Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile solvothermal synthesis of plate-like submicron NaNbO3 particles†
Shingo
Machida
 *a,
Shoma
Niwa
a,
Sho
Usuki
*a,
Shoma
Niwa
a,
Sho
Usuki
 b,
Kazuya
Nakata
b,
Kazuya
Nakata
 c,
Makoto
Ogawa
c,
Makoto
Ogawa
 de,
Atsuo
Yasumori
de,
Atsuo
Yasumori
 a and
Ken-ichi
Katsumata
a and
Ken-ichi
Katsumata
 *a
*a
aDepartment of Material Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: shingo.machida@rs.tus.ac.jp; k.katsumata@rs.tus.ac.jp
bGraduate School of Bio-Application and Systems Engineering, Tokyo University of Agriculture and Technology, 2-24-16 Naka-cho, Koganei, Tokyo 184-0012, Japan
cDivision of Sciences for Biological System, Institute of Agriculture, Tokyo University of Agriculture and Technology, 2-24-16 Naka-cho, Koganei, Tokyo 184-0012, Japan
dSchool of Energy Science and Engineering, Vidyasirimedhi Institute of Science and Technology, 555 Moo 1 Tumbol Payupnai, Amphoe Wangchan, Rayong 21210, Thailand
eJapan Advanced Institute of Science & Technology, 1-1 Asahidai, Nomi, Ishikawa 923-1292, Japan
First published on 6th July 2022
Abstract
Plate-like particles of orthorhombic NaNbO3 were obtained via a solvothermal reaction using a methanol/ethanol mixed solvent, in contrast to the formation of cubic NaNbO3 particles from methanol alone. Control of the submicron NaNbO3 particle morphology was achieved by simply changing the solvent, suggesting the role of ethanol in suppressing the growth in one direction.
The discovery of the Honda–Fujishima effect, i.e., the decomposition of water by titanium dioxide (TiO2) photocatalysis, prompted researchers to investigate the fundamental properties and practical applications of photocatalysts.1–4 Titanium oxide (TiO2) has been widely studied because of its high activity, photo-induced hydrophilicity utilized for the self-cleaning effect, and low cost.1–4 Additionally, TiO2 is well-known as a white pigment2,5–7 that requires submicron-sized particles to achieve its hiding power,7,8 which is enhanced when the particle shape is platy.9 One drawback of TiO2 as a white pigment is its photocatalytic activity, which causes degradation of fabrics and resins.1,2 The photo-induced hydrophilicity of sodium niobate (NaNbO3) was first discovered by Katsumata and co-authors,10,11 and NaNbO3 shows potential as a photocatalyst, though with weaker activity than TiO2,10 and as a white pigment. The former case is promising for investigating the mechanisms of photo-induced hydrophilicity suppressed by photo-oxidation, while the latter case has high expectations for the particle shape and size of NaNbO3, which possesses a perovskite-type (ABO3) structure with an orthorhombic unit cell.12 Well-defined cubic particles are thus typically obtained.12–22 Submicron particles are prepared by solvothermal syntheses using synthesized niobium pentoxide (Nb2O5) and methanol as the raw materials.16,17 In contrast, micron-sized and/or aggregated plate-like NaNbO3 particles are prepared via hydrothermal syntheses20–24 or topochemical ion exchange reactions of Bi2.5Na3.5Nb5O18, an Aurivillius phase layered perovskite, in molten sodium salts.25–27 Submicron NaNbO3 particles with a plate-like morphology are advantageous not only for photocatalytic activity through exposed surface design but also for higher hiding power in white pigment applications.
In this study, we devoted special attention to the facile preparation of plate-like submicron NaNbO3 particles. The products were prepared by a modified version of the procedure reported in previous studies.18,19 After dispersing 500 mg of Nb2O5 (obtained from Kojundo Chemical Laboratory Co., Ltd.) in 25 mL of methanol (MeOH)/ethanol (EtOH) mixed solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 vol%), the dispersion was mixed with 5 mL of sodium hydroxide (NaOH) aqueous solution (10 mol L−1). The obtained dispersion was placed in a Teflon vessel with a stainless jacket and then heated at 180 °C for 6, 24, or 48 h. After the reaction, the resultant solids were obtained by centrifugation, exhaustively washed with distilled water and methanol, and then dried at 80 °C for 1 h, to form the products denoted herein as NaNbO3-(1Me+10Et)-6h, NaNbO3-(1Me+10Et), and NaNbO3-(1Me+10Et)-48h, respectively. For comparison, these procedures with a reaction time of 24 h were applied to cases using 25 mL of MeOH, EtOH, or MeOH/EtOH mixed solvent (1
10 vol%), the dispersion was mixed with 5 mL of sodium hydroxide (NaOH) aqueous solution (10 mol L−1). The obtained dispersion was placed in a Teflon vessel with a stainless jacket and then heated at 180 °C for 6, 24, or 48 h. After the reaction, the resultant solids were obtained by centrifugation, exhaustively washed with distilled water and methanol, and then dried at 80 °C for 1 h, to form the products denoted herein as NaNbO3-(1Me+10Et)-6h, NaNbO3-(1Me+10Et), and NaNbO3-(1Me+10Et)-48h, respectively. For comparison, these procedures with a reaction time of 24 h were applied to cases using 25 mL of MeOH, EtOH, or MeOH/EtOH mixed solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vol%) instead of the MeOH/EtOH mixed solvent (1
4 vol%) instead of the MeOH/EtOH mixed solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 vol%) to generate products denoted herein as NaNbO3-Me, NaNbO3-Et, and NaNbO3-(1Me+4Et), respectively. In addition, the reactions for MeOH and MeOH/EtOH mixed solvent (1
10 vol%) to generate products denoted herein as NaNbO3-Me, NaNbO3-Et, and NaNbO3-(1Me+4Et), respectively. In addition, the reactions for MeOH and MeOH/EtOH mixed solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 vol%) were conducted by changing the amount of Nb2O5 from 500 mg to 5 g, to form the products denoted herein as NaNbO3-Me-5g and NaNbO3-(1Me+10Et)-5g, respectively. Moreover, the reactions for NaNbO3-(1Me+10Et) were modified by decreasing the reaction temperature to 150 °C or the NaOH aqueous solution (5 mL) to 2 mol L−1, to generate the products denoted herein as NaNbO3-(1Me+10Et)-150°C and NaNbO3-(1Me+10Et)-low-NaOH, respectively. The crystalline phases of the raw material and the products were characterized by X-ray diffraction (XRD) analyses (XRD-6100, Shimadzu). The particle shapes of the raw material and products were characterized by field-emission scanning electron microscopy (FE-SEM) (spra40, Zeiss). The photocatalytic properties of the products were briefly examined by performing decomposition of methylene blue (MB) using 50 mL of MB aqueous solution (1.0 × 10−5 mol L−1) containing 50 mg of NaNbO3-Me or NaNbO3-(1Me+10Et) under 365 nm irradiation for 1 hour. Prior to these experiments, the dispersions were stirred for 2 h in the dark. The photocatalytic experiments were also performed using Degussa P25 standard for comparison purposes. The absorption spectra of NaNbO3-Me and NaNbO3-(1Me+10Et) were recorded using a JASCO v-670 spectrophotometer. Before these analyses, the samples were mixed with barium sulfate. The surface areas of NaNbO3-Me and NaNbO3-(1Me+10Et) were determined to be 4.8 and 6.0 m2 g−1, respectively, from the nitrogen adsorption isotherms measured at −196 °C (Belsorp MINI, MicrotracBEL) using the Brunauer–Emmett–Teller (BET) method.28
10 vol%) were conducted by changing the amount of Nb2O5 from 500 mg to 5 g, to form the products denoted herein as NaNbO3-Me-5g and NaNbO3-(1Me+10Et)-5g, respectively. Moreover, the reactions for NaNbO3-(1Me+10Et) were modified by decreasing the reaction temperature to 150 °C or the NaOH aqueous solution (5 mL) to 2 mol L−1, to generate the products denoted herein as NaNbO3-(1Me+10Et)-150°C and NaNbO3-(1Me+10Et)-low-NaOH, respectively. The crystalline phases of the raw material and the products were characterized by X-ray diffraction (XRD) analyses (XRD-6100, Shimadzu). The particle shapes of the raw material and products were characterized by field-emission scanning electron microscopy (FE-SEM) (spra40, Zeiss). The photocatalytic properties of the products were briefly examined by performing decomposition of methylene blue (MB) using 50 mL of MB aqueous solution (1.0 × 10−5 mol L−1) containing 50 mg of NaNbO3-Me or NaNbO3-(1Me+10Et) under 365 nm irradiation for 1 hour. Prior to these experiments, the dispersions were stirred for 2 h in the dark. The photocatalytic experiments were also performed using Degussa P25 standard for comparison purposes. The absorption spectra of NaNbO3-Me and NaNbO3-(1Me+10Et) were recorded using a JASCO v-670 spectrophotometer. Before these analyses, the samples were mixed with barium sulfate. The surface areas of NaNbO3-Me and NaNbO3-(1Me+10Et) were determined to be 4.8 and 6.0 m2 g−1, respectively, from the nitrogen adsorption isotherms measured at −196 °C (Belsorp MINI, MicrotracBEL) using the Brunauer–Emmett–Teller (BET) method.28
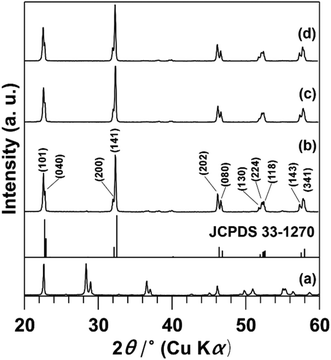
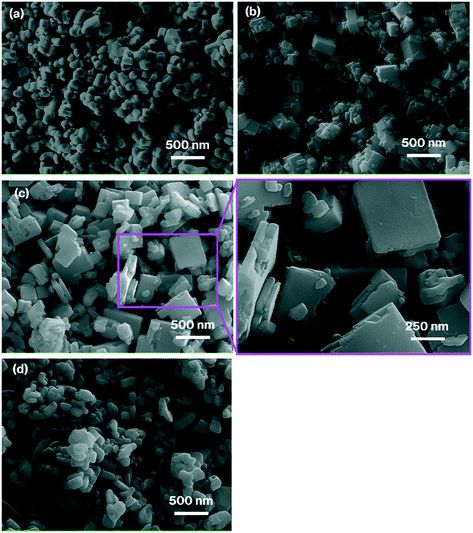
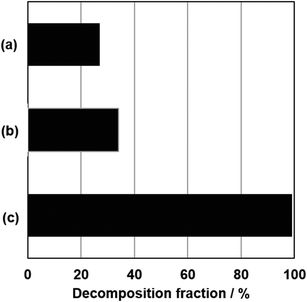
Fig. 1 shows XRD patterns for Nb2O5 and the products. All the patterns (Fig. 1b–d) show diffraction lines attributed to the orthorhombic phase of NaNbO3,13–19 along with the disappearance of reflections due to the orthorhombic phase of Nb2O5 (Fig. 1a).13–19 Notably, the space group for the orthorhombic phase of NaNbO3 is classified as Pbcm12,13 or Pbma,14,15 both are equivalent in different settings. For simplicity, we thus tentatively index the reflections observed in the XRD patterns of the products to those of Pbcm.12Fig. 2 presents the FE-SEM images of Nb2O5 and the products. The image of NaNbO3-Me exhibits cube-like particles in the 50–500 nm range along with minute amounts of rod-like particles, while those of NaNbO3-(1Me+10Et) and NaNbO3-Et exhibit plate-like particles in the 100–800 nm range (Fig. 2b–d). Compared to the image of NaNbO3-Et (Fig. 2d), that of NaNbO3-(1Me+10Et) shows well-defined shapes and plate-like particles (Fig. 2c). Irregular-shaped particles in the 50–400 nm range observed in the image of Nb2O5 (Fig. 2a) are absent in all the product images. The absorption spectra of NaNbO3-Me or NaNbO3-(1Me+10Et) (Fig. S1†) exhibit broad signals at 320 nm, which is consistent with those reported previously.16,17 Based on the MB decomposition fractions (Fig. 3), the photocatalytic activities of the present NaNbO3-Me and NaNbO3-(1Me+10Et) products were lower than that of P25. In previous studies,10,11 NaNbO3 films displayed a lower MB decomposition fraction than TiO2 films. Also, the 34% MB decomposition fraction for NaNbO3-(1Me+10Et) was approximately 1.25 times higher than that for NaNbO3-Me (Fig. 3), in agreement with the BET surface areas for NaNbO3-(1Me+10Et) (6.0 m2 g−1) and NaNbO3-Me (4.8 m g−1). Therefore, these results show that all the products are truly orthorhombic NaNbO3 particles.
Notably, the plate-like particles form at a relatively early state of the reaction as suggested in the FE-SEM image of NaNbO3-(1Me+10Et)-6h, which shows smaller and thinner plate-like particles than those of NaNbO3-(1Me+10Et) (Fig. 2c and S2a†). The plate size reaches a maximum at about 24 h, since there are no major differences in the particle size range or shape observed between the FE-SEM images of NaNbO3-(1Me+10Et) and NaNbO3-(1Me+10Et)-48h (Fig. 2c and S2b†). When the MeOH/EtOH ratio in the mixed solvent was changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 1
9 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vol%, the thickness of the plate-like particles increased from 20–80 nm to 50–250 nm, as shown in the FE-SEM images of NaNbO3-(1Me+10Et) and NaNbO3-(1Me+4Et) (Fig. 2c and S2c†). Thus, the thickness of plate-like particles can be controlled by the reaction time or the MeOH/EtOH mixing ratio. In contrast, an increase in the amount of Nb2O5 as a raw material causes the formation of cube-like particles as exhibited in the FE-SEM image of NaNbO3-(1Me+10Et)-5g, which is similar to NaNbO3-Me-5g (Fig. S2d and e†). Cube-like particles also form when the reaction temperature or the NaOH concentration is decreased, based on the FE-SEM images of NaNbO3-(1Me+10Et)-150°C and NaNbO3-(1Me+10Et)-low-NaOH (Fig. S2f and g†). Based on these results, crystal growth in certain directions is suppressed for cubic NaNbO3 particles under the reaction conditions that form plate-like particles. Feasible mechanisms are discussed below.
4 vol%, the thickness of the plate-like particles increased from 20–80 nm to 50–250 nm, as shown in the FE-SEM images of NaNbO3-(1Me+10Et) and NaNbO3-(1Me+4Et) (Fig. 2c and S2c†). Thus, the thickness of plate-like particles can be controlled by the reaction time or the MeOH/EtOH mixing ratio. In contrast, an increase in the amount of Nb2O5 as a raw material causes the formation of cube-like particles as exhibited in the FE-SEM image of NaNbO3-(1Me+10Et)-5g, which is similar to NaNbO3-Me-5g (Fig. S2d and e†). Cube-like particles also form when the reaction temperature or the NaOH concentration is decreased, based on the FE-SEM images of NaNbO3-(1Me+10Et)-150°C and NaNbO3-(1Me+10Et)-low-NaOH (Fig. S2f and g†). Based on these results, crystal growth in certain directions is suppressed for cubic NaNbO3 particles under the reaction conditions that form plate-like particles. Feasible mechanisms are discussed below.
It is well-known that hydrothermal reactions of the orthorhombic phase of Nb2O5 in the presence of a strong base generate cubic NaNbO3 with the same phase, with sizes of 50 nm–20 μm,15–22 while those in the 50–200 nm range are highly aggregated22 and are prepared using relatively small amounts of Nb2O5 and dilute NaOH solution.14 In addition, cubic particles of NaNbO3 typically form via fibrous sodium niobate hydrate (Na2Nb2O6·nH2O) as the intermediate.16,17,20,22 In the literature, possible mechanisms for converting fiber- into cubic-particles have been presented,16 but there is scant direct evidence for the process involved. Unfortunately, to the best of our knowledge, there have been no reports on the formation of fibrous sodium niobate hydrate (Na2Nb2O6·nH2O) in solvothermal syntheses using alcohols. In addition, rod-like and orthorhombic NaNbO3 particles have been synthesized hydrothermally, however, detailed formation mechanism is also absent.22 A prior study found that methanol, with a lower dielectric constant than water, affected the raw material solubility for the solvothermal syntheses of NaNbO3 as follows,19 a large number of nuclei form, and the crystal growth is retarded when methanol is used instead of water, causing a decrease in the maximum sizes of cubic NaNbO3 particles from 600 nm to 2 μm to 100–300 nm.19 As mentioned above,17 the particle size of Nb2O5 is a possible factor to determine its solubility in a strongly basic solvent. The size of the Nb2O5 particles used in the previous studies was 100–500 nm as judged from the FE-SEM images.19 This size range is similar to that of the present Nb2O5 particles, although smaller particles are also present, as shown in the SEM image (Fig. 2a). Therefore, the present results for NaNbO3-Me in the 50–500 nm range (Fig. 2b) are mostly consistent with previously reported values18,20 and show that the size distribution of cubic NaNbO3 particles is wider than the reported values.17 In the previous reports on the hydrothermal synthesis of plate-like NaNbO3 particles in the 200 nm to 1 μm range,20–22 aggregated particles form at relatively short reaction times, whereas longer reaction times generate cubic NaNbO3 particles. Plate-like particles likely form at an early stage of the hydrothermal synthesis, and the growth rate in the direction to convert plate-like particles into cubic particles seems to be rapid. Judging from the SEM images of the plate-like particles obtained by hydrothermal synthesis,20–22 they are thinner than those in the present study using solvothermal synthesis (Fig. 2b and c). In the present study, plate-like orthorhombic NaNbO3 particles form when ethanol is used as the solvent. The dielectric constant of ethanol is lower than that of methanol.27 In addition, the dielectric constant of a mixture of methanol and ethanol decreases with an increasing ethanol content.29 Based on the aforementioned discussion on the solvothermal synthesis of NaNbO3, the rapid formation and preservation of plate-like particles, and the decrease in their thickness with an increasing ethanol content in the mixed solvent shown in the FE-SEM images (Fig. 2c and S2a–c†),19 ethanol likely acted to retard the crystal growth more compared to methanol. The unit cell of orthorhombic NaNbO3 is longer in one direction than in the other directions, i.e., four NbO6 octahedra bridged via the vertices are longer in one direction.12,30,31 Based on the crystal structure of NaNbO3 determined in a previous study,12 the electron density in the longer direction for orthorhombic NaNbO3 is lower than that in other directions.12 Additionally, step- and kink-like structures30 are present on the surfaces of the plate-like particles of NaNbO3-(1Me+10Et) (Fig. 2c). Therefore, the crystal growth of NaNbO3 in the ethanol and methanol mixture is likely to be suppressed in certain directions, causing the formation of plate-like particles. The plate-like particles in the present study are thus unlikely to grow into cubic particles, which is consistent with the formation of cube-like particles with increasing amount of raw material that could cause particle to grow in the thickness direction (Fig. 2c and S2d†). In addition, a decrease in the reaction temperature and base concentration may retard the growth of plate-like particles in the lateral direction, thus forming cube-like particles (Fig. 2c and S2f and g†). Such growth suppression in one direction could be utilized in further studies on the design and control of both the thickness and the lateral size of particles with a plate-like morphology.32 In addition, compared to the ethanol and methanol mixture, a larger number of nuclei and a lower solubility of Nb2O5, which leads to less availability of the Nb source, may likely cause the formation of smaller and irregular plate-like particles when ethanol is used solely as the solvent. A decrease in the particle size of NaNbO3 by ethanol addition is feasible judging from previous reports,18,19,33 although the experimental conditions including the niobium sources in these previous studies are not identical. In addition, the particle size in other ABO3 perovskites, such as potassium niobate (KNbO3) and barium titanate, prepared by solvothermal synthesis increases by changing the solvent from methanol to ethanol.34,35 Thus, reports on the solvothermal syntheses of other ABO3 perovskites including those using a mixture of methanol and ethanol36,37 are not very helpful for clarifying the growth mechanisms involved in the present study. Interestingly, although in previous studies the particle morphology of KNbO3 changed very little when using a mixed solvent,34 in the present study, the NaNbO3 particle shape was successfully changed from cubic to plate-like. Note that there are non-linear relationships between specific solvent parameters for methanol–ethanol mixtures and the methanol or ethanol content.29 Therefore, the effects of methanol–ethanol mixed solvents on the morphology of NaNbO3 particles are worth further investigating, and it is thought that fibrous sodium niobate hydrate (Na2Nb2O6·nH2O) could play a role. Nevertheless, the cubic- and plate-like NaNbO3 particles identified in the present study, exhibiting photo-inducted properties, may find practical applications.10,11,38–40 The photocatalytic activity of plate-like NaNbO3 could be especially dependent on its morphology because there is a relationship between the MB decomposition fraction and the BET surface area for cubic- and plate-like NaNbO3, despite the lower water dispersibility of plate-like NaNbO3 (Fig. S3†). Such studies are ongoing in our laboratory, and the results will be reported in due course.
In summary, we have demonstrated the formation of platy submicron-sized NaNbO3 particles via solvothermal synthesis using a methanol/ethanol mixed solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 vol%). The results of the present study could potentially lead to a method for producing a wide range of perovskite compounds41 with a plate-like morphologies.
10 vol%). The results of the present study could potentially lead to a method for producing a wide range of perovskite compounds41 with a plate-like morphologies.
Author contributions
Shingo Machida: conceptualization, investigation, data curation, writing – original draft, preparation, and project administration; Shoma Niwa: data curation; Sho Usuki: writing – review and editing; Kazuya Nakata: writing – review and editing; Makoto Ogawa: writing – review and editing; Atsuo Yasumori: project administration; Ken-ichi Katsumata: conceptualization, writing – review and editing, and supervision.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was supported by a Grant-in-Aid from the moon-shot project (JPNP18016) “Development of photo-switching ocean-degradable plastics with edibility” of New Energy and Industrial Technology Development Organization (NEDO), Japan.References
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1–21 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- W. Lei, N. Suzuki, C. Terashima and A. Fujishima, Front. Energy, 2021, 15, 577–595 CrossRef.
- C. F. Goodeve, Trans. Faraday Soc., 1937, 33, 340–347 RSC.
- S. Bourtillier, S. Fourmentin and B. Laperche, Environ. Chem. Lett., 2022, 20(1033), 1017–1033 CrossRef.
- J. H. Braun, A. Baidins and R. E. Marganski, Prog. Org. Coat., 1992, 20, 105–138 CrossRef CAS.
- G. K. van der Wel and O. C. G. Adan, Prog. Org. Coat., 1999, 37, 1–44 CrossRef CAS.
- M. J. A. Ruszala, N. A. Rowson, L. M. Grover and R. A. Choudhery, Int. J. Chem. Eng. Appl., 2015, 6, 331–340 CAS.
- K. Katsumata, C. E. J. Cordonier, T. Shichi and A. Fujishima, J. Am. Chem. Soc., 2009, 131, 3856–3857 CrossRef CAS PubMed.
- K. Katsumata, C. E. J. Cordonier, T. Shichi and A. Fujishima, Mater. Sci. Eng., B, 2010, 172, 267–270 CrossRef.
- A. M. Hamilton, S. O'Donnell, B. Zoellner, I. Sullivan and P. A. Maggard, J. Am. Ceram. Soc., 2020, 103, 454–464 CrossRef CAS.
- H. Ge, Y. Hou, C. Xia, M. Zhu, H. Wang and H. Yan, J. Am. Ceram. Soc., 2011, 94, 4329–4334 CrossRef CAS.
- B. Zou, T. Wang, S. Li, R. Kang, G. Li, S. A. El-Khodary, D. H. L. Ng, X. Liu, J. Qiu, Y. Zhao, J. Lian and L. Huang, J. Energy Chem., 2021, 57, 109–117 CrossRef.
- S. Ji, H. Liu, Y. Sang, W. Liu, G. Yu and Y. Leng, CrystEngComm, 2014, 16, 7598–7604 RSC.
- Q. Liu, L. Zhang, Y. Chai and W.-L. Dai, J. Phys. Chem. C, 2017, 121, 25898–25907 CrossRef CAS.
- H. Zhu, Z. Zheng, X. Gao, Y. Huang, Z. Yan, J. Zou, H. Yin, S. H. Kable, J. Zhao, Y. Xi, W. N. Martens and R. L. Frost, J. Am. Chem. Soc., 2006, 128, 2373–2384 CrossRef CAS PubMed.
- K. Nakashima, Y. Toshima, Y. Kobayashi and M. Kakihana, J. Asian Ceram. Soc., 2019, 7, 36–41 CrossRef.
- K. Nakashima, Y. Toshima, Y. Kobayashi, Y. Ishikawa and M. Kakihana, J. Asian Ceram. Soc., 2019, 7, 544–550 CrossRef.
- T. Y. Ke, H. A. Chen, H. S. Sheu, J.-W. Yeh, H.-N. Lin, C.-Y. Lee and H. T. Chiu, J. Phys. Chem. C, 2008, 112, 8827–8831 CrossRef CAS.
- H. Song and W. Ma, Ceram. Int., 2011, 37, 877–882 CrossRef CAS.
- G. F. Teixeira, E. Silva Junior, A. Z. Simões, E. Longo and M. A. Zaghete, CrystEngComm, 2017, 19, 4378–4392 RSC.
- Y. Lu, T. Karaki and T. Fujii, J. Am. Ceram. Soc., 2015, 98, 1668–1672 CrossRef CAS.
- L. Jiang, Y. Zhang, Y. Qiu and Z. Yi, RSC Adv., 2014, 4, 3165–3170 RSC.
- Y. Yan, D. Liu, W. Zhao and H. Zhou, J. Am. Ceram. Soc., 2007, 90, 2399–2403 CrossRef CAS.
- X. Li, G. Li, S. Wu, X. Chen and W. Zhang, J. Phys. Chem. Solids, 2014, 75, 491–494 CrossRef CAS.
- Z. Pan, B. Liu, J. Zhai, L. Yao, K. Yang and B. Shen, Nano Energy, 2017, 40, 587–595 CrossRef CAS.
- S. Brunauner, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 407, 309–319 CrossRef.
- B. G. Lone, P. B. Undre, S. S. Patil, P. W. Khirade and S. C. Mehrotara, J. Mol. Liq., 2008, 141, 47 CrossRef CAS.
- M. Mohsen-Nia, H. Amiri and B. Jazi, J. Solution Chem., 2010, 39, 701–708 CrossRef CAS.
- P. G. Vekilov, Cryst. Growth Des., 2007, 7, 2796–2810 CrossRef CAS.
- S. Machida, T. Yoshida, R. Hashimoto and M. Ogawa, J. Colloid Interface Sci., 2014, 420, 66–69 CrossRef CAS PubMed.
- K. Oshima, K. Nakashima, S. Ueno and S. Wada, Trans. Mater. Res. Soc. Jpn., 2015, 40, 413–416 CrossRef CAS.
- K. Nakashima, S. Ueno and S. Wada, J. Ceram. Soc. Jpn., 2014, 122, 547–551 CrossRef.
- K. Nakashima, K. Onagi, Y. Kobayashi, T. Ishigaki, Y. Ishikawa, Y. Yoneda, S. Yin, M. Kakihana and T. Sekino, ASC Omega, 2020, 6, 9410 CrossRef PubMed.
- Y. Yoneda, R. Kunisada, T. Chikata, S. Ueno, I. Fujii, H. Nagata, K. Ohara and S. Wada, Jpn. J. Appl. Phys., 2019, 58, SLLA03 CrossRef CAS.
- Y. Yoneda, R.-I. Kunisada, T. Chikada, S. Ueno, K. Ohara and S. Wada, Trans. Mater. Res. Soc. Jpn., 2018, 43, 93–96 CrossRef CAS.
- K. Matsui, M. Karasaki, M. Segawa, S. Y. Hwang, T. Tanaka, C. Ogino and A. Kondo, Med. Chem. Commun., 2010, 1, 209–211 RSC.
- R. Nakano, M. Hara, H. Ishiguro, Y. Yao, T. Ochiai, K. Nakata, T. Murakami, J. Kajioka, K. Sunada, K. Hashimoto, A. Fujishima and Y. Kubota, Catalysts, 2013, 3, 310–323 CrossRef CAS.
- S. F. Silva, F. A. Silva, A. P. Martins de Souza, T. S. Rodrigues and R. R. Teixeira, J. Mater. Sci., 2022, 57, 1669–1688 CrossRef.
- K. Huang, L. Yuan and S. Feng, Inorg. Chem. Front., 2015, 2, 965–981 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ce00665k |
| This journal is © The Royal Society of Chemistry 2022 |