure of ITO/PEDOT:PSS/perovskite/C60/BCP/Ag (Fig. S12–S17 and Table S3, ESI†). Benefiting from outstanding ferroelectric and semiconductor properties, the manufactured MPSnI3-based solar-cell device showed initial photoelectric performance, and achieved a moderate PCE of 2.231%.
In summary, we reported a new 3D lead-free OIHP ferroelectric semiconductor of MPSnI3 that showed clear ferroelectricity with a Tc of 298 K and a narrow bandgap of 1.43 eV. MPSnI3 was a multiaxial ferroelectric with the Aizu notion of ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3mFmm2, and the ferroelectric domains and polarization switching confirmed its ferroelectricity. The considerable initial energy conversion efficiency, combined with easy processing of solutions, suggests that MPSnI3 has great potential for photovoltaic, photoelectric, and ferroelectric applications.
3mFmm2, and the ferroelectric domains and polarization switching confirmed its ferroelectricity. The considerable initial energy conversion efficiency, combined with easy processing of solutions, suggests that MPSnI3 has great potential for photovoltaic, photoelectric, and ferroelectric applications.
We thank Professor Yiqiang Zhan and his co-workers (Fudan University) for their significant contribution to photoelectric experiments. This work was supported by the seventh Youth Elite Scientist Sponsorship Program by the China Association for Science and Technology, the Ten Science and Technology Problem of Southeast University, and the National Natural Science Foundation of China (21991142 and 21831004).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef PubMed.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef PubMed.
- W. Li, Z. Wang, F. Deschler, S. Gao, R. H. Friend and A. K. Cheetham, Nat. Rev. Mater., 2017, 2, 16099 CrossRef.
- M. Konstantakou and T. Stergiopoulos, J. Mater. Chem. A, 2017, 5, 11518–11549 RSC.
- K. Liu, Y. Jiang, Y. Jiang, Y. Guo, Y. Liu and E. Nakamura, J. Am. Chem. Soc., 2019, 141, 1406–1414 CrossRef PubMed.
- Y.-M. You, W.-Q. Liao, D. Zhao, H.-Y. Ye, Y. Zhang, Q. Zhou, X. Niu, J. Wang, P.-F. Li, D.-W. Fu, Z. Wang, S. Gao, K. Yang, J.-M. Liu, J. Li, Y. Yan and R.-G. Xiong, Science, 2017, 357, 306–309 CrossRef CAS PubMed.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- J. Tong, Z. Song, D. H. Kim, X. Chen, C. Chen, A. F. Palmstrom, P. F. Ndione, M. O. Reese, S. P. Dunfield, O. G. Reid, J. Liu, F. Zhang, S. P. Harvey, Z. Li, S. T. Christensen, G. Teeter, D. Zhao, M. M. Al-Jassim, M. F. A. M. van Hest, M. C. Beard, S. E. Shaheen, J. J. Berry, Y. Yan and K. Zhu, Science, 2019, 364, 475–479 CrossRef CAS PubMed.
- H. J. Snaith, Nat. Mater., 2018, 17, 372–376 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- H. Lu, Y. Liu, P. Ahlawat, A. Mishra, W. R. Tress, F. T. Eickemeyer, Y. Yang, F. Fu, Z. Wang, C. E. Avalos, B. I. Carlsen, A. Agarwalla, X. Zhang, X. Li, Y. Zhan, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, L. Zheng, A. Hagfeldt and M. Grätzel, Science, 2020, 370, eabb8985 CrossRef CAS PubMed.
- B. Chen, J. Shi, X. Zheng, Y. Zhou, K. Zhu and S. Priya, J. Mater. Chem. A, 2015, 3, 7699–7705 RSC.
- H. Röhm, T. Leonhard, A. D. Schulz, S. Wagner, M. J. Hoffmann and A. Colsmann, Adv. Mater., 2019, 31, 1806661 CrossRef.
- J. Breternitz, F. Lehmann, S. A. Barnett, H. Nowell and S. Schorr, Angew. Chem., Int. Ed., 2020, 59, 424–428 CrossRef CAS.
- C. Baeumer, D. Saldana-Greco, J. M. P. Martirez, A. M. Rappe, M. Shim and L. W. Martin, Nat. Commun., 2015, 6, 6136 CrossRef CAS.
- T. Choi, S. Lee, Y. J. Choi, V. Kiryukhin and S.-W. Cheong, Science, 2009, 324, 63–66 CrossRef CAS.
- X. Han, Y. Ji and Y. Yang, Adv. Funct. Mater., 2022, 32, 2109625 CrossRef CAS.
- T. Rojac, A. Bencan, B. Malic, G. Tutuncu, J. L. Jones, J. E. Daniels and D. Damjanovic, J. Am. Ceram. Soc., 2014, 97, 1993–2011 CrossRef.
- Y. B. Yuan, Z. G. Xiao, B. Yang and J. S. Huang, J. Mater. Chem. A, 2014, 2, 6027–6041 RSC.
- Z. Shi, J. Guo, Y. Chen, Q. Li, Y. Pan, H. Zhang, Y. Xia and W. Huang, Adv. Mater., 2017, 29, 1605005 CrossRef.
- C. C. Stoumpos and M. G. Kanatzidis, Acc. Chem. Res., 2015, 48, 2791–2802 CrossRef.
- B. Lee, C. C. Stoumpos, N. J. Zhou, F. Hao, C. Malliakas, C. Y. Yeh, T. J. Marks, M. G. Kanatzidis and R. P. H. Chang, J. Am. Chem. Soc., 2014, 136, 15379–15385 CrossRef.
- S. J. Lee, S. S. Shin, Y. C. Kim, D. Kim, T. K. Ahn, J. H. Noh, J. Seo and S. I. Seok, J. Am. Chem. Soc., 2016, 138, 3974–3977 CrossRef PubMed.
- S. Y. Shao, J. Liu, G. Portale, H. H. Fang, G. R. Blake, G. H. ten Brink, L. J. A. Koster and M. A. Loi, Adv. Energy Mater., 2018, 8, 1702019 CrossRef.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. H. Chang and M. G. Kanatzidis, Nat. Photonics, 2014, 8, 489–494 CrossRef CAS.
- K. Nishimura, M. A. Kamarudin, D. Hirotani, K. Hamada, Q. Shen, S. Iikubo, T. Minemoto, K. Yoshino and S. Hayase, Nano Energy, 2020, 74, 104858 CrossRef CAS.
- T. B. Song, T. Yokoyama, S. Aramaki and M. G. Kanatzidis, ACS Energy Lett., 2017, 2, 897–903 CrossRef CAS.
- A. Abate, Joule, 2017, 1, 659–664 CrossRef CAS.
- W. J. Ke and M. G. Kanatzidis, Nat. Commun., 2019, 10, 965 CrossRef PubMed.
- E. Jokar, C. H. Chien, C. M. Tsai, A. Fathi and E. W. G. Diau, Adv. Mater., 2019, 31, 1804835 CrossRef PubMed.
- W. J. Ke, C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2019, 31, 1803230 CrossRef CAS.
- Y. Dang, Y. Zhou, X. Liu, D. Ju, S. Xia, H. Xia and X. Tao, Angew. Chem., Int. Ed., 2016, 55, 3447–3450 CrossRef CAS.
- I. Swainson, L. Chi, J.-H. Her, L. Cranswick, P. Stephens, B. Winkler, D. J. Wilson and V. Milman, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2010, 66, 422–429 CrossRef CAS.
- M. T. Weller, O. J. Weber, J. M. Frost and A. Walsh, J. Phys. Chem. Lett., 2015, 6, 3209–3212 CrossRef CAS.
- S. Wang, X. Liu, L. Li, C. Ji, Z. Sun, Z. Wu, M. Hong and J. Luo, J. Am. Chem. Soc., 2019, 141, 7693–7697 CrossRef CAS PubMed.
- D. Li, J. Shi, Y. Xu, Y. Luo, H. Wu and Q. Meng, Natl. Sci. Rev., 2017, 5, 559–576 CrossRef.
- W. A. Dunlap-Shohl, Y. Zhou, N. P. Padture and D. B. Mitzi, Chem. Rev., 2019, 119, 3193–3295 CrossRef CAS.
- S. Körbel, M. A. L. Marques and S. Botti, J. Mater. Chem. A, 2018, 6, 6463–6475 RSC.
- H. Y. Zhang, X. G. Chen, Z. X. Zhang, X. J. Song, T. Zhang, Q. Pan, Y. Zhang and R. G. Xiong, Adv. Mater., 2020, 32, 2005213 CrossRef CAS PubMed.
- D. B. Mitzi, S. Wang, C. A. Feild, C. A. Chess and A. M. Guloy, Science, 1995, 267, 1473–1476 CrossRef PubMed.
- C. R. Kagan, D. B. Mitzi and C. D. Dimitrakopoulos, Science, 1999, 286, 945–947 CrossRef PubMed.
- K. Aizu, J. Phys. Soc. Jpn., 1969, 27, 387–396 CrossRef.
- T. Besara, P. Jain, N. S. Dalal, P. L. Kuhns, A. P. Reyes, H. W. Kroto and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6828–6832 CrossRef.
- C. Qiu, B. Wang, N. Zhang, S. Zhang, J. Liu, D. Walker, Y. Wang, H. Tian, T. R. Shrout, Z. Xu, L.-Q. Chen and F. Li, Nature, 2020, 577, 350–354 CrossRef PubMed.
- D. Di Sante, A. Stroppa, P. Jain and S. Picozzi, J. Am. Chem. Soc., 2013, 135, 18126–18130 CrossRef PubMed.
- A. Stroppa, P. Barone, P. Jain, J. M. Perez-Mato and S. Picozzi, Adv. Mater., 2013, 25, 2284–2290 CrossRef PubMed.
- R. Resta and D. Vanderbilt, in Physics of Ferroelectrics: A Modern Perspective, ed. K. M. Rabe, C. H. Ahn and J. M. Triscone, 2007, vol. 105, pp. 31–68 Search PubMed.
- N. A. Spaldin, J. Solid State Chem., 2012, 195, 2–10 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, NMR, crystal data, PFM, and solar cells. CCDC 2031849–2031850 and 2036182. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06408a |
| This journal is © The Royal Society of Chemistry 2023 |