Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational design via dual-site aliovalent substitution leads to an outstanding IR nonlinear optical material with well-balanced comprehensive properties†
He-Di
Yang‡
ab,
Mao-Yin
Ran‡
ac,
Sheng-Hua
Zhou
ac,
Xin-Tao
Wu
 ad,
Hua
Lin
ad,
Hua
Lin
 *ad and
Qi-Long
Zhu
*ad and
Qi-Long
Zhu
 *ad
*ad
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: linhua@fjirsm.ac.cn; qlzhu@fjirsm.ac.cn
bCollege of Chemistry, Fuzhou University, Fujian 350002, China
cUniversity of the Chinese Academy of Sciences, Beijing 100049, China
dFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350002, China
First published on 7th September 2022
Abstract
The acquisition of a non-centrosymmetric (NCS) structure and achieving a nice trade-off between a large energy gap (Eg > 3.5 eV) and a strong second-harmonic generation (SHG) response (deff > 1.0 × benchmark AgGaS2) are two formidable challenges in the design and development of infrared nonlinear optical (IR-NLO) candidates. In this work, a new quaternary NCS sulfide, SrCdSiS4, has been rationally designed using the centrosymmetric SrGa2S4 as the template via a dual-site aliovalent substitution strategy. SrCdSiS4 crystallizes in the orthorhombic space group Ama2 (no. 40) and features a unique two-dimensional [CdSiS4]2− layer constructed from corner- and edge-sharing [CdS4] and [SiS4] basic building units (BBUs). Remarkably, SrCdSiS4 displays superior IR-NLO comprehensive performances, and this is the first report on an alkaline-earth metal-based IR-NLO material that breaks through the incompatibility between a large Eg (>3.5 eV) and a strong phase-matching deff (>1.0 × AgGaS2). In-depth mechanism explorations strongly demonstrate that the synergistic effect of distorted tetrahedral [CdS4] and [SiS4] BBUs is the main origin of the strong SHG effect and large birefringence. This work not only provides a high-performance IR-NLO candidate, but also offers a feasible chemical design strategy for constructing NCS structures.
Introduction
The infrared (IR) laser occupies a more and more important position in military weapons, information storage, precision micromanufacturing and other scientific research.1–5 Generally, the IR nonlinear optical (NLO) crystal is an integral part for IR laser generation, which requires a phase-matching (PM) feature, a broad energy gap (Eg), a strong second-harmonic-generation (SHG) intensity (deff), a large laser-induced damage threshold (LIDT), an appropriate birefringence (Δn), and a favorable physical and chemical stability.6 Chalcopyrite-type materials, AgGaS2,7 AgGaSe2,8 and ZnGeP2,9 are commercially available and exhibit sufficient deff and wide transmittance in the IR region. However, they still suffer from several fatal drawbacks, e.g. low LIDT of AgGaS2, non-phase-matching (NPM) behavior of AgGaSe2 and unexpected multi-phonon absorption of ZnGeP2, which hinder their further applications in far-IR regions and high-power lasers. Therefore, it is necessary and urgent to explore new IR-NLO candidates with excellent comprehensive properties.A non-centrosymmetric (NCS) structure is the prerequisite for a NLO crystal, which is also the first challenge in the design and synthesis of IR-NLO candidates. In order to overcome this problem, various strategies have been developed in the past decades. Among them, chemical substitution is considered to be the simplest and most effective method.10,11 On one hand, it can greatly improve the IR-NLO properties of chalcogenides, such as, Li0.6Ag0.4GaS2 (deff = 1.1 × AgGaS2) versus LiGaS2 (deff = 0.4 × AgGaS2),12 Cu5Zn0.5P2S8 (deff = 0.3 × AgGaS2) versus Cu3PS4 (deff = 0.03 × AgGaS2),13 and Sr1.3Pb0.7GeSe4 (deff = 16 × SiO2) versus Pb2GeSe4 (deff = 2 × SiO2).14 On the other hand, it can realize centrosymmetric (CS)-to-NCS structure evolution in the chalcogenide system. Some classic examples include CS La2CuInS5versus NCS La2CuSbS5,15 CS Ba2GaAsSe5versus NCS Ba2As2Se5,16 CS BaGa2Se4versus NCS K0.38Ba0.81Ga2Se4,17 CS K2Sb4S7versus NCS K2Ag3Sb3S7,18 CS Rb4Hg2Ge2S8versus NCS (Na3Rb)Hg2Ge2S8,19 CS SrGeO3versus NCS SrGeOSe2,20 CS SnBr2versus NCS Sn7Br10S2,21 and CS Rb4P2S6versus NCS RbBiP2S6.22 Notably, compared to a large number of structural transformations achieved by single-site substitution mentioned above, examples of dual-site and multi-site substitution are rarely reported.23,24
Among the essential conditions for a promising IR-NLO candidate, a large Eg and a strong deff are not only the most vital factors but also the most challenging to achieve concurrently due to their incompatibility. Metal chalcogenides have been considered as promising candidates for IR-NLO materials, and nearly a thousand novel NLO-active chalcogenides have been discovered in the past few decades.25–31 Unfortunately, there are only 6 PM chalcogenides that can meet the preferred requirement for a useful IR-NLO crystal, that is, a nice trade-off between a large Eg (> 3.5 eV) and strong deff (> 1.0 × benchmark AgGaS2), see Table S1 in the ESI for details.† As summarized in Table S1,† some useful information can be obtained as follows: (1) all of them are sulfides; (2) most significant structural features are two-dimensional (2D) or three-dimensional (3D) structures that are constructed from tetrahedral [MS4] basic building units (BBUs) (M = metal elements); (3) the filled cations are mainly alkali metals (A) or polycations. Nevertheless, a similar example based on an alkaline-earth metal (AE) as a filled cation is still not reported to date.
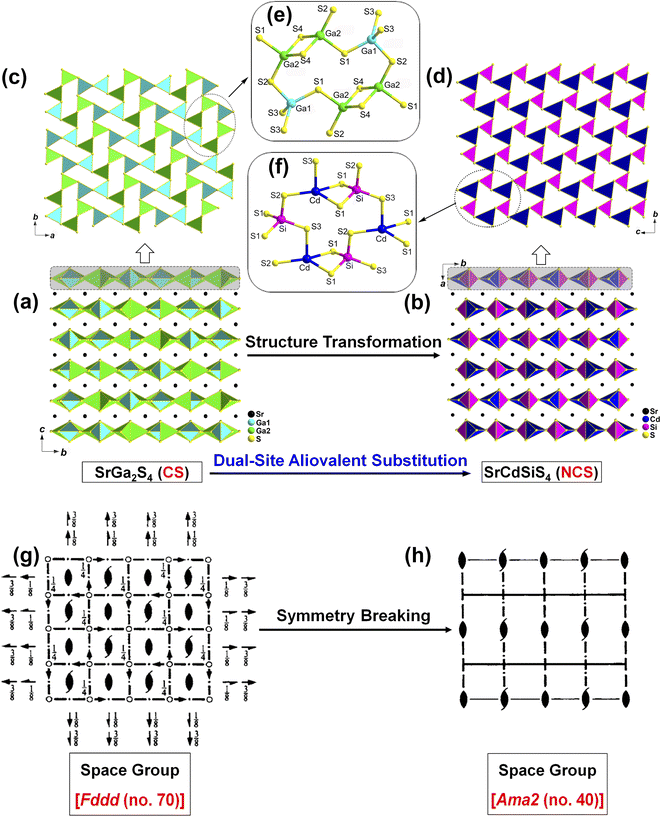
Recently, our research focuses on the ternary AE–MIII–Q system (MIII = group IIIA metal Ga, In), hoping to obtain NCS chalcogenides. The tetrahedral [MIIIQ4] BBUs are the beneficial NLO-active units for achieving a large deff, while the introduction of AE elements into this system may have the additional advantage of enlarging the Eg, which may help to increase the LIDT once an IR-NLO crystal is obtained.32–34 Our systematic exploratory efforts have led to the discovery of a known ternary sulfide in this family, namely, SrGa2S4.35 It exhibits a unique 2D [Ga2S4]2− layer that is constructed from common NLO-active [GaS4] units and possesses a wide optical Eg (3.93 eV) and a large theoretical birefringence (Δn = 0.147@2050 nm). Unfortunately, the CS space group of Fddd (no. 70) makes this sulfide NLO inert, that is, it does not display any SHG signal under laser irradiation. Inspired by the aforementioned chemical substitution strategy and detailed structural analysis, we are eager to realize the CS-to-NCS structural evolution via the replacement of two GaIII sites by “MI + MV” or “MII + MIV” in such a 2D layer. We term this the “dual-site aliovalent substitution” strategy.
Guided by a dual-site aliovalent substitution strategy, a novel quaternary NCS sulfide SrCdSiS4 was successfully discovered herein. Remarkably, SrCdSiS4 exhibits the PM feature and excellent IR-NLO performances, including a strong deff (1.1 × AgGaS2), wide Eg (3.61 eV), ultra-high LIDT (20.4 × AgGaS2), broad transmission range (0.33–18.19 μm) and suitable Δn (0.158@2050 nm), which indicates that it is a promising candidate for IR-NLO materials and eliminates the disadvantageous factors of commercial chalcopyrite-type chalcogenides. Moreover, SrCdSiS4 is also the first example of an alkaline-earth metal-based IR-NLO material that breaks through the incompatibility between a large Eg (>3.5 eV) and a strong PM deff (>1.0 × AgGaS2). In this work, a systematic study of the syntheses, structural evolution, NLO and linear optical properties, and the in-depth mechanism is reported as well.
Results and discussion
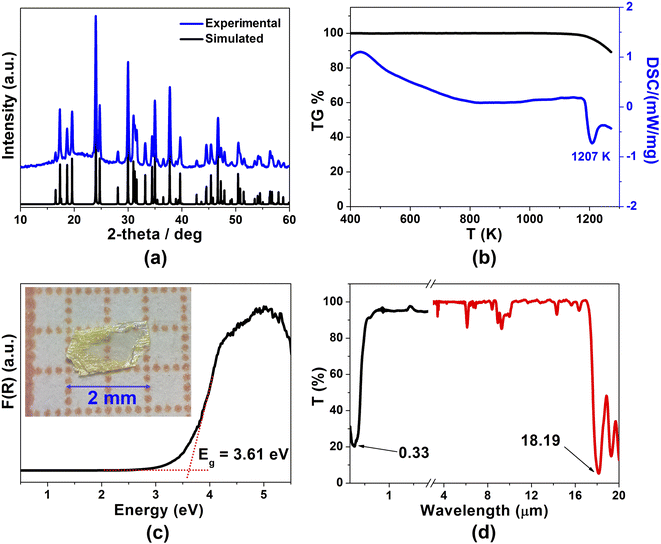
In this study, light-yellow crystals of SrCdSiS4 were prepared by a high-temperature solid-state reaction between stoichiometric SrS, CdS, Si, and S at 1123 K using CsI as the flux. The purity of the polycrystalline sample was checked by powder X-ray diffraction (XRD) analysis (Fig. 1a), and energy-dispersive X-ray spectroscopy (EDX) provides average atomic ratios of 1.09/1.10/1/4.13 for Sr, Cd, Si, and S elements (Fig. S1†), which are close to theoretical values determined from single-crystal XRD results. As shown in Fig. 1b, SrCdSiS4 exhibits desirable thermal stability below 1207 K under N2 condition and decomposes to Sr2SiS4 and CdS at higher temperatures (Fig. S2†). The UV-vis and near-IR absorption spectra of SrCdSiS4 reveal an optical Eg of 3.61 eV (see Fig. 1c) based on the Kubelka Munk function,36 which is not only keeping the advantage of the wide Eg of the parent compound SrGa2S4 (3.93 eV, as plotted in Fig. S3†) but is also considerably wider than those of commercial IR-NLO materials AgGaS2 (2.56 eV),37 AgGaSe2 (1.83 eV)38 and ZnGeP2 (2.0 eV).39 Notably, such an ultra-wide Eg of SrCdSiS4 can effectively avoid two- or multi-photon absorptions under the incident normal laser, which is helpful to obtain a high LIDT. In addition, the transmittance spectrum (Fig. 1d) recorded from a well-polished single crystal piece indicates that SrCdSiS4 exhibits a wide transparent window from 0.33 μm (UV-vis region) to 18.19 μm (far-IR region), which can cover two notable atmospheric windows (3–5 μm and 8–12 μm). Remarkably, such a transparent range is wider than those of distinguished IR-NLO materials AgGaS2 (0.48–11.4 μm),39 ZnGeP2 (0.74–12 μm),39 AgGaSe2 (0.76–17 μm)39 and other recently reported IR-NLO candidates.40–45The structural evolution from CS SrGa2S4 to NCS SrCdSiS4 based on the dual-site aliovalent substitution strategy is illustrated in Fig. 2. Comparison of their structures shows that they belong to the same orthorhombic system and possess tetrahedral [MS4] BBUs in their 2D layered structures. However, they still have several significantly different characteristics in their structures: (i) SrCdSiS4 crystallizes in the space group of Ama2 (no. 40), while SrGa2S4 adopts the space group of Fddd (no. 70), see Table 1 for details; (ii) the asymmetric unit of SrCdSiS4 has 6 crystallographically independent sites (i.e., 1 Sr, 1 Cd, 1 Si, and 3 S atoms) and the Z value (number of molecules in a unit cell) is 4, which are different from those of SrGa2S4 [9 unique sites, namely, 3 Sr, 2 Ga, and 4 S atoms) and Z = 32], see Tables 1 and 2 for details; (iii) note that the repeated functional primitive, namely the 12-member-ring [Cd3Si3S16] (including 3 [CdS4] and 3 [SiS4] BBUs, see the dashed part in Fig. 2f) exists in each 2D [CdSiS4]2− layer of SrCdSiS4 (Fig. 2d), but in SrGa2S4, the repeated 12-member-ring [Ga6S16] in each 2D [Ga2S4]2− layer consists of 2 [Ga(1)S4] and 4 [Ga(2)S4] BBUs (see the dashed part in Fig. 2c and e); (iv) the [SrS8] polyhedra are more highly distorted in SrCdSiS4 than those in SrGa2S4, e.g., the larger difference (Δd) between the Sr–S bonds (Δd (Sr–S) = 0.15 Å) in SrCdSiS4 than that (0.03 Å) in SrGa2S4, and a similar trend also occurred in the tetrahedral [MS4] BBUs, see Fig. S4 and Tables S2 and S3 for details.† In a word, the dual-site aliovalent substitution led to the above-mentioned obvious changes in their crystal structures, thus realizing the CS-to-NCS structural transformation from ternary SrGa2S4 to quaternary SrCdSiS4. Moreover, the detailed symmetric operation change shown in Fig. 2g and h clearly displays the evolution of symmetry breaking, that is, the loss of the different glide planes and the inversion centre from CS SrGa2S4 [high symmetry Fddd (no. 70)] to NCS SrCdSiS4 [low symmetry Ama2 (no. 40)].
| Empirical formula | SrCdSiS4 | SrGa2S4 |
|---|---|---|
| a R 1 = Σ‖Fo| − |Fc‖/Σ|Fo|, wR2 = [Σw(Fo2 − Fc2)2/Σw(Fo2)2]1/2 | ||
| Formula weight | 356.35 | 355.30 |
| Temperature (K) | 293(2) | 293(2) |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | Ama2 (no. 40) | Fddd (no. 70) |
| a (Å) | 10.2821(7) | 12.2216(5) |
| b (Å) | 10.1551(9) | 20.5008(9) |
| c (Å) | 6.3699(5) | 20.8426(10) |
| V (Å3) | 665.12(9) | 5222.2(4) |
| Z | 4 | 32 |
| D c (g cm−3) | 3.559 | 3.615 |
| μ (mm−1) | 12.520 | 17.481 |
| GOOF on F2 | 1.204 | 1.174 |
| R 1, wR2 (I > 2σ (I)) a | 0.0212, 0.0533 | 0.0228, 0.0591 |
| R 1, wR2 (all data) | 0.0217, 0.0594 | 0.0338, 0.0627 |
| Largest diff. peak and hole (e Å−3) | 0.81, −0.49 | 0.69, −1.03 |
| Flack parameter | 0.007(12) | |
| Atom | Wyckff | X | y | Z | U eq(Å)a |
|---|---|---|---|---|---|
| a U eq is defined as one third of the trace of the orthogonalized Uij tensor. | |||||
| SrCdSiS 4 | |||||
| Sr | 4a | 0 | 0 | 0 | 0.0165(4) |
| Cd | 4b | 0.75 | 0.83086(9) | 0.4430(2) | 0.0251(4) |
| Si | 4b | 0.75 | 0.7178(3) | 0.9879(5) | 0.0124(7) |
| S1 | 8c | 0.4166(2) | 0.7754(2) | 0.6887(3) | 0.0165(5) |
| S2 | 4b | 0.75 | 0.8972(3) | 0.8119(4) | 0.0150(7) |
| S3 | 4b | 0.75 | 0.5662(2) | 0.7620(5) | 0.0171(7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| SrGa 2 S 4 | |||||
| Sr1 | 16g | 0.875 | 0.375 | 0.62685(2) | 0.01154(9) |
| Sr2 | 8b | 0.625 | 0.625 | 0.625 | 0.0111(2) |
| Sr3 | 8a | 0.375 | 0.375 | 0.875 | 0.01104(2) |
| Ga1 | 32h | 0.37362(2) | 0.51258(2) | 0.74942(2) | 0.00896(6) |
| Ga2 | 32h | 0.66403(2) | 0.44634(2) | 0.74984(2) | 0.00917(6) |
| S1 | 32h | 0.49464(4) | 0.59489(3) | 0.74888(4) | 0.01004(9) |
| S2 | 32h | 0.48491(4) | 0.42197(2) | 0.74897(3) | 0.00918(9) |
| S3 | 32h | 0.25159(6) | 0.50054(3) | 0.83226(3) | 0.0099(9) |
| S4 | 32h | 0.75071(7) | 0.49983(3) | 0.66738(3) | 0.00993(9) |
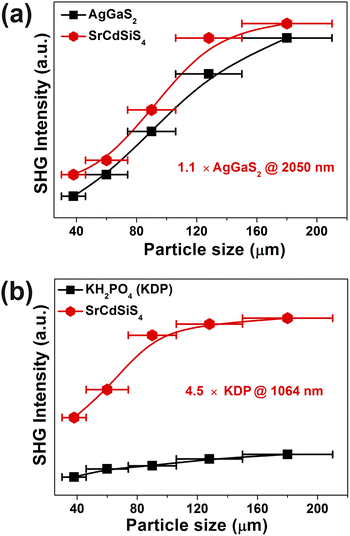
Owing to SrCdSiS4 possessing the NCS polar structure, we exhaustively investigated and analyzed the NLO performance. Size-dependent SHG effect measurements were performed by using the Kurtz-Perry method46 at five different particle size ranges. As illustrated in Fig. 3a, the SHG intensity strength increases with the increase of particle size, indicating that SrCdSiS4 can achieve type-I PM in the IR region. Under the same particle size of 150–210 μm, the deff is around 1.1 times that of AgGaS2 under a 2050 nm Q-switched laser. We also measured the SHG signals under a 1064 nm laser due to the shorter UV absorption edge of SrCdSiS4 (ca. 330 nm), giving it the potential to be applied in the UV-vis range. As indicated in Fig. 3b, SrCdSiS4 shows a large SHG effect of 4.5 × KH2PO4 (KDP) with type-I PM nature. Therefore, SrCdSiS4 is an excellent dual-band NLO candidate that can be used in both the IR and UV-vis regions. Apart from an adequate SHG response, a large LIDT is also vitally important for an IR-NLO material. So, its LIDT was measured by a single-pulse power technology.47 As shown in Fig. S5,† the experimental LIDT of SrCdSiS4 of 57.14 MW cm−2 in the particle size range of 150–210 μm is around 20.4 times higher than that of benchmark AgGaS2 (2.8 MW cm−2) under the same condition (1064 nm, 1 Hz, 10 ns). Such a value shows the outstanding laser tolerance of SrCdSiS4, indicating its potential in high-power laser applications. As a new member of the XMIIMIVQ4 (X = Eu, Sr, Ba; MII = Mn, Zn, Cd, and Hg; MIV = group-14 elements; and Q = chalcogen) system,24,48–63 it is necessary to make a detailed comparison with other compounds. A summary of the two key performance parameters (i.e., deff and Eg) of the XMIIMIVQ4 family is provided in Fig. 4 and details are listed in Table S4.† Remarkably, SrCdSiS4 displays superior IR-NLO comprehensive performances, and this is the first report on an alkaline-earth metal-based IR-NLO material that breaks through the incompatibility between a large Eg (>3.5 eV) and a strong phase-matching deff (>1.0 × AgGaS2) in this system. Furthermore, a more comparative study with other state-of-the-art IR-NLO candidates is worthwhile.32,64–67 As shown in Fig. S6 and Table S1,† there are 7 PM IR-NLO chalcogenides with Eg > 3.5 eV and deff > 1.0 × AgGaS2 (Fig. S6a†), which have been selected on the basis of literature research. From the perspective of structural dimension, they are mainly constructed in 3D framework (43%) and 2D layered (43%) structures, and only K2BaP2S667 possess a zero-dimensional (0D) cluster structure (14%) (Fig. S6b†). In addition, they can be divided into four categories according to the kind of filled cation: polycation-based (43%), alkali-metal-based (29%), mixed-cation-based (14%) and alkaline-earth-metal-based (14%) (Fig. S6c†). Note that the central atoms in most of the BBUs are main group elements [e.g., Ga (20%), P (20%), Si (13%), Li (13%) and Ge (7%)] and transition metal elements [e.g., Zn (20%), and Cd (7%)] (Fig. S6d†). The production of SrCdSiS4 not only enlarges the proportion of Cd and Si acting as favorable framework cations but also represents the first report of an alkaline-earth metal-based IR-NLO material that breaks through the wall of Eg > 3.5 eV and deff > 1 × AgGaS2.
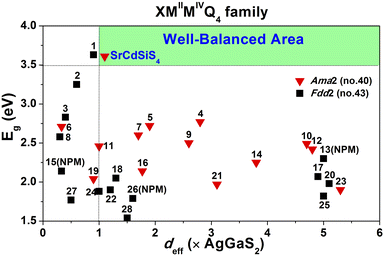 | ||
| Fig. 4 Comparison of deff and Eg of the XMIIMIVQ4 system (X = Eu, Sr, Ba; MII = Mn, Zn, Cd, and Hg; MIV = group-14 elements; and Q = chalcogen) and the green shaded region represents the well-balanced (Eg > 3.5 eV and deff > 1.0 × AgGaS2) area for IR-NLO materials. Details are listed in Table S4.† | ||
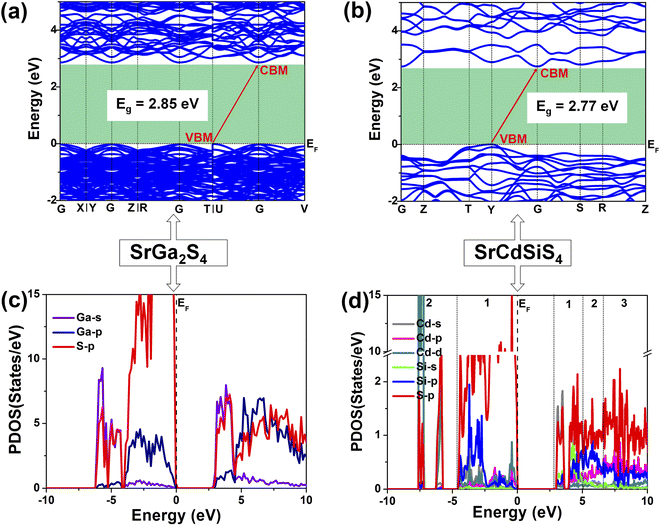
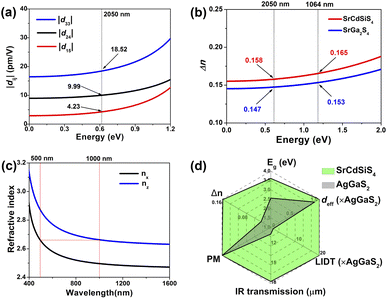
Theoretical computations were adopted to better understand the structure–activity relationships of the title compound. According to the electronic structures, the valence band minimum (VBM) and the conduction band maximum (CBM) are at different k-points for SrGa2S4 (Fig. 5a) and SrCdSiS4 (Fig. 5b), which indicates that they are indirect Eg semiconductors. Theoretical results exhibit that the calculated Eg values are 2.85 eV for SrGa2S4 and 2.77 eV for SrCdSiS4. Such values are smaller than the experimental ones (3.93 eV for SrGa2S4 and 3.61 eV for SrCdSiS4, respectively), which is mainly due to the discontinuity of the exchange correlation energy of the GGA functional.68–70 In addition, their partial density of states (PDOSs) in the energy field from −10 to 10 eV are shown in Fig. 5c and d. From the PDOSs, it is found that the contribution in the VBM is mainly from Ga-4s, S-3p orbitals for SrGa2S4 and S-3p, Si-3p orbitals for SrCdSiS4, while the CBM consists of Ga-4p, S-3p orbitals for SrGa2S4 and Cd-5s, S-3p orbitals for SrCdSiS4. The main source of contribution transformed from Ga-4s to Si-3p for the VB and Ga-4p to Cd-5s for the CB after dual-site aliovalent substitution. Accordingly, diverse orbital states finally account for the little difference in optical Eg and the electron transfer mainly depends on [GaS4] (for SrGa2S4) converting to [CdS4] and [SiS4] (for SrCdSiS4). Moreover, the origin of the SHG response and birefringence (Δn) as the two important NLO indexes were also analyzed in detail. As seen from Fig. 6a, SrCdSiS4 has three nonzero independent second-order susceptibility tensors based on the rule of Kleinman's symmetry,71 namely, d33, d24 and d15. The calculated values at 2050 nm are d33 = 18.52, d24 = 9.99, and d15 = 4.23 pm V−1, respectively. In general, a larger Eg is usually accompanied by a smaller deff, but the largest one is 1.4 times that of reference AgGaS2 (d14 = 13.6 pm V−1 at 2050 nm), which is basically consistent with experimental results (about 1.1 times that of AgGaS2). In addition, the theoretical static Δn values for SrCdSiS4 are 0.158@2050 nm and 0.165@1064 nm, which are higher than those of SrGa2S4 (Δn = 0.147@2050 nm and 0.153@1064 nm) and sufficiently large to ensure PM features in both UV-vis and IR regions (Fig. 6b). Typically, a significant anisotropic structure is beneficial to produce a large Δn, that is, dual-site aliovalent substitution induces greater structural distortion from SrGa2S4 to SrCdSiS4. Meanwhile, these calculated values are larger than those of commercialized NLO materials, such as AgGaS2 (Δn = 0.039@2050 nm),72 ZnGeP2 (Δn = 0.04@2050 nm)72 and KDP (Δn = 0.034@1064 nm).20 Besides, the frequency-dependent refractive index diagrams mean that under the premise of PM determined at 2050 nm, the lower limit of the SHG output wavelength is 500 nm (Fig. 6c). Based on theoretical studies and experimental observations, we compared SrCdSiS4 with the illustrious IR-NLO crystal AgGaS2. As illustrated in the radar chart (Fig. 6d), the green colored shadow is larger than the gray indicating the superior performance of SrCdSiS4, including the PM feature, large Eg (ca. 3.61 eV), strong deff (ca. 1.1 × AgGaS2 at 2050 nm), giant LIDT (20.4 × AgGaS2), beneficial Δn (0.158@2050 nm) and broad transparent region (0.33–18.19 μm).
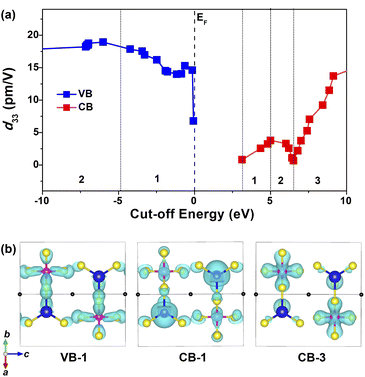
Furthermore, the cut-off energy dependences of the largest static d33 were analyzed based on a length-gauge formalism method73,74 with the purpose of revealing the intrinsic source of the SHG response. As shown in Fig. 7a, d33 values are trending upward in the range of VB-1 (dominated by the S-3p and Si-3p states, CB-1 (dominated by the S-3p and Cd-5s states) and CB-3 (dominated by the S-3p and Si-3p states). Distinctly, these three regions have a predominant impact on the overall NLO response. Considering the PDOS (Fig. 5d) and the relevant partial charge density profiles (Fig. 7b), the splendid SHG response comes from the collaborative effect of NLO-active [CdS4] and [SiS4] BBUs, i.e., the 2D [CdSiS4]2− alternating arrangement layer.
Conclusions
In conclusion, employing the ternary CS SrGa2S4 as the parent structure, a new NCS quaternary SrCdSiS4 was successfully designed and synthesized via a dual-site aliovalent substitution strategy, whose 2D layered structure consisted of alternately connected [CdS4] and [SiS4] BBUs through corner- and edge-sharing S atoms. Detailed performance analyses indicated that SrCdSiS4 could be a promising candidate for the UV-vis and IR-NLO crystal due to its advantages including a strong SHG intensity (deff = 4.5 × KDP at 1064 nm, or 1.1 × AgGaS2 at 2050 nm) with PM feature, a suitable birefringence (Δn(cal.) = 0.165 at 1064 nm, or 0.158 at 2050 nm), a wide transmission window (0.33–18.19 μm), a large Eg (3.61 eV), and an ultra-high LIDT (20.4 × AgGaS2). In addition, theoretical calculations reveal that the large Δn and strong deff are mainly contributed by the tetrahedral [CdS4] and [SiS4] NLO-active motifs that are nicely arranged in a most favorable stacking. Hopefully, such a simple and effective chemical design strategy can accelerate the discovery of novel NCS materials with advanced NLO properties.Data availability
Supporting data for this article is presented in the ESI.†Author contributions
Synthesis, characterization and original manuscript: H. D. Yang and M. Y. Ran; theoretical calculations: S. H. Zhou; experimental conception, supervision and manuscript editing: X. T. Wu, H. Lin and Q. L. Zhu. H. D. Yang and M. Y. Ran contributed equally to this work. All authors provided comments and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22175175, 21771179 and 21901246), Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR118), the Natural Science Foundation of Fujian Province (2019J01133) and the Youth Innovation Promotion Association CAS (2022303). The authors thank Prof. Bing-Xuan Li at FJIRSM for helping with the NLO measurements and Prof. Yong-Fan Zhang at Fuzhou University for helping with the DFT calculations.Notes and references
- F. J. Duarte, in Tunable Laser Applications, CRC Press, Boca Raton, FL, 2nd edn, 2008, ch. 2, pp. 9 and 12 Search PubMed.
- V. Petrov, Prog. Quantum Electron., 2015, 44, 1–106 CrossRef.
- V. A. Serebryakov, E. V. Boiko, N. N. Petrishchev and A. V. Yan, J. Opt. Technol., 2010, 77, 6–17 CrossRef CAS.
- X.-T. Wu and L. Chen, Structure–Property Relationships in Nonlinear Optical Crystals II The IR Region, Struct. Bonding, 2012, 145, 1–42 CrossRef.
- N. L. B. Sayson, T. Bi, V. Ng, H. Pham, L. S. Trainor, H. G. L. Schwefel, S. Coen, M. Erkintalo and S. G. Murdoch, Nat. Photonics, 2019, 13, 701–706 CrossRef CAS.
- L. Kang, M. Zhou, J. Yao, Z. Lin, Y. Wu and C. Chen, J. Am. Chem. Soc., 2015, 137, 13049–13059 CrossRef CAS PubMed.
- A. Harasaki and K. Kato, Appl. Phys., 1997, 36, 700–703 CAS.
- G. C. Catella, L. R. Shiozawa, J. R. Hietanen, R. C. Eckardt, R. K. Route, R. S. Feigelson, D. G. Cooper and C. L. Marquardt, Appl. Opt., 1993, 32, 3948–3951 CrossRef CAS PubMed.
- G. D. Boyd, E. Buehler and F. G. Storz, Appl. Phys. Lett., 1971, 18, 301–304 CrossRef CAS.
- H. Lin, W.-B. Wei, H. Chen, X.-T. Wu and Q.-L. Zhu, Coord. Chem. Rev., 2020, 406, 213150 CrossRef CAS.
- G. Zou and K. M. Ok, Chem. Sci., 2020, 11, 5404–5409 RSC.
- H. M. Zhou, L. Xiong, L. Chen and L. M. Wu, Angew. Chem., Int. Ed., 2019, 58, 9979–9983 CrossRef CAS.
- B. J. Song, Z. Ma, B. Li, X. T. Wu, H. Lin and Q. L. Zhu, Inorg. Chem., 2021, 60, 4357–4361 CrossRef CAS PubMed.
- L. T. Menezes, A. Assoud, W. Zhang, P. S. Halasyamani and H. Kleinke, Inorg. Chem., 2020, 59, 15028–15035 CrossRef CAS PubMed.
- H. Lin, Y.-Y. Li, M.-Y. Li, Z. Ma, L.-M. Wu, X.-T. Wu and Q.-L. Zhu, J. Mater. Chem. C, 2019, 7, 4638–4643 RSC.
- M.-M. Chen, Z. Ma, B.-X. Li, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, J. Mater. Chem. C, 2021, 9, 1156–1163 RSC.
- Y. N. Li, Y. Chi, Z. D. Sun, H. Xue, N. T. Suen and S. P. Guo, Chem. Commun., 2019, 55, 13701–13704 RSC.
- C. Liu, S. H. Zhou, Y. Xiao, C. Zhang, H. Lin and Y. Liu, J. Mater. Chem. C, 2021, 9, 15407–15414 RSC.
- C. Tang, W. Xing, F. Liang, M. Sun, J. Tang, Z. Lin, J. Yao, K. Chen, J. Wu, W. Yin and B. Kang, J. Mater. Chem. C, 2022, 10, 3300–3306 RSC.
- M.-Y. Ran, Z. Ma, H. Chen, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Mater., 2020, 32, 5890–5896 CrossRef CAS.
- X. H. Li, Z. H. Shi, M. Yang, W. Liu and S. P. Guo, Angew. Chem., Int. Ed., 2022, 61, e202115871 CAS.
- M.-M. Chen, S.-H. Zhou, W. Wei, M.-Y. Ran, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, ACS Mater. Lett., 2022, 4, 1264–1269 CrossRef CAS.
- Z.-X. Chen, Y.-N. Li, W.-D. Yao, W. Liu and S.-P. Guo, J. Alloys Compd., 2022, 899, 163255 CrossRef CAS.
- Y.-N. Li, Z.-X. Chen, W.-D. Yao, R.-L. Tang and S.-P. Guo, J. Mater. Chem. C, 2021, 9, 8659–8665 RSC.
- I. Chung and M. G. Kanatzidis, Chem. Mater., 2013, 26, 849–869 CrossRef.
- S.-P. Guo, Y. Chi and G.-C. Guo, Coord. Chem. Rev., 2017, 335, 44–57 CrossRef CAS.
- K. Wu and S. Pan, Coord. Chem. Rev., 2018, 377, 191–208 CrossRef CAS.
- P. Gong, F. Liang, L. Kang, X. Chen, J. Qin, Y. Wu and Z. Lin, Coord. Chem. Rev., 2019, 380, 83–102 CrossRef CAS.
- W. Wang, D. Mei, F. Liang, J. Zhao, Y. Wu and Z. Lin, Coord. Chem. Rev., 2020, 421, 213444 CrossRef CAS.
- L. Gao, J. Huang, S. Guo, Z. Yang and S. Pan, Coord. Chem. Rev., 2020, 421, 213379 CrossRef CAS.
- H. Chen, W.-B. Wei, H. Lin and X.-T. Wu, Coord. Chem. Rev., 2021, 448, 214154 CrossRef CAS.
- H. Chen, Y.-Y. Li, B. Li, P.-F. Liu, H. Lin, Q.-L. Zhu and X.-T. Wu, Chem. Mater., 2020, 32, 8012–8019 CrossRef CAS.
- Y. Zhang, Q. Bian, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Angew. Chem., Int. Ed., 2021, 61, e202115374 Search PubMed.
- Q. G. Yue, S. H. Zhou, B. Li, X. T. Wu, H. Lin and Q. L. Zhu, Inorg. Chem., 2022, 61, 1797–1804 CrossRef CAS PubMed.
- T. E. Peters and J. A. Baglio, J. Electrochem. Soc., 1972, 119, 230–236 CrossRef CAS.
- E. L. Simmons, Appl. Opt., 1975, 14, 1380–1386 CrossRef CAS PubMed.
- H. Lin, L. Chen, L. J. Zhou and L. M. Wu, J. Am. Chem. Soc., 2013, 135, 12914–12921 CrossRef CAS PubMed.
- M. C. Ohmer and R. Pandey, MRS Bull., 1998, 23, 16–22 CrossRef CAS.
- L. Bai, Z. Lin, Z. Wang, C. Chen and M. H. Lee, J. Chem. Phys., 2004, 120, 8772–8778 CrossRef CAS.
- M.-Y. Li, B. Li, H. Lin, Z. Ma, L.-M. Wu, X.-T. Wu and Q.-L. Zhu, Chem. Mater., 2019, 31, 6268–6275 CrossRef CAS.
- Y. Chu, P. Wang, H. Zeng, S. Cheng, X. Su, Z. Yang, J. Li and S. Pan, Chem. Mater., 2021, 33, 6514–6521 CrossRef CAS.
- J. Yao, D. Mei, L. Bai, Z. Lin, W. Yin, P. Fu and Y. Wu, Inorg. Chem., 2010, 49, 9212–9216 CrossRef CAS PubMed.
- M.-Y. Li, Z. Ma, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Mater., 2020, 32, 4331–4339 CrossRef CAS.
- Z. Li, S. Zhang, Z. Huang, L.-D. Zhao, E. Uykur, W. Xing, Z. Lin, J. Yao and Y. Wu, Chem. Mater., 2020, 32, 3288–3296 CrossRef CAS.
- M.-Y. Ran, S.-H. Zhou, B. Li, W. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Mater., 2022, 34, 3853–3861 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- M.-J. Zhang, X.-M. Jiang, L.-J. Zhou and G.-C. Guo, J. Mater. Chem. C, 2013, 1, 4754–4760 RSC.
- Q. Q. Liu, X. Liu, L. M. Wu and L. Chen, Angew. Chem., Int. Ed., 2022, 62, e202205587 Search PubMed.
- Y. Zhang, D. Mei, Y. Yang, W. Cao, Y. Wu, J. Lu and Z. Lin, J. Mater. Chem. C, 2019, 7, 8556–8561 RSC.
- Y. Guo, F. Liang, Z. Li, W. Xing, Z. S. Lin, J. Yao, A. Mar and Y. Wu, Inorg. Chem., 2019, 58, 10390–10398 CrossRef CAS PubMed.
- W. Yin, A. K. Iyer, C. Li, J. Yao and A. Mar, J. Alloys Compd., 2017, 708, 414–421 CrossRef CAS.
- Y. Dou, Y. Chen, Z. Li, A. K. Iyer, B. Kang, W. Yin, J. Yao and A. Mar, Cryst. Growth Des., 2019, 19, 1206–1214 CrossRef CAS.
- Y.-J. Lin, R. Ye, L.-Q. Yang, X.-M. Jiang, B.-W. Liu, H.-Y. Zeng and G.-C. Guo, Inorg. Chem. Front., 2019, 6, 2365–2368 RSC.
- W. Xing, N. Wang, Y. Guo, Z. Li, J. Tang, K. Kang, W. Yin, Z. Lin, J. Yao and B. Kang, Dalton Trans., 2019, 48, 17620–17625 RSC.
- Y. Guo, F. Liang, W. Yin, Z. Li, X. Luo, Z.-S. Lin, J. Yao, A. Mar and Y. Wu, Chem. Mater., 2019, 31, 3034–3040 CrossRef CAS.
- N. Zhen, K. Wu, Y. Wang, Q. Li, W. Gao, D. Hou, Z. Yang, H. Jiang, Y. Dong and S. Pan, Dalton Trans., 2016, 45, 10681–10688 RSC.
- X. Pang, R. Wang, X. Che and F. Huang, J. Solid State Chem., 2021, 297, 122092 CrossRef CAS.
- W. Xing, C. Tang, N. Wang, C. Li, Z. Li, J. Wu, Z. Lin, J. Yao, W. Yin and B. Kang, Inorg. Chem., 2020, 59, 18452–18460 CrossRef CAS.
- Y.-J. Lin, B.-W. Liu, R. Ye, X.-M. Jiang, L.-Q. Yang, H.-Y. Zeng and G.-C. Guo, J. Mater. Chem. C, 2019, 7, 4459–4465 RSC.
- M. Yan, Z.-D. Sun, W.-D. Yao, W. Zhou, W. Liu and S.-P. Guo, Inorg. Chem. Front., 2020, 7, 2451–2458 RSC.
- F. Hou, D. Mei, Y. Zhang, F. Liang, J. Wang, J. Lu, Z. Lin and Y. Wu, J. Alloys Compd., 2022, 904, 163944 CrossRef CAS.
- K. Wu, X. Su, Z. Yang and S. Pan, Dalton Trans., 2015, 44, 19856–19864 RSC.
- F.-Y. Yuan, C.-S. Lin, Y.-Z. Huang, H. Zhang, A.-Y. Zhou, G.-L. Chai and W.-D. Cheng, J. Solid State Chem., 2021, 302, 122352 CrossRef CAS.
- G. Li, Y. Chu and Z. Zhou, Chem. Mater., 2018, 30, 602–606 CrossRef CAS.
- J.-H. Zhang, D. J. Clark, J. A. Brant, K. A. Rosmus, P. Grima, J. W. Lekse, J. I. Jang and J. A. Aitken, Chem. Mater., 2020, 32, 8947–8955 CrossRef CAS.
- B. W. Liu, H. Y. Zeng, X. M. Jiang, G. E. Wang, S. F. Li, L. Xu and G. C. Guo, Chem. Sci., 2016, 7, 6273–6277 RSC.
- V. Nguyen, B. Ji, K. Wu, B. Zhang and J. Wang, Chem. Sci., 2022, 13, 2640–2648 RSC.
- K. Burke, J. Chem. Phys., 2012, 136, 150901 CrossRef PubMed.
- K. Govaerts, R. Saniz, B. Partoens and D. Lamoen, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 235210 CrossRef.
- N. E. Christensen, A. Svane and E. L. Peltzer y Blancá, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 014109 CrossRef.
- D. A. Kleinman, Phys. Rev., 1962, 126, 1977–1979 CrossRef CAS.
- Z. Qian, Q. Bian, H. Wu, H. Yu, Z. Lin, Z. Hu, J. Wang and Y. Wu, J. Mater. Chem. C, 2022, 10, 96–101 RSC.
- C. Aversa and J. E. Sipe, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 14636–14645 CrossRef CAS.
- S. N. Rashkeev, W. Lambrecht and B. Segall, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 3905–3919 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2167628 and 2168083. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc03760b |
| ‡ H. D. Yang and M. Y. Ran contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |