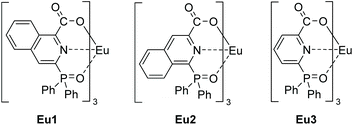
Luminescent europium(III) complexes based on tridentate isoquinoline ligands with extremely high quantum yield†
Zelun
Cai
a,
Chen
Wei
 *a,
Boxun
Sun
a,
Huibo
Wei
*b,
Zhiwei
Liu
*a,
Boxun
Sun
a,
Huibo
Wei
*b,
Zhiwei
Liu
 a,
Zuqiang
Bian
a,
Zuqiang
Bian
 *a and
Chunhui
Huang
a
*a and
Chunhui
Huang
a
aCollege of Chemistry and Molecular Engineering, Peking University, 202 Chengfu Road, Beijing 100871, P. R. China. E-mail: bianzq@pku.edu.cn; c.wei@pku.edu.cn
bJiangsu JITRI Molecular Engineering Institute Co., Ltd., 88 Xianshi Road, Changshu 215500, P.R. China. E-mail: weihuibo@163.com
First published on 21st October 2020
Abstract
Here, we report two europium(III) complexes (Eu1 and Eu2) with high photoluminescence quantum yield, based on tridentate isoquinoline derivatives (HL1 and HL2). The single crystal structures of Eu1 and Eu2 show that three ligands coordinate with an Eu(III) ion through diphenylphosphoryl oxygen, pyridyl nitrogen and carboxyl oxygen atoms (O^N^O) with no solvent in the first coordination sphere. An appropriate triplet energy level (about 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) ensures efficient energy transfer from the ligands to the Eu(III) ion. As a result, the two complexes exhibited significantly high quantum yields in the solid state (94% and 87% for Eu1 and Eu2, respectively) with excitation bands extending to 350 nm.
000 cm−1) ensures efficient energy transfer from the ligands to the Eu(III) ion. As a result, the two complexes exhibited significantly high quantum yields in the solid state (94% and 87% for Eu1 and Eu2, respectively) with excitation bands extending to 350 nm.
Introduction
On account of the unique photophysical properties of f–f transitions, such as characteristic narrow-band spectra and long luminescence lifetimes, inorganic rare earth compounds (oxides or fluorides) have been widely used as trichromatic phosphors and up-conversion imaging agents.1,2 But direct excitation from the Laporte forbidden f–f transitions results in very low absorption intensities (<3 M−1 cm−1).3 Alternatively, using organic ligands with high absorption coefficients to sensitize the emission of lanthanide ions can reduce the consumption of lanthanide materials, and show great potential in applications such as organic light emitting diodes (OLEDs),4 bio-imaging,5 pH sensing,6,7 and light conversion for photovoltaic devices.8,9High photoluminescence quantum yield (PLQY) is indispensable for lanthanide complexes to be used in the above applications, which requires appropriate design of the ligand structure. On the one hand, the ligands should have appropriate triplet energy levels for efficient energy transfer and on the other hand, they need to afford saturated coordination environments to protect the lanthanide ions from quenching by the O–H and N–H oscillators originating from coordinated solvents.10 A variety of luminescent lanthanide complexes have been synthesized, such as those based on β-diketonate derivatives,11 pyridyl derivatives,12 and dipyridyl derivatives.13 Recently, we have reported the use of a diphenylphosphoryl (DPPO) group to construct a series of highly emissive lanthanide(III) complexes.12,14 Among them, the Eu(III) complex based on 6-(diphenylphosphoryl)picolinic acid (denoted as Eu3 in this work) displayed very high PLQY in the solid state (81%). But the short excitation band (<280 nm) of the complex limits its further application in bio-imaging and light conversion for photovoltaic devices.
In this work, aiming at extending the excitation wavelength while maintaining the high PLQY of the complex, two isoquinoline derivatives, 3-diphenylphosphoryl-1-carboxyisoquinoline (HL1) and 1-diphenylphosphoryl-3-carboxyisoquinoline (HL2), were designed with an enlarged π conjugation system and reserved (O^N^O) coordination sites. Their corresponding Eu(III) complexes Eu1 and Eu2 were synthesized and characterized by X-ray diffraction (Scheme 1). The absorption spectra showed a large red-shift of the absorption edge for Eu1 and Eu2 compared with that of Eu3. Besides, Eu1 and Eu2 displayed bright red emissions under UV-A excitation (edge to 350 nm) with PLQY/lifetime values of 94%/2.18 ms and 87%/2.32 ms in the solid state, respectively. Sm(III), Nd(III), and Yb(III) complexes based on HL1 were also prepared and they showed favorable photophysical properties.
Experimental
Materials and methods
All the reagents and solvents are commercially available and used without additional purification except when otherwise noted.1H, 13C, and 31P NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer. Both 13C and 31P NMR spectra were obtained as proton-decoupled data. Electrospray ionization mass spectra (ESI-MS) were recorded in positive or negative ion mode on a Bruker Apex IV Fourier transform ion cyclotron resonance mass spectrometer. Elemental analyses (C, H, and N) were performed with a VARIO elemental analyzer from Elementar Analysensysteme GmbH. Thermo-gravimetric analyses were carried out using Q600SDT instruments at an elevation temperature rate of 10 °C min−1 under a 100 ml min−1 nitrogen flow. All the lanthanide complex samples were dried at 120 °C for 6 hours and then placed under ambient conditions for 1 day before EA and TGA measurements. Single crystals of Eu1 and Eu2 were obtained by slow evaporation of their methanol/dichloromethane solutions. The single crystal data were collected using a RAPID-S image plate diffractometer, and determined and refined using the SHELXL program package.
UV-Vis absorption spectra were measured on a PerkinElmer Lambda 35 spectrometer. Photoluminescence spectra and decay curves of the lanthanide complexes, and low temperature phosphorescence spectra of the gadolinium complexes were recorded on an Edinburgh FLS 980 spectrometer. Absolute PLQYs of the complexes were measured on a Hamamatsu C9920-02 PLQY measurement system (for emissions in the visible region) or an Edinburgh FLS980 with an integrating sphere (for emissions in the NIR region). The photophysical properties of the complexes were obtained in CH2Cl2 solutions (1 × 10−5 M) and in the solid states. All measurements were carried out at room temperature except the phosphorescence experiments at 77 K.
Because of the low absorption intensity of the f–f transition, the intrinsic quantum yield (ΦEuEu) of the Eu(III) complex cannot be determined experimentally, but can be estimated from eqn (1).
| ΦEuEu = τobs/τrad | (1) |
The radiative lifetime (τrad) of Eu (5D0) can be calculated using eqn (2),15 in which n represents the refractive index of the medium (1.46 for dichloromethane), AMD,0 is the spontaneous emission probability for the 5D0 → 7F1 transition under vacuum (14.65 s−1), Itot/IMD is the ratio of the total integrated intensity of the corrected Eu(III) emission spectrum to the integrated intensity of the magnetic dipole 5D0 → 7F1 transition.
| 1/τrad = AMD,0 × n3 × (Itot/IMD) | (2) |
Sensitization efficiency (ηsens) can be determined using eqn (3), where Φtot is the PLQY of the complex.
| Φtot = ΦEuEu × ηsens | (3) |
Synthetic procedures
Results and discussion
Syntheses of ligands and complexes
For the preparation of the ligand HL1, a five-step synthetic route was used (Scheme 2). The C–P bond was formed by the cross-coupling of 4 with diphenylphosphine oxide, catalyzed by Ni(dppp)Cl2 in the presence of K2CO3.16 The ligand HL2 was synthesized in three steps similar to the method of synthesizing quinolin-2-ylphosphonates by deoxygenative phosphorylation of the corresponding N-oxides (Scheme 3).17 First, 3-carboxyisoquinoline was treated with urea hydrogen peroxide (UHP) and trifluoroacetic anhydride (TFAA) in CH2Cl2 to afford the N-oxide 7 without further purification. Then 7 was isopropylcarbonylated by using isobutyric chloride to give an isoquinolinium salt 8. An Arbuzov reaction of 8 and the ethyl diphenylphosphinite was conducted, followed by the elimination of isobutyric acid to afford the final product HL2 (the assumed reaction mechanism is shown in Scheme S1†).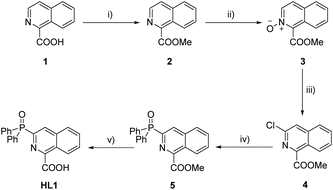 | ||
| Scheme 2 Synthetic route of HL1: (i) CH3OH, H2SO4, reflux; (ii) m-CPBA, CH2Cl2, RT; (iii) POCl3, reflux; (iv) Ph2P(O)H, K2CO3, NiCl2(dppp), toluene, reflux; and (v) TMAOH, reflux; concentrated HCl. | ||
The lanthanide complexes were prepared by the reaction of the corresponding deprotonated ligands and one-third equivalents of lanthanide chloride salt in methanol.
Crystal structure characterization
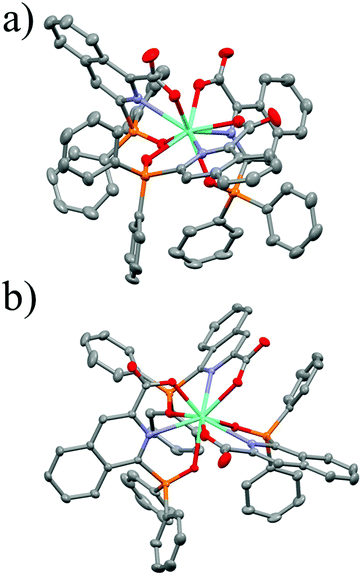
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of the diphenylphosphoryl group and the carboxyl group are found to be located nearly in the same plane with the isoquinoline segment (the dihedral angles are 4.93° and 9.88° for Eu1, and 6.37° and 8.32° for Eu2). The selected bond lengths for Eu1 and Eu2 are listed in Table 2. The average coordination bond length of both Eu1 and Eu2 is 2.49 Å, being suitable for energy transfer from the ligands to the centered ion. Eu1 crystallized in the trigonal space group P3, featuring a chiral trimeric structure, which contained one Λ complex and two Δ enantiomers in one repeating unit (Fig. S8†). Eu2 crystallizes in the triclinic space group P
O bonds of the diphenylphosphoryl group and the carboxyl group are found to be located nearly in the same plane with the isoquinoline segment (the dihedral angles are 4.93° and 9.88° for Eu1, and 6.37° and 8.32° for Eu2). The selected bond lengths for Eu1 and Eu2 are listed in Table 2. The average coordination bond length of both Eu1 and Eu2 is 2.49 Å, being suitable for energy transfer from the ligands to the centered ion. Eu1 crystallized in the trigonal space group P3, featuring a chiral trimeric structure, which contained one Λ complex and two Δ enantiomers in one repeating unit (Fig. S8†). Eu2 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and consists of centrosymmetric complexes. The difference between the packing structures of Eu1 and Eu2 may be due to the different aspects of the isoquinoline moiety.
and consists of centrosymmetric complexes. The difference between the packing structures of Eu1 and Eu2 may be due to the different aspects of the isoquinoline moiety.
| Compound | Eu1 | Eu2 |
|---|---|---|
| Empirical formula | C66H45EuN3O9P3 | C71H65EuN3O14P3 |
| Formula weight/g mol−1 | 1268.92 | 1429.13 |
| Crystal size/mm | 0.22 × 0.18 × 0.16 | 0.32 × 0.28 × 0.26 |
| Temperature/K | 113 | 113 |
| Crystal system | Trigonal | Triclinic |
| Space group | P3 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 19.351(4) | 11.452(2) |
| b/Å | 19.351(4) | 15.657(3) |
| c/Å | 13.859(2) | 20.993(4) |
| α/° | 90.00 | 77.41(3) |
| β/° | 90.00 | 89.17(3) |
| γ/° | 120.00 | 75.35(3) |
| V/Å3 | 4494.6(18) | 3551.0(14) |
| Z | 3 | 2 |
| D calcd/g cm−3 | 1.406 | 1.337 |
| F(000) | 1926 | 1464 |
| Absorption coefficient/mm−1 | 1.186 | 1.014 |
| Data/restraints/parameters | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 646/1/739 646/1/739 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850/0/840 850/0/840 |
| Goodness-of-fit on F2 | 1.033 | 1.061 |
| R 1/wR2 [I > 2σ(I)] | 0.0389/0.0993 | 0.0338/0.0902 |
| R 1/wR2 (all data) | 0.0413/0.1005 | 0.0377/0.0926 |
| Eu1 | Bond lengths/Å | Eu2 | Bond lengths/Å |
|---|---|---|---|
| Eu–O(C) | 2.367 | Eu–O(C) | 2.394 |
| Eu–N | 2.669 | Eu–N | 2.664 |
| Eu–O(P) | 2.428 | Eu–O(P) | 2.403 |
| Average | 2.488 | Average | 2.487 |
Photophysical properties
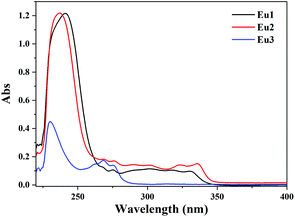
The UV-vis absorption spectra of Eu1 and Eu2 were investigated in dichloromethane solutions (1 × 10−5 M, Fig. 2). Two absorption bands could be observed, with one narrow band at about 240 nm with a molar absorption coefficient (ε) of 1.2 × 105 M−1 cm−1 and the other weaker broad band between 280 nm and 350 nm with an ε of 1.0 × 104 M−1 cm−1. Both bands originate from π → π* transitions of the ligands. The singlet energy gap (Eg) of the ligands can be estimated from the edge of the absorption spectra of the complexes, calculated to be 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and 28
000 cm−1 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 for L1−1 and L2−1, respectively. For comparison, the absorption spectra of Eu3 are also shown in Fig. 2. Two absorption bands at 230 nm (ε = 4.5 × 104 M−1 cm−1) and 270 nm (ε = 1.7 × 104 M−1 cm−1) are observed for Eu3, and the Eg is calculated to be as high as 35
600 cm−1 for L1−1 and L2−1, respectively. For comparison, the absorption spectra of Eu3 are also shown in Fig. 2. Two absorption bands at 230 nm (ε = 4.5 × 104 M−1 cm−1) and 270 nm (ε = 1.7 × 104 M−1 cm−1) are observed for Eu3, and the Eg is calculated to be as high as 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1.13 The expansion of the conjugating system in these isoquinoline derivatives decreases their singlet energy gap of more than 6200 cm−1 compared with their pyridine analogue.
200 cm−1.13 The expansion of the conjugating system in these isoquinoline derivatives decreases their singlet energy gap of more than 6200 cm−1 compared with their pyridine analogue.
The phosphorescence spectra of the corresponding gadolinium(III) complexes in the glass state at 77 K were measured to evaluate the triplet state energy levels (ET) of HL1 and HL2 (Fig. S9†), which were obtained from the 0-phonon component of the phosphorescence spectra (0 → 0 transition, fitted with gaussian distributions).21 The ET of HL1 and HL2 is 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and 20
000 cm−1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1, respectively, lying about 2500 cm−1 higher than the 5D0 energy level (17
200 cm−1, respectively, lying about 2500 cm−1 higher than the 5D0 energy level (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) of the Eu(III) ion, which is beneficial for efficient energy transfer.22
500 cm−1) of the Eu(III) ion, which is beneficial for efficient energy transfer.22
The excitation and emission spectra of the title Eu(III) complexes in dichloromethane solution and in the solid state are shown in Fig. 3 and Fig. S10.† The excitation spectra were recorded by monitoring the 5D0 → 7F2 (615 nm) transition. Similar to the absorption spectra, the excitation spectra of Eu1 and Eu2 show a broad band with edge to 350 nm while that of Eu3 displays a band tailed to about 290 nm. Upon excitation, Eu1 and Eu2 displayed characteristic emissions with peaks at 580, 593, 615, 650 and 700 nm, which are attributed to the f–f transitions of 5D0 → 7FJ with J = 0, 1, 2, 3 and 4, respectively. The emissions derived from the ligands are not observed, which indicates the effective energy transfer from the ligands to the Eu(III) ions. The emission spectra for Sm1, Nd1 and Yb1 were also measured and are shown in Fig. S11.†
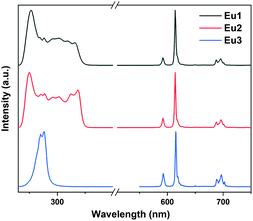 | ||
| Fig. 3 The excitation and emission spectra of the Eu(III) complexes in CH2Cl2 solution (1 × 10−5 M) at room temperature. | ||
The luminescence lifetimes (τobs) of Eu1, Eu2, Sm1, and Yb1 were obtained in both dichloromethane solution and the solid state by fitting the luminescence decay curves (Fig. S12–S14†) and the results are shown in Table 3. Long lifetime values of 2.2/2.3 ms were observed for Eu1/Eu2 while Sm1 and Yb1 showed much shortened ones. The τobs of Nd1 was not able to be obtained due to weak emission intensity. The monoexponential lifetime indicates that the lanthanide ions are locate in a similar environment for each complex.
| Complex | PLQYa (%) | τ obs (ms) | τ rad (ms) | Φ EuEu | η sens |
T
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (°C) d (°C) |
|
|---|---|---|---|---|---|---|---|
| Solid | Solution | ||||||
| a Photoluminescence quantum yield (PLQY) measured in the visible region using the Hamamatsu C9920-02 PLQY measurement system with an integrating sphere, Ex = 330 nm and 350 nm for solutions and solids, respectively. b PLQY measured in the NIR region using Edinburgh FLS980 with an integrating sphere, Ex = 350 nm. c Observed lifetime τobs and calculated radiative lifetime τrad in the solution. d Decomposition temperature determined by the loss of 5% desolvated weight. | |||||||
| Eu1 | 94 | 69 | 2.2 | 2.7 | 0.80 | 0.87 | 352 |
| Eu2 | 87 | 62 | 2.3 | 3.0 | 0.76 | 0.82 | 438 |
| Sm1 | 3.2 | n.a. | 0.11 | n.a. | n.a. | n.a. | 354 |
| Nd1 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 354 |
| Yb1 | 2.0b | n.a. | 0.048 | n.a. | n.a. | n.a. | 356 |
The photoluminescence quantum yields (PLQYs) of Eu1 and Eu2 were determined and the results are shown in Table 3. Eu1 and Eu2 show extremely high PLQYs in the solid state (94% and 87%, respectively) and in CH2Cl2 solution (69% and 62%, respectively). We also calculated the intrinsic quantum yield of the Eu(III)-centered emission (ΦEuEu) and the sensitization efficiency (ηsens) of these complexes in solution according to the literature.15 Both complexes showed high sensitization efficiencies (over 80%) and intrinsic quantum yields (over 75%), benefiting from the appropriate energy level of the ligands and suppressed quenching pathways. Sm1 and Yb1 showed much lower PLQYs compared with Eu1 in the solid states, as these complexes showed NIR emissions which were easily quenched by the small energy oscillators such as the C–H vibration.
Thermal stability study
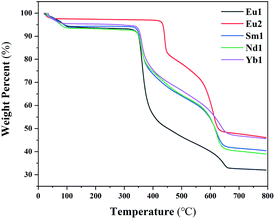
The thermo-gravimetric analyses (TGA) of Eu1, Eu2, Sm1, Nd1 and Yb1 were carried out to evaluate the thermal stability of the complexes (Fig. 4). The weight loss below 150 °C is attributed to the loss of the lattice solvent, which is consistent with the results of elemental analyses in the Experimental part. Taking Eu1 and Eu2 for example, the solvent weight loss values of the two complexes are 6.2% and 2.6%, respectively, which correspond to about 4.7 water molecules for one Eu1 and 1.9 water molecules for one Eu2. Anal. calcd for Eu1·4.7H2O: C 58.56, N 3.10, H 4.05; found, C 58.66, N 3.04, H 3.84. Anal. calcd for Eu2·1.9H2O: C 60.83, N 3.22, H 3.77; found, C 60.57, N 3.17, H 3.62. The decomposition temperature (Td) for all the complexes are higher than 350 °C (Table 3), which benefits from the rigid structures of the tridentate isoquinoline ligands.Conclusions
In summary, two structurally rigid europium(III) complexes based on diphenylphosphoryl modified tridentate isoquinoline derivatives were synthesized. Because isoquinoline has an enlarged π conjugation system compared with its pyridine analogue, broad band excitation tailed to 350 nm is obtained. The tridentate ligands present appropriate triplet energy levels for efficient intramolecular energy transfer and can saturate the coordination spheres of the Eu(III) ion when forming 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, thus avoiding quenching by solvents. Consequently, the title complexes display extremely high PLQYs in the solid state (94% and 87% for Eu1 and Eu2, respectively), showing that it is an effective way to construct highly luminescent lanthanide(III) complexes by employing diphenylphosphoryl tridentate ligands.
1 complexes, thus avoiding quenching by solvents. Consequently, the title complexes display extremely high PLQYs in the solid state (94% and 87% for Eu1 and Eu2, respectively), showing that it is an effective way to construct highly luminescent lanthanide(III) complexes by employing diphenylphosphoryl tridentate ligands.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported through grants from the National Key R&D Program of China (Grant 2017YFA0205100), National Natural Science Foundation of China (Grant 21621061), Key Project of Science and Technology Plan of Beijing Education Commission (Grant KZ201910028038), and Natural Science Foundation of Beijing Municipality (Grant 2172017).References
- H. Dong, L.-D. Sun, Y.-F. Wang, J. Ke, R. Si, J.-W. Xiao, G.-M. Lyu, S. Shi and C.-H. Yan, Efficient Tailoring of Upconversion Selectivity by Engineering Local Structure of Lanthanides in NaxREF3+x Nanocrystals, J. Am. Chem. Soc., 2015, 137, 6569–6576 CrossRef CAS.
- J.-C. G. Bünzli and C. Piguet, Taking advantage of luminescent lanthanide ions, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- S. V. Eliseeva and J.-C. G. Bünzli, Lanthanide luminescence for functional materials and bio-sciences, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- L. Wang, Z. Zhao, C. Wei, H. Wei, Z. Liu, Z. Bian and C. Huang, Review on the Electroluminescence Study of Lanthanide Complexes, Adv. Opt. Mater., 2019, 7, 1801256 CrossRef.
- J.-C. G. Bünzli, Lanthanide Luminescence for Biomedical Analyses and Imaging, Chem. Rev., 2010, 110, 2729–2755 CrossRef.
- A. P. de Silva, H. Q. N. Gunaratne and T. E. Rice, Proton-Controlled Switching of Luminescence in Lanthanide Complexes in Aqueous Solution: pH Sensors Based on Long-Lived Emission, Angew. Chem., Int. Ed. Engl., 1996, 35, 2116–2118 CrossRef CAS.
- L. K. Truman, S. Comby and T. Gunnlaugsson, pH-Responsive Luminescent Lanthanide-Functionalized Gold Nanoparticles with “On–Off” Ytterbium Switchable Near-Infrared Emission, Angew. Chem., Int. Ed., 2012, 51, 9624–9627 CrossRef CAS.
- J. Liu, K. Wang, W. Zheng, W. Huang, C.-H. Li and X.-Z. You, Improving spectral response of monocrystalline silicon photovoltaic modules using high efficient luminescent down-shifting Eu3+ complexes, Prog. Photovoltaics, 2013, 21, 668–675 CAS.
- C. Wei, L. Ma, H. Wei, Z. Liu, Z. Bian and C. Huang, Advances in luminescent lanthanide complexes and applications, Sci. China: Technol. Sci., 2018, 61, 1265–1285 CrossRef CAS.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. Gareth Williams and M. Woods, Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- D. B. A. Raj, B. Francis, M. L. P. Reddy, R. R. Butorac, V. M. Lynch and A. H. Cowley, Highly Luminescent Poly(Methyl Methacrylate)-Incorporated Europium Complex Supported by a Carbazole-Based Fluorinated β-Diketonate Ligand and a 4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene Oxide Co-Ligand, Inorg. Chem., 2010, 49, 9055–9063 CrossRef CAS.
- C. Wei, B. Sun, Z. Cai, Z. Zhao, Y. Tan, W. Yan, H. Wei, Z. Liu, Z. Bian and C. Huang, Quantum Yields over 80% Achieved in Luminescent Europium Complexes by Employing Diphenylphosphoryl Tridentate Ligands, Inorg. Chem., 2018, 57, 7512–7515 CrossRef CAS.
- G. S. Kottas, M. Mehlstäubl, R. Fröhlich and L. De Cola, Highly Luminescent, Neutral, Nine-Coordinate Lanthanide(III) Complexes, Eur. J. Inorg. Chem., 2007, 22, 3465–3468 CrossRef.
- C. Wei, B. Sun, Z. Zhao, Z. Cai, J. Liu, Y. Tan, H. Wei, Z. Liu, Z. Bian and C. Huang, A Family of Highly Emissive Lanthanide Complexes Constructed with 6-(Diphenylphosphoryl)picolinate, Inorg. Chem., 2020, 59, 8800–8808 CrossRef CAS.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes, Phys. Chem. Chem. Phys., 2002, 4, 1542–1548 RSC.
- Y.-L. Zhao, G.-J. Wu, Y. Li, L.-X. Gao and F.-S. Han, [NiCl2(dppp)]-Catalyzed Cross-Coupling of Aryl Halides with Dialkyl Phosphite, Diphenylphosphine Oxide, and Diphenylphosphine, Chem. – Eur. J., 2012, 18, 9622–9627 CrossRef CAS.
- S.-J. Lee, H.-S. Kim, H.-W. Yang, B.-W. Yoo and C. M. Yoon, Synthesis of Diethyl Pyridin-2-ylphosphonates and Quinolin-2-ylphosphonates by Deoxygenative Phosphorylation of the Corresponding N-Oxides, Bull. Korean Chem. Soc., 2014, 35, 2155–2158 CrossRef CAS.
- H. Zabrodsky, S. Peleg and D. Avnir, Continuous symmetry measures. 2. Symmetry groups and the tetrahedron, J. Am. Chem. Soc., 1993, 115, 8278–8289 CrossRef CAS.
- M. Pinsky and D. Avnir, Continuous Symmetry Measures. 5. The Classical Polyhedra, Inorg. Chem., 1998, 37, 5575–5582 CrossRef CAS.
- A. Ruiz-Martinez, D. Casanova and S. Alvarez, Polyhedral structures with an odd number of vertices: nine-atom clusters and supramolecular architectures, Dalton Trans., 2008, 2583–2591 RSC.
- W. F. Sager, N. Filipescu and F. A. Serafin, Substituent Effects on Intramolecular Energy Transfer. I. Absorption and Phosphorescence Spectra of Rare Earth β-Diketone Chelates, J. Phys. Chem., 1965, 69, 1092–1100 CrossRef CAS.
- M. Latva, H. Takalo, V.-M. Mukkala, C. Matachescu, J. C. Rodríguez-Ubis and J. Kankare, Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield, J. Lumin., 1997, 75, 149–169 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1814236 and 2009015. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qi00894j |
| This journal is © the Partner Organisations 2021 |