The rare-earth derivant of mixed-polyoxoniobate clusters with high proton release capacity†
Haiying
Wang‡
a,
Xiaohan
Xu‡
b,
Qianqian
Shang
a,
Kexing
Xiao
b,
Jiahui
Chen
b,
Yuqing
Yang
b,
Ehsan
Raee
b,
Dongdi
Zhang
 *a,
Jingyang
Niu
*a,
Jingyang
Niu
 *a and
Tianbo
Liu
*a and
Tianbo
Liu
 *b
*b
aHenan Key Laboratory of Polyoxometalate Chemistry, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, P. R. China. E-mail: ddzhang@henu.edu.cn; jyniu@henu.edu.cn
bSchool of Polymer Science and Polymer Engineering, The University of Akron, Akron, Ohio 44325, USA. E-mail: tliu@uakron.edu
First published on 31st October 2022
Abstract
A family of rare-earth derivant mixed-polyoxoniobate clusters K12(NH4)10[{Nb12P4W24O122}2{Ln(H2O)5}4{Nb4O4(OH)6}]·xH2O (Ln = Sm, Eu, Tb, Dy, Er, Tm and Yb for 1–7, abbreviated as {Ln4Nb28}) were synthesized and structurally characterized by single-crystal X-ray diffraction, elemental analyses, IR spectroscopy and TG analyses. Containing four Wells–Dawson [Nb6P2W12O62]12−, a {Nb4O6} core, and four LnIII ions, the polyanions in 1–7 are a group of rare-earth derivants of phosphoniobotungstates. These water-soluble clusters behave as weak acids with good stability and high proton release capacity depending on the pH. Each cluster carries ∼22 negative charges in the aqueous solution without any deprotonation with the pH the same as that used for deionized water. Upon the introduction of bases, they get deprotonated gradually and each anion cluster can release up to 20 protons from its 20 coordinated water ligands. The pKa values of these acidic clusters with different degrees of deprotonation range from ∼8.3 to ∼10.5. Moreover, these clusters demonstrate an increasing deprotonation efficiency with the decreasing ionic radius of incorporated LnIII ions, which could be attributed to the lanthanide contraction. In other words, the {Ln4Nb28} macroanion clusters with smaller LnIII centers are easier to deprotonate due to their shorter and stronger Ln–O bonds. This is the first study that focuses on the effect of lanthanide contraction on proton release in polyoxometalate chemistry. The {Ln4Nb28} clusters with good stability, high proton release capacity, and controllable deprotonation efficiency provide models for the understanding of protonated polyelectrolyte solutions, and the design and applications of polyoxometalate-based protonated materials.
Introduction
Polyoxometalates (POMs), a family of molecular clusters composed of metal oxide polyhedra as building blocks, have been applied broadly in catalysis, photo-electronic/magnetic materials, POMs/polymer composite materials, and biologically active materials.1,2 Many types of POM clusters are macroanions in the aqueous solution, while some others are neutral or macrocations. It is particularly interesting that besides the inherent charges on the clusters, they can sometimes experience additional protonation or deprotonation in the aqueous solution, with their coordinated water ligands being a major source. Such clusters can behave like weak Brønsted acids, which affects their solution behaviors including solubility, oxidation and reduction, and their consequent self-assembly into “blackberry”-type structures. For instance, we systematically investigated the pH-dependent deprotonations of a series of Keplerate-clusters including {Mo72V30}, {Mo72Cr30} and {Mo72Fe30}, and their self-assembly processes in solution.3,4 In addition, the catalytic activities of some POM clusters could be triggered by and rely on the deprotonation/protonation of their aqua and/or hydroxyl ligands, due to the effect of deprotonation/protonation on the surrounding coordination bonds.5,6Moreover, with deprotonation/protonation as one of the most widely applied and fundamental stimuli, the clusters can be used as model systems to help understand and design the deprotonation/protonation of functional stimuli-responsive materials, such as protonated polymers and proteins,7–9 and the molecular hybrid of POMs and metal nanoclusters.10
Some POM molecular clusters with coordinated water ligands can reversibly release protons from their water ligands into the aqueous solution. They can therefore be treated as weak acids with multiple deprotonation sites. Such a deprotonation process is usually pH dependent, as a feature of weak acids.3,4,6,11,12 In some cases, with a large number of water ligands, the deprotonation capacity could be rather high. For example, a molecular cluster with the formula of K41[(P2W12Nb6O62)6{Mn3(OH)3(H2O)6}4{Mn3Na(H2O)16}]·26H2O, is an extremely weak acid with a high proton release capacity of up to 40 protons.11 Moreover, the deprotonation potential relates to the geometry of the acidic sites. For some Keplerate-type clusters, the water ligands staying on the external surface of the cluster demonstrate pH-dependent deprotonation, while those ligands inside the Keplerate capsules could not release free protons into the solution because protons could not pass through the negatively charged surface pores on the surface of clusters.3 Another type of deprotonation is enabled by the cavity of clusters that can accommodate a few protons via hydrogen bonds.13
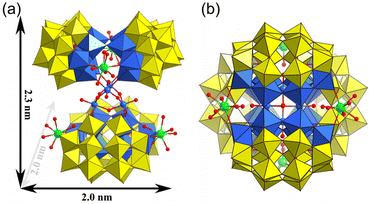
Herein, a family of rare-earth derivant mixed-polyoxoniobate clusters has been presented as a tetramer with a general formula of K12(NH4)10[{Nb12P4W24O122}2{Ln(H2O)5}4-{Nb4O4(OH)6}]·xH2O (Ln = Sm x = 85, Eu x = 65, Tb x = 85, Dy x = 85, Er x = 85, Tm x = 85, Yb x = 85 for 1–7, abbreviated as {Ln4Nb28}), and a dimension of 2.3 × 2.0 × 2.0 nm3 (Fig. 1). As far as we know, they are the rare examples of rare-earth derivatives of phosphoniobotungstates. It would be interesting to examine if such clusters can deprotonate their coordinated water ligands, and if they can, whether such processes are pH dependent as speculated. Moreover, this series of samples provide a valuable opportunity to compare the effect of lanthanide ions incorporated into the clusters – they are expected to demonstrate very similar chemical features; a noticeable difference among them would be their ionic sizes due to the so-called lanthanide contraction effect – leading to smaller ionic size for heavier Ln-series elements.
Results and discussion
Structure characterization
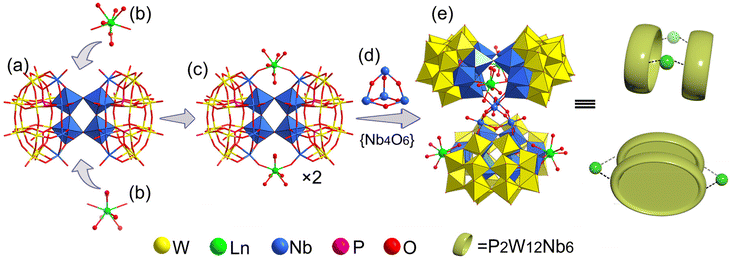
Single-crystal X-ray diffraction analysis (Table S1†) reveals that the polyanion {Ln4Nb28} crystallizes in the orthorhombic system Fddd space-group. The polyanion in 1–7 is a rare-earth derivant tetramer based on mixed-addendum niobotungstate containing four Wells–Dawson [Nb6P2W12O62]12− (Nb6P2W12), a {Nb4O6} core, four Ln3+ ions, 12 K+, 10 NH4+ and crystal molecules. The six lacunary sites of [P2W12O48]14− are occupied by six {NbO6} octahedra forming the saturated mixed-Wells–Dawson {Nb6P2W12} unit (Fig. S1†). Two [Nb6P2W12O62]12− units are linked together by equatorial Nb–O–Nb bridges forming a {Nb12P4W24O122} dimer (Fig. 2a). The terminal oxygens (Ot) of {NbO6} octahedra have higher nucleophilicity relative to {WO6} octahedra because of the uneven charge density distribution which is caused by the different oxidation states of W6+ and Nb5+, resulting in the easier coordination ability with Ln3+ ions. Two Ln3+ ions are located at both ends of the dimer and are connected to the polar {NbO6} octahedron by Nb–O–Ln bridges (Fig. 2b and c). The Ln3+ derived dimers face each other, crisscross, and are connected by a central {Nb4O6} core (Fig. 2d) via eight Nb–O–Nb bridges, and finally forming the tetrameric structure of the polyanion (Fig. 2e). Actually, the polyanion can also be viewed as a Ln3+ ion derivant of the structure we reported in 2014.14 To our knowledge, this is a rare report about the example of a rare-earth derivant based on niobotungstate chemistry.15 In addition, the polyanion can be extended into a purely inorganic three-dimensional metal frame if the peripheral K+ cations act as linkers (Fig. S2†). All of the Nb atoms in the polyanion show hexa-coordinate octahedron configuration. For the Ln3+ ions, all of them are coordinated with two bridge oxygen (μ2-O) and six terminal aqua ligands resulting in the eight-coordinate two-capped triangular prism geometry (Fig. S3†). However, crystallographic refinement indicates that the occupancy factors of O5 W and O6 W are both 0.5, leading to [Ln(H2O)5]3+. The Ln–O bond lengths are reduced, which are generally in agreement with the ion radius trend in the lanthanide elements (Table S2†).Bond valence sum (BVS) calculations14 are carried out on all the Ln, Nb, P and W centers (Table S3†) and the results show the 3+, 5+, 5+ and 6+ oxidation states, respectively. The BVS values of all the O oxygen atoms indicate that the μ2-O of O64 and O65 are monoprotonated with values in the range of 0.79–1.44 resulting in the {Nb4O4(OH)6} core. It should be noted that the molecular formula was finally determined according to the crystal analysis, charge balance, ICP-OES, CHN elemental analysis and TGA.
Deprotonation in aqueous solution
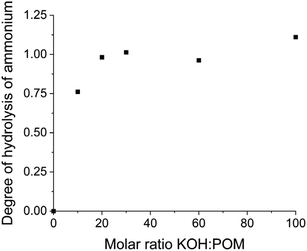
Each {Ln4Nb28} cluster carries ∼22 negative charges with 12 K+ and 10 NH4+ counterions. When dissolved in water, they release all their counterions into the solution (Table S4†). The pH of these solutions (at 1.0 mg mL−1) is ∼5.70, suggesting that there is no protonation/deprotonation from the clusters in pure water. This is further confirmed by conductivity measurements (Table S4†). When KOH was gradually introduced into the solutions of the clusters, the pH increment during this titration was slower than introducing the same amount of KOH into pure water. For example, the pH of 1.0 mg mL−1 {Sm4Nb28} solution containing 10 equiv. of KOH (molar ratio of KOH/clusters = 10) is 8.39; while the same amount of KOH in pure water leads to a pH of 10.73 (Fig. 3). This indicates that some protons are released from the clusters to neutralize the OH−, i.e., the clusters act as weak acids with a buffering effect by showing a pH-dependent proton release feature.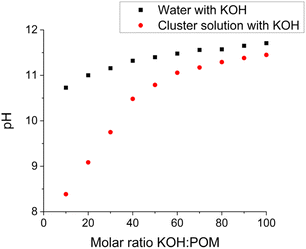 | ||
| Fig. 3 The pH response of introducing KOH into pure water and 1.0 mg mL−1 {Sm4Nb28} cluster solution. | ||
The number of protons released per cluster can be calculated by the pH difference between the aqueous solution of the clusters and pure water (reference) when the same amount of KOH is added. The solution preparation and pH measurements were conducted under a N2 atmosphere (in a glovebox). The deionized water used in experiments was boiled in advance to avoid CO2 dissolution neutralizing KOH. The deprotonation process of the {Sm4Nb28} cluster is given in Fig. 4 as an example. With the introduction of up to 30 equiv. of KOH, the titration curve shows that the majority of hydroxide introduced is neutralized quickly and linearly. In this stage (0–30 equiv. of KOH), there is a linear relationship between the amount of KOH introduced and the number of deprotonations from each cluster with a slope of 0.73. With the further addition of KOH, 40 equiv. of KOH leads to the final deprotonation of ∼30 ± 3 protons, and the clusters do not further release any protons even with excess KOH. All solutions do not display any colour change or precipitation during the experiments for up to one year, which suggests the good stability of these acidic clusters in highly basic environments. Besides, the polyanions can remain intact throughout the titration experiment, as demonstrated by negative-ion ESI mass spectra (Fig. S5–S7, Table S13†). It is noted that the deprotonation process of clusters during the period of addition of 30–40 equiv. of KOH is not a linear growth anymore, showing that it is more difficult for the highly negatively charged clusters to release protons. Nevertheless, not all of the 30 released protons stem from the anion cluster. As shown in Fig. 5, the hydrolysis of NH4+ ions would be expected to occur in a basic solution. The degree of hydrolysis can be determined by the difference in pH between the aqueous solution of NH4Cl containing KOH (with a concentration of NH4+ the same as that in {Ln4Nb28} solution) and water containing the same amount of KOH. Assuming that the NH4+ ions experience the same extent of hydrolysis in the NH4Cl solution and the cluster solutions, the number of protons released from the anion in the {Sm4Nb28} cluster can be calculated by subtracting the contribution of NH4+, as shown in Table S5.† Consequently, 10 of the 30 protons are attributed to the hydrolysis of NH4+ ions, indicating that each anion cluster can release up to ∼20 protons eventually.
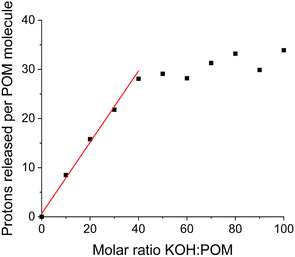 | ||
| Fig. 4 The number of protons released per molecular cluster (including the protons contributed by the hydrolysis of ammonium ions) versus the molar ratio of KOH introduced to {Sm4Nb28} clusters. | ||
All members of this family of rare-earth clusters demonstrate a similar deprotonation behaviour: they release protons linearly in an early stage with up to 30 equiv. of KOH added and stop deprotonating after releasing ∼20 protons from the original anion with the introduction of ∼40 equiv. of KOH (Fig. S4†). These 20 protons released easily from the macroanion suggest the most possible deprotonation sites, i.e., the 20 coordinated water ligands on the cluster.
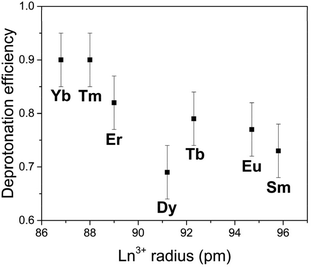
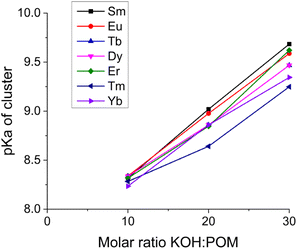
The deprotonation capacity of these rare-earth derivant clusters was qualitatively explored by comparing their deprotonation efficiency in the linear stage of deprotonation (with the addition of 0–30 equiv. of KOH). The deprotonation efficiency is calculated by the ratio of the number of protons released per entire POM molecule (from both the anionic cluster and the ammonium ions) over the molar ratio of KOH/cluster, i.e., the slope of the linear part in Fig. 4. The deprotonation efficiency suggests the number of protons that an entire cluster can provide with the introduction of 1 equiv. of KOH. As shown in Fig. 6, with the decreasing ionic radius of the LnIII center in the cluster, the deprotonation efficiency increases monotonically from 0.73 (Sm) to 0.90 (Yb). In other words, a smaller LnIII center leads to a higher deprotonation efficiency. We attribute this observation to the continuously increasing charge density, i.e., Lewis acidity, of LnIII in the coordination complex [LnIII(H2O)5]3+ due to the well-known lanthanide contraction.16 A higher charge density of LnIII leads to stronger coordination between LnIII and O, making the protons linked to the oxygen atoms easier to be released from the cluster. This trend of deprotonation efficiency is consistent with our observation that the Ln–O bond length decreases with the decreasing ionic radius of LnIII (Table S2†). It also agrees with some earlier reports that smaller LnIII results in a shorter Ln–O bond length in POM clusters.17,18
The deprotonation processes of the {Ln4Nb28} clusters are quite different from typical polyprotic acids which have multiple distinguishable titration stages that may overlap with each other. The linearly increasing pH during the titration shown in Fig. 3 suggests the small and continuous increment in the successive acid dissociation constant (pKa) of the clusters and the overlap between the pH ranges of the dissociation equilibria of the clusters with different degrees of deprotonation. In other words, the multiple deprotonation sites on each cluster are independent of each other. However, with more and more deprotonations occurring on the same cluster, the departure of future protons becomes slightly more difficult. This deprotonation feature is consistent with our observation of another POM cluster.11 Overall, it reveals a feature that the cluster's deprotonation behavior can be briefly described by one pKa value; or to be more accurate, a series of increasing pKa values corresponding to an increasing amount of the added base. Assuming that the deprotonation of clusters has no impact on the hydrolysis of the NH4+, the pKa values of clusters can be calculated based on the deprotonation efficiency after subtracting the contribution of NH4+, as shown in Fig. 7. The clusters with a few deprotonations have acidities slightly higher than NH4+ (pKa of NH4+ = 9.25), while the clusters after releasing a number of protons, e.g., ∼12 protons with 30 equiv. of KOH, are less acidic than NH4+. The cluster solution with 40 equiv. of KOH at pH = 10.5 can be used as an approximation of pKa of the cluster carrying one acidic proton, because 40 equiv. of KOH is just right for each anion cluster to release ∼20 protons and all NH4+ ions have already hydrolyzed with ∼20 equiv. of KOH. Overall, the pKa values of these clusters with different deprotonations are in the range of 8.3–10.5 roughly. A sample pKa calculation is provided in the ESI.†
Conclusions
In summary, the synthesis and structures of the rare examples of rare-earth derivants based on hexalacunary niobotungstate are reported. The polyanions in this new family are rare-earth derivant tetramers based on Nb/W mixed-addendum POM clusters. Depending on the pH, the clusters exhibit a high proton release capacity. Each cluster can deprotonate up to 20 protons from their coordinated water ligands in the basic solution. Moreover, these clusters with different LnIII centers demonstrated an increased deprotonation efficiency with a smaller ionic radius of LnIII due to lanthanide contraction. A smaller LnIII center with a higher charge density forms shorter/stronger coordination bonds with the oxygen in the water molecule, thus making the protons in the coordinated water ligands easier to be released. The pKa values of these clusters slightly increase from ∼8.3 to ∼10.5 when more base was added (and consequently more protons were deprotonated), with the clusters having smaller LnIII centers exhibiting pKa values slightly lower than the others. The understanding of deprotonation features of such POM clusters provides a reference for both fundamental studies and applications of (POM-based) protonated materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21671056 and 22071045), the Excellent Youth Science Fund Project of Henan Province (202300410042) and Henan University. T. L. acknowledges support from the National Science Foundation (CHE2132178) and The University of Akron.References
- Y.-F. Song and R. Tsunashima, Recent advances on polyoxometalate-based molecular and composite materials, Chem. Soc. Rev., 2012, 41, 7384–7648 RSC.
- D.-L. Long, E. Burkholder and L. Cronin, Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices, Chem. Soc. Rev., 2007, 36, 105–121 RSC.
- M. L. Kistler, T. Liu, P. Gouzerh, A. M. Todea and A. Müller, Molybdenum-oxide based unique polyprotic nanoacids showing different deprotonations and related assembly processes in solution, Dalton Trans., 2009, 26, 5094–5100 RSC.
- T. Liu, B. Imber, E. Diemann, G. Liu, K. Cokleski, H. Li, Z. Chen and A. Müller, Deprotonations and Charges of Well-Defined {Mo72Fe30} Nanoacids Simply Stepwise Tuned by pH Allow Control/Variation of Related Self-Assembly Processes, J. Am. Chem. Soc., 2006, 128, 15914–15920 CrossRef CAS PubMed.
- A. Sartorel, M. Carraro, A. Bagno, G. Scorrano and M. Bonchio, Asymmetric Tetraprotonation of γ-[(SiO4)W10O32]8− Triggers a Catalytic Epoxidation Reaction: Perspectives in the Assignment of the Active Catalyst, Angew. Chem., Int. Ed., 2007, 46, 3255–3258 CrossRef CAS PubMed.
- K. Sugahara, S. Kuzuya, T. Hirano, K. Kamata and N. Mizuno, Reversible Deprotonation and Protonation Behaviors of a Tetra-Protonated γ-Keggin Silicodecatungstate, Inorg. Chem., 2012, 51, 7932–7939 CrossRef CAS PubMed.
- D. Patni and S. K. Jha, Protonation–Deprotonation Switch Controls the Amyloid-like Misfolding of Nucleic-Acid-Binding Domains of TDP-43, J. Phys. Chem. B, 2021, 125, 8383–8394 CrossRef CAS PubMed.
- Q. Zhang, F. Xia, T. Sun, W. Song, T. Zhao, M. Liu and L. Jiang, Wettability switching between high hydrophilicity at low pH and high hydrophobicity at high pH on surface based on pH-responsive polymer, Chem. Commun., 2008, 10, 1199–1201 RSC.
- J. Alves da Silva, J. Felcman, C. C. Lopes, R. S. C. Lopes and J. D. F. Villar, Study of the protonation/deprotonation sequence of two polyamines: bis-[(2S)-2-pyrrolidinylmethyl] ethylenediamine and spermidine by 1H and 13C nuclear magnetic resonance, Spectrosc. Lett., 2002, 35, 643–661 CrossRef.
- S. K. Yamaguchi, K. Yonesato, S. Yamazoe, S. Kikkawa, D. Yokogawa, K. Yamaguchi and K. Suzuki, Variable control of the electronic states of a silver nanocluster via protonation/deprotonation of polyoxometalate ligands, Chem. Sci., 2022, 13, 5557–5561 RSC.
- D. Zhang, H. Li, C. Li, Z. Wang, T. Li, N. Li, M. Cheng, J. Wang, J. Niu and T. Liu, A large molecular cluster with high proton release capacity, Chem. Commun., 2020, 56, 12849–12852 RSC.
- L. Ruhlmann, L. Nadjo, J. Canny, R. Contant and R. Thouvenot, Di- and Tetranuclear Dawson-Derived Sandwich Complexes: Synthesis, Spectroscopic Characterization, and Electrochemical Behavior, Eur. J. Inorg. Chem., 2002, 2002, 975–986 CrossRef.
- T. Minato, K. Suzuki, K. Kamata and N. Mizuno, Synthesis of α-Dawson-Type Silicotungstate [α-Si2W18O62]8− and Protonation and Deprotonation Inside the Aperture through Intramolecular Hydrogen Bonds, Chem. – Eur. J., 2014, 20, 5946–5952 CrossRef CAS PubMed.
- D. Zhang, Z. Liang, S. Xie, P. Ma, C. Zhang, J. Wang and J. Niu, A New Nb28 Cluster Based on Tungstophosphate, [{Nb4O6(OH)4}{Nb6P2W12O61}4]36−, Inorg. Chem., 2014, 53, 9917–9922 CrossRef CAS PubMed.
- Z. Weng, Y. Ren, X. Li, B. Yue and H. He, Aggregation of Nb/W mixed addenda Dawson-type polyoxometalates units and lanthanide ions: Synthesis, characterization and electrocatalysis, Polyhedron, 2021, 195, 114974 CrossRef CAS.
- Y. Wu, S. Morton, X. Kong, G. S. Nichol and Z. Zheng, Hydrolytic synthesis and structural characterization of lanthanide-acetylacetonato/hydroxo cluster complexes – A systematic study, Dalton Trans., 2011, 40, 1041–1046 RSC.
- K. Y. Wang, B. S. Bassil, Z. Lin, I. Römer, S. Vanhaecht, T. N. Parac-Vogt, C. Sáenz De Pipaón, J. R. Galán-Mascarós, L. Fan, J. Cao and U. Kortz, Ln12-Containing 60-Tungstogermanates: Synthesis, Structure, Luminescence, and Magnetic Studies, Chem. – Eur. J., 2015, 21, 18168–18176 CrossRef CAS PubMed.
- R. Copping, A. J. Gaunt, I. May, M. J. Sarsfield, D. Collison, M. Helliwell, I. S. Denniss and D. C. Apperley, Trivalent lanthanide lacunary phosphomolybdate complexes: a structural and spectroscopic study across the series [Ln(PMo11O39)2]11−, Dalton Trans., 2005, 1256–1262 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2194738–2194744. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi01953a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |