 Open Access Article
Open Access ArticleZirconia-based nanomaterials: recent developments in synthesis and applications
Nisha
Kumari
a,
Shweta
Sareen
b,
Meenakshi
Verma
ac,
Shelja
Sharma
a,
Ajay
Sharma
ac,
Harvinder Singh
Sohal
 a,
S. K.
Mehta
a,
S. K.
Mehta
 b,
Jeongwon
Park
b,
Jeongwon
Park
 *d and
Vishal
Mutreja
*d and
Vishal
Mutreja
 *a
*a
aDepartment of Chemistry, University Institute of Science, Chandigarh University, Mohali, Punjab-140 413, India. E-mail: vishal.mutreja@gmail.com
bDepartment of Chemistry, Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh-160 014, India
cDepartment of UCRD, Chandigarh University, Gharuan, Mohali, Punjab-140 413, India
dDepartment of Electrical and Biomedical Engineering, University of Nevada, Reno, NV 89557, USA. E-mail: jepark@unr.edu
First published on 23rd August 2022
Abstract
In the last decade, the whole scientific community has witnessed great advances and progress in the various fields of nanoscience. Among the different nanomaterials, zirconia nanomaterials have found numerous applications as nanocatalysts, nanosensors, adsorbents, etc. Additionally, their exceptional biomedical applications in dentistry and drug delivery, and interesting biological properties, viz. anti-microbial, antioxidant, and anti-cancer activity, have further motivated the researchers to explore their physico-chemical properties using different synthetic pathways. With such an interest in zirconia-based nanomaterials, the present review focuses systematically on different synthesis approaches and their impact on the structure, size, shape, and morphology of these nanomaterials. Broadly, there are two approaches, viz., chemical synthesis which includes hydrothermal, solvothermal, sol–gel, microwave, solution combustion, and co-precipitation methods, and a greener approach which employs bacteria, fungus, and plant parts for the preparation of zirconia nanoparticles. In this review article, the aforementioned methods have been critically analyzed for obtaining specific phases and shapes. The review also incorporates a detailed survey of the applications of zirconia-based nanomaterials. Furthermore, the influence of specific phases, morphology, and the comparison with their counterpart composites for different applications have also been included. Finally, the concluding remarks, prospects and possible scope are given in the last section.
1 Introduction
Zirconium is a tough transition metal which has found boundless applications when present in its oxide form, known as zirconia or zirconium oxide. Zirconia is also known as ceramic steel owing to its intrinsic hardness, abrasiveness, high melting point, and low frictional resistance. In the nanoscale, it becomes immensely valuable owing to its high thermal stability, luminescence, refractive index, chemical stability, high specific area, biocompatibility, and exhibiting significant antibacterial, antioxidant, and antifungal properties. Such outstanding characteristics have motivated the scientific community to explore zirconia-based nanomaterials in a wide range of technological fields such as functional materials, viz., catalysts,1 sensors2 and semiconductor devices,3 and structural materials, viz., coating on cutting tools, ceramics,4 implants,5etc. Apart from that, it can also be employed as a dielectric, electro-optic, and piezoelectric material due to its favourable optical and electrical properties.Zirconia exists in three crystal phases, cubic, tetragonal, and monoclinic, in a typical air atmosphere and at distinct temperature ranges. The pure monoclinic phase is stable up to 1100 °C, the tetragonal phase is stable in the range of 1100–2370 °C and the cubic phase exists at temperatures over 2370 °C.6 Among the different phases, the cubic and tetragonal crystals are found to be unstable in bulk forms at ambient temperature. Therefore, considerable efforts have been dedicated to stabilizing the unstable cubic and tetragonal crystal phases by doping with several divalent or trivalent cationic stabilizers such as Ca2+, Mg2+, Sc3+, and Y3+ or by the reduction of particle/grain size to the nanometre scale.7–10 Moreover, the interconversion of phases such as cubic to tetragonal and subsequently tetragonal to monoclinic takes place at higher temperatures and also affects the volume of zirconia nanomaterials significantly which ultimately limits their applicability in different fields such as catalysis, coatings etc. Another limitation of zirconia nanoparticles has been their tendency to undergo agglomeration during their synthesis. Furthermore, limited methods have been reported for the synthesis of anisotropic zirconia nanoparticles with the desired size range. In this regard, it appears quite worthwhile to critically analyze the various methods, routes, and reaction conditions for the synthesis of such versatile nanomaterials. Therefore, the present review article contributes toward a comprehensive analysis of the state-of-art specific synthetic methods for the preparation of zirconia nanomaterials for the desired applications and provides a database for novel future research perspectives. There are two major sections in this review paper; the first section involves details about different synthesis methods of zirconia-based nanomaterials and a critical analysis of the effect of various reaction parameters and methods on size, shape, and morphology, and in the second part, a variety of applications of these materials have been included. Finally, perspectives and scope of further developments of such materials have been given.
1.1 Synthesis of zirconia nanomaterials
The research interest in synthesizing ZrO2 nanoparticles by various methods has increased considerably in the last decade owing to their diverse applications. There have been interesting findings in terms of shape, size, morphology, yield, and purity when different methods were employed. Broadly, there are two approaches: the chemical method and greener synthesis which are discussed below:| Chemical method | Materials | Solvent | pH | Reaction temperature/power and time | Thermal treatment | Crystal system, shape, size range, average size | Ref. |
|---|---|---|---|---|---|---|---|
| a RT: room temperature. | |||||||
| Microwave-assisted method | Zirconium oxychloride octahydrate, sodium hydroxide | — | — | 80 °C, 12 min | 400 °C, 2 h | Mixed phase (monoclinic and tetragonal), spherical (8–10) nm, 8.8 nm, – | 41 |
| Zirconium oxychloride, anhydrous citric acid | — | 2 | –, 2 min | 450 °C, 4 h | Tetragonal, –, –,∼5–10 nm | 42 | |
| Zirconium oxychloride, sodium hydroxide | — | 12 | –, 6 min | 600 °C, 4 h | Monoclinic, –, 32–38 nm, – | 43 | |
| Zirconyl nitrate monohydrate, L-serine amino acid | Water | — | 800 W, 60 s | 400 °C, 600 °C, 800 °C, – | Cubic, –, –, 70, 76 and 77 nm | 44 | |
| Zirconium oxychloride octahydrate | Water | — | 100–1000 W, – | –, – | Tetragonal, –, –, – | 19 | |
| Zirconium oxychloride octahydrate, honey | Water | — | ∼100 W, – | –, – | Tetragonal, –, –, ∼26 nm | 18 | |
| Zirconyl oxynitrate | 1,4-Butanediol | — | 800 W, 10 min | –, – | Tetragonal, –, –, ∼10 nm | 20 | |
| Hydrothermal method | ZrO(NO3)2·xH2O, sodium hydroxide | — | — | 150 °C, 4 h | 110 °C, 90 min | Monoclinic, –, –25 nm | 23 |
| Zirconyl chloride octahydrate, glucose monohydrate | Water | — | 180 °C, 20 h | 550 °C, 4 h | –, sphere, –8 nm, – | 26 | |
| Commercial zirconia, sodium hydroxide | — | — | 150 °C, 85 h | –, – | Mixed phases (monoclinic, tetragonal and cubic), –, –, 15–30 nm | 12 | |
| Zirconium oxychloride, sodium hydroxide | Ethanol and water | 14 | –, – | 400 °C and 500 °C, 2 h and 0.5 h | Tetragonal, –, 2–11 and 3–12 nm, 4.5 and 6 nm | 21 | |
| Zirconyl nitrate, CTAB/SDS/triton, ammonium hydroxide | — | 12 | 90 °C, 12 h | 500 °C, 2 h | –, varied, varied, – | 27 | |
| Sol–gel | Zirconium(IV) propoxide, acetic acid, nitric acid | Isopropanol | 2.5 | –, – | 700 °C, 1 h | Monoclinic, –, 25.39 nm, – | 32 |
| Zirconium(IV) propoxide, nitric acid | — | — | –, 1 h | 500 °C, 2 h | Tetragonal, –, –, 30–60 nm | 33 | |
| Zirconium n-propoxide, glucose, fructose, aqueous ammonia | — | 9–10 | RT, 1 h | 700 °C, 3 h | Tetragonal and monoclinic, –, 10–30 nm, – | 13 | |
| Zr(SO4)2·H2O, sodium lauryl sulfate | Benzyl alcohol | — | — | 600 °C, 5 h | Tetragonal and monoclinic, –, 9.7 nm, – | 45 | |
| Zr(acac)4, sucrose | Ethanol | ∼12 | 110 °C, 10 min | 300–650 °C, – | Tetragonal and monoclinic, –, –, – | 35 | |
| Co-precipitation | Zirconium nitrate hexahydrate, potassium hydroxide | Water | >10 | RT, – | 900 °C, 4 h | –, spherical (193 nm), –, – | 38 |
| Zirconyl chloride octahydrate, diammonium oxalate monohydrate, ethylene glycol | Water | — | Varied, varied | Varied, varied | Monoclinic, spherical, –, 12.85 nm | 39 | |
| Zirconium(IV) nitrate, tetraethyl ortho-silicate, ammonium hydroxide | Water and ethanol | 7 | 348 K, 10 min | 1173 K, 10 min | Tetragonal, –, 3.3 nm, – | 40 | |
(i) Microwave-assisted approach. Microwave-assisted approaches are quite popular as they are facile, advantageous, rapid, and energy efficient and hence, have been extensively employed in nanomaterial synthesis. In addition, microwave heating proves to be beneficial over conventional heating approaches in a way that microwaves could achieve elevated temperatures quickly in a pressure-controlled environment and exhibit a tendency to attain optimum crystallinity. The microwave technique depends on the effective heating of matter by microwave dielectric heating which is the capacity of a solvent and reagent to retain microwave energy and to convert it into heat. In this technique, heating is generally accomplished through dipolar polarization and ionic conduction.15 Different zirconia salts, viz. nitrate, sulphate, chloride, and chelating agents have been employed in this method. Singh and Nakate16 reported the synthesis of zirconia nanoparticles by using the microwave method at a low temperature (80 °C) and studied their photoluminescence (PL) properties. Powder X-ray diffraction analysis revealed the formation of combined phases of monoclinic and tetragonal ZrO2. The microscopic analysis confirmed that the sizes of nanoparticles were less than 10 nm. The specific surface area of ZrO2 NPs was found to be 65.85 m2 g−1. These particles exhibited excellent optical properties such as an optical band gap of 2.49 eV and an intense emission peak at 414 nm in the photoluminescence spectrum.
Recently, Yousaf et al.17 demonstrated the microwave synthesis of zirconia nanoparticles by using honey as a capping agent, for optical applications. The bandgap of the prepared tetragonal zirconia was found to be in the range of 4.7–4.82 eV. The structural characterization revealed the formation of single-phase tetragonal zirconium oxide with high purity under optimized conditions.
Similarly, Bukhari et al.18 prepared zirconia nanofibers by a microwave-assisted sol–gel approach. In this study, honey was used as a structure-directing and capping agent to enhance the stability, reduce the crystallite size of nanofibers, and prevent the hard agglomeration of nanoparticles. Consequently, soft or less agglomeration resulted in a stabilized tetragonal phase of zirconia by inhibiting its interphase transformation from tetragonal into monoclinic (Fig. 1).
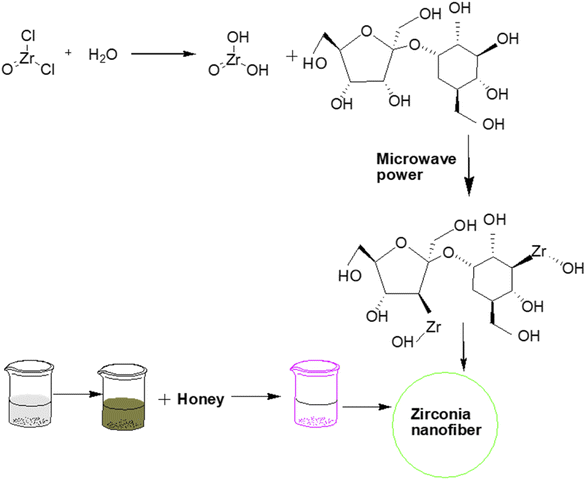 | ||
| Fig. 1 Honey mediated microwave assisted sol–gel synthesis of stabilized zirconia nanofibers.18 | ||
The microwave wattage (100–900 W) also significantly affected the stabilization of zirconia nanoparticles. Powder X-ray diffraction study revealed the formation of highly pure tetragonal zirconia at low microwave power ∼100 W with a smaller crystallite size of approximately 26 nm due to the presence of an optimum quantity of honey to coat the zirconia particles.
In another interesting report by Batool et al.,19 the preparation of zirconia nanoparticles and the dilemma of long-term stability were addressed. The obtained tetragonal zirconia nanoparticles were found to be stable even after a period of 6 to 12 months at room temperature.
Recently, Mishra et al.20 demonstrated the synthesis of tetragonal zirconia nanoparticles having an average crystal size of 10 nm using a microwave-assisted solvothermal route. In this study, the bandgap of the prepared nanoparticles was found to be 3.67 eV.
(ii) Hydrothermal and solvothermal approach. The hydrothermal method is a promising approach for synthesizing nanomaterials and involves heating the mixture of precursors, appropriate agents (reducing, oxidizing, hydrolyzing), and suitable solvent in a closed vessel which facilitates the fabrication of nanocrystals with controlled surface alteration, morphology, size, and stability. Apart from that, such kinds of synthesis methods are also useful for doping heteroatoms in nanoparticles or fabrication of nanocomposites owing to the generation of high pressure in a closed vessel. These methods have been used for the preparation of zirconia nanoparticles as well as for the doping of zirconia with foreign species. For instance, Behbahani et al.12 reported the hydrothermal synthesis of zirconia nanoparticles by using commercial monoclinic zirconia in an alkaline medium. Some of the parameters such as the concentration of the initial solution and pH were varied accordingly to obtain novel nanoparticles with desired shape and size. All three phases of zirconia, viz., cubic, tetragonal, and monoclinic phases, were observed and the corresponding sizes were found to be in the range of 15–30 nm.
Usually, attempting the synthesis of t-ZrO2 nanoparticles by using an inorganic precursor without any surfactant results in the agglomeration of particles. Under such circumstances, the hydrothermal corrosion method, which involves a corroding medium such as H2SO4, HCl etc. to break down hard aggregates into dispersed fine nanoparticles during the hydrothermal treatment, could be employed. Zhou et al.21 demonstrated the hydrothermal–corrosion method to produce highly dispersed tetragonal zirconia nanoparticles (less than 10 nm) without using any surfactants. It was stated that the dispersity and crystallinity of the prepared tetragonal ZrO2 powders can be modified by using reaction conditions such as calcination of the precipitates followed by hydrothermal HCl corrosion at different temperatures. In the follow-up study, Sagadevan et al.22 investigated the synthesis of spherical zirconia nanoparticles by the hydrothermal technique. The optical band gap of 5.02 eV and photoluminescence measurements confirmed the presence of oxygen vacancies accompanied by intrinsic defects in such NPs.
Ahmad et al.23 successfully prepared ZrO2 nanoparticles in an alkaline medium by employing the hydrothermal technique. Investigation of the structure and phase composition of the ZrO2 nanoparticles by powder X-ray diffraction indicated the presence of pure monoclinic zirconia. The dielectric investigations suggested that ZrO2 nanoparticles could be used for storage and electronic devices owing to the values of dielectric constant and dielectric loss, which were 7.5 and 0.0094, respectively.
Meetei et al.24 demonstrated the synthesis of white light-emitting nanocrystals composed of cubic ZrO2 doped with Eu3+via the hydrothermal method. In this study, the dopant Eu3+ was employed to stabilize the cubic phase with simultaneous production of the red counterpart from the white light. The wide range of photoluminescence emission arose due to the oxygen vacancies which could coalesce with a dopant to produce white light within the sample. The prolonged lifetime of the red counterpart of the sample could efficiently rectify the issue of the brief lifetime of traditional phosphors. Such materials could be beneficial in simulating the daylight of the sun by preparing light-emitting diodes, electronic flash, and lights for theatres and operas.
Noh et al.25 showcased the synthesis of anisotropic-shaped zirconia nanocrystals by using amorphous zirconium hydroxide powder and tetragonal zirconia powder at 150–250 °C along with varying concentrations of sodium hydroxide as an additive. The higher temperature facilitated the transformation of nanocrystals from spherical into spindle/rod-like structures with increase in the monoclinic phase fraction. Additionally, the fraction of the monoclinic phase was also found to increase with increasing sodium hydroxide concentration.
Abdelaal et al.26 reported a one-pot method for the fabrication of hollow sub-microspheres of zirconia having a diameter of 300 nm and a wall of thickness of 8 nm. In the first step, in situ preparation of the core@shell composite was achieved via autoclaving of glucose monohydrate as a core and sacrificial template, and zirconyl chloride octahydrate as the oxide precursor. Subsequently, calcination at 550 °C for 4 h resulted in the formation of a hollow sphere of ZrO2 due to the removal of the template.
In another study, Nath et al.27 reported the synthesis of highly thermally stable zirconia nanoparticles by a surfactant-assisted hydrothermal route (Fig. 2). Different types of surfactants such as cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), and Triton X-100 were used in the synthesis. Consequently, different morphologies of nanocrystals, for example, rod and mortar–pestle-shaped particles with different sizes were obtained. The distinct modifications in phase composition and morphology can also be achieved by tuning the reaction conditions during the preparation of the nanoparticles.
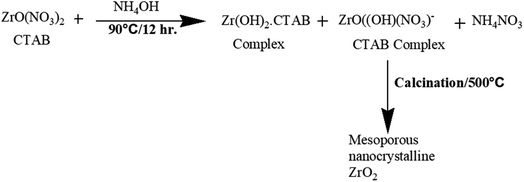 | ||
| Fig. 2 Formation mechanism of mesoporous nanocrystalline zirconia.27 | ||
Stolzenberg et al.28 demonstrated the fractal growth of ZrO2 nanocrystals by varying the temperature and concentrations of the zirconia precursor and benzyl alcohol during their synthesis. A possible reaction mechanism for the non-aqueous preparation of ZrO2 NPs under solvothermal conditions was proposed. The growth mechanism involved two steps, in the first step, the precursor reacted with benzyl alcohol (solvent) through a ligand exchange to convert it into an intermediate which finally reacts in a subsequent ether condensation reaction to form zirconia nanoparticles (Fig. 3). During the growth of NPs, the size increases from 2.7 nm to 7 nm along with phase transformation from tetragonal into monoclinic. Transmission electron microscopic images revealed that the nanoparticles grew from a round shape to a dendritic form along with the transformation of phase. In contrast to the supposition that only thermodynamics could control phase transformation, the reported study proposed that phase transition could be controlled by kinetics as well.
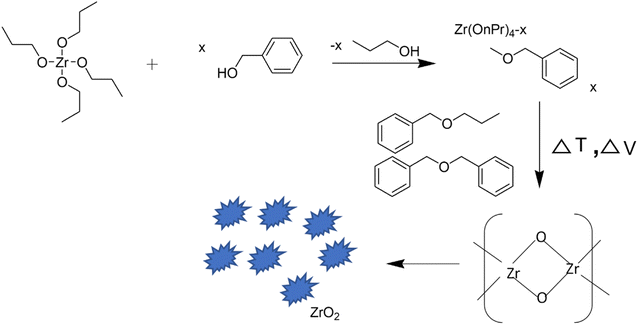 | ||
| Fig. 3 Reaction mechanism of the nonaqueous synthesis of ZrO2 nanoparticles.28 | ||
Zirconia nanotubes in the range of 60–90 nm were also prepared by hydrothermal treatment. For this, 50 mL aqueous solution of each of ZrO(NO3)2·xH2O (0.5 M) and NaOH (5 M) were uniformly mixed followed by the addition of 2 mL of absolute ethanol as a buffering agent. The final mixture was taken in a hydrothermal vessel which was heated at 250 °C for 14 h.29
Shu et al.30 also demonstrated the synthesis of unique star-shaped particles in the range of 40–100 nm having a tetragonal phase via hydrothermal treatment. For this, ZrOCl2 and sodium acetate were dissolved in water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and subjected to hydrothermal treatment at 240 °C for 6 h. Subsequently, the product was separated via centrifugation and dried at 90 °C.
2 molar ratio and subjected to hydrothermal treatment at 240 °C for 6 h. Subsequently, the product was separated via centrifugation and dried at 90 °C.
Reddy et al.31 demonstrated that hydrothermal treatment of a zirconyl chloride octahydrate precursor and NH4OH at 200 °C for 12 h could result in pure tetragonal zirconia NPs without using any template. The prepared nanoparticles were spherical.
(iii) Sol–gel approach. The sol–gel method involves the formation of a sol which is a colloidal solution of solid particles, followed by the formation of a gel which is an interconnected network of polymerized particles in a separate phase (solvent). Zirconia nanoparticles could also be prepared by the sol–gel method wherein, the sol can be obtained by dissolving a metal alkoxide or an organo-metallic precursor in water or alcohol which, under suitable reaction conditions (temperature, aging time, etc.), undergoes phase transformation into a gel-like structure. In the gel, owing to the network structure, polymeric particles remain separated, therefore the sizes of the particles can be controlled and agglomeration can be prevented. Finally, the gel upon suitable heat treatment could lead to the formation of nanoparticles. The principal advantage of the sol–gel method is the formation of uniform nanostructures at comparatively low temperatures as can be seen in Table 1. In this method, the reactivity of the precursors can be modified by alteration with chelation agents, consequently the gelation process could also be influenced, and ultimately the size and shape of the particles can also be modified. The different chelating reagents that have been employed for the preparation of nano zirconia are acetylacetone, acetic acid, citric acid, and different sugars (e.g., sucrose, maltose, glucose).
Madhusudhana et al.32 reported the synthesis of monoclinic zirconia nanoparticles by a facile sol–gel technique using acetic acid as a chelating agent. The resultant nanoparticles were found to be suitable for thermal applications due to their high resistance behavior to crack propagation. Lim et al.33 developed a synthesis of tetragonal phase zirconia nanoparticles. Different concentrations of NH3 solution and nitric acid were used with zirconium isopropoxide to analyze the impact on the crystallinity and particle size of ZrO2. In contrast, smaller sizes of nanoparticles were obtained when nitric acid was used instead of ammonia in the reaction mixture.
Bahari et al.34 investigated the preparation of ZrO2 at a relatively lower temperature. It was observed that some amount of the monoclinic phase gets converted into metastable tetragonal ZrO2 during heating. Heshmatpour et al.13 reported a simple sol–gel synthesis of zirconia nanoparticles of uniform size. Glucose and fructose were used as organic additives which could prevent the phase transition from the monoclinic to the tetragonal phase.
Likewise, the synthesis of zirconia thin films having a pure tetragonal phase was reported by Majedi et al.35via a sucrose-mediated sol–gel method. Initially, for preparing the sol, the zirconium(IV) acetylacetonate precursor was stirred in ethanol at 70 °C for 30 min followed by the addition of an ammoniacal solution as the hydrolyzing agent. Subsequently, sucrose was added to the solution as a gelation agent under stirring at 110 °C which results in the formation of the sol. The as-obtained sol was transformed into a xerogel by drying in an oven at 80 °C for 24 h. Furthermore, calcination at 350–500 °C results in t-zirconia, and calcination at 650 °C results in the formation of a mixture of the tetragonal and monoclinic phases. Such findings were expected as the post-calcination temperature could influence phase formation as well as phase transition.
Sponchia et al.36 demonstrated a simple and efficient neutral surfactant-assisted sol–gel method for the synthesis of spherical mesoporous zirconia nanoparticles by using a zirconium propoxide precursor, surfactant, ethanol, and alkali halide, followed by solvothermal thermal treatment using ethanol and water. The obtained dried powder was subjected to heat treatment under vacuum conditions to extract the surfactant without any structural modification. The prepared material exhibited a high surface area in comparison to literature reports. The effect of the addition of different alkali halides on the shape and size of the nanoparticle was also investigated. The salt's solubility plays a crucial role in governing the particle size. Biological experiments demonstrated that mesoporous zirconia nanoparticles were biocompatible, degradable, and cell-permeable, therefore they could be employed for theranostic applications.
For instance, Davar et al.37 synthesized pure monoclinic zirconia nanosheets by the polymeric sol–gel method. In this method, zirconium acetylacetonate was used as an organic metal precursor (Zr4+), and citric acid and ethylene glycol were used as the source of chelation and polymerization agents respectively. By varying the molar ratio of ethylene glycol to citric acid in the synthesis procedure, the phase as well as morphology of the nanoparticles could be modified. With increase in mole ratio from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 90
5 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the phase changes from cubic to monoclinic, and morphology varies from semi-spherical to nanosheets.
5, the phase changes from cubic to monoclinic, and morphology varies from semi-spherical to nanosheets.
(iv) Co-precipitation technique. Co-precipitation is an easy approach to prepare metal oxide nanoparticles using an aqueous solution of metal salts (chloride and nitrate metal salts) and bases such as ammonia or sodium hydroxide followed by heating or calcination at a suitable temperature. It involved nucleation followed by growth upon the addition of a precipitating agent; subsequently, heat treatment (calcination) led to the formation of metal oxide nanoparticles.
Ramachandran et al.38 reported a co-precipitation method for the synthesis of zirconia nanoparticles using zirconium nitrate as the precursor and KOH as the precipitating agent. The ratio of zirconium nitrate and KOH (0.5, 1, and 1.5 M) was optimized to obtain crystalline zirconium oxide with spherical morphology. At the same line, Foo et al.39 demonstrated the preparation of uniformly shaped zirconia nanoparticles having crystal sizes in the range of 6 to 35 nm. In this study, it was demonstrated that the calcination temperature in the reaction procedure could significantly impact the surface morphology, crystal size, and purity of the resulting ZrO2 NPs. A higher calcination temperature increased the degree of agglomeration and size of crystals. Similarly, Huang et al.40 also demonstrated that calcination temperature and time could drastically influence the crystallite size of tetragonal ZrO2. In this study, when the freeze-dried precursor powder was calcined at 1173 K, crystallites of size 3.3 nm were obtained; however, comparatively bigger crystallites of 20 nm were observed upon calcination at 1473 K.
(v) Solution combustion method. The solution combustion method involves the propagation of self-sustained and coupled complex exothermic reactions in an aqueous solution which could result in the formation of a ZrO2 powder along with emission of various gases such as N2, CO, CO2,etc. It starts with the burning/oxidation of a fuel which is an organic compound employed as a reactant along with a metal precursor. This method has been used in the synthesis of a variety of nanomaterials, including zirconia nanoparticles. Manjunatha et al.44 synthesized the pure cubic phase of ZrO2 mesoporous nanoparticles via a microwave-assisted combustion method. In this procedure, zirconyl nitrate monohydrate (ZrO(NO3)2·H2O) was used as the metal precursor and L-serine amino acid was employed as the fuel. Both components were dissolved in a minimum quantity of de-ionized water. Subsequently, the excess water was allowed to evaporate to obtain a viscous gel which upon microwave irradiation at 800 W for 60 seconds led to vigorous boiling followed by dehydration. Subsequently, the material gets burnt leading to the liberation of gases such as CO2, N2, and water vapour, and the formation of a white ZrO2 powder having a cubic phase. This study indicated that both the crystallinity and band gap (∼5.21 eV) of the prepared nanoparticles increases with increase in calcination temperature. An increase in band gap was observed due to a decline in the number of intermediate energy levels owing to an increase in orderliness with a smaller number of defects. The photoluminescence spectrum displayed an intense violet emission band peak centred at 387 nm, owing to the existence of oxygen vacancies in the zirconia matrix. The surface area analysis revealed that such nanomaterials were mesoporous in nature and the pore size also tends to decrease with an increase in the calcination temperature.
Dwivedi et al.42 prepared ZrO2 NPs by a microwave-driven citrate sol–gel technique. In this study, the prepared zirconia nanoparticles exhibited a smaller size due to gelation and rapid combustion.
Prakash Babu et al.46 also introduced a simple low-temperature solution combustion method without any stabilizing agent and calcination. In this method, zirconyl nitrate as the metal precursor and oxlayl di-hydrazide as the fuel were dissolved in water. The aqueous solution was taken in a dish and subjected to heat treatment at 400 °C in a preheated muffle furnace. Zirconia nano-powder having a cubic phase and crystallite size in the range from 6 to 12 nm was obtained.
Similarly, Tang et al.47 reported the synthesis of hollow mesoporous zirconia nanocapsules with a porous shell structure. In this study, first, monodispersed silica nanoparticles were prepared via Stober's method using concentrated ammonia, water, ethanol, and TEOS followed by the formation of the SiO2@ZrO2 composite using Zr(OBu)4. Finally, the composite was treated with NaOH (5 M) to remove silica which resulted in the formation of hexagonal mesoporous ZrO2.
Though different methods for the formation of specific phases have been reported, microwave-assisted sol–gel and solution combustion methods can be identified as promising choices for the formation of specific phases. Particularly, with the solution combustion method, formation of the cubic phase has been observed predominantly. This could be due to the attainment of high temperature that favors the formation of the cubic phase which is also a high-temperature stable form of zirconia. Interestingly, the use of microwave treatment in the case of the sol–gel has also resulted in the formation of selective phases, particularly the tetragonal phase. Apart from that, employing a corrosive solvent (HCl, H2SO4) during the hydrothermal treatment of zirconia nanoparticles could be a useful approach for obtaining monodisperse nanoparticles.
In most of the published literature studies on zirconia nanomaterials, the formation of spherical particles has been reported. However, in some studies, the formation of unique shapes has also been reported using unusual reaction conditions and precursors. For instance, the formation of nanosheets has been reported via the sol–gel method using zirconium acetylacetonate as the metal precursor, and the formation of cubic and star-like shapes has been reported via the hydrothermal method at a comparatively higher temperature of 240–250 °C.29,30,48 Interestingly, comparatively smaller particle sizes have been observed along with the pure tetragonal phase in all types of chemical synthesis methods.
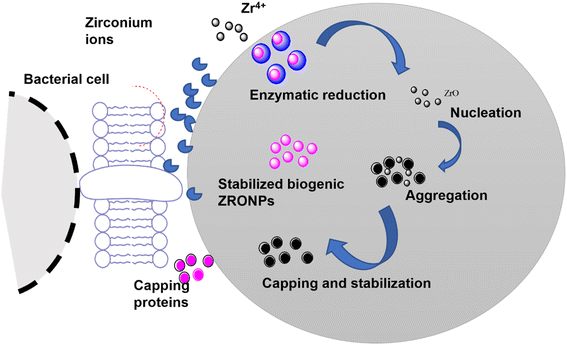 | ||
| Fig. 4 Schematic representation of proposed extracellular biosynthesis mechanisms of ZrO2 NPs. Nitrate reductase enzyme is responsible for the reduction of metal ions to their respective metal atoms that lead to nucleation and formation of NPs.53 | ||
| Microorganism | Zirconia source | pH | Size range, average size (nm) | Crystal system, morphology | Ref. |
|---|---|---|---|---|---|
| Humicola sp. (fungus) | Potassium hexafluoro zirconate | 9 | 13 nm | –, quasi-spherical | 54 |
| Fusarium oxysporum (fungus) | Potassium hexafluoro zirconate | 3.6 | 7.3 ± 2.0 nm | Monoclinic, – | 55 |
| Acinetobacter sp. KCSI1 (bacteria) | Zirconium oxychloride octahydrate | 2 | 44 ± 7 nm | — | 56 |
| Pseudomonas aeruginosa (bacteria) | Zirconium oxychloride octahydrate | — | 6.41 nm | Monoclinic and tetragonal, – | 66 |
| Enterobacter sp. (bacteria) | Zirconium oxychloride octahydrate | — | 33–75 nm | –, spherical | 53 |
Nowadays, extracts of different parts of plants such as roots, leaves, flowers, and fruits are also being used for the preparation of nanomaterials. Various phytochemicals, viz. flavones, terpenoids, sugars, ketones, aldehydes, carboxylic acids, and amides, are found to be responsible for the reduction of metal precursors. These species act as both stabilizing and reducing agents. In general, the plant-mediated synthesis of zirconia NPs is a simple and facile process, in which a zirconia salt, such as zirconium oxychloride or zirconia nitrate is mixed and stirred with an appropriate plant extract at a suitable temperature followed by heat treatment. However, there are limited reports on the mechanism of formation and stabilization of zirconia NPs from such biomolecules. Different reaction parameters, and resultantly different findings in terms of crystal structure and size distribution from recent reports on plant-mediated synthesis have been summarized in Table 3.
| Plant | Zirconia source | Conc. of plant extract, precursor, ratio | Reaction temperature, time | Thermal treatment (drying and calcination) | Size range, average size (nm) | Crystal system, shape | Ref. |
|---|---|---|---|---|---|---|---|
| Euclea natalensis roots | Zirconyl chloride octahydrate | 50 g L−1, 0.03 mol L−1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
–, 3 h | 105 °C for 24 h, 550 °C for 3 h | 5.25–41.71 nm, – | Monoclinic and tetragonal, spherical | 57 |
| Acalypha indica leaves | Zirconyl chloride octahydrate | 100 g L−1, 0.1 mol L−1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
80 °C, 2 h | 200 °C for 2 h, – | 58–72 nm, – | Monoclinic, – | 60 |
| Azadirachta indica leaves | Zirconyl chloride octahydrate | 100 g L−1, 50 mol L−1, – | 50–60 °C, – | 120 °C, 600 °C for 5–6 h | –, – | –, – | 61 |
| Aloe vera leaves | Zirconium oxychloride octahydrate | 500 g L−1, 0.4 mol L−1, – | Room temperature, 4 h | –, 500 °C | 27.4 nm, 50 nm | Tetragonal, spherical | 67 |
| Eucalyptus globulus leaves | Zirconium oxychloride | 100 g L−1, 0.0098 mol L−1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
–, 2–3 h | 80 °C, 600 °C for 3 h | 9–11 nm, – | Monoclinic and cubic, spherical | 68 |
| Lagerstroemia speciosa leaves | Zirconium nitrate | 150 g L−1, 0.2 N, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 °C, 3 h | –, – | –, 56.8 nm | Tetragonal, – | 65 |
| Curcuma longa tuber | Potassium hexafluoro zirconate | 25 g L−1, 0.00098 mol L−1, – | 25 °C, 72 h | –, – | –, 41–45 nm | Monoclinic, – | 62 |
| Citrus aurantiifolia fruit | Zirconium(IV) acetate | 10–30 mL, 0.038 mol L−1, – | 90 °C, 3 h | –, 750 °C for 2 h | 21 nm, 20 nm | Cubic, – | 69 |
Silva et al.57 demonstrated a green approach for the preparation of zirconia nanoparticles, exhibiting monoclinic and tetragonal phases by using Euclea natalensis plant extract. In this study, factorial design suggested that a higher concentration of precursor, lower amount of extract, and higher calcination temperature supported the synthesis of the tetragonal phase. Moreover, a possible mechanism (Fig. 5) for the preparation of ZrO2 NPs using phytochemicals of Euclea natalensis such as pentacyclic terpenoids (lupeol, betulin, and β-sitosterol) and naphthoquinones (diospirine, shinanolone, and 7-methyl juglone) is also given in this report. However, there is ambiguity in the mechanism of formation of ZrO2 NPs. It involves three steps: the reduction of zirconyl ions, the grouping of nanoparticles, and the growth of nanoparticles. But, only the reduction of zirconium ions could not lead to the formation of ZrO2 as oxidation is also a necessary step for its formation. In another report,58 bioreduction of zirconium ions, followed by complexation with water molecules, chelation with phytochemicals and finally oxidation were the major suggested steps in the plant-mediated synthesis of zirconia. As per our recent investigation on the plant-mediated synthesis of ZrO2, which is in the process of publication, a change in the oxidation state of zirconium from +4 to +2 followed by complexation with phytocomponents was observed. The complex upon heat treatment regains its oxidation state of +4 in zirconia as that of the precursor (ZrOCl2). These findings were supported by DFT calculations.
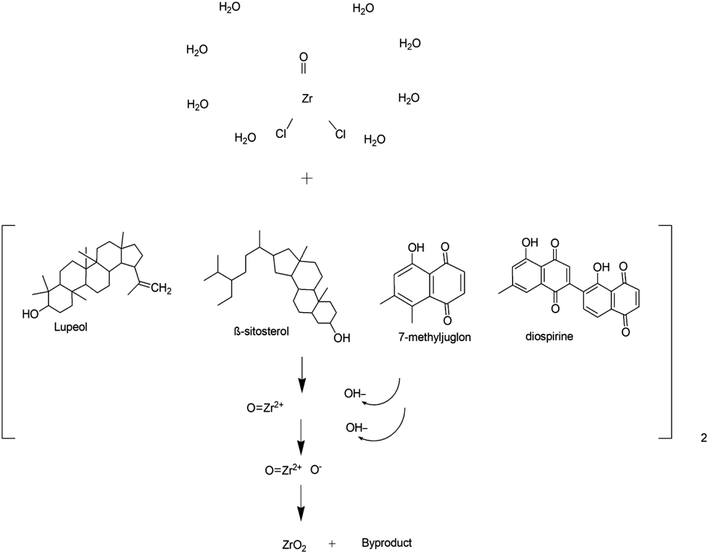 | ||
| Fig. 5 Proposed reaction mechanism of synthesis of zirconia nanoparticles.57 | ||
Another study on the green fabrication of ZrO2NPs was reported by Jalill et al.59 and the prepared nanoparticles exhibited excellent antifungal and antibacterial activity. Shanthi et al.60 synthesized ZrO2 nanoparticles by employing aqueous leaf extracts of Acalypha indica as a reducing agent. Encouraged by previous studies, Padma et al.61 fabricated zirconium dioxide nanoparticles using Azadirachta indica leaves. Similarly, Sathish et al.62 synthesized zirconia nanoparticles using Curcuma longa tuber. In this study, a higher pH was found to be favorable for the fabrication of zirconia NPs. Similarly, Gowri et al.63 investigated the greener synthesis of spherical zirconia nanoparticles using Aloe vera plant extract. The nanoparticles were found to have sizes in the range of 50–100 nm. Majedi et al.64 reported the synthesis of cubic phase zirconia nanoparticles using zirconium acetate as the precursor and lemon juice as the reducing agent. The impacts of sucrose addition on enhancing the particle size and the yield of the product were examined. A mixture of 20 mL lemon juice and sucrose was found to show a positive effect on uniform morphology having an average particle size of 21 nm. The as-prepared nanoparticles were employed in electrolyte materials for intermediate or low-temperature solid oxide fuel cells. Saraswathi et al.65 demonstrated the fabrication of tetragonal zirconia nanoparticles (2 nm) using Lagerstroemia speciosa leaves. The authors investigated the photocatalytic activity of the nanoparticles by monitoring the degradation of an azo dye using sunlight and reported excellent photocatalytic degradation of up to 94.58%.
It can be pointed out from the data listed in Tables 2 and 3 that zirconia nanomaterials prepared via greener routes (plant and microorganism-mediated methods) were found to have mainly pure phases either monoclinic or tetragonal. However, small particles having sizes in the range of 6–9 nm can be observed in all kinds of crystal systems known for zirconia. Interestingly, in almost all the instances of microorganism-mediated synthesis, heat treatment was not given which is surprising as merely the use of enzymes and proteins for the transformation of the zirconium precursor into zirconia could not yield fruitful results under ex situ conditions. However, in most of the plant-mediated synthesis, heat treatment in the temperature range of 60–120 °C of the reaction mixtures as well as in the range of 300–400 °C of the reaction product was given to obtain ZrO2.
1.2 Applications of zirconia and its nanocomposites
New emerging and greener pathways for the preparation of metal-based nanomaterials have also unwrapped the significant applications of nanoscale zirconia in catalysis, adsorption, sensing, and dentistry. These applications have been discussed below.| Reactants | Catalyst | Methanol to oil molar ratio | Reaction temperature (°C) and time | Catalyst loading | Type of reaction | Conversion/yield | Ref. |
|---|---|---|---|---|---|---|---|
| Waste cooking oil and methanol | Sr/ZrO2 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
115.5 °C, 169 min | 2.7% | Transesterification, esterification | 79.7% | 70 |
| Soybean oil and methanol | C4H4O6HK/ZrO2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 °C, 2.0 h | 6% | Transesterification | 98.03% | 71 |
| Oleic acid and methanol | Chlorosulfonic acid modified zirconia | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 °C, 12 h | 3% | Esterification | ∼100% | 73 |
| Refined soybean oil and methanol | CdO/ZrO2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
135 °C, 3 h | 7% | Transesterification | 97% | 75 |
| Silybum marianum oil and methanol | KOH/ZrO2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 °C, 2 h | 6% | Transesterification | 90.8% | 74 |
| Tannery waste sheep fat and methanol | Ferric-manganese-doped sulfated zirconia | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 °C, 300 min | 8% | Transesterification | 98.7% | 76 |
| Oleic acid and jatropha oil | SO42−/Zr-KIT-6 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 °C, 6 h | 4% | Esterification, transesterification | 95%, 85% | 78 |
| Soybean oil and methanol | Mesoporous sulfated zirconia | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 °C, 4 h | 4% | Transesterification | 94.9% | 79 |
| Soybean oil and methanol | Sulfated zirconia | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 °C, 6 h | 3% | Transesterification | 84.6% | 80 |
| Sunflower oil | Sulphated ZrO2-MCM-41 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 °C, 30 min | 5% | Esterification | 96.9% | 81 |
| Soybean oil and methanol | ZrO2-supported bamboo leaf ash (ZrO2/BLA) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
–, 30 min | 12% | Transesterification | 92.75% | 82 |
| Oleic acid and methanol | SO3H@ZrP | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 °C, – | 5.0% | Esterification | 89% | 95 |
| Nannochloropsis sp. (lipid) and methanol | Bi2O3/ZrO2(CTAB) | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 °C, 6 h | 20% | Transesterification, esterification | 73.21% | 83 |
| Soybean oil/tributyrin, and methanol | UiO-66 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1/52 1.1/52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 respectively 1.3 respectively |
140 °C, 5 h/120 °C, 5 h respectively | 0.1 g | Transesterification | 98.5%, 99.3% | 90 |
| Oleic acid and methanol | UiO-66(Zr)-green, UiO-66(Zr)-NO2-green, and UiO-66(Zr)-NH2 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 °C, 4 h | 6% | Esterification | 86%, 90%, 97% respectively | 91 |
| Soybean oil and methanol | AILs/POM/UiO-66-2COOH | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 °C, 6 h | 10% | Esterification, transesterification | 95.8% | 92 |
| Tributyrin and methanol | Zr-MOFs-S | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
140 °C, 6 h | 0.1 g | Transesterification | — | 93 |
| Oleic acid with methanol | ZrSiW/UiO-66 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 °C, 4 h | 0.24 g | Esterification | 98.0% | 94 |
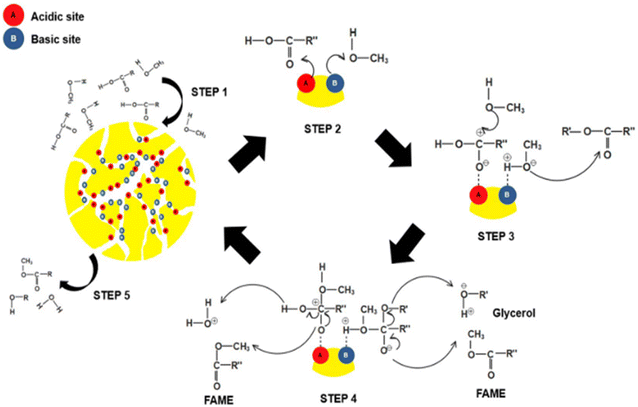 | ||
| Fig. 6 The reaction mechanism of biodiesel production using a bifunctional acid–base catalyst.83 | ||
Metal organic frameworks (MOFs) constitute a new emerging class of porous materials. However, these materials are still in early stages of development and gradually progressing towards diverse applications such as catalysis, drug delivery, fluorescence sensing, etc. Zr based metal organic frameworks (Zr-MOFs) are most likely considered as encouraging MOF materials owing to their excellent physico-chemical properties and outstanding chemical stability. In 2008, an extraordinary discovery was made by Cavka et al.,84 wherein 12-coordinated Zr6(μ3-O)4(μ3-OH)4(CO2)12 clusters were synthesized, commonly referred to as UiO-66. It was found that the chemical and hydrothermal stability of UiO-66 was much higher than that of the other reported MOFs.85–88 Since then, extensive efforts have been dedicated for the synthesis of Zr based MOFs, and the investigation of their properties, structures, functions and applications has been carried out intensively.
Schaate et al.89 described the very first example of employing a modulated fabrication approach to prepare Zr based MOFs and studied the effects of different modulators such as acetic acid, water and benzoic acid on the formation of Zr-MOFs. Interestingly, it was found out that among all modulators, only benzoic acid affected the synthesis process; as its concentration was increased, bigger and quite distinct crystals were expected to form from the intermediates of UiO-66. Hence, the first large single crystals of Zr-MOF were obtained by this strategy, as confirmed by the single-crystal XRD technique. An interesting study by Zhou et al.90 revealed the catalytic activity of UiO-66 prepared by using terephthalic acid and ZrCl4. The catalyst demonstrated excellent recyclability for the preparation of biodiesel using tributyrin and soybean oil with methanol. The performance of the catalyst was significantly dependent upon linker defects under different reaction parameters. Similarly, Abou-Elyazed et al.91 reported the use of UiO-66(Zr)-green, UiO-66(Zr)-NO2-green, and UiO-66(Zr)-NH2 green for the preparation of biodiesel. Among all these MOFs, UiO-66(Zr)-NH2 is found to be an efficient catalyst with an activation energy of 15.13 kJ mol−1. Xie et al.92 demonstrated a one-pot preparation of biodiesel from low-cost acidic vegetable oils by using the AILs/POM/UiO-66-2COOH catalyst with 95.8% conversion of oil under optimized conditions. The obtained catalyst exhibited various merits such as good activity, higher surface area, reusability up to 5 cycles, and tolerance to high amounts of free fatty acid (9 wt%) and water (3 wt%) in the feedstock. Lu et al.93 synthesized sulphated Zr-based MOFs which had flower-like mesoporous nanosheets of zirconium and sulphate. In this study, the catalyst exhibited excellent activity, larger surface area (186.1 m2 g−1), and recyclability for the transesterification reaction. Recently Zhang et al.94 prepared a ZrSiW/UiO-66 metal–organic framework by a hydrothermal method and used it for the biodiesel production. The prepared catalyst exhibited good activity, high surface area, and showcased heterogeneous nature as per the mechanism depicted in Fig. 7.
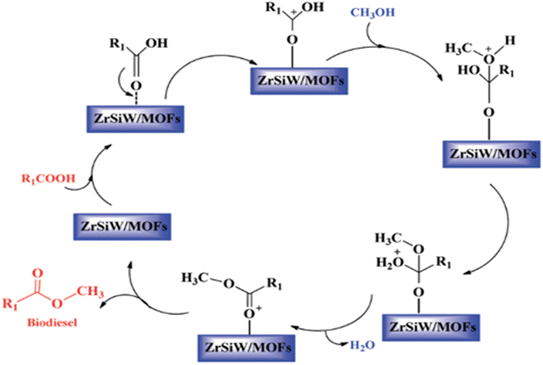 | ||
| Fig. 7 Possible mechanism of esterification.94 | ||
It is manifested from the literature cited in Table 4 that zirconia-based nanomaterials with enhanced acidity (sulphated zirconia) have been investigated extensively as a catalyst for simultaneous transesterification and esterification. However, a comparatively higher temperature and molar ratio of methanol to oil is required for the high yield of the reaction in contrast to the catalyst with enhanced basic sites (zirconia modified with KOH). Therefore, exploring Zr-based MOF and composites with basic sites could result in an efficient catalyst for transesterification.
1.2.1.1 Photocatalysis. ZrO2, which is a semiconductor having a broad direct bandgap and high negative value of the conduction band, has also been used as a photocatalyst due to its suitable thermal stability, and optical and electrical properties. It has been used as a photocatalyst for the degradation of organic water pollutants such as antibiotics and dyes. In Table 5, key parameters for the photocatalytic reactions using ZrO2-based photocatalysts have been summarized. As can be seen in Table 5, there is variation in the photocatalytic activity of zirconia even for the same dye owing to different particle sizes and consequent different band gaps. Therefore, lights from both UV and visible region have been employed with ZrO2 for the photocatalytic reactions. For instance, Ajabshir et al.96 demonstrated the photocatalytic oxidation of the Eriochrome Black T dye using sphere-like ZrO2 NPs under UV light. On the other hand, star-like ZrO2 NPs exhibited selective photocatalytic activity for the degradation of rhodamine B, methyl orange, and Congo red under visible light.30 The mesoporous form of zirconia also demonstrated photocatalytic activity under the illumination of visible light for the degradation of tetracycline.97 The ˙O2− and h+ radicals were found to be accountable for the degradation of tetracycline. In another interesting report, Basahel et al.98 proposed a mechanism (Fig. 8) for the degradation of methyl orange using zirconia nanostructures in varied crystalline forms, viz., monoclinic, tetragonal, and cubic. The monoclinic zirconia showed relatively higher photocatalytic activity than the other phases (tetragonal and cubic) due to the high crystallinity, large pore size distribution, oxygen-deficient zirconium oxide phase, and high density of surface hydroxyl groups. It involved the adsorption of methyl orange onto the surface of the nanoparticles. The exposure to UV light resulted in the formation of electron–hole pairs which react with the dissolved oxygen molecules and surface hydroxyl groups to form superoxide anion radicals and reactive hydroxyl radicals respectively.
| Nanomaterial | Band gap (eV) | Average size (nm), shape, size range (nm) | Light source | Dye | Time of irradiation (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| ZrO2 | 3.78 | 17, spherical, – | Sunlight | RY 160 | 120 | 94 | 99 |
| ZrO2 | 4.9 | 15, spherical, 10–18 | UV light | Methylene blue and methyl orange | 240 | 91, 69 | 100 |
| ZrO2 | — | 56.8, oval, – | Sunlight | Methyl orange | 290 | 94.58 | 65 |
| ZnO–ZrO2 | 3.96, 3.99, 3.97, and 4.01 | 79.56, 98.78, 54.86, and 67.43, –, – | Solar light | Rhodamine 6G | 330 | 98.94 | 101 |
| Mg-doped ZrO2 | Varied | –, aggregated spherical, tetragonal and hexagonal, – | UV light | Rhodamine B | 60 | 93 | 8 |
| Reduced graphene oxide–ZrO2 | 3.25 | –, –, – | Sunlight | Reactive Blue 4 | 120 | 93 | 102 |
| V2O5/ZrO2 | 3.93 | 34–50 | Solar light | Methyl orange and picloram | 75 | 76.94% and 86% | 103 |
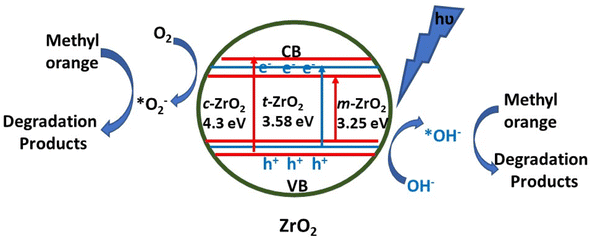 | ||
| Fig. 8 Schematic representation of the processes leading to photocatalytic degradation.98 | ||
As can be seen in Table 5, different sizes of ZrO2 NPs and their nanocomposites have been used for the degradation of different dyes. Therefore, a clear conclusion about their photocatalytic activity can't be drawn directly. However, a generalization on the band-gap which is responsible for photocatalysis can be made. The lowest band gap of ZrO2 was observed in its composites with reduced graphene oxide followed by that for the pure ZrO2 NPs having a spherical shape and an average size of 17 nm. Thus, composites of small size ZrO2 could further result in promising photocatalytic activities.
Gowri et al.67 synthesized zirconia nanoparticles of 50–100 nm using leaves of aloe vera and investigated their antimicrobial and antifungal properties. Abdul et al.59 demonstrated excellent antifungal and antibacterial properties of ZrO2 NPs against F. moniliforme, F. graminearum, E. coli, and S. aureus. Similarly, Imran et al.104 prepared Fe3O4-doped ZrO2 nanoparticles of average size ∼23 nm by the microwave method and investigated their antimicrobial activity. These nanoparticles (NPs) were used as dental fillers. Kumaresan et al.105 demonstrated the antibacterial activity of zirconia NPs against Bacillus subtilis, Escherichia coli, and Salmonella typhi. The authors used marine brown alga, Sargassum wightii for the synthesis of zirconia nanoparticles having the size of ∼4.8 nm. Masim et al.106 prepared a polyaniline zirconia (PANI-ZrO2) composite and employed it for the treatment of wastewater. The obtained composite showed antibacterial and anti-corrosion activity and acted as a phosphate adsorbent material.
Fathima et al.107 reported the fabrication of zirconia nanoparticles having sizes in the range of 15–21 nm and investigated their antimicrobial activity. In this study, they identified antimicrobial activity against Gram-positive (Bacillus subtilis and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), and found superior inhibitory activity against Pseudomonas aeruginosa at the concentration of 100 μg mL−1. The antimicrobial activity of ZrO2 NPs for different bacterial species is listed in Table 6. In another study, ZrO2–ZnO nanoparticles were prepared by the sol–gel method and further evaluated for their antibacterial activity.108 The mechanism of antibacterial activity by ZrO2 NPs was proposed by Asha et al.43 against Pseudomonas aeruginosa due to the rapid attraction between the negatively charged cell walls of Pseudomonas aeruginosa and positively charged zirconia NPs. Khan et al.109 prepared glutamic acid-functionalized ZrO2 NPs by using a solvothermal method and studied their antimicrobial activity against four strains of oral bacteria, viz., Streptococcus mutans, Rothia dentocariosa, Streptococcus mitis, and Rothia mucilaginosa. The alteration of the surface of ZO2 with glutamic acid increases the dispersion characteristics of nanoparticles in an aqueous medium which in turn possibly increases interactions between bacteria and nanoparticles.
| Zirconia-based nanomaterial, phase, shape, average size (nm), size range (nm) | Method | Microorganism | % Activity | Inhibition zone | Applications | Ref. |
|---|---|---|---|---|---|---|
| ZrO2, tetragonal, spherical, –, 50–100 | Agar diffusion method | S. aureus, E. coli (bacteria) and C. albicans, A. niger (fungus) | — | 23, 32, 22, 18 mm respectively | — | 67 |
| Fe3O4-doped ZrO2, tetragonal, spherical, 30 | Agar well diffusion method | Bacillus subtilis (bacteria) | — | 32 mm | Dental fillers | 104 |
| ZrO2, –, spherical, 5, – | Agar well diffusion method | Bacillus subtilis, E. coli, and Salmonella typhi (bacteria) | — | 21, 19, 19 mm respectively | — | 105 |
| Polyaniline-ZrO2, –, –, –, – | Agar diffusion method | E. coli and Staphylococcus aureus (bacteria) | — | 14, 18 mm respectively | Used in the treatment of wastewater effluent and decontamination of rearing water | 106 |
| ZrO2, monoclinic, spherical, 35, 32–38 | Disc diffusion method | Pseudomonas aeruginosa, E. coli, Staphylococcus aureus, and Bacillus subtilis (bacteria) | — | 10 mm in Pseudomonas aeruginosa | — | 43 |
| ZrO2–ZnO, –, spherical, 26–34, – | Disc diffusion method | Bacillus subtilis, Streptococcus mutans, Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, and Klebsiella oxytoca (bacteria) | — | — | — | 108 |
| Glutamic acid functionalized zirconia (GA-ZrO2), cubic, spherical, 2.5, – | Autoclaved brain heart infusion broth (BHI) or agar | Rothia mucilaginosa, Rothia dentocariosa, Streptococcus mitis, and Streptococcus mutans (bacteria) | 58% ± 4.9% (R. dentocariosa) 52% ± 2.9% (Streptococcus mutans) | — | — | 109 |
| ZrO2, –, spherical, –, 15–21 | Disc diffusion method | Bacillus subtilis, Staphylococcus aureus, E. coli and Pseudomonas aeruginosa (bacteria) | — | 20 mm in Pseudomonas aeruginosa | Anti-tooth decay applications | 107 |
| ZrO2, tetragonal, –, 17, – | Agar dilution method | Rhizoctonia solani (fungus) | 86.6%, 100 μg L−1 | — | Improved cucumber growth | 110 |
| ZrO2, –, spherical, –, 33–75 | Agar well diffusion | Pestalotiopsis versicolor (fungus) | — | 25.18 ± 1.52 mm | Protects bayberry (twig blight disease pathogen) plants against antifungal agents | 53 |
Derbalah et al.110 reported antifungal activity against Rhizoctonia solani which is known to be responsible for root rot in cucumber. The induction of resistance was examined using a real-time polymerase chain reaction. A concentration of 100 μg L−1 of zirconia nanoparticles showed better inhibition to improve the growth of the cucumber plant than the untreated cucumber plant. Ahmed et al.53 reported the synthesis of biogenic ZrO2 NPs by using Enterobacter sp. and studied their antifungal activity against P. versicolor (Fig. 9). In this study, nanoparticles ruptured the cells of pathogens by getting absorbed on the cell membrane and demonstrated a significant inhibition zone at the concentration level of 20 μg mL−1. Similarly, Balaji et al.68 demonstrated the synthesis of spherical zirconia nanoparticles (∼9 to 11 nm) by using the leaf extract of Eucalyptus globulus. In this study, the antioxidant activity of the prepared material was also investigated using human colon carcinoma (HCT-116) and human lung carcinoma (A-549) cell lines by DPPH assay. The antioxidant studies of the prepared zirconia NPs displayed significant inhibition (85.6%) at the concentration level of 158.15 μg mL−1. The cytotoxicity of the prepared ZrO2 was also investigated against HCT-116 colon cancer and A-549 lung cancer cell lines. A mechanism for the action of ZrO2 towards cytotoxicity is also given in this report where ROS have been identified in key roles (Fig. 10). Furthermore, the anticancer activity of zirconia-based nanomaterials against various cell lines, and their % reduction in cell viability are summarized in Table 7.
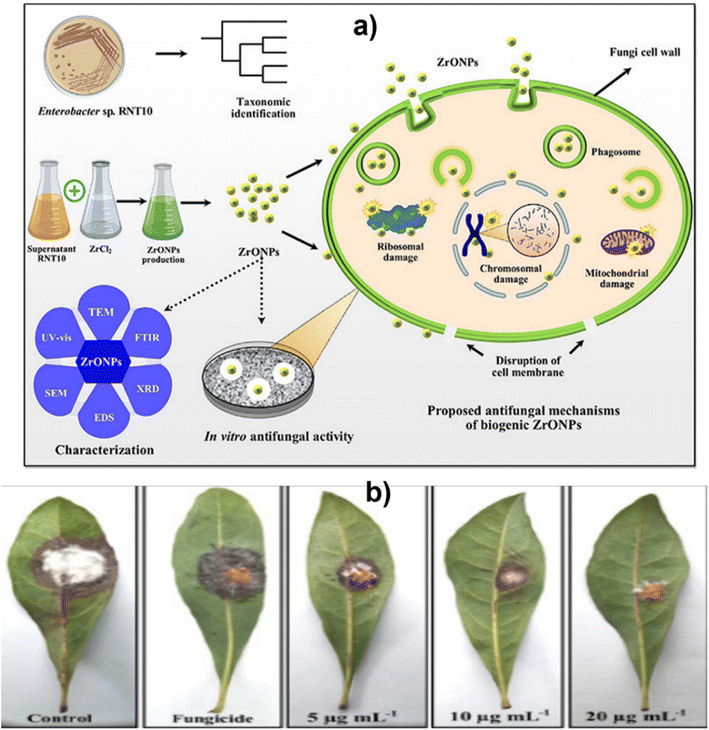 | ||
| Fig. 9 (a) Green synthesis and characterization of zirconium oxide nanoparticles by using a native Enterobacter sp. and its antifungal activity against bayberry twig blight disease pathogen Pestalotiopsis versicolor and (b) effect of various concentrations of ZrO NPs on bayberry leaves infected with P. versicolor strain XJ27. Adapted from ref. 53 with permission from Elsevier, copyright 2020. | ||
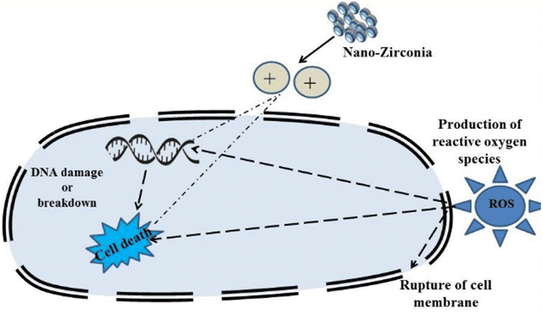 | ||
| Fig. 10 Plausible mechanism of action by ZrO2 NPs towards cytotoxicity. Adapted from ref. 68 with permission from Elsevier, copyright 2017. | ||
| Zirconia-based nanomaterial, phase, shape, average size (nm), size range (nm) | Method | Cell line | % Reduction in cell viability | Ref. |
|---|---|---|---|---|
| ZrO2, tetragonal, spherical, –, 9–11 | MTT | Human colon carcinoma (HCT-116) and human lung carcinoma (A-549) cell lines | 50% | 68 |
| ZrO2, tetragonal, tetragonal, 56.8, – | MTT | MCF-7 cell lines | 18% | 65 |
| Fe3O4@ZrO2, –, spherical, 155.23 ± 7.1, – | CCK-8 method | HepG2 cell line | 54.76% ± 5.88% to 17.72% ± 3.66% | 111 |
| AgNPs@UiO-66(DMF), –, cubical, 140, – | MTT | SMMC-7721 and HeLa cells | 92 and 89% respectively | 112 |
| Y2O3/ZrO2, –, –, 33.9 ± 2.1, – | MTS and NRU | Human skin keratinocyte (HaCaT) cells | 94%, 90%, 78% and 65% (in 24 h) | 113 |
| Sulphated zirconia, –, tetragonal, 43, 37–54 | MTT | HT29, human breast cancer MCF-7, and human liver cancer HepG2 | 50% | 114 |
Based upon data listed in Tables 3 and 5, it can't be concretely concluded that a particular phase of zirconia would be highly efficient for antibacterial and anticancer activity as most of the studies have been carried out on different microorganisms and cell lines by different methods. However, prima facie, the tetragonal phase seems to be more efficient as it had shown antibacterial activity on a range of microorganisms and significant anticancer activity on a range of cell lines. But, composites of zirconia with silver or yttria or iron oxide have shown promising results for both antibacterial as well as anti-cancer activities. Probably, composites of the tetragonal phase of zirconia could show enhanced superior activities.
| Adsorption activity | Zirconia nanomaterial type | Experimental conditions (temperature, pH, and equilibrium time) | Adsorption capacity | Isotherm | Ref. |
|---|---|---|---|---|---|
| Tetracycline from wastewater | ZrO2 | –, 6, 15 min | 526.32 mg g−1 | Langmuir isotherm | 66 |
| Phosphate in treated wastewater | PANI-ZrO2 | –, 25 °C, 6.9, – | 32.4 mg g−1 | Langmuir isotherm | 106 |
| Phospholipid adsorption from jatropha oil used for biofuel | ZrO2 | — | 13.92 mg g−1 | Langmuir isotherm | 77 |
| Adsorption of methylene blue and tetracycline hydrochloride from water | UiO-66-(OH)2/graphene oxide (GO) | 298 K, 9, – | 99.96% and 94.88%, respectively | Langmuir, Freundlich, Sips isotherm | 116 |
| Adsorption of arsenic(III) from the water | Ceria incorporated zirconia nanocomposites | 303 K, 7.0, – | 17.07 mg g−1 | Langmuir isotherm | 115 |
| Adsorption of chromium and dyes RB-21, RR-141, and Rh-6 G | Chitosan-zirconium phosphate nanostructures | 303 K, –, – | 311.53 mg g−1, 457 mg g−1, 435.1 mg g−1 and 438.596 mg g−1 respectively | Langmuir isotherm | 117 |
| Adsorption of vanadium ion | CNZ@NCT/Zr | 298 K, <5, – | 277.75 mg g−1 | Langmuir isotherm | 118 |
| Adsorption of phosphate from water | ZrO2/Fe3O4 | –, neutral, – | 29.5 mg g−1 | Langmuir isotherm | 119 |
| Adsorption of norfloxacin in water | UiO-66-NH2 | 298 K, 6–8.5, 6 h | 222.5 mg g−1 | Langmuir isotherm | 120 |
| Adsorption of toxic arsenite (As(III)) and arsenate (As(V)) from aqueous solutions | Mesoporous zirconia nanostructure | –, neutral, – | 105.03 and 110.29 mg g−1, respectively | Langmuir isotherm | 121 |
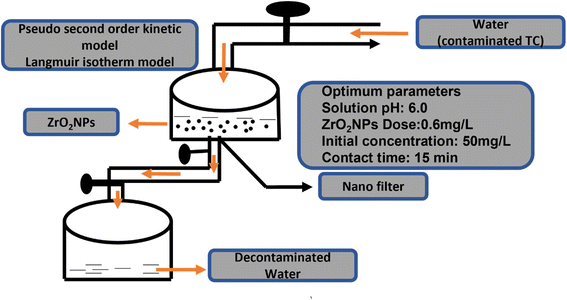 | ||
| Fig. 11 Schematic diagram of batch adsorption of TC on the surface of biosynthesized ZrO2 NPs.66 | ||
It can be suggested from the literature summarized in Table 8 that both bare ZrO2 and its composites could be employed for the adsorption of pollutants from effluents effectively. Specifically, designing composites of ZrO2 NPs with graphene oxide could be a useful approach for fabricating economical and efficient adsorbents for a range of pollutants and industrial-by-products.
Recently, an yttria-stabilized t-zirconia polycrystal material has been used for implants and dental applications due to its excellent mechanical properties.7 Interestingly, zirconia-based nanomaterials can also be used to improve the physical and mechanical properties of polymethyl methacrylate (PMMA) which is a denture repair material. Gad et al.126 also demonstrated that the tensile strength of PMMA could be increased using zirconia nanoparticles and can be used as a denture base with reduced translucency. Lohbauer et al.127 demonstrated the preparation of spherical ZrO2 NPs in the range of 20–50 nm using laser vaporization. These nanomaterials were used as fillers in the dental adhesive.
Fathima et al.107 synthesized zirconia nanoparticles and demonstrated their excellent antibacterial activity and anti-decay application on teeth. Bacteria may generate acids during their metabolic activities which result in the demineralization of teeth (Fig. 12). However, coating with zirconia NPs has shown resistance to acid attacks as well as remarkable activity against oral pathogens bearing negatively charged cell walls. The electrostatic interactions between positively charged nanoparticles and charged microorganisms inhibit the activities of microbes. Gad et al.128 reported a novel method for denture stomatitis inhibition by using ZrO2 nanoparticles owing to their antifungal activity. The authors investigated the inhibitory effect of ZrO2 nanoparticles on Candida albicans adhesion to restore polymethyl methacrylate denture bases. Similarly, in another investigation by Oshima et al.,129 a zirconia/alumina composite nanomaterial (Ce-TZP/Al2O3) was found to increase the osseointegration capability and osteogenic response of implants, where the surface of the nanostructured Ce-TZP/Al2O3 was modified by HF treatment.
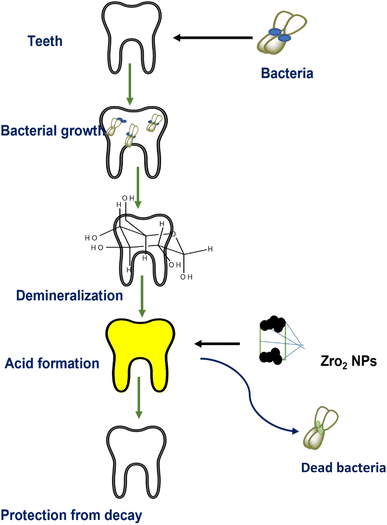 | ||
| Fig. 12 Role of ZrO2 NPs in dental care.107 | ||
Yamada et al.130 prepared silver NP-coated yttria-stabilized ZrO2 and investigated its antibacterial activity against oral pathogens such as Staphylococcus aureus, Streptococcus mutans, Escherichia coli, and Aggregatibacter actinomycetemcomitans (Fig. 13). The composite shows antimicrobial activity, particularly against Escherichia coli. The efficacy of the prepared material was tested via an accelerated aging test. In this test, the release profile of silver NPs was investigated which was found to be less than 1 percent, hence the prepared material was found to be thermally stable. Therefore, it was suggested that the bactericidal action of the composite was due to its contact with the bacteria and not owing to the release of silver NPs. Thus, the prepared material could be further investigated for the treatment of dental caries and periodontal disease.
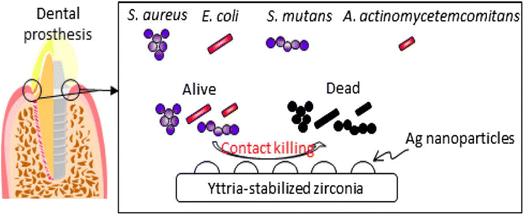 | ||
| Fig. 13 Silver NP-coated zirconia for antibacterial prosthesis. Adapted from ref. 130 with permission from Elsevier, copyright 2017. | ||
| Sensor | Synthesis method | Analyte | Working temperature | Recovery time, response time | Sensing technique | Limit of detection/linear response | Ref. |
|---|---|---|---|---|---|---|---|
| ZrO2 on a silicon substrate | — | Humidity | 10–30 °C | 60 s, 130 s | Measurement of impedance | — | 134 |
| ZrO2 NP decorated graphene nanosheets | Electrochemical approach | Methyl parathion | — | –, – | Square-wave voltammetry | 0.6 ng mL−1 | 135 |
| Zirconia nanocubes | Hydrothermal method | Arsenic(III) | — | –, below 2 s | Cyclic voltammetry | 5 ppb | 29 |
| Zirconia NPs | Precipitation method | Dimethyl amine | 330 °C | –, less than 50 s | Cataluminescence | 6.47 × 10−4 ng mL−1 | 138 |
| ZrO2 5%Y/ZrO2 Ag-5% Y/ZrO2 | Hydrothermal method | CO2 | 300–400 °C | 22 s, – | Measurement of current | — | 139 |
| Polyaniline zirconia nanocomposite | Polymerization method | Esomeprazole | — | –, 9 s | Electrochemical impedance spectroscopy and cyclic voltammetry | 97.21 ng mL−1 | 140 |
| Au-NPs/ZrO2 nanofiber | Nonthermal spin-coated sol–gel method | Pesticide | — | –, – | Surface-enhanced Raman scattering | 10−6 to 10−8 M | 137 |
| GQDs@La3+@ZrO2 nanoparticles | Bottom-up and sintering approach | Flutamide | Room temperature | — | Electrochemical signal | 0.00082 μM | 136 |
| ZrO2 NP modified carbon paste electrode | Hydrothermal method | Gallic acid | Room temperature | — | Differential pulse voltammetry (DPV) | 1.24 × 10−7 mol L−1 | 133 |
| SiO2ZrO2 composite | Sol–gel method | NO2 | 25 °C | Varied | Measurement of resistance | — | 141 |
Garadkar et al.132 fabricated thick film resistors using nanostructured ZrO2 (25 nm) via the screen printing technique. The as-prepared resistor was used for the selective detection of H2 gas and showed a significant signal at room temperature even in the presence of a smaller amount of H2. It was reported that films exhibited a clear response to 50 ppm of H2 in contrast to other gases at a higher amount of 1000 ppm. Chikere et al.133 fabricated a zirconia nanoparticle modified carbon paste electrode and employed it for the electrochemical detection of gallic acid in a concentration range of 1 × 10−6 to 1 × 10−3 mol L−1. The weak ionic interactions between the oxygen anions of zirconia nanoparticles (monoclinic) and positively charged graphite sheets, as depicted in Fig. 14, were found to stabilize the sensor. The designed sensor generated a signal via electrochemical oxidation of gallic acid and was employed for detection of the latter in red and white wine.
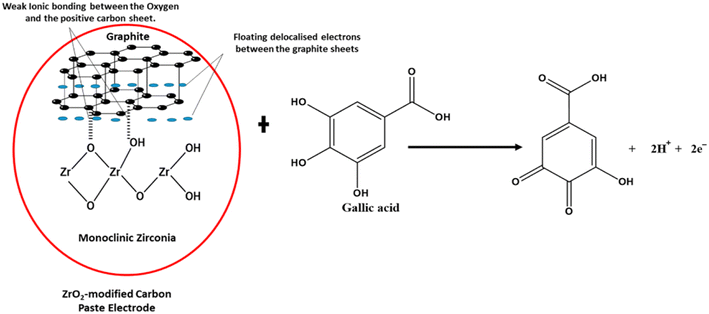 | ||
| Fig. 14 Proposed interaction between ZrO2 NPs and graphite, demonstrating a weak ionic bond between some of the oxygen and the positive graphite sheet in the electrochemical determination of gallic acid.133 | ||
Wang et al.134 prepared zirconia nanoparticles having an average size of 20 nm and employed them to make a thick film humidity sensor using a substrate. The prepared sensor showed a change in impedance from 106 to 102 Ω with an increase in relative humidity (RH) from 11 to 98%. In this report, the impact of temperature on the impedance of the sensor was evaluated and the conduction process of the sensor was explained using an equivalent circuit with resistors and capacitors. Gong et al.135 fabricated a nanostructured composite of zirconia and graphene by solid-phase extraction. The prepared material afforded high recognition and enrichment for phosphoric moieties due to the NPs of ZrO2, large surface area, and high conductivity owing to graphene nanosheets. The prepared material was successfully employed for the detection of methyl parathion via square-wave voltammetry.
Trinadh et al.136 prepared an electrochemical sensor using graphene quantum dots (GQDs) and lanthanum doped zirconia nanoparticles for the detection of flutamide in urine samples as per the scheme depicted in Fig. 15. For designing this sensor, La3+@ZrO2 was prepared by the coprecipitation method using a solution of zirconyl chloride and lanthanum nitrate as metal precursors and NH4OH as the precipitating agent. The addition of NH4OH decreases the pH of the solution which leads to the formation of precipitates. The obtained precipitates were washed with methanol and water, dried, and calcined at 600 °C. Finally, the glass carbon electrode-based sensor was fabricated by drop casting a suspension of 1.0 mg of GQDs and 1.0 mg of La3+@ZrO2 on a pre-treated GCE. In this study, the signal was generated due to the electrochemical reduction of flutamide (12 μM) in phosphate buffer solution (pH = 7).
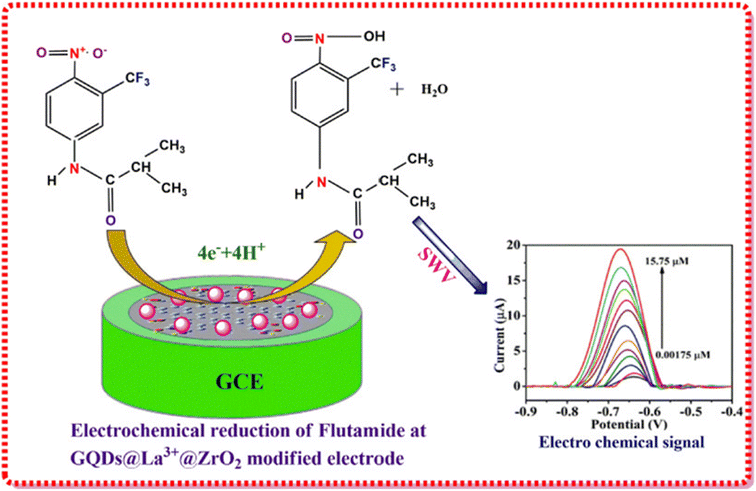 | ||
| Fig. 15 Pictorial representation of the fabrication of GQDs@La3+@ZrO2@GCE. Adapted from ref. 136 with permission from Elsevier, copyright 2017. | ||
Lee et al.137 demonstrated the preparation of Au nanoparticle coated zirconia nanofibers and employed them as a surface-enhanced Raman scattering sensor for the detection of a variety of pesticide (carbaryl, phosmet, cypermethrin, and permethrin) residues in fruits or vegetables. In this study, nanofibers of zirconia were fabricated on a silicon wafer via the spin coating technique using a solution of ZrCl4 in isopropanol. Afterward, gold NPs were deposited on ZrO2 nanofibers via an electron beam evaporator. The prepared sensor shows excellent selectivity to organophosphates even in the presence of multiple pesticides. It was successfully employed for a practical application using diluted juice containing sliced pesticide-containing apple peels and could differentiate different pesticides.
The literature summarized in Table 9 revealed that ZrO2 or its composites could be employed for designing a range of sensors based upon different techniques such as electrochemical, luminescence etc. However, significant LODs have been displayed by them via electrochemical signals. Specifically, a cyclic voltammetry-based signal from such sensors seems to be a more promising one owing to the electrical properties of ZrO2.
2 Outlook and futuristic scope
Recently, there have been substantial efforts and significant developments in the field of synthesis of nanomaterials for better exploitation in numerous applications. With the aid of a modified outlook of nanomaterials and the comprehension of their potential in various industries, including, but not limited to, pharmaceuticals, textiles, paper, pulp, and drug delivery, the influx of zirconia nanoparticles has offered innumerable benefits and provided solutions for several continuing problems related to clinical diagnosis, catalysis, and dentistry, and can indefinitely offer long-term economic benefits. In the particular interest towards the synthesis of zirconia nanoparticles, several chemical and green methods have been developed and their plausible mechanisms have been studied in detail. Furthermore, efforts have also been made to achieve monodispersity, desired particle size, shape, and stability via tuning the reaction conditions and parameters. However, there are challenges associated with improving the efficiency of the synthesis and achieving the specific particle sizes and morphology, a desirable cubic phase, long-term stability, and reduced aggregation of particles. Moreover, expansion of the volume of particles with the cubic phase has also been observed when employed in high-temperature applications due to its transformation into the tetragonal phase and subsequently tetragonal to monoclinic phase which results in the generation of stress and cracks and makes the material unfit for applications. Therefore, a long journey is still pending in this direction to overcome the above-mentioned limitations. To gain an insight, a significant number of theoretical studies using density functional theory have been performed to understand the structure, nature of surface sites, and stability of zirconia nanoparticles. Recently, Maleki et al.142 performed DFT calculations to obtain the 17O solid-state NMR chemical shifts for studying the nature of surface sites on zirconia. In an interesting study, Puigdollers et al.143 showed that nanoparticles with partly truncated octahedral morphology display the highest stability. Moreover, nanoparticles with only stable facets {101} were found to be less stable due to the presence of reduced Zr, thereby confirming that low-coordinated sites create defective states in the electronic structure and reduce the effective bandgap, which can result in enhanced interaction with deposited species and could modify the photocatalytic activity. In another report, the presence of acidic and basic sites on zirconia was investigated using DFT calculations for obtaining the IR and NMR spectra of adsorbed molecules (carbon monoxide and pyrrole). This study confirms the presence of acidic Zr sites and basic O− sites in nanoparticles. However, further theoretical studies for obtaining stronger acidic and basic sites and achieving the stability of the cubic phase in zirconia nanoparticles could enhance the suitability of zirconia in various applications as discussed earlier. To ensure the commercial applicability of such materials in various technological applications, future perspectives should focus not only on an in-depth understanding of the surface structure and active sites but also on the design and development of well-defined chemical and green systems for the substantially controllable site environment and attractive characteristics.Experimentally, two approaches have been employed to enhance the stability and applications of zirconia nanoparticles. The first one involves in situ modification with stabilizers and other metal oxides such as CaO, MgO, and Y2O3 during the preparation of the nanomaterials. Modification with another metal oxide could generate vacancies stimulating changes in the electronic and geometric environment that can further enhance the optical and electrical properties which is also supported by several theoretical studies.143–145 The second strategy involves exploring greener methods with novel additives that could be employed. Although the synthesis of zirconia nanomaterials using plant extracts and microbes is considered to be clean, nontoxic, simple, and safe, there are only a few reports in this regard and sufficient efforts have not been made with diverse varieties of plants and microorganisms with different possible reaction pathways. Moreover, the combination of the sol–gel method and greener reactants is rarely reported. Specifically, the sol–gel method offers unique advantages such as good control over the structure and kinetics of the process. Moreover, fine control of the composition of a multicomponent nanomaterial could be achieved via this method as small quantities of dopants can be introduced in the sol and uniformly dispersed in the final product. Therefore, further improvising this method using greener ingredients by keeping in view the motive of increasing the strength and biocompatibility could result in the invention of new materials suitable for various applications such as dentistry, catalysis, sensors, etc.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Vishal Mutreja is thankful to SERB for the National Post-Doctoral Fellowship (File No: PDF/2017/002750). Shweta Sareen is grateful to SERB for the National Post-Doctoral Fellowship (File No: PDF/2017/002828).References
- J. L. Gole, S. M. Prokes, J. D. Stout, O. J. Glembocki and R. Yang, Adv. Mater., 2006, 18, 664–667 CrossRef CAS.
- S. Zhuiykov and N. Miura, Sens. Actuators, B, 2007, 121, 639–651 CrossRef CAS.
- G. D. Wilk and R. M. Wallace, Appl. Phys. Lett., 2000, 76, 112–114 CrossRef CAS.
- X. J. Jin, Curr. Opin. Solid State Mater. Sci., 2005, 9, 313–318 CrossRef CAS.
- S. Gupta, S. Noumbissi and M. F. Kunrath, Med. Devices Sens., 2020, 3, 1–8 Search PubMed.
- B. Tyagi, K. Sidhpuria, B. Shaik and R. V. Jasra, Ind. Eng. Chem. Res., 2006, 45, 8643–8650 CrossRef CAS.
- E. Camposilvan, F. G. Marro, A. Mestra and M. Anglada, Acta Biomater., 2015, 17, 36–46 CrossRef CAS PubMed.
- L. Renuka, K. S. Anantharaju, S. C. Sharma, H. P. Nagaswarupa, S. C. Prashantha, H. Nagabhushana and Y. S. Vidya, J. Alloys Compd., 2016, 672, 609–622 CrossRef CAS.
- E. Mustafa, M. Wilhelm and W. Wruss, Ceram. Int., 2003, 29, 189–194 CrossRef CAS.
- K. Anandan, K. Rajesh, K. Gayathri, S. Vinoth Sharma, S. G. Mohammed Hussain and V. Rajendran, Phys. E, 2020, 124, 114342 CrossRef CAS.
- J. Liang, Z. Deng, X. Jiang, F. Li and Y. Li, Inorg. Chem., 2002, 41, 3602–3604 CrossRef CAS PubMed.
- A. Behbahani, S. Rowshanzamir and A. Esmaeilifar, Procedia Eng., 2012, 42, 908–917 CrossRef.
- F. Heshmatpour and R. B. Aghakhanpour, Powder Technol., 2011, 205, 193–200 CrossRef CAS.
- E. Geuzens, G. Vanhoyland, J. D'Haen, M. K. Van Bael, H. Van Den Rul, J. Mullens and L. C. Van Poucke, Key Eng. Mater., 2004, 264–268, 343–346 CAS.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed.
- A. K. Singh and U. T. Nakate, Sci. World J., 2014, 2014, 349457 CAS.
- H. Yousaf, M. Azhar, M. Bashir, S. Riaz, Z. N. Kayani and S. Naseem, Optik, 2020, 222, 165297 CrossRef CAS.
- B. S. Bukhari, M. Imran, M. Bashir, S. Riaz and S. Naseem, J. Sol-Gel Sci. Technol., 2018, 87, 554–567 CrossRef CAS.
- T. B. Riaz, K. B. Hadi and S. Naseem, J. Mech. Behav. Biomed. Mater., 2020, 112, 104012 CrossRef PubMed.
- S. Mishra, A. K. Debnath, K. P. Muthe, N. Das and P. Parhi, Colloids Surf., A, 2021, 608, 125551 CrossRef CAS.
- H. Zhou, S. Pu, J. Huo, W. Cao, B. Wang and J. Li, Ceram. Int., 2016, 42, 15005–15011 CrossRef CAS.
- S. Sagadevan, J. Podder and I. Das, J. Mater. Sci.: Mater. Electron., 2016, 27, 5622–5627 CrossRef CAS.
- T. Ahmad, Mater. Sci. Eng. Int., 2017, 1, 4–8 Search PubMed.
- S. D. Meetei and S. D. Singh, J. Alloys Compd., 2014, 587, 143–147 CrossRef CAS.
- H. J. Noh, D. S. Seo, H. Kim and J. K. Lee, Mater. Lett., 2003, 57, 2425–2431 CrossRef CAS.
- H. M. Abdelaal, Mater. Lett., 2018, 212, 218–220 CrossRef CAS.
- S. Nath, A. Biswas, P. P. Kour, L. S. Sarma, U. K. Sur and B. G. Ankamwar, J. Nanosci. Nanotechnol., 2018, 18, 5390–5396 CrossRef CAS PubMed.
- P. Stolzenburg, A. Freytag, N. C. Bigall and G. Garnweitner, CrystEngComm, 2016, 18, 8396–8405 RSC.
- G. Bhanjana, N. Dilbaghi, S. Chaudhary, K. H. Kim and S. Kumar, Analyst, 2016, 141, 4211–4218 RSC.
- Z. Shu, X. Jiao and D. Chen, CrystEngComm, 2013, 15, 4288–4294 RSC.
- C. V. Reddy, B. Babu, I. N. Reddy and J. Shim, Ceram. Int., 2018, 44, 6940–6948 CrossRef CAS.
- R. Madhusudhana, M. A. Sangamesha, R. G. Krishne Urs, L. Krishnamurthy and G. L. Shekar, Int. J. Adv. Res., 2014, 2, 433–436 Search PubMed.
- H. S. Lim, A. Ahmad and H. Hamzah, AIP Conf. Proc., 2013, 1571, 812–816 CrossRef CAS.
- A. Bahari and M. Ghanbari, Int. J. ChemTech Res., 2011, 3, 1686–1691 CAS.
- A. Majedi, F. Davar, A. Abbasi and A. Ashrafi, J. Inorg. Organomet. Polym. Mater., 2016, 26, 932–942 CrossRef CAS.
- G. Sponchia, E. Ambrosi, F. Rizzolio, M. Hadla, A. Del Tedesco, C. R. Spena, G. Toffoli, P. Riello and A. Benedetti, J. Mater. Chem. B, 2015, 3, 7300–7306 RSC.
- F. Davar and M. R. Loghman-Estarki, Ceram. Int., 2014, 40, 8427–8433 CrossRef CAS.
- M. Ramachandran, R. Subadevi, W. R. Liu and M. Sivakumar, J. Nanosci. Nanotechnol., 2018, 18, 368–373 CrossRef CAS PubMed.
- Y. T. Foo, A. Z. Abdullah, B. Amini Horri and B. Salamatinia, Ceram. Int., 2019, 45, 22930–22939 CrossRef CAS.
- H. J. Huang and M. C. Wang, Ceram. Int., 2013, 39, 1729–1739 CrossRef CAS.
- A. K. Singh and U. T. Nakate, Sci. World J., 2014, 2014 Search PubMed.
- R. Dwivedi, A. Maurya, A. Verma, R. Prasad and K. S. Bartwal, J. Alloys Compd., 2011, 509, 6848–6851 CrossRef CAS.
- S. Baby Asha, D. Muthuraj, E. Kumar and V. Veeraputhiran, J. Nanosci. Nanotechnol., 2019, 5, 642–644 Search PubMed.
- S. Manjunatha and M. S. Dharmaprakash, J. Lumin., 2016, 180, 20–24 CrossRef CAS.
- M. R. H. Siddiqui, A. I. Al-Wassil, A. M. Al-Otaibi and R. M. Mahfouz, Mater. Res., 2012, 15, 986–989 CrossRef CAS.
- D. Prakash babu, R. Hari Krishna, B. M. Nagabhushana, H. Nagabhushana, C. Shivakumara, R. P. S. Chakradar, H. B. Ramalingam, S. C. Sharma and R. Chandramohan, Spectrochim. Acta, Part A, 2014, 122, 216–222 CrossRef CAS PubMed.
- S. Tang, X. Huang, X. Chen and N. Zheng, Adv. Funct. Mater., 2010, 20, 2442–2447 CrossRef CAS.
- F. Davar, A. Hassankhani and M. R. Loghman-Estarki, Ceram. Int., 2013, 39, 2933–2941 CrossRef CAS.
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Res. Pharm. Sci., 2014, 9, 385–406 CAS.
- R. K. Das, V. L. Pachapur, L. Lonappan, M. Naghdi, R. Pulicharla, S. Maiti, M. Cledon, L. M. A. Dalila, S. J. Sarma and S. K. Brar, Nanotechnol. Environ. Eng., 2017, 2, 1–21 CrossRef CAS.
- K. N. Thakkar, S. S. Mhatre and R. Y. Parikh, Nanomedicine, 2010, 6, 257–262 CrossRef CAS PubMed.
- N. I. Hulkoti and T. C. Taranath, Colloids Surf., B, 2014, 121, 474–483 CrossRef CAS PubMed.
- T. Ahmed, H. Ren, M. Noman, M. Shahid, M. Liu, M. A. Ali, J. Zhang, Y. Tian, X. Qi and B. Li, NanoImpact, 2021, 21, 100281 CrossRef CAS PubMed.
- I. Uddin and A. Ahmad, J. Mater. Environ. Sci., 2016, 7, 3068–3075 CAS.
- V. Bansal, D. Rautaray, A. Ahmad and M. Sastry, J. Mater. Chem., 2004, 14, 3303–3305 RSC.
- S. P. Suriyaraj, G. Ramadoss, K. Chandraraj and R. Selvakumar, Mater. Sci. Eng., C, 2019, 105, 110021 CrossRef PubMed.
- A. F. V. da Silva, A. P. Fagundes, D. L. P. Macuvele, E. F. U. de Carvalho, M. Durazzo, N. Padoin, C. Soares and H. G. Riella, Colloids Surf., A, 2019, 583, 123915 CrossRef CAS.
- T. Van Tran, D. T. C. Nguyen, P. S. Kumar, A. T. M. Din, A. A. Jalil and D. V. N. Vo, Environ. Chem. Lett., 2022, 20, 1309–1331 CrossRef PubMed.
- R. D. Abdul Jalill, M. M. H. M. Jawad and A. N. Abd, J. Genet. Environ. Resour. Conserv., 2017, 5, 6–23 Search PubMed.
- S. Shanthi and S. Sri Nisha Tharani, Int. J. Eng. Appl. Sci., 2016, 3, 257689 Search PubMed.
- P. Nimare and A. A. Koser, Int. J. Eng. Technol., 2016, 3, 1910–1912 Search PubMed.
- M. Sathishkumar, K. Sneha and Y. S. Yun, Mater. Lett., 2013, 98, 242–245 CrossRef CAS.
- S. Gowri, R. R. Gandhi and M. Sundrarajan, J. Mater. Sci. Technol., 2014, 30(8), 782–790 CrossRef CAS.
- A. Majedi, A. Abbasi and F. Davar, J. Sol-Gel Sci. Technol., 2016, 77, 542–552 CrossRef CAS.
- V. S. Saraswathi and K. Santhakumar, J. Photochem. Photobiol., B, 2017, 169, 47–55 CrossRef PubMed.
- B. Debnath, M. Majumdar, M. Bhowmik, K. L. Bhowmik, A. Debnath and D. N. Roy, J. Environ. Manage., 2020, 261, 110235 CrossRef CAS PubMed.
- S. Gowri, R. Rajiv Gandhi and M. Sundrarajan, J. Mater. Sci. Technol., 2014, 30, 782–790 CrossRef CAS.
- S. Balaji, B. K. Mandal, S. Ranjan, N. Dasgupta and R. Chidambaram, J. Photochem. Photobiol., B, 2017, 170, 125–133 CrossRef CAS PubMed.
- A. Majedi, A. Abbasi and F. Davar, J. Sol-Gel Sci. Technol., 2016, 77, 542–552 CrossRef CAS.
- W. N. N. Wan Omar and N. A. S. Amin, Fuel Process. Technol., 2011, 92, 2397–2405 CrossRef CAS.
- F. Qiu, Y. Li, D. Yang, X. Li and P. Sun, Bioresour. Technol., 2011, 102, 4150–4156 CrossRef CAS.
- S. K. Das and S. A. El-Safty, ChemCatChem, 2013, 5, 3050–3059 CrossRef CAS.
- Y. Zhang, W. T. Wong and K. F. Yung, Appl. Energy, 2014, 116, 191–198 CrossRef CAS.
- M. Takase, M. Zhang, W. Feng, Y. Chen, T. Zhao, S. J. Cobbina, L. Yang and X. Wu, Energy Convers. Manage., 2014, 80, 117–125 CrossRef CAS.
- P. Patil and A. Pratap, J. Oleo Sci., 2016, 65, 331–337 CrossRef CAS PubMed.
- V. K. Booramurthy, R. Kasimani, S. Pandian and B. Ragunathan, Environ. Sci. Pollut. Res., 2020, 27, 20598–20605 CrossRef CAS PubMed.
- Y. F. Lin, J. H. Chen, S. H. Hsu, H. C. Hsiao, T. W. Chung and K. L. Tung, J. Colloid Interface Sci., 2012, 368, 660–662 CrossRef CAS PubMed.
- S. Gopinath, P. S. M. Kumar, K. A. Y. Arafath, K. V. Thiruvengadaravi, S. Sivanesan and P. Baskaralingam, Fuel, 2017, 203, 488–500 CrossRef CAS.
- Y. Luo, Z. Mei, N. Liu, H. Wang, C. Han and S. He, Catal. Today, 2017, 298, 99–108 CrossRef CAS.
- G. L. Shi, F. Yu, X. L. Yan and R. F. Li, J. Fuel Chem. Technol., 2017, 45, 311–316 CrossRef CAS.
- S. Dehghani and M. Haghighi, Ultrason. Sonochem., 2017, 35, 142–151 CrossRef CAS.
- I. Fatimah, D. Rubiyanto, A. Taushiyah, F. B. Najah, U. Azmi and Y. L. Sim, Sustainable Chem. Pharm., 2019, 12, 100129 CrossRef.
- N. J. A. Rahman, A. Ramli, K. Jumbri and Y. Uemura, Sci. Rep., 2019, 9, 1–12 CrossRef.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- J. B. Decoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y. G. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 RSC.
- X. Liu, N. K. Demir, Z. Wu and K. Li, J. Am. Chem. Soc., 2015, 137, 6999–7002 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS.
- F. Zhou, N. Lu, B. Fan, H. Wang and R. Li, J. Energy Chem., 2016, 25, 874–879 CrossRef.
- A. S. Abou-Elyazed, G. Ye, Y. Sun and A. M. El-Nahas, Ind. Eng. Chem. Res., 2019, 58, 21961–21971 CrossRef CAS.
- W. Xie and F. Wan, Chem. Eng. J., 2019, 365, 40–50 CrossRef CAS.
- N. Lu, X. Zhang, X. Yan, D. Pan, B. Fan and R. Li, CrystEngComm, 2019, 22, 44–51 RSC.
- Q. Zhang, D. Lei, Q. Luo, J. Wang, T. Deng, Y. Zhang and P. Ma, RSC Adv., 2020, 10, 8766–8772 RSC.
- Y. Zhou, I. Noshadi, H. Ding, J. Liu, R. S. Parnas, A. Clearfield, M. Xiao, Y. Meng and L. Sun, Catalysts, 2018, 8(1), 17 CrossRef.
- S. Zinatloo-Ajabshir and M. Salavati-Niasari, J. Mol. Liq., 2016, 216, 545–551 CrossRef CAS.
- J. Zhang, Y. Gao, X. Jia, J. Wang, Z. Chen and Y. Xu, Sol. Energy Mater. Sol. Cells, 2018, 182, 113–120 CrossRef CAS.
- S. N. Basahel, T. T. Ali, M. Mokhtar and K. Narasimharao, Nanoscale Res. Lett., 2015, 10(1), 1–13 CrossRef CAS.
- N. Al-Zaqri, A. Muthuvel, M. Jothibas, A. Alsalme, F. A. Alharthi and V. Mohana, Inorg. Chem. Commun., 2021, 127, 108507 CrossRef CAS.
- H. M. Shinde, T. T. Bhosale, N. L. Gavade, S. B. Babar, R. J. Kamble, B. S. Shirke and K. M. Garadkar, J. Mater. Sci.: Mater. Electron., 2018, 29, 14055–14064 CrossRef CAS.
- S. Haq, H. Afsar, I. U. Din, P. Ahmad, M. U. Khandaker, H. Osman, S. Alamri, M. I. Shahzad, N. Shahzad, W. Rehman and M. Waseem, Catalysts, 2021, 11(12), 1481 CrossRef CAS.
- K. Gurushantha, K. S. Anantharaju, L. Renuka, S. C. Sharma, H. P. Nagaswarupa, S. C. Prashantha, Y. S. Vidya and H. Nagabhushana, RSC Adv., 2017, 7, 12690–12703 RSC.
- P. Rasheed, S. Haq, M. Waseem, S. U. Rehman, W. Rehman, N. Bibi and S. A. A. Shah, Mater. Res. Express, 2020, 7(2), 025011 CrossRef CAS.
- M. Imran, S. Riaz, I. Sanaullah, U. Khan, A. N. Sabri and S. Naseem, Ceram. Int., 2019, 45, 10106–10113 CrossRef CAS.
- M. Kumaresan, K. Vijai Anand, K. Govindaraju, S. Tamilselvan and V. Ganesh Kumar, Microb. Pathog., 2018, 124, 311–315 CrossRef CAS.
- F. C. P. Masim, C. H. Tsai, Y. F. Lin, M. L. Fu, M. Liu, F. Kang and Y. F. Wang, Environ. Technol., 2019, 40, 226–238 CrossRef CAS PubMed.
- J. B. Fathima, A. Pugazhendhi and R. Venis, Microb. Pathog., 2017, 110, 245–251 CrossRef CAS PubMed.
- A. Precious Ayanwale and S. Y. Reyes-López, ACS Omega, 2019, 4, 19216–19224 CrossRef CAS PubMed.
- M. Khan, M. R. Shaik, S. T. Khan, S. F. Adil, M. Kuniyil, M. Khan, A. A. Al-Warthan, M. R. H. Siddiqui and M. Nawaz Tahir, ACS Omega, 2020, 5, 1987–1996 CrossRef CAS PubMed.
- A. Derbalah, M. M. Elsharkawy, A. Hamza and A. El-Shaer, Pestic. Biochem. Physiol., 2019, 157, 230–236 CrossRef CAS PubMed.
- L. Chen, H. Zhong, X. Qi, H. Shao and K. Xu, RSC Adv., 2019, 9, 13220–13233 RSC.
- C. Han, J. Yang and J. Gu, J. Nanopart. Res., 2018, 20(3), 1–11 CrossRef CAS.
- F. M. Alzahrani, K. M. S. Katubi, D. Ali and S. Alarifi, Int. J. Nanomed., 2019, 14, 7003–7016 CrossRef CAS PubMed.
- A. Mftah, F. H. Alhassan, M. S. Al-Qubaisi, M. E. El Zowalaty, T. J. Webster, M. Sh-Eldin, A. Rasedee, Y. H. Taufiq-Yap and S. S. Rashid, Int. J. Nanomed., 2015, 10, 765–774 CrossRef PubMed.
- A. Ghosh, S. Kanrar, D. Nandi, P. Sasikumar, K. Biswas and U. C. Ghosh, J. Chem. Eng. Data, 2020, 65, 885–895 CrossRef CAS.
- Y. Sun, M. Chen, H. Liu, Y. Zhu, D. Wang and M. Yan, Appl. Surf. Sci., 2020, 525, 146614 CrossRef CAS.
- R. Bhatt, V. Ageetha, S. B. Rathod and P. Padmaja, Carbohydr. Polym., 2019, 208, 441–450 CrossRef CAS PubMed.
- S. Salehi, S. Alijani and M. Anbia, Int. J. Biol. Macromol., 2020, 164, 105–120 CrossRef CAS PubMed.
- J. Wang, X. Shao, J. Liu, Q. Zhang, J. Ma and G. Tian, Mater. Chem. Phys., 2020, 249, 123024 CrossRef CAS.
- X. Fang, S. Wu, Y. Wu, W. Yang, Y. Li, J. He, P. Hong, M. Nie, C. Xie, Z. Wu, K. Zhang, L. Kong and J. Liu, Appl. Surf. Sci., 2020, 518, 146226 CrossRef CAS.
- K. Shehzad, M. Ahmad, C. Xie, D. Zhan, W. Wang, Z. Li, W. Xu and J. Liu, J. Hazard. Mater., 2019, 373, 75–84 CrossRef CAS PubMed.
- A. Hafezeqoran and R. Koodaryan, BioMed Res. Int., 2017, 2017, 9246721 Search PubMed.
- M. Aivazi, M. Fathi, F. Nejatidanesh, V. Mortazavi, B. H. Beni and J. P. Matinlinna, J. Lasers Med. Sci., 2018, 9, 87–91 CrossRef PubMed.
- T. Tahsili, W. Park, M. Hirota, N. M. Rezaei, M. Hasegawa, M. Ishijima, K. Nakhaei, T. Okubo, T. Taniyama, A. Ghassemi, T. Tahsili, W. Park, M. Hirota and T. Ogawa, Int. J. Nanomed., 2018, 13, 3381–3395 CrossRef.
- P. Yu, C. Wang, J. Zhou, L. Jiang, J. Xue and W. Li, BioMed Res. Int., 2016, 2016, 8901253 Search PubMed.
- M. M. Gad, R. Abualsaud, A. Rahoma, A. M. Al-Thobity, K. S. Al-Abidi and S. Akhtar, Int. J. Nanomed., 2018, 13, 283–292 CrossRef CAS.
- U. Lohbauer, A. Wagner, R. Belli, C. Stoetzel, A. Hilpert, H. D. Kurland, J. Grabow and F. A. Müller, Acta Biomater., 2010, 6, 4539–4546 CrossRef CAS.
- M. M. Gad, A. M. Al-Thobity, S. Y. Shahin, B. T. Alsaqer and A. A. Ali, Int. J. Nanomed., 2017, 12, 5409–5419 CrossRef CAS PubMed.
- Y. Oshima, F. Iwasa, K. Tachi and K. Baba, Int. J. Oral. Maxillofac. Implants, 2017, 32, 81–91 CrossRef PubMed.
- R. Yamada, K. Nozaki, N. Horiuchi, R. Nemoto, H. Miura and A. Nagai, Mater. Sci. Eng., C, 2017, 78, 1054–1060 CrossRef CAS PubMed.
- Z. Zhang, C. Zhang and X. Zhang, Analyst, 2002, 127, 792–796 RSC.
- K. M. Garadkar, B. S. Shirke, Y. B. Patil, D. R. Patil, Sensors and transducers, 2009, Vol. 110, Iss. 11, pp. 17–25 Search PubMed.
- C. O. Chikere, N. H. Faisal, P. Kong-Thoo-Lin and C. Fernandez, Nanomaterials, 2020, 10(3), 537 CrossRef CAS PubMed.
- J. Wang, M. Y. Su, J. Q. Qi and L. Q. Chang, Sens. Actuators, B, 2009, 139, 418–424 CrossRef CAS.
- J. Gong, X. Miao, H. Wan and D. Song, Sens. Actuators, B, 2012, 162, 341–347 CrossRef CAS.
- T. Trinadh, H. Khuntia, T. Anusha, K. S. Bhavani, J. V. S. Kumar and P. K. Brahman, Diamond Relat. Mater., 2020, 110, 108143 CrossRef CAS.
- J. Der Liao, K. Sivashanmugan, B. H. Liu, W. E. Fu, C. C. Chen, G. D. Chen and Y. Der Juang, Nanomaterials, 2018, 8(6), 402 CrossRef.
- C. Yu, G. Liu, B. Zuo and R. Tang, Luminescence, 2009, 24, 282–289 CrossRef CAS PubMed.
- A. V. Borhade, D. R. Tope and J. A. Agashe, J. Mater. Sci.: Mater. Electron., 2018, 29, 7551–7561 CrossRef CAS.
- R. Jain, D. C. Tiwari and S. Shrivastava, J. Electrochem. Soc., 2014, 161, B39–B44 CrossRef CAS.
- T. N. Myasoedova, T. S. Mikhailova, G. E. Yalovega and N. K. Plugotarenko, Chemosensors, 2018, 6(4), 67 CrossRef CAS.
- F. Maleki and G. Pacchioni, Top. Catal., 2020, 63, 1717–1730 CrossRef CAS.
- A. R. Puigdollers, F. Illas and G. Pacchioni, J. Phys. Chem. C, 2016, 120, 4392–4402 CrossRef CAS.
- L. Liu, X. Su, H. Zhang, N. Gao, F. Xue, Y. Ma, Z. Jiang and T. Fang, Appl. Surf. Sci., 2020, 528, 146900 CrossRef CAS.
- A. Ruiz Puigdollers, F. Illas and G. Pacchioni, Rend. Lincei., 2017, 28, 19–27 CrossRef.
- N. Kumari, M. K. Aulakh, S. Sareen, A. Sharma, H. S. Sohal, M. Verma, S. K. Mehta and V. Mutreja, Top. Catal., 2022 DOI:10.1007/s11244-022-01652-z.
| This journal is © The Royal Society of Chemistry 2022 |






