Open Access Article
Open Access ArticleElement-doped graphitic carbon nitride: confirmation of doped elements and applications
Wenjun
Zhang
,
Datong
Xu
,
Fengjue
Wang
and
Meng
Chen
 *
*
Department of Materials Science, Fudan University, Shanghai 200433, PR China. E-mail: chenmeng@fudan.edu.cn
First published on 17th June 2021
Abstract
Doping is widely reported as an efficient strategy to enhance the performance of graphitic carbon nitride (g-CN). In the study of element-doped g-CN, the characterization of doped elements is an indispensable requirement, as well as a huge challenge. In this review, we summarize some useful characterization methods which can confirm the existence and chemical states of doped elements. The advantages and shortcomings of these characterization methods are discussed in detail. Various applications of element-doped g-CN and the function of doped elements are also introduced. Overall, this review article aims to provide helpful information for the research of element-doped g-CN.
1. Introduction
In modern society, energy shortages, environmental issues, and diseases have been prominent problems. To solve these problems, photocatalysts have been developed for different applications, such as hydrogen evolution, pollutant treatment, antibiosis, cancer therapy, and so on.1–9 Graphitic carbon nitride, a polymeric semiconductor, has become one of the most promising photocatalysts since Wang's group reported this material for hydrogen production from water under visible light.10 It should be noted that graphitic carbon nitride is often wrongly named “g-C3N4” in the literature, but “perfect” g-C3N4 has not been synthesized so far.11 Therefore, we decided to denote graphitic carbon nitride as “g-CN” in this review.Up to now, g-CN, which is a low-cost, non-toxic, and environment-friendly material, has been a star photocatalyst because of its simple synthesis, tunable bandgap, and visible-light absorption.12 g-CN can be facilely prepared via thermal condensation of nitrogen-rich precursors, such as urea, thiourea, cyanamide, dicyandiamide, and melamine.13,14 The bandgap of g-CN is ∼2.7 eV with the conduction band (CB) minimum at ∼−1.1 eV and the valence band (VB) maximum at ∼+1.6 eV vs. normal hydrogen electrode (NHE).15,16 Such an energy band structure allows g-CN to absorb visible light and provide active groups for reactions. However, pristine g-CN suffers from several disadvantages including the low specific surface area, narrow optical absorption range, and high recombination efficiency of charge carriers,17–19 which limit the practical applications of g-CN. Therefore, tremendous efforts have been made to overcome the limitations mentioned above, including the preparation of g-CN-based composites, modification of precursors, post-treatment of g-CN, exfoliation, element doping, and so on.20–29
Element doping is a potential strategy to enhance the performance of g-CN, thus the doping of various elements has been reported in recent years. Different from g-CN-based composites, element-doped g-CN always involves the changes in the microstructure of g-CN, such as the loss of the graphitic structure30 and the impact on the in-plane ordering of tri-s-triazine units.31 Therefore, accurate characterization results are required to determine the existence of heteroatoms in element-doped g-CN.
In this review, we focus on the characterization methods that can provide evidence of successful element doping in g-CN. Relevant characterization techniques and their functions, namely how they can determine the doping of exotic elements will be discussed. Furthermore, the applications of element-doped g-CN will be introduced to show the positive influence of dopants on the performance of element-doped g-CN.
2. Confirmation of doped elements in g-CN
As the dopants in element-doped g-CN cannot be observed directly in electron microscopy images, comprehensive characterization methods should be applied to confirm the presence and chemical states of doped elements. The advantages and shortcomings of the techniques that can be used for the characterization of element-doped g-CN will be discussed below. Furthermore, the effect of doped elements on the band structure of g-CN will also be introduced because of the importance of band structure to the performance of g-CN materials.2.1 X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) measurements are widely used in the characterization of non-metallic element-doped g-CN. The oxidation states of the doped elements are understood by the XPS peak positions in their core-level spectra; therefore, the bond types of heteroatoms are determined. For example, Wang's group obtained fluorine-doped g-CN (F/g-CN) by evaporating the mixed precursor solution of dicyandiamide and NH4F and then calcining the resultant solid.31 A peak at 686.2 eV is shown in the XPS spectrum of F 1s (Fig. 1(a)), which can be assigned to the C–F bonds according to the previous report.34 Therefore, the XPS results indicate that F atoms are successfully introduced into the g-CN matrix as C–F bonds. Similar conclusions are also reported by other research groups.35–37 Doping of other non-metallic elements, such as B,38–48 P,49–59 S,60–66 and so on, can also be characterized by XPS measurements.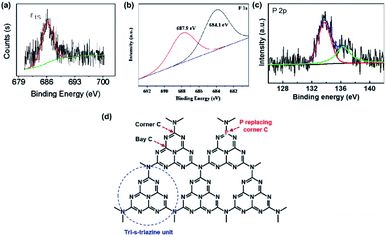 | ||
| Fig. 1 (a) F 1s XPS spectrum of F/g-CN. Reprinted with permission from ref. 31, copyright 2010, American Chemical Society. (b) F 1s XPS spectrum of F/g-CN. Reprinted with permission from ref. 32, copyright 2018, Elsevier. (c) P 2p XPS spectrum of P/g-CN and (d) illustration of replacement of corner C by P. Reprinted with permission from ref. 33, copyright 2018, Elsevier. | ||
Although the bond types of heteroatoms can be easily determined by the XPS peak positions, these characteristic peaks can be affected by some factors. The work of Chew's group, which prepared F/g-CN via the hydrothermal treatment of g-CN with NH4F, indicated that the XPS peak positions of C–F bonds can be altered by the strength of bonds.32 The XPS spectrum of their sample is shown in Fig. 1(b). The XPS peaks in the F 1s spectrum are divided into two components due to binding strength. One peak at 684.1 eV, whose binding energy is lower, is attributed to the weak bonds of isolated F atoms with C atoms; the other peak at 687.9 eV, whose binding energy is higher, is attributed to covalent C–F bonds. Another research shows that the doping position of heteroatoms can lead to some special XPS peaks.33 A new peak at 136.3 eV appears in the XPS spectrum of P-doped g-CN (Fig. 1(c)), which is quite different from the value of P–N bonds (133.7 eV) or P–C bonds (∼1–2 eV lower than that of P–N bonds). This peak can be attributed to the replacement of corner C atoms by P atoms (Fig. 1(d)) according to the computation results of the in-planar distance of nitride pores.
XPS measurements are also applicable to verify the doping of metallic elements in element-doped g-CN, such as Fe,30,67–75 Co,76–80 Mn,76,81–83 and so on. But, unlike the replacement of C or N atoms by non-metallic elements, metallic elements tend to anchor to the cavities between tri-s-triazine units and form coordination bonds with N atoms (Fig. 2).77,84–88 Hence, the characteristic peak positions of metallic elements correspond to their valence states. For instance, Fe is one of the commonest elements used in g-CN doping. Typically, Fe-doped g-CN (Fe/g-CN) can be synthesized via evaporating the mixed precursor solution of dicyandiamide and ferric salt and then calcining the resultant solid.30,67,70,71 The XPS spectra of Fe in Fe/g-CN are shown in Fig. 3. The XPS peak of Fe 2p3/2 at ∼710.5 eV, which lies within the range of the binding energy of Fe3+ valence state (710.3–711.8 eV),89 indicates the presence of Fe3+ in Fe/g-CN. Fe3+ can also exist in the composites of g-CN and iron species,90 so more evidence of doping should be provided: the absence of iron species can be observed via the X-ray diffraction (XRD) patterns of Fe/g-CN samples;30 elemental mapping is also utilized to display the uniform distribution of Fe atoms in Fe/g-CN, namely the successful doping of Fe.91
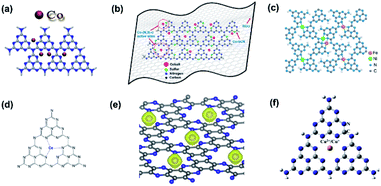 | ||
| Fig. 2 (a) Chemical structure of Co-doped g-CN. Reprinted with permission from ref. 77, copyright 2019, Elsevier. (b) Chemical structure of Co-coordinated S-doped g-CN on reduced graphene oxide (RGO). Reprinted with permission from ref. 84, copyright 2019, American Chemical Society. (c) Chemical structure of Fe–Ni co-doped g-CN. Reprinted with permission from ref. 85, copyright 2019, Royal Society of Chemistry. (d) Chemical structure of Ce-doped g-CN. Reprinted with permission from ref. 86, copyright 2015, Wiley. (e) Chemical structure of 3d transition-metal doped g-CN (the yellow balls represent Fe, Co, and Ni atoms). Reprinted with permission from ref. 87, copyright 2017, Elsevier. (f) Chemical structure of Cu2+/Cu+ doped g-CN. Reprinted with permission from ref. 88, copyright 2018, Elsevier. | ||
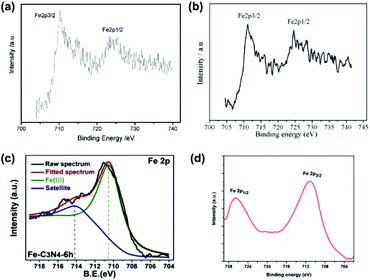 | ||
| Fig. 3 (a) XPS spectra of Fe in Fe/g-CN. Reprinted with permission from ref. 30, copyright 2009, Wiley. (b) XPS spectra of Fe in Fe/g-CN. Reprinted with permission from ref. 70, copyright 2010, Elsevier. (c) XPS spectra of Fe in Fe/g-CN. Reprinted with permission from ref. 67, copyright 2020, Elsevier. (d) XPS spectra of Fe in Fe/g-CN. Reprinted with permission from ref. 71, copyright 2014, Royal Society of Chemistry. | ||
To facilitate further research on g-CN doping, we summarize the reported bond types or oxidation states of heteroatoms and their XPS peak positions in element-doped g-CN materials in Table 1, thus the doping condition of a novel g-CN-based material can be quickly determined.
| Element | Bond type or oxidation state | XPS peak (eV) | Ref. |
|---|---|---|---|
| B | B–N | 190.8 | 38 |
| B–N–C | 191.1–192.4 | 39–44 | |
| B–N–C2 | 190.1 | 44 | |
| B–C3 | 190.9 | 45 and 46 | |
| B–C2O | 191.8 | 45 and 46 | |
| N–B–N | 191.7 | 47 and 48 | |
| B–O | 192.3 | 44 | |
| F | C–F2 | 692.0 | 37 |
| Weak C–F bonds | 684.1 | 32 | |
| Covalent C–F bonds | 687.9 | 32 | |
| O | C–O–C | 531.5–533.2 | 92 and 93 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
532.3 | 93 | |
| N–C–O | 531.45 | 94 | |
| Na | Na+ | 1070.3–1071.7 | 59 and 95–98 |
| Mg | Mg2+ | 305.2 (Auger) | 99 |
| Al | Al3+ | 76.0 (Al 2p3/2) | 100 |
| 72.1 (Al 2p1/2) | |||
| Si | Si–N | 102.2–102.8 | 101 |
| P | P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
132.8 | 49 |
| P–N | 133.3–134.0 | 49–54, 56 and 59 | |
| P–C | 131.5–132.6 | 50, 55 and 58 | |
| P–O–C | 133.5 | 57 | |
| P–O | 136.0 | 57 | |
| Replacement of corner C by P | 136.3 | 33 | |
| S | C–S–C | 161.8–164.0 | 60, and 62–66 |
| S–C–N | 165.6 | 63 | |
| S–N | 165.4 | 66 | |
| S–O | 168.0 | 62 | |
| C–S | 165.4 (S 2p3/2) | 61 | |
| 164.3 (S 2p1/2) | |||
| Cl | Cl–N | 197.6–198.1 (Cl 2p3/2) | 102 and 103 |
| 199.5–200.0 (Cl 2p1/2) | |||
| K | K+ | 295.2–295.5 (K 2p3/2) | 104–110 |
| 292.5–293.1 (K 2p1/2) | |||
| Ti | Ti4+ | 457.4 (Ti 2p3/2) | 111 |
| 462.8 (Ti 2p1/2) | |||
| V | V4+–5+ | 516.7 (V 2p3/2) | 112 |
| 524.1 (V 2p1/2) | |||
| Cr | Cr2+ | 575.7–575.9 (Cr 2p) | 113 |
| Cr3+ | 576.7–577.2 (Cr 2p) | 113 | |
| Cr6+ | 578.2–579.3 (Cr 2p) | 113 | |
| Mn | Mn2+ | 640.8–641.3 (Mn 2p3/2) | 81 and 82 |
| 653.3 (Mn 2p1/2) | |||
| Mn3+ | 639.5–642.1 (Mn 2p3/2) | 76, 82 and 83 | |
| 651.7–652.7 (Mn 2p1/2) | |||
| Mn4+ | 646.5 (Mn 2p3/2) | 83 | |
| 656.4 (Mn 2p1/2) | |||
| Fe | Fe2+ | 709.2–715.9 (Fe 2p3/2) | 67–69 |
| 723.7 (Fe 2p1/2) | |||
| Fe3+ | 710.3–711.8 (Fe 2p3/2) | 30, 67, 68, 70–75 and 114 | |
| 723.9–725.7 (Fe 2p1/2) | |||
| Co | Co3+ | 781.2–782.5 (Co 2p3/2) | 76–80 |
| 797.0–799.0 (Co 2p1/2) | |||
| Ni | Ni2+ | 853.5 (Ni 2p3/2) | 115 |
| 859.2 (Ni 2p1/2) | |||
| Ni3+ | 855.8–856.1 (Ni 2p3/2) | 76 and 116 | |
| 873.2–874.1 (Ni 2p1/2) | |||
| Cu | Cu+ | 932.1–932.8 (Cu 2p3/2) | 117–119 |
| 951.9–952.6 (Cu 2p1/2) | |||
| Cu2+ | 932.5–933.3 (Cu 2p3/2) | 76, 117, 120 and 121 | |
| 959.1–953.3 (Cu 2p1/2) | |||
| Zn | Zn2+ | 1022.1–1022.4 (Zn 2p3/2) | 122 and 123 |
| 1045.1–1045.4 (Zn 2p1/2) | |||
| Se | Se–C | 53.8–57.8 | 124–126 |
Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NH2)2 C(NH2)2 |
55.0 | 124 | |
| Se–N | 52.3–53.4 | 125 and 126 | |
| Br | Br− | 67.5–67.6 (Br 3d5/2) | 127 and 128 |
| 68.3–68.5 (Br 3d5/2) | |||
| Br–C | 68.3–69.3 | 127, 129 and 130 | |
| Sr | Sr2+ | 133.4 (Sr 3d5/2) | 131 |
| 135.0 (Sr 3d3/2) | |||
| Y | Y3+ | 154.6 (Y 3d5/2) | 132 |
| 159.7 (Y 3d3/2) | |||
| Zr | Zr4+ | 181.6 (Zr 3d5/2) | 133 |
| 184.0 (Zr 3d3/2) | |||
| Mo | Mo6+ | 231.8–232.1 (Mo 3d5/2) | 134–137 |
| 235.1–235.3 (Mo 3d3/2) | |||
| Pd | Pd2+ | 338.3 (Pd 3d5/2) | 138 |
| 343.6 (Pd 3d3/2) | |||
| In | In3+ | 445.1 (In 3d5/2) | 139 |
| 452.6 (In 3d3/2) | |||
| Sb | Sb3+ | 530.6 (Sb 3d5/2) | 140 |
| 539.9 (Sb 3d3/2) | |||
| Te | Te4+ | 575.0 (Te 3d5/2) | 141 |
| 585.5 (Te 3d3/2) | |||
| Te6+ | 577.0 (Te 3d5/2) | 141 | |
| 588.0 (Te 3d3/2) | |||
| I | C–I | 617.8–619.3 (I 3d5/2) | 142–146 |
| 629.8–630.7 (I 3d3/2) | |||
| C–I+–C | 619.8–619.9 (I 3d5/2) | 142 and 144–146 | |
| Ba | Ba2+ | 779.6–780.1 (Ba 3d5/2) | 147 and 148 |
| 795.0–795.4 (Ba 3d3/2) | |||
| La | La3+ | 834.3 and 837.8 (La 3d5/2) | 149 |
| 851.4 and 854.5 (La 3d3/2) | |||
| Ce | Ce3+ | 882.3, 885.6–886.0, and 888.4 (Ce 3d5/2) | 150–152 |
| 899.4–900.8, 904.4, and 907.3 (Ce 3d3/2) | |||
| Ce4+ | 881.9–882.4, 885.7, and 888.4 (Ce 3d5/2) | 150–152 | |
| 900.5–900.8, 904.1, 907.2, and 916.3 (Ce 3d3/2) | |||
| Sm | Sm3+ | 1084.0 (Sm 3d5/2) | 153 |
| 1105.0 (Sm 3d3/2) | |||
| Eu | Eu3+ | 1124.3 and 1135.8 (Eu 3d5/2) | 154 |
| 1165.6 (Eu 3d3/2) | |||
| Gd | Gd3+ | 142.4 and 148.8 (Gd 4d5/2) | 155 |
| 171.0 (Gd 4d3/2) | |||
| Er | Er3+ | 167.0–171.0 (Er 4d5/2) | 156 |
| Pt | Pt2+ | 73.0–73.3 (Pt 4f7/2) | 157 and 158 |
| 76.4–76.5 (Pt 4f5/2) |
2.2 Nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a powerful technique applied for the characterization of non-metallic element-doped g-CN. Peaks in NMR spectra reflect the chemical environment of a doped element, thus the chemical structure of element-doped g-CN can be speculated. B-doped carbon nitride microspheres (B-SSCN) were synthesized by Xue's group via a one-pot solvothermal method using ammonia borane, cyanuric chloride, and cyanuric acid as precursors.41 The 11B solid-state NMR spectrum of B-SSCN is shown in Fig. 4(a). The peaks at 15.9 and 6.72 ppm are considered as the result of sp2-coordinated B at the bay and corner C sites in the tri-s-triazine rings, and the peak at 2.97 ppm can be assigned to sp3-coordinated borane such as BH(N3).159–161 These results suggest that B atoms have been successfully doped into the microstructure of g-CN (Fig. 4(b)). Similar results are also discussed by Wang's group (Fig. 4(c)).40 The NMR spectra and proposed microstructures of some other element-doped g-CN materials are also determined by researchers (Fig. 4(d) and (e)).31,50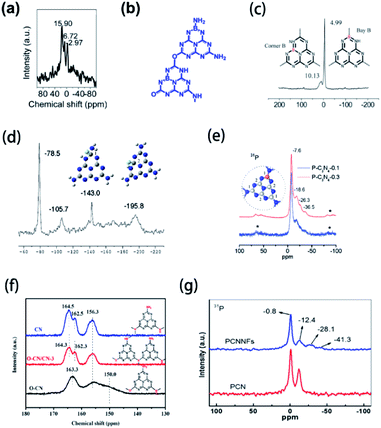 | ||
| Fig. 4 (a) 11B NMR spectrum and (b) microstructure of B-SSCN. Reprinted with permission from ref. 41, copyright 2016, Wiley. (c) 11B NMR spectra and microstructures of B-doped g-CN. Reprinted with permission from ref. 40, copyright 2011, Royal Society of Chemistry. (d) 19F NMR spectrum and microstructures of F-doped g-CN (blue, grey, white, and green balls represent N, C, H, and F atoms, respectively). Reprinted with permission from ref. 31, copyright 2010, American Chemical Society. (e) 31P NMR spectra and microstructure of P-doped g-CN (blue, grey, and orange balls represent N, C, and P atoms, respectively). Reprinted with permission from ref. 50, copyright 2010, American Chemical Society. (f) 13C NMR spectra and microstructures of CN, O–CN/CN-3, and O–CN. Reprinted with permission from ref. 92, copyright 2020, Elsevier. (g) 31P NMR spectra of PCN and PCNNFs. Reprinted with permission from ref. 33, copyright 2018, Elsevier. | ||
Notably, some structural details can be obtained via NMR, while other characterization methods have a limited effect. NMR can be used to determine the doping of oxygen, which widely exists on the surface of pristine g-CN,162 thus it becomes difficult to confirm the presence of O atoms in the microstructure of g-CN. Sun's group prepared O-doped g-CN (O–CN) via the solvothermal treatment of g-CN with cyanuric chloride.92 To prove the existence of O atoms in the microstructure of g-CN, solid-state 13C NMR spectra of pristine g-CN (CN), composite of O–CN and CN (O–CN/CN-3), and O–CN were obtained (Fig. 4(f)). Compared with CN, O–CN shows a unique peak at 163.3 ppm, which is attributed to the replacement of NHx species by O atoms.163 This result suggests that O atoms have been introduced into g-CN. Furthermore, NMR measurements are beneficial to discover the special chemical environment of doped elements. Tao's group synthesized P-doped g-CN (PCN) via annealing the mixed precursor of phytic acid and urea and then prepared P-doped g-CN nanoflakes (PCNNFs) via the post-treatment of PCN.33 The 31P NMR spectra of PCN and PCNNFs are shown in Fig. 4(g). Two peaks at −28.1 and −41.3 ppm, which only exist in the spectrum of PCNNFs, can be attributed to the terminal P species formed in the process of preparing PCNNFs.
2.3 Fourier transform infrared spectroscopy
Typically, with the introduction of non-metallic heteroatoms, some new vibration modes may be created, or some inherent vibration modes may be influenced. Therefore, Fourier transform infrared spectroscopy (FTIR) measurements can be used in the characterization of non-metallic element-doped g-CN materials. For example, Huang's group prepared Cl-doped g-CN via the thermal treatment for the mixed precursor of melamine and cyanuric chloride.164Fig. 5(a) shows the FTIR spectra of bulk g-CN (sample I) and Cl-doped g-CN (samples II and III). A new vibration band at ∼667 cm−1 exists only in the spectra of samples II and III, which is related to the stretching vibration of C–Cl and thus implies the successful doping of Cl atoms. But, in the FTIR spectra of some samples, instead of the appearance of the new bands, doped elements can influence the characteristic band positions. As shown in Fig. 5(b), the FTIR spectra of mesoporous g-CN (MCN) and B-doped mesoporous g-CN (BMCN) prepared by Badiei's group are consistent except for the band at 1600 cm−1 or MCN slightly shifting to 1580 cm−1 or BMCN, which suggests that B doping changed the tri-s-triazine units.165 Herein, we collect the data of the reported bond types related to the doped elements in g-CN and their characteristic FTIR bands in Table 2.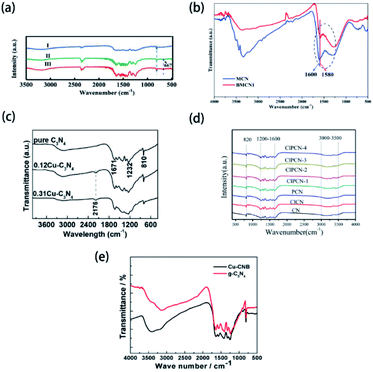 | ||
| Fig. 5 (a) FTIR spectra of bulk g-CN(I) and Cl-doped g-CN (II & III). Reprinted with permission from ref. 164, copyright 2019, Elsevier. (b) FTIR spectra of mesoporous g-CN (MCN) and B-doped mesoporous g-CN (BMCN). Reprinted with permission from ref. 165, copyright 2019, Elsevier. (c) FTIR spectra of pure g-CN, 0.12Cu–C3N4, and 0.31Cu–C3N4. Reprinted with permission from ref. 119, copyright 2015, Elsevier. (d) FTIR spectra of g-CN (CN), Cl-doped g-CN (ClCN), P-doped g-CN (PCN), and P–Cl co-doped g-CN (ClPCN-1–ClPCN-4). Reprinted with permission from ref. 103, copyright 2020, Elsevier. (e) FTIR spectra of g-CN and Cu and B co-doped g-CN (Cu-CNB). Reprinted with permission from ref. 170, copyright 2015, MDPI. | ||
| Element | Bond type | FTIR band (cm−1) | Ref. |
|---|---|---|---|
| B | B–N | 1370–1398 (stretching vibration) | 41, 43, 48 and 166 |
| Cl | C–Cl | 667 (stretching vibration) | 164 |
| F | C–F | 1220 (stretching vibration) | 31, 35 and 166 |
| I | C–I | 539.9 (stretching vibration) | 143 |
| P | P–N | 502 (bending vibration) | 51 and 167 |
| 950 (stretching vibration) | |||
| P–OH | 978 (stretching vibration) | 167 | |
| S | C–S | 1050–1200 (stretching vibration) | 64 and 168 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
1126–1185 (stretching vibration) | 61 and 169 | |
| S–H | 2380–2400 (stretching vibration) | 168 | |
| Se | Se–N | 2250 (stretching vibration) | 124 |
| Si | Si–N | 760 (stretching vibration) | 101 |
Interestingly, though the vibration modes of metal-contained bonds are unable to be reflected in FTIR spectra, the doping of metallic elements can result in some new vibration modes and further suggest the successful doping of heteroatoms. Asefa's group reported the synthesis of Cu-doped g-CN (Cu-C3N4) via annealing the mixed precursor of copper(II) salt and dicyandiamide.119 As shown in Fig. 5(c), the new band of Cu-C3N4 at 2176 cm−1, which is assigned to the stretching mode of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, demonstrates that the doping of Cu atoms leads to the formation of defect sites.88,171
N bond, demonstrates that the doping of Cu atoms leads to the formation of defect sites.88,171
However, FTIR spectroscopy suffers the following disadvantages in the characterization of element-doped g-CN: (1) according to some reports, doping may make no difference between the FTIR spectra of pristine g-CN and element-doped g-CN because the heteroatoms do not change the microstructure of g-CN;38,42,56,62,64,66 (2) FTIR bands can be influenced by the content of heteroatoms. Yang's group synthesized P–Cl co-doped g-CN via annealing the mixed precursor of ammonium chloride, ammonium phosphate, and melamine.103 The FTIR spectra of their samples exhibit bands at 820, 1200–1600 and 3000–3500 cm−1, which are attributed to the breathing mode of tri-s-triazine rings, stretching vibration modes of CN heterocycles, and uncondensed terminal amino groups, respectively; while no vibration bands associated with P or Cl groups are discovered due to the low content of P and Cl (Fig. 5(d)). (3) FTIR bands of doped elements may not appear due to overlapping. Han's group prepared Cu and B co-doped g-CN (Cu-CNB) via calcining the mixed precursor of 1-cyanopropyl-3-methylimidazolium tetrafluoroborate, Cu(NO3)2·3H2O, and urea.170 The FTIR spectra of g-CN and Cu-CNB are shown in Fig. 5(e). The typical vibration bands of B–N bonds should appear at 1370 cm−1, but they were presumably overlapped by the C–N stretching vibrations.172 The FTIR spectra of B-doped g-CN samples prepared by other research groups also showed the same result.48,173
2.4 X-ray absorption spectroscopy
X-ray absorption spectroscopy (XAS) measurements, including X-ray absorption near-edge structure (XANES), near-edge X-ray absorption fine structure (NEXAFS) and extended X-ray absorption fine structure (EXAFS), can be used to characterize the chemical states of doped elements in g-CN-based materials.60,85,114,174,175 The bond types of doped elements can be easily distinguished according to EXAFS peaks. For instance, as shown in the Fourier-transformed EXAFS (FT-EXAFS) spectra (Fig. 6(a)), a novel peak of Fe–g-CN at 1.54 Å implies the backscattering of the first coordination shell of Fe–N bonds.174 To gain more insight, the oxidation states of doped elements can be determined via XANES spectra in Fig. 6(b): the normalized Fe K-edge XANES spectra of Fe-g-CN, Fe2O3, and the reference Fe foil were measured. The absorption edge of Fe-g-CN possesses higher energy than Fe2O3 and lower energy than the Fe foil, which indicates that the oxidation state of Fe in the sample is between Fe(0) and Fe3+. Based on the evidence shown above, they proved the successful doping of Fe atoms. Similar methods were also used in the characterization of other element-doped g-CN. Sun's group prepared Ni-doped g-CN (Ni@PCN) and Ni-Fe co-doped g-CN (NiFe@PCN) for the oxygen evolution reaction.85 FT-EXAFS spectra of these samples are shown in Fig. 6(c); the peak of both Ni@PCN and Ni0.65Fe0.35@PCN at 1.8 Å corresponds to the Ni–N bond, which indicates the doping of Ni in these samples. Liu's group synthesized S-doped g-CN, and the S K-edge XANES spectrum of this material is shown in Fig. 6(d).60 Two pre-edge features at 2472.6 and 2482.0 eV can be assigned to two kinds of functional groups, Sθ− (0 < θ ≤ 2) and SO42−, respectively. Sθ− species is the result of the replacement of N atoms with S atoms, namely the formation of C–S bond; SO42− species exist on the surface of S-doped g-CN.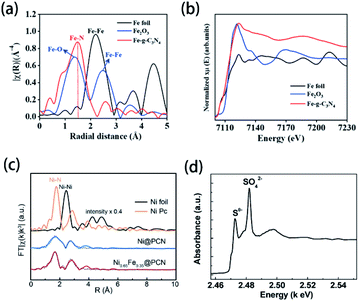 | ||
| Fig. 6 (a) FT-EXAFS spectra and (b) Fe K-edge XANES spectra of the Fe foil, Fe2O3, and Fe-g-CN. Reprinted with permission from ref. 174, copyright 2020, American Chemical Society. (c) FT-EXAFS spectra of the Ni foil, Ni Pc, Ni@PCN, and Ni0.65Fe0.35@PCN. Reprinted with permission from ref. 85, copyright 2019, Royal Society of Chemistry. (d) S K-edge XANES spectrum of S-doped g-CN. Reprinted with permission from ref. 60, copyright 2010, American Chemical Society. | ||
Compared with other characterization techniques, XAS measurement is considered a more powerful and efficient technique to gain direct insights into the chemical environments of target atoms. In general, XAS measurement exhibits the following advantages:176 (1) fitting to the investigation of amorphous materials; (2) potent detection at a low concentration; and (3) high spatial and temporal resolutions for an in situ experiment. Hence, XAS has been extensively utilized in the study of materials science in recent years,182,183 especially in the research of some element-doped materials, such as Cu-exchanged zeolites,184 P-doped hematite,185 Ta-doped α-Fe2O3 nanorods,186 and P–B co-doped amorphous porous Ni–Fe-based material.187 Therefore, the future looks bright for the use of XAS measurement in the characterization of element-doped g-CN, though very little work about this has been carried out so far.
2.5 Elemental mapping and electron energy-loss spectroscopy
Elemental mapping is suitable for the characterization of element-doped g-CN. Generally, the uniform distribution of the target element can be directly observed in elemental mapping results, which demonstrates the successful doping combined with the absence of nanoparticles in electron microscopy images. The vast majority of doped elements, such as Cu (Fig. 7(a–c)),88 S (Fig. 7(d–f)),177 F (Fig. 7(g)),35 Ce (Fig. 7(h–i)),86 and so on, can be characterized easily by elemental mapping. However, it is noteworthy that we can only confirm the ‘existence’ of elements but not the ‘doping’ of elements through elemental mapping. For example, as shown in Fig. 7(j–m), the uniform distribution of Co can also be observed in the elemental mapping results of a co-catalyst system containing g-CN and the cobalt-based cubane molecular, which is not an element-doped g-CN material.178 Therefore, more characterization information, such as electron microscopy images and the characterization techniques mentioned above, has to be provided to certify the successful doping.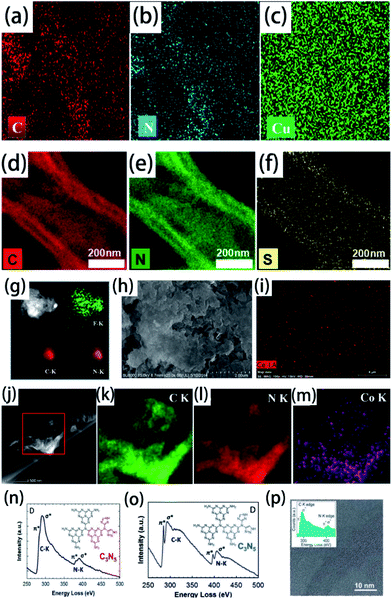 | ||
| Fig. 7 Elemental mapping images of (a) C, (b) N, and (c) Cu for Cu2+-doped g-CN. Reprinted with permission from ref. 88, copyright 2018, Elsevier. Elemental mapping images of (d) C, (e) N, and (f) S for S-doped g-CN. Reprinted with permission from ref. 177, copyright 2020, Elsevier. (g) Elemental mapping images of F, C, and N for F-doped g-CN nanosheets. Reprinted with permission from ref. 35, copyright 2015, Royal Society of Chemistry. (h) SEM image of Ce-doped g-CN and (i) elemental mapping image of Ce for this material. Reprinted with permission from ref. 86, copyright 2015, Wiley. (j) HAADF-STEM image of cobalt-based cubane molecular and g-CN co-catalysts and elemental mapping images of (k) C, (l) N, and (m) Co for this material. Reprinted with permission from ref. 178, copyright 2018, Elsevier. (n) EELS spectrum of highly ordered mesoporous C3N5. Reprinted with permission from ref. 179, copyright 2017, Wiley. (o) EELS spectrum of highly ordered nitrogen-rich mesoporous C3N5. Reprinted with permission from ref. 180, copyright 2017, Wiley. (p) EELS of g-CN. Reprinted with permission from ref. 181, copyright 2011, Royal Society of Chemistry. | ||
Electron energy-loss spectroscopy (EELS) is another available tool for elemental analysis. EELS is especially useful for the analysis of light elements, and its spatial resolution is higher than that of elemental mapping, meaning that it is particularly suitable for the detection of doped elements.188 This technique has been widely used in the elemental analysis of carbon nitride materials.179–181 As shown in (n–p), the characteristic peaks in the EELS spectra illustrate the presence of C and N in g-CN and C3N5. Therefore, we believe that EELS can also be applied for the characterization of element-doped g-CN—although it is rarely used in the research of element-doped g-CN nowadays.
2.6 Band structure
It is well known that the band structure has a decisive impact on the properties of g-CN materials. Therefore, it is very necessary to mention the effect of element doping on the band structure of g-CN. The values of the CB minimum, VB maximum, and band gap are the most important information about the band structure of element-doped g-CN. Generally, the value of the band gap can be determined via UV-vis diffuse reflectance spectroscopy (DRS).37,102,132 The values of the CB minimum and VB maximum can be evaluated using Mott–Schottky plots or XPS valence band spectra.44,78,94,154As the situations of non-metallic element and metallic element doping are quite different, they will be discussed separately in this section. According to related research results, the influence of diverse non-metallic elements doping on the band structure varies greatly. As shown in Fig. 8(a), the majority of non-metallic element-doped g-CN possess a narrower band gap than that of pristine g-CN, which makes them have better optical absorption ability and thus more excellent photocatalytic performance.38,55,62,130 Such a change of band gap can be attributed to the following reasons: (1) defects are formed during the synthesis process due to the doping of elements, which can enhance the optical absorption38 and (2) doped elements lead to electronic integration, which can also influence the band gap.50,62Fig. 8(a) also illustrates that the VB and CB positions of different non-metallic element-doped g-CN materials are located in a wide range, which may be ascribed to the electronegativity difference among C, N, and doped elements.44,60 Sometimes, this variation of VB and CB positions can cause a wider band gap.101,103
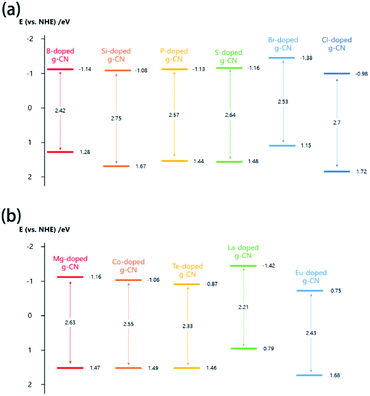 | ||
| Fig. 8 (a) Band structures of B-doped g-CN from ref. 38, Si-doped g-CN from ref. 101, P-doped g-CN from ref. 55, S-doped g-CN from ref. 62, Br-doped g-CN from ref. 130, and Cl-doped g-CN from ref. 103; (b) band structures of Mg-doped g-CN from ref. 99, Co-doped g-CN from ref. 80, Te-doped g-CN from ref. 141, La-doped g-CN from ref. 149, and Eu-doped g-CN from ref. 154. | ||
The band structures of some metallic element-doped g-CN materials are shown in Fig. 8(b). Although their VB and CB positions are various, all of their band gaps are narrower than that of pristine g-CN. The charge-transfer transition between the electrons of the doped metallic element and the VB or CB of g-CN can result in the redshift of absorption edge in the DRS spectrum of a metallic element-doped g-CN material, which means the narrower band gap.132 In addition, the doping of metallic elements can produce a midgap state in the band gap. The midgap state can hold the electrons that are photo-excited from the valence band, which is beneficial to the transfer of photogenerated electrons.141
2.7 Potential characterization techniques
With the development of technology, more and more novel characterization techniques are used for the investigation of element doping. Although these techniques have not been mentioned in the literature of element-doped g-CN, they are already applied in the investigation of other materials. Herein, we will introduce some potential characterization techniques which can be used for the confirmation of doped elements in the element-doped g-CN materials.Atom probe tomography (APT) can provide a 2D projection or 3D reconstruction of elements with great spatial resolution, which makes it suitable for the analysis of doped elements.191 For instance, Wang's group used APT to study the details of transition-metal single atoms in a graphene shell.189 As shown in Fig. 9(a) and (b), the 3D tomography and the projected 2D image of Ni-doped graphene shells, which reveal the distribution of C, Ni, and N atoms, are obtained through APT. To better understand the doping of Ni, the statistics of Ni atoms inside the yellow circle in Fig. 9(b) are analyzed in Fig. 9(c). Among all Ni atoms, 83% are single atoms, which means the successful doping of Ni in graphene shells.
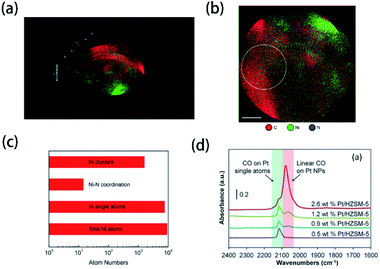 | ||
| Fig. 9 (a) 3D tomography and (b) projected 2D image of Ni-doped graphene shells; (c) statistics of Ni atoms inside the yellow circle in (b). Reprinted with permission from ref. 189, copyright 2017, Elsevier. (d) FTIR spectra of CO adsorbed at saturation coverage and room temperature onto different Pt/HZSM-5 catalysts containing varying Pt weight loading. Reprinted with permission from ref. 190, copyright 2017, Elsevier. | ||
Probe molecule FTIR spectroscopy is another potential technique that can characterize doped elements. Probe molecules, such as CO and CO2, can be absorbed by certain microstructures, thus some characteristic FTIR bands are generated.192 In this method, characteristic FTIR bands of single atoms combined with probe molecules may be observed. Christopher's group characterized Pt-group metal based single atom catalysts via probe molecule FTIR spectroscopy (CO as probe molecules).190 The FTIR spectra in Fig. 9(d) illustrate that a unique band at ∼2120 cm−1 belongs to the absorption of CO on Pt single atoms. Therefore, probe molecule FTIR spectroscopy may be usable in the characterization of element-doped g-CN.
3. Applications of element-doped g-CN materials
In recent years, numerous novel materials involved in element-doped g-CN are investigated in-depth due to their excellent performances in various fields, such as photocatalysis, electrocatalysis, sensors, sonocatalysis, supercapacitors, and adsorbents. The effects of element-doped g-CN and the mechanisms of performance enhancement in practical applications will be discussed below.3.1 Photocatalysis
Element-doped g-CN materials are considered as an up-and-coming metal-free photocatalyst since the early stage of related research. Up to now, the applications of photocatalysis, including the degradation of organics, hydrogen generation, and gas treatment, have been widely studied.Most element-doped g-CN materials are efficient for the degradation of organic contaminants, such as organic dyes and antibiotics. To display the excellent performance of element-doped g-CN materials intuitively, some results of photocatalytic degradation measurements are provided in Table 3. As shown in Table 3, compared with pristine g-CN, element-doped g-CN materials have better performance for the degradation of various organic contaminants. The effects of element-doped g-CN and the mechanisms of performance enhancement are usually revealed by various measurements. For example, the photocatalytic mechanism of P–Cl co-doped g-CN was investigated by scavenger experiments and electron spin response (ESR) experiments.103 The results of scavenger experiments in Fig. 10(a) illustrate that the degradation ability of rhodamine B is reduced when p-benzoquinone (BQ, ·O2− scavenger) was added into the reaction solution, which indicates that ·O2− played an important role in the degradation reaction. To further confirm the effect of ·O2−, ESR experiments were used with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a trapping agent at room temperature. As shown in Fig. 10(b), the response of ·O2− species appeared under light irradiation. The above findings demonstrate that the photocatalytic activity of P–Cl co-doped g-CN is mainly attributed to ·O2− species. Therefore, the photocatalytic mechanism of P–Cl co-doped g-CN can be implied as follows: the energy band structure of g-CN can also be regulated by the P–Cl co-doping and thus a stronger reducing ability to form ·O2− is obtained. Photoluminescence (PL) analysis can also be used to demonstrate the mechanisms of performance enhancement. The PL spectra of pristine g-CN and Ba-doped g-CN are displayed in Fig. 10(c):147 the lower intensity of a series of Ba-doped g-CN samples directly points out that the doping of Ba reduced the recombination of photogenerated electron–hole pairs.
| Sample | Organic contaminant | Degeneration performance (according to the rate constant k, compared with pristine g-CN) | Ref. |
|---|---|---|---|
| B–P co-doped g-CN nanosheets | Oxytetracycline | 2.9 times | 193 |
| Ba-doped g-CN | Tetracycline | 3.25 times | 147 |
| Ce-doped g-CN | Rhodamine B | 2.12 times | 86 |
| Cl-doped g-CN | Rhodamine B | 4 times | 164 |
| Cu-doped g-CN | Methylene blue | 2.3 times | 88 |
| Cu-doped mesoporous g-CN | Methyl orange | 10.5 times | 194 |
| Fe-doped g-CN | Trimethoprim | 1.33 times | 195 |
| Na-doped g-CN | Rhodamine B | 3.56 times | 95 |
| P-doped g-CN | Rhodamine B | 18 times | 55 |
| P-doped g-CN | Rhodamine B | 2.7 times | 54 |
| P–Cl co-doped g-CN | Rhodamine B | 5.9 times | 103 |
| Antibiotic norfloxacin | 2 times | ||
| P–S co-doped g-CN | Rhodamine B | 3.54 times | 49 |
| Si-doped g-CN | Rhodamine B | 2.5 times | 101 |
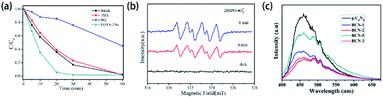 | ||
| Fig. 10 (a) Species trapping experiments for the degradation of rhodamine B; (b) DMPO spin-trapping ESR spectra of P–Cl co-doped g-CN. Reprinted with permission from ref. 103, copyright 2020, Elsevier. (c) PL spectra of pristine g-CN and Ba-doped g-CN. Reprinted with permission from ref. 147, copyright 2020, Elsevier. | ||
In addition, to cope with the increasing requirements of environmental issues, composites of element-doped g-CN and nanostructure have become a hotspot most recently. Element-doped g-CN-based composites and their excellent photocatalytic performance were reported, including Ag2CO3/P–S co-doped g-CN,196 Ag3VO4/P–S co-doped g-CN,198 MoS2/S-doped g-CN,197 S-doped rGO/S-doped g-CN,64 carbon quantum dots supported AgI/ZnO/P-doped g-CN,57 Zn3In2S6/F-doped g-CN,32 and so on. Typically, one or several kinds of nanostructures can be loaded on the element-doped g-CN matrix to obtain a composite (Fig. 11(a), (c), and (e)). Characterization of the band structures of these composites reveals that Z-scheme heterojunctions, which are conducive to the performance of photocatalysis, are formed between element-doped g-CN and the nanostructure (Fig. 11(b), (d), and (f)).
 | ||
| Fig. 11 (a) TEM image and (b) band structure of Ag2CO3/P–S co-doped g-CN. Reprinted with permission from ref. 196, copyright 2020, Elsevier. (c) TEM image and (d) band structure of MoS2/S-doped g-CN. Reprinted with permission from ref. 197, copyright 2020, Elsevier. (e) TEM image and (f) band structure of carbon quantum dots supported AgI/ZnO/P-doped g-CN. Reprinted with permission from ref. 57, copyright 2019, Elsevier. | ||
Besides the degradation of organic contaminants, photocatalysis of water splitting (including hydrogen evolution and water oxidation) is another encouraging practical application of element-doped g-CN-based materials. As shown in Fig. 12(a), B–S co-doped g-CN (CNBS),199 (c) P-doped g-CN nanoflakes (PCNNFs),33 (e) F-doped g-CN (GCNF),36 (h) S-doped g-CN (MTCN),62 (k) Co-doped g-CN (Co–g-CN),78 and (m) Bi-doped g-CN (Bi–g-C3N4),200 compared with pristine g-CN and element-doped g-CN-based materials, always possess spectacularly better H2 evolution performance or water oxidation performance. The enhancement can be assigned to one or more of the following reasons: (1) lower recombination of photogenerated electron and hole pairs caused by the element doping according to the lower intensity in PL spectra (Fig. 12(b), (d), (i), (l), and (n)) or a stronger signal in ESR spectra (Fig. 12(f)); (2) the nitrogen adsorption–desorption isotherms (Fig. 12(g) and (j)) reveal that, in some samples, the doping process can lead to larger specific areas, which is also effective for enhancing the photocatalytic performance; (3) the dopants modify the band structure of g-CN, especially their CB positions (for H2 evolution) or VB positions (for water oxidation), so that element-doped g-CN materials can have a better photocatalytic performance.
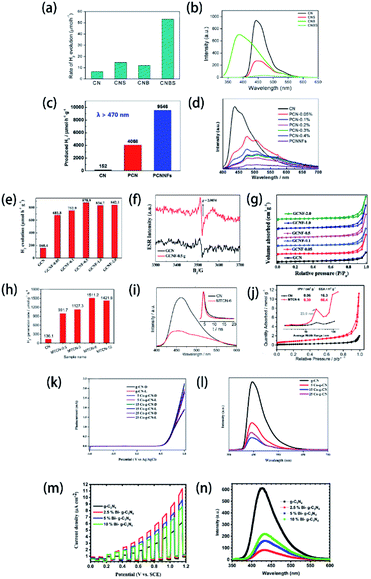 | ||
| Fig. 12 (a) Photocatalytic H2 evolution efficiency and (b) PL spectra of g-CN (CN), S-doped g-CN (CNS), B-doped g-CN (CNB), and B–S co-doped g-CN (CNBS). Reprinted with permission from ref. 199, copyright 2018, American Chemical Society. (c) Photocatalytic H2 evolution efficiency and (d) PL spectra of g-CN (CN), P-doped g-CN (PCN), and P-doped g-CN nanoflakes (PCNNFs). Reprinted with permission from ref. 33, copyright 2018, Elsevier. (e) Photocatalytic H2 evolution efficiency, (f) ESR spectra, and (g) N2 adsorption–desorption isotherms of g-CN (GCN) and F-doped g-CN (GCNF). Reprinted with permission from ref. 36, copyright 2018, Royal Society of Chemistry. (h) Photocatalytic H2 evolution efficiency, (i) PL spectra, and (j) N2 adsorption–desorption isotherms (the inset shows the corresponding pore size distributions) of g-CN (CN) and S-doped g-CN (MTCN). Reprinted with permission from ref. 62, copyright 2018, Elsevier. (k) Linear-sweep voltammetry plots and (l) PL spectra of g-CN and Co-doped g-CN (Co-g-CN). Reprinted with permission from ref. 78, copyright 2020, Elsevier. (m) Linear-sweep voltammetry plots and (n) PL spectra of g-CN and Bi-doped g-CN (Bi-g-C3N4). Reprinted with permission from ref. 200, copyright 2020, Elsevier. | ||
Under light irradiation, element-doped g-CN materials are capable of photocatalytic gas treatments. In some element-doped g-CN materials, doping can create vacancies, which are able to trap the gas molecules. Yao's group reported the photocatalytic N2 fixation performance of I-doped g-CN, which was prepared by annealing the mixed precursor of dicyandiamide and KIO3.143 The introduction of I atoms produces many vacancies, which can capture photogenerated electrons (Fig. 13(a)). These electrons can reduce N2 to form NH4+, which can be fixed by plants. Because of such a mechanism, the ammonia evolution rate of I-doped g-CN reaches 200.8 mg L−1 gcat−1, which is 2.8 times higher than that of pristine g-CN. For other element-doped g-CN materials, dopants can promote the separation of photogenerated electrons and holes and thereby catalyze the gas reactions. Such a mechanism (Fig. 13(b)) can demonstrate the NOx removal by B-doped g-CN nanotubes (their NO removal rate is 1.5 times higher than that of pristine g-CN)48 and the reduction of CO2 by P-doped g-CN nanotubes (their CO2 reduction rate is 13.92 times higher than that of pristine g-CN).201
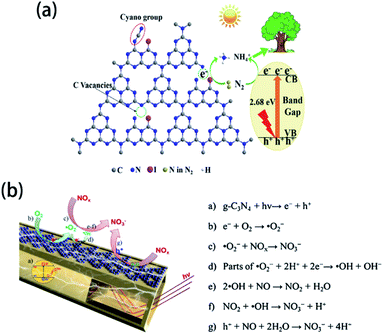 | ||
| Fig. 13 (a) Mechanism of N2 fixation on I-doped g-CN. Reprinted with permission from ref. 143, copyright 2020, Elsevier. (b) Mechanism of NOx reduction on B-doped g-CN nanotubes. Reprinted with permission from ref. 48, copyright 2018, Elsevier. | ||
3.2 Electrocatalysis
It is well known that semiconductors are capable of electrocatalysis, including the oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), and oxygen evolution reaction (OER), so element-doped g-CN-based materials have great potential as candidates for electrocatalysts. Typically, element-doped g-CN can provide many active sites, leading to the catalysis effect for electrochemical reactions on electrodes. To show the excellent performance of element-doped g-CN materials directly, some results of electrocatalytic measurements are provided in Table 4.| Sample | Reaction type | Tafel slope of sample (mV dec−1) | Tafel slope of reference (mV dec−1) | Ref. |
|---|---|---|---|---|
| S-doped g-CN | HER | 196 | 238 (g-CN) | 135 |
| Mo-doped g-CN | HER | 180 | 238 (g-CN) | 135 |
| Mo–S co-doped g-CN | HER | 110 | 238 (g-CN) | 135 |
| RhP@P-doped g-CN | HER | 38.4 | 38.1 (Pt/C) | 58 |
| Cu-doped g-CN | HER | 76 | 164 (g-CN) | 119 |
| S-doped g-CN/RGO | OER | 115 | 158 (RGO) | 84 |
| Fe-doped g-CN | ORR | 73 | 84.5 (g-CN) | 174 |
| 76.5 (Pt/C) |
Many efforts have been devoted to electrocatalysis for ORR, which is one of the promising applications of element-doped g-CN. For example, Dey's group reported transition-metal-doped g-CN for highly efficient ORR.174 Tafel plots of Fe-g-CN, g-CN, and commercial Pt/C are shown in Fig. 14(a); it is obvious from the figure that the Tafel slope of Fe-g-CN (73 mV dec−1) is much smaller than those of g-CN (84.5 mV dec−1) and commercial Pt/C (76.5 mV dec−1), which suggests the better electrocatalytic activity of Fe-g-CN. Its enhanced performance results from the absorption of O2 on Fe–Nx active sites of Fe-doped g-CN, which in turn promotes the process of ORR (the mechanism is shown in Fig. 14(b)). To further improve the electrocatalytic performance, the preparation of element-doped g-CN-contained composites is considered a useful strategy. Zhou's group prepared the composite of Co-doped g-CN and ordered mesoporous carbon (Co–C3N4/OMC) as the electrocatalyst for ORR.77 The Tafel slopes in Fig. 14(c) show that the electrocatalytic activity of Co–C3N4/OMC (84 mV dec−1) is significantly higher than that of Co–C3N4 (189 mV dec−1), which implies that the conductivity of OMC plays a crucial role in the improvement of electrocatalytic performance.
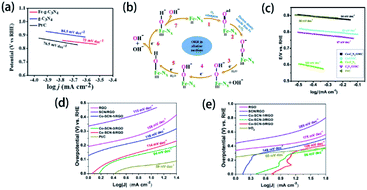 | ||
| Fig. 14 (a) Tafel plots of the Fe-g-CN, g-CN, and Pt/C catalysts and (b) ORR mechanism of the Fe–Nx active sites of Fe-g-CN. Reprinted with permission from ref. 174, copyright 2020, American Chemical Society. (c) Tafel plots of Co–C3N4, C3N4/OMC, Pt/C, Co–C3N4/OMC-2, and Co/OMC. Reprinted with permission from ref. 77, copyright 2019, Elsevier. Tafel plots of RGO, SCN/RGO, Co–SCN-1/RGO, Co–SCN-3/RGO, Co–SCN-5/RGO, and Pt/C catalysts for (d) HER and (e) OER, respectively. Reprinted with permission from ref. 84, copyright 2019, American Chemical Society. | ||
Electrocatalysis for water splitting is also an important application of element-doped g-CN due to the demand for sustainable energy. Generally, electrochemical water splitting consists of two electrode half-reactions: HER at the cathode and OER at the anode; and element-doped g-CN can serve as the electrocatalyst for the above two reactions. Tonda's group prepared Co-coordinated S-doped g-CN on reduced graphene oxide (RGO) as an efficient bifunctional electrocatalyst for water splitting.84 The Tafel plots of various samples for HER and OER are shown in Fig. 14(d) and (e), respectively. As is seen from the Tafel slopes, the optimized Co–C3N4/OMC shows a better electrocatalytic performance than RGO and SCN/RGO in both HER and OER due to the conductivity of RGO and the active sites provided by Co–(N, S)–C.
3.3 Sensors
Element-doped g-CN can be used to detect certain substances due to its unique structure and properties. Therefore, element-doped g-CN-contained materials are fascinating choices for sensitive and selective sensors.Organics in the solution can be involved in the electrochemical process on electrodes. The electrochemical reactions of organics lead to the current response, and the current density is closely related to the concentration of organics. Based on this phenomenon, an electrochemical sensor can be achieved to detect organics. Additionally, element-doped g-CN-contained materials modified electrodes can largely enhance the current density in the presence of a small concentration of organics. For example, Mohammad's group prepared S-doped g-CN modified fluorine-doped tin oxide (S-g-C3N4/FTO) for electrochemical sensing of hydrazine (the mechanism diagram is shown in Fig. 15(a)).202 Linear-sweep voltammetry (LSV) measurements reveal that the S-g-C3N4/FTO/hydrazine system has much higher current density than other systems (Fig. 15(b)), and its current density changes with the concentrations of hydrazine (Fig. 15(c)), which show a detection limit as low as 0.06 μM with a linear range of 60–475 mM. The above results determine the feasibility of the S-g-CN/FTO sensor for hydrazine detection. Similar methods can be used to detect other organics, such as acetaminophen,204 nitrofurantoin,173 metronidazole,205 and 4-nitrophenol.61
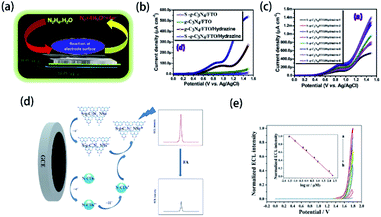 | ||
| Fig. 15 (a) Mechanism diagram of the S-g-CN/FTO sensor for hydrazine, (b) LSV measurements for S-g-CN/FTO, g-CN/FTO, g-CN/FTO/hydrazine, and S-g-CN/FTO/hydrazine, and (c) LSV measurements at varying concentrations of hydrazine. Reprinted with permission from ref. 202, copyright 2020, Elsevier. (d) Mechanism diagram of S-doped g-CN nanosheets/nitrogen-doped carbon dot system and (e) the ECL response of folic acid at (a) 0.05 μM, (b) 0.1 μM, (c) 0.5 μM, (d) 1 μM, (e) 5 μM, (f) 10 μM, (g) 100 μM, and (h) 200 μM concentrations (the inset shows a linear calibration plot for folic acid). Reprinted with permission from ref. 203, copyright 2019, Springer. | ||
Besides current density, electrochemiluminescence (ECL) intensity can be a characteristic response too. Li's group reported a sensor system including S-doped g-CN nanosheets for the detection of folic acid according to the ECL behavior (the mechanism diagram is shown in Fig. 15(d)).203 It was found that the ECL signal decreased gradually with the addition of folic acid to the S-doped g-CN nanosheet/N-doped carbon dot system (Fig. 15(e)). Therefore, a novel sensor for detecting folic acid, whose detection limit is 16 nM with a linear range of 0.05–200 μM, was successfully prepared. The sensing of other organics, such as L-cysteine,206 and K-RAS gene,169 can be achieved via ECL measurements.
Element-doped g-CN-based sensor systems are also useful to detect heavy metal ions, which are ubiquitous in environmental pollutants. In some sensor systems, doped elements can play a crucial role in detecting ions. According to Jiang's group, the high-sensitive, selective, repeatable, and stable electrochemical sensor of S-doped g-CN nanoflakes modified glassy carbon electrode (S-g-CN–GCE) is able to detect Pb2+ ions.207 Based on the strong interaction between the doped S atoms and Pb2+ ions, S-g-CN–GCE can detect Pb2+ ions via differential pulse voltammetry (DPV) measurements, which exhibited a detection limit of 3.0 × 10−9 mol L−1 within the Pb2+ concentration range of 7.5 × 10−8 to 2.5 × 10−6 mol L−1 and 2.5 × 10−6 to 1 × 10−3 mol L−1. Moreover, fixing metal nanoparticles on element-doped g-CN is another feasible method to detect ions. Ramaraj's group has reported a new system of chitosan functionalized Au nanoparticles assembled S-doped g-CN as the sensitive and selective detector of trace Hg2+ ions due to the interaction between Au nanoparticles and Hg2+, and this system possessed a lower limit of detection of 0.275 nM than other systems.168
Furthermore, based on detection requirements, many more techniques can be applied for sensors, such as the chemiluminescent platform applied for the sensing of H2S in the human plasma by efficient Cu2+ modified g-CN nanosheets enhanced luminol–H2O2 system,120 and UV-vis absorption spectra for the detection of glucose by Fe-doped g-CN nanoparticles.68
3.4 Sonocatalysis
Recently, sonodegradation has attracted great attention in the field of wastewater treatment. In wastewater, with the breaking of bubbles under ultrasonic treatment, extreme pressure and temperature are generated around the bubbles, which is positive for the oxidation of organic pollutants.208,209 As a result, sonocatalysis, a state-of-the-art technique, is considered a promising way to solve environmental problems.Element-doped g-CN-based materials are promising candidates for sonocatalysts. Yang's group prepared the composite of S-doped g-CN and CoFe2O4 (SCN/CoFe2O4) via the calcination of H2SO4-treated dicyandiamide, followed by the reaction of Co(NO3)2·6H2O, Fe(NO3)3·9H2O and NaOH in a SCN-dispersed solution.210 The samples were applied to degrade methylene blue, and the sonocatalytic results of various systems are shown in Fig. 16(a). The SCN/CoFe2O4/H2O2/US system exhibited the highest degradation performance of 96% in 20 min. Additionally, the pseudo-first-order kinetic constant of the SCN/CoFe2O4/H2O2/US system is 4.84 times higher than that of the CoFe2O4/H2O2/US system, which suggested the positive effect of SCN. Remarkably, the degradation performances of rhodamine B and Congo red were lower than that of methylene blue (Fig. 16(b)), indicating that the performance can be influenced by the dye sizes, structural compositions, and electric charges. Therefore, the modification and mechanism of sonocatalysts should be investigated further.
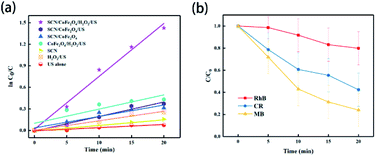 | ||
| Fig. 16 (a) Plot of ln(C0/C) vs. ultrasonication time in the sonocatalysis measurements of various systems for methylene blue (US: ultrasonication); (b) effect of different type of organic dye on sonodegradation (RhB: rhodamine B, CR: Congo red, and MB: methylene blue). Reprinted with permission from ref. 210, copyright 2020, MDPI. | ||
3.5 Supercapacitors
Inspired by the application of two-dimensional graphitic carbon materials as supercapacitors due to their unique electronic properties, Shen's group reported the utility of porous B-doped g-CN nanosheet electrodes as supercapacitors.45 Electrochemical measurements exhibited that porous B-doped g-CN nanosheets had a better capacitive performance than g-CN: the cyclic voltammetry (CV) results of porous B-doped g-CN nanosheets and g-CN in Fig. 17(a) show that the enclosed area of the CV curve of porous B-doped g-CN nanosheets is much larger than that of g-CN, suggesting the better capacitive performance of the former; and the calculated results of their specific capacitances at different current densities in Fig. 17(b) show that porous B-doped g-CN nanosheets have a higher capacitance than that of g-CN; the results of repeated charge–discharge measurement in Fig. 17(c) show that the specific capacitance of porous B-doped g-CN nanosheets is stable after 2500 cycles. The above results demonstrate the high electrochemical capacitive properties of porous B-doped g-CN nanosheets. Moreover, a further study of Shen's group modified the B-doped g-CN material and thus enhanced its specific capacitance (∼660.6 F g−1), which is 3.02 times higher than that of pristine g-CN (∼218.7 F g−1) and have exceeded most of the reported carbon electrode materials.46 So, this work may boost the development of high-performance supercapacitor materials.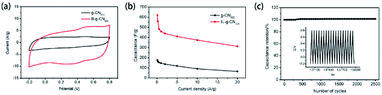 | ||
| Fig. 17 (a) CV curves of B-doped g-CN nanosheets and g-CN; (b) specific capacitances of B-doped g-CN nanosheets and g-CN at different current densities; (c) repeated charge–discharge measurement of B-doped g-CN nanosheets. Reprinted with permission from ref. 45, copyright 2017, Elsevier. | ||
3.6 Adsorbents
Generally, there are some hazardous heavy metal ions in industrial wastewater. As a result, various adsorption materials have been developed to remove these ions due to the interaction between the functional groups and ions.211–213 Element-doped g-CN, as a kind of functionalized porous material, possesses promising adsorption performance to ions. Wang's group provided an ideal tool for mercury (Hg2+) removal via S-doped g-CN nanotubes/graphene oxide aerogel (SGA) adsorbent.177 As shown in Fig. 18(a), under certain conditions (pH = 5, 25 °C, [SGA] = 1.0 g L−1, [Hg2+] = 50 mg L−1, and 120 min), the removal efficiency of SGA towards Hg2+ initially and after four cycles are 83.3% and 58.1%, respectively, which suggests that this adsorbent has great potential in ion removal. The investigation of the adsorption mechanism reveals that the excellent performance can be attributed to the specific binding of S atoms to Hg2+ (Fig. 18(b)) and the high mobility of Hg2+ in S-doped g-CN nanotubes (Fig. 18(c)).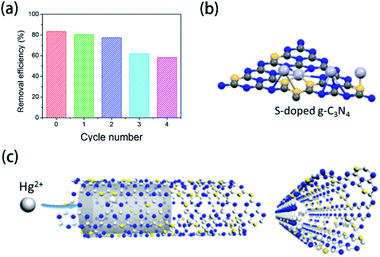 | ||
| Fig. 18 (a) Removal efficiency of Hg2+ by SGA at different regeneration cycles; (b) specific binding of S atoms to Hg2+; and (c) mobility of Hg2+ in S-doped g-CN nanotubes. Reprinted with permission from ref. 177, copyright 2020, Elsevier. | ||
4. Conclusions
Herein, we have highlighted the significant characterization techniques for the determination of heteroatoms in element-doped g-CN. Typically, XPS, which can certify the presence of the doped elements in the microstructure of g-CN, is the most frequently used method in the characterization of element-doped g-CN. To achieve a better understanding of the doped elements, more details can be obtained by NMR, FTIR spectroscopy, XAS, elemental mapping, and EELS. Moreover, some advanced characterization techniques, such as APT and probe molecule FTIR spectroscopy, offer exciting opportunities for the characterization of doped elements in g-CN.With the study of element doping in g-CN, the application fields of g-CN-based materials are increasingly enlarged. Conventionally, g-CN-based materials, including element-doped g-CN, are ideal choices for photocatalysts. Therefore, the vast majority of research results of element-doped g-CN have been focused on photocatalysis in recent years. Meanwhile, these materials are also expected to have potential applications in other applications, such as electrocatalysis, sensors, sonocatalysis, supercapacitors, and adsorbents.
Overall, the characterization of element-doped g-CN and related materials is a significant work, as well as a huge challenge. The excellent properties of these materials are a great impetus to research efforts to explore practical applications. Last but not least, we hope that the information provided in this review can play a role in the future research of element-doped g-CN-based materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Key Research and Development Program of China (2017YFA0207301) and the National Natural Science Foundation of China (21890751, 21471036).Notes and references
- X. Zhang, X. Yuan, L. Jiang, J. Zhang, H. Yu, W. Hou and G. Zeng, Chem. Eng. J., 2020, 390, 124475 CrossRef CAS.
- I. Som, M. Roy and R. Saha, ChemCatChem, 2020, 12, 3409–3433 CrossRef CAS.
- T. Su, Z. Qin, H. Ji and Z. Wu, Nanotechnology, 2019, 30, 502002 CrossRef CAS.
- S. Y. Lim, C. S. Law, L. Liu, M. Markovic, C. Hedrich, R. H. Blick, A. D. Abell, R. Zierold and A. Santos, Catalysts, 2019, 9, 988 CrossRef CAS.
- I. J. Ani, U. G. Akpan, M. A. Olutoye and B. H. Hameed, J. Clean. Prod., 2018, 205, 930–954 CrossRef CAS.
- H. Abdullah, M. M. R. Khan, H. Ong and Z. Yaakob, J. CO2 Util., 2017, 22, 15–32 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- Y. Cheng, R. Song, K. Wu, N. Peng, M. Yang, J. Luo, T. Zou, Y. Zuo and Y. Liu, J. Hazard. Mater., 2020, 383, 121166 CrossRef CAS PubMed.
- J. Wang, D. Liu, Y. Zhu, S. Zhou and S. Guan, Appl. Catal., B, 2018, 231, 251–261 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- I. F. Teixeira, E. C. M. Barbosa, S. C. E. Tsang and P. H. C. Camargo, Chem. Soc. Rev., 2018, 47, 7783–7817 RSC.
- H. Wang, M. Li, H. Li, Q. Lu, Y. Zhang and S. Yao, Mater. Des., 2019, 162, 210–218 CrossRef CAS.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- C. Liu, Y. Zhang, F. Dong, X. Du and H. Huang, J. Phys. Chem. C, 2016, 120, 10381–10389 CrossRef CAS.
- H. Fan, N. Wang, Y. Tian, S. Ai and J. Zhan, Carbon, 2016, 107, 747–753 CrossRef CAS.
- W. Ong, L. Tan, Y. Ng, S. Yong and S. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- J. Zhou, J. Xue, Q. Pan, X. Yang, Q. Shen, T. Ma, X. Liu and H. Jia, J. Photochem. Photobiol., A, 2019, 372, 147–155 CrossRef CAS.
- S. Zhang, C. Hu, H. Ji, L. Zhang and F. Li, Appl. Surf. Sci., 2019, 478, 304–312 CrossRef CAS.
- E. Murugan, S. Santhosh Kumar, K. M. Reshna and S. Govindaraju, J. Mater. Sci., 2018, 54, 5294–5310 CrossRef.
- T. Arumugham, R. G. Amimodu, N. J. Kaleekkal and D. Rana, J. Environ. Sci., 2019, 82, 57–69 CrossRef PubMed.
- X. Zhang, L. Li, Y. Zeng, F. Liu, J. Yuan, X. Li, Y. Yu, X. Zhu, Z. Xiong, H. Yu and Y. Xie, ACS Appl. Nano Mater., 2019, 2, 7255–7265 CrossRef CAS.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L. Wu, C. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- M. Yousefi, S. Villar-Rodil, J. I. Paredes and A. Z. Moshfegh, J. Alloys Compd., 2019, 809, 151783 CrossRef CAS.
- G. Lei, Y. Cao, W. Zhao, Z. Dai, L. Shen, Y. Xiao and L. Jiang, ACS Sustainable Chem. Eng., 2019, 7, 4941–4950 CrossRef CAS.
- D. K. Chauhan, S. Jain, V. R. Battula and K. Kailasam, Carbon, 2019, 152, 40–58 CrossRef CAS.
- M. Inagaki, T. Tsumura, T. Kinumoto and M. Toyoda, Carbon, 2019, 141, 580–607 CrossRef CAS.
- C. Xu, W. Zhang, K. Deguchi, S. Ohki, T. Shimizu, R. Ma and T. Sasaki, J. Mater. Chem. A, 2020, 8, 13299–13310 RSC.
- N. Zhang, C. Chen, Y. Chen, G. Chen, C. Liao, B. Liang, J. Zhang, A. Li, B. Yang, Z. Zheng, X. Liu, A. Pan, S. Liang and R. Ma, ACS Appl. Energy Mater., 2018, 1, 2016–2023 CrossRef CAS.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- Y. Wang, Y. Di, M. Antonietti, H. Li, X. Chen and X. Wang, Chem. Mater., 2010, 22, 5119–5121 CrossRef CAS.
- Y. Wu, H. Wang, W. Tu, Y. Liu, S. Wu, Y. Tan and J. Chew, Appl. Catal., B, 2018, 233, 58–69 CrossRef CAS.
- H. Fang, X. Zhang, J. Wu, N. Li, Y. Zheng and X. Tao, Appl. Catal., B, 2018, 225, 397–405 CrossRef CAS.
- I. Palchan, M. Crespin, H. Estradeszwarckopf and B. Rousseau, Chem. Phys. Lett., 1989, 157, 321–327 CrossRef CAS.
- D. Gao, Y. Liu, M. Song, S. Shi, M. Si and D. Xue, J. Mater. Chem. C, 2015, 3, 12230–12235 RSC.
- Y. Liu, J. Wang, C. Yin, H. Duan, S. Kang and L. Cui, RSC Adv., 2018, 8, 27021–27026 RSC.
- A. Mirzaei, Z. Chen, F. Haghighat and L. Yerushalmi, Appl. Catal., B, 2019, 242, 337–348 CrossRef CAS.
- X. Guo, L. Rao, P. Wang, L. Zhang and Y. Wang, Int. J. Environ. Res. Publ. Health, 2019, 16, 581 CrossRef CAS PubMed.
- S. Yan, Z. Li and Z. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- Y. Wang, H. Li, J. Yao, X. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446–450 RSC.
- Q. Gu, J. Liu, Z. Gao and C. Xue, Chem. - Asian J., 2016, 11, 3169–3173 CrossRef CAS PubMed.
- S. Thaweesak, S. Wang, M. Lyu, M. Xiao, P. Peerakiatkhajohn and L. Wang, Dalton Trans., 2017, 46, 10714–10720 RSC.
- J. Zhu, T. Diao, W. Wang, X. Xu, X. Sun, S. A. C. Carabineiro and Z. Zhao, Appl. Catal., B, 2017, 219, 92–100 CrossRef CAS.
- Y. Shiraishi, K. Chishiro, S. Tanaka and T. Hirai, Langmuir, 2020, 36, 734–741 CrossRef CAS PubMed.
- L. Kong, Q. Chen, X. Shen, C. Xia, Z. Ji and J. Zhu, Electrochim. Acta, 2017, 245, 249–258 CrossRef CAS.
- L. Kong, Q. Chen, X. Shen, G. Zhu and J. Zhu, J. Colloid Interface Sci., 2018, 532, 261–271 CrossRef CAS PubMed.
- J. Zhao, Y. Liu, Y. Wang, H. Li, J. Wang and Z. Li, Appl. Surf. Sci., 2019, 470, 923–932 CrossRef CAS.
- Z. Wang, M. Chen, Y. Huang, X. Shi, Y. Zhang, T. Huang, J. Cao, W. Ho and S. Lee, Appl. Catal., B, 2018, 239, 352–361 CrossRef CAS.
- J. Li, Y. Qi, Y. Mei, S. Ma, Q. Li, B. Xin, T. Yao and J. Wu, J. Colloid Interface Sci., 2020, 566, 495–504 CrossRef CAS PubMed.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- T. Ma, J. Ran, S. Dai, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2015, 54, 4646–4650 CrossRef CAS PubMed.
- J. Su, P. Geng, X. Li, Q. Zhao, X. Quan and G. Chen, Nanoscale, 2015, 7, 16282–16289 RSC.
- S. Guo, Z. Deng, M. Li, B. Jiang, C. Tian, Q. Pan and H. Fu, Angew. Chem., Int. Ed., 2016, 55, 1830–1834 CrossRef CAS PubMed.
- B. Chai, J. Yan, C. Wang, Z. Ren and Y. Zhu, Appl. Surf. Sci., 2017, 391, 376–383 CrossRef CAS.
- P. Wang, C. Guo, S. Hou, X. Zhao, L. Wu, Y. Pei, Y. Zhang, J. Gao and J. Xu, J. Alloys Compd., 2018, 769, 503–511 CrossRef CAS.
- X. Wang, X. Li, W. Chen, R. Wang, W. Bian and M. M. F. Choi, Spectrochim. Acta, Part A, 2018, 198, 1–6 CrossRef CAS PubMed.
- V. Hasija, A. Sudhaik, P. Raizada, A. Hosseini-Bandegharaei and P. Singh, J. Environ. Chem. Eng., 2019, 7, 103272 CrossRef CAS.
- C. Xu, Q. Wang, R. Ding, Y. Wang, Y. Zhang and G. Fan, Appl. Surf. Sci., 2019, 489, 796–801 CrossRef CAS.
- S. Cao, Q. Huang, B. Zhu and J. Yu, J. Power Sources, 2017, 351, 151–159 CrossRef CAS.
- G. Liu, P. Niu, C. Sun, S. C. Smith, Z. Chen, G. Lu and H. Cheng, J. Am. Chem. Soc., 2010, 132, 11642–11648 CrossRef CAS PubMed.
- C. Rajkumar, P. Veerakumar, S. M. Chen, B. Thirumalraj and K. C. Lin, ACS Sustainable Chem. Eng., 2018, 6, 16021–16031 CrossRef CAS.
- H. Wang, Y. Bian, J. Hu and L. Dai, Appl. Catal., B, 2018, 238, 592–598 CrossRef CAS.
- W. Cha, I. Y. Kim, J. M. Lee, S. Kim, K. Ramadass, K. Gopalakrishnan, S. Premkumar, S. Umapathy and A. Vinu, ACS Appl. Mater. Interfaces, 2019, 11, 27192–27199 CrossRef CAS PubMed.
- S. Joseph, S. Abraham, T. Abraham, R. N. Priyanka and B. Mathew, Appl. Surf. Sci., 2019, 495, 143478 CrossRef CAS.
- Z. Li, G. Gu, S. Hu, X. Zou and G. Wu, Chin. J. Catal., 2019, 40, 1178–1186 CrossRef CAS.
- H. Qin, W. Lv, J. Bai, Y. Zhou, Y. Wen, Q. He, J. Tang, L. Wang and Q. Zhou, J. Mater. Sci., 2018, 54, 4811–4820 CrossRef.
- W. Miao, Y. Liu, X. Chen, Y. Zhao and S. Mao, Carbon, 2020, 159, 461–470 CrossRef CAS.
- J. Xian, Y. Weng, H. Guo, Y. Li, B. Yao and W. Weng, Spectrochim. Acta, Part A, 2019, 215, 218–224 CrossRef CAS PubMed.
- H. Li, C. Shan and B. Pan, Sci. Total Environ., 2019, 675, 62–72 CrossRef CAS PubMed.
- J. Zhu, S. A. C. Carabineiro, D. Shan, J. L. Faria, Y. Zhu and J. L. Figueiredo, J. Catal., 2010, 274, 207–214 CrossRef CAS.
- S. Tonda, S. Kumar, S. Kandula and V. Shanker, J. Mater. Chem. A, 2014, 2, 6772–6780 RSC.
- W. Luo, W. Huang, X. Feng, Y. Huang, X. Song, H. Lin, S. Wang and G. Mailhot, RSC Adv., 2020, 10, 21876–21886 RSC.
- T. Ma, Q. Shen, B. Zhao, J. Xue, R. Guan, X. Liu, H. Jia and B. Xu, Inorg. Chem. Commun., 2019, 107, 107451 CrossRef CAS.
- J. Hu, P. Zhang, W. An, L. Liu, Y. Liang and W. Cui, Appl. Catal., B, 2019, 245, 130–142 CrossRef CAS.
- X. Yang, X. Cao, B. Tang, B. Shan, M. Deng and Y. Liu, J. Photochem. Photobiol., A, 2019, 375, 40–47 CrossRef CAS.
- Z. Ding, X. Chen, M. Antonietti and X. Wang, ChemSusChem, 2011, 4, 274–281 CAS.
- M. Hassan, T. Liu, X. Bo and M. Zhou, J. Phys. Chem. Solids, 2019, 131, 111–118 CrossRef CAS.
- B. Babu, J. Shim and K. Yoo, Ceram. Int., 2020, 46, 16422–16430 CrossRef CAS.
- X. Cao, X. Chi, X. Deng, Q. Sun, X. Gong, B. Yu, A. C. Y. Yuen, W. Wu and R. K. Y. Li, Polymers, 2020, 12, 1106 CrossRef CAS PubMed.
- Z. Zhu, X. Tang, W. Fan, Z. Liu, P. Huo, T. Wang, Y. Yan and C. Li, J. Alloys Compd., 2019, 775, 248–258 CrossRef CAS.
- Z. Honda, M. Saito, F. Takenaka, N. Kamata, Y. Sawada, T. Kida and M. Hagiwara, Solid State Sci., 2019, 98, 106017 CrossRef CAS.
- J. Fan, H. Qin and S. Jiang, Chem. Eng. J., 2019, 359, 723–732 CrossRef CAS.
- X. Xu, S. Wang, T. Hu, X. Yu, J. Wang and C. Jia, Dyes Pigments, 2020, 175, 108107 CrossRef CAS.
- W. K. Jo, S. Moru and S. Tonda, ACS Sustainable Chem. Eng., 2019, 7, 15373–15384 CrossRef CAS.
- C. Wu, X. Zhang, Z. Xia, M. Shu, H. Li, X. Xu, R. Si, A. I. Rykov, J. Wang, S. Yu, S. Wang and G. Sun, J. Mater. Chem. A, 2019, 7, 14001–14010 RSC.
- R. Jin, S. Hu, J. Gui and D. Liu, Bull. Korean Chem. Soc., 2015, 36, 17–23 CrossRef CAS.
- Y. Yang, M. Guo, G. Zhang and W. Li, Carbon, 2017, 117, 120–125 CrossRef CAS.
- H. Dou, L. Chen, S. Zheng, Y. Zhang and G. Xu, Mater. Chem. Phys., 2018, 214, 482–488 CrossRef CAS.
- T. Choudhury, S. O. Saied, J. L. Sullivan and A. M. Abbot, J. Phys. D: Appl. Phys., 1989, 22, 1185–1195 CrossRef CAS.
- D. P. Ojha, H. P. Karki, J. H. Song and H. J. Kim, Composites, Part B, 2019, 160, 277–284 CrossRef CAS.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- Y. Chen, X. Liu, L. Hou, X. Guo, R. Fu and J. Sun, Chem. Eng. J., 2020, 383, 123132 CrossRef CAS.
- C. Zhang, M. Zhang, Y. Li and D. Shuai, Appl. Catal., B, 2019, 248, 11–21 CrossRef CAS.
- L. K. Putri, B. J. Ng, W. J. Ong, H. W. Lee, W. S. Chang and S. P. Chai, J. Mater. Chem. A, 2018, 6, 3181–3194 RSC.
- J. Zhang, S. Hu and Y. Wang, RSC Adv., 2014, 4, 62912–62919 RSC.
- L. Zhang, N. Ding, M. Hashimoto, K. Iwasaki, N. Chikamori, K. Nakata, Y. Xu, J. Shi, H. Wu, Y. Luo, D. Li, A. Fujishima and Q. Meng, Nano Res., 2018, 11, 2295–2309 CrossRef CAS.
- Y. Shang, Y. Ma, X. Chen, X. Xiong and J. Pan, Mol. Catal., 2017, 433, 128–135 CrossRef CAS.
- Z. Shu, Y. Wang, W. Wang, J. Zhou, T. Li, X. Liu, Y. Tan and Z. Zhao, Int. J. Hydrogen Energy, 2019, 44, 748–756 CrossRef CAS.
- S. B. Vuggili, S. K. Khanth, K. Kadiya, U. K. Gaur and M. Sharma, J. Environ. Chem. Eng., 2019, 7, 103440 CrossRef CAS.
- M. M. Islam, R. D. Tentu, M. A. Ali and S. Basu, ChemistrySelect, 2018, 3, 11241–11250 CrossRef CAS.
- Z. Liang, G. Ba, H. Li, N. Du and W. Hou, J. Alloys Compd., 2020, 815, 152488 CrossRef CAS.
- C. Li, S. Yu, X. Zhang, Y. Wang, C. Liu, G. Chen and H. Dong, J. Colloid Interface Sci., 2019, 538, 462–473 CrossRef CAS PubMed.
- Y. Yang, H. Jin, C. Zhang, H. Gan, F. Yi and H. Wang, J. Alloys Compd., 2020, 821, 153439 CrossRef CAS.
- M. Zhang, X. Bai, D. Liu, J. Wang and Y. Zhu, Appl. Catal., B, 2015, 164, 77–81 CrossRef CAS.
- S. Hu, F. Li, Z. Fan, F. Wang, Y. Zhao and Z. Lv, Dalton Trans., 2015, 44, 1084–1092 RSC.
- M. Wu, J. Yan, X. Tang, M. Zhao and Q. Jiang, ChemSusChem, 2014, 7, 2654–2658 CrossRef CAS PubMed.
- Y. Guo, Q. Liu, Z. Li, Z. Zhang and X. Fang, Appl. Catal., B, 2018, 221, 362–370 CrossRef CAS.
- X. Wu, D. Long, X. Rao and Y. Zhang, J. Photochem. Photobiol., A, 2020, 401, 112759 CrossRef CAS.
- L. Chen, D. Zhu, J. Li, X. Wang, J. Zhu, P. S. Francis and Y. Zheng, Appl. Catal., B, 2020, 273, 9 CrossRef.
- S. Wang, J. Zhan, K. Chen, A. Ali, L. Zeng, H. Zhao, W. Hu, L. Zhu and X. Xu, ACS Sustainable Chem. Eng., 2020, 8, 8214–8222 CrossRef CAS.
- R. Zhang, S. Niu, X. Zhang, Z. Jiang, J. Zheng and C. Guo, Appl. Surf. Sci., 2019, 489, 427–434 CrossRef CAS.
- I. N. Reddy, L. V. Reddy, N. Jayashree, C. V. Reddy, M. Cho, D. Kim and J. Shim, Chemosphere, 2021, 264, 128593 CrossRef CAS PubMed.
- T. Cao, M. Cai, L. Jin, X. Wang, J. Yu, Y. Chen and L. Dai, New J. Chem., 2019, 43, 16169–16175 RSC.
- G. Lei, W. Zhao, L. Shen, S. Liang, C. Au and L. Jiang, Appl. Catal., B, 2020, 267, 12 CrossRef.
- Q. Chen, H. Dou, S. Zheng, X. Rao and Y. Zhang, J. Photochem. Photobiol., A, 2019, 382, 111931 CrossRef CAS.
- P. Deng, J. Xiong, S. Lei, W. Wang, X. Ou, Y. Xu, Y. Xiao and B. Cheng, J. Mater. Chem. A, 2019, 7, 22385–22397 RSC.
- L. Li, M. Liang, J. Huang, S. Zhang, Y. Liu and F. Li, Environ. Sci. Pollut. Res., 2020, 27, 29391–29407 CrossRef CAS PubMed.
- S. Hu, X. Qu, P. Li, F. Wang, Q. Li, L. Song, Y. Zhao and X. Kang, Chem. Eng. J., 2018, 334, 410–418 CrossRef CAS.
- X. Zou, R. Silva, A. Goswami and T. Asefa, Appl. Surf. Sci., 2015, 357, 221–228 CrossRef CAS.
- J. Cao, W. Zhang, X. Fu, H. Wang, S. Ma and Y. Liu, Spectrochim. Acta, Part A, 2020, 230, 118040 CrossRef CAS.
- S. Hu, X. Qu, J. Bai, P. Li, Q. Li, F. Wang and L. Song, ACS Sustainable Chem. Eng., 2017, 5, 6863–6872 CrossRef CAS.
- Y. Wang, X. Zhou, W. Xu, Y. Sun, T. Wang, Y. Zhang, J. Dong, W. Hou, N. Wu, L. Wu, B. Zhou, Y. Wu, Y. Du and W. Zhong, Appl. Catal., A, 2019, 582, 117118 CrossRef CAS.
- Z. Wang, J. Xu, H. Zhou and X. Zhang, Rare Met., 2019, 38, 459–467 CrossRef CAS.
- F. Qian, J. Wang, S. Ai and L. Li, Sens. Actuators, B, 2015, 216, 418–427 CrossRef.
- A. Kumar, R. K. Yadav, N. J. Park and J. O. Baeg, ACS Appl. Nano Mater., 2018, 1, 47–54 CrossRef CAS.
- Y. Rangraz, F. Nemati and A. Elhampour, Appl. Surf. Sci., 2020, 507, 145164 CrossRef CAS.
- Y. Wang, S. Hu, Q. Li, G. Gu, Y. Zhao, H. Liang and W. Li, RSC Adv., 2018, 8, 36903–36909 RSC.
- M. Wang, Y. Zeng, G. Dong and C. Wang, Chin. J. Catal., 2020, 41, 1498–1510 CrossRef CAS.
- J. Hong, D. K. Hwang, R. Selvaraj and Y. Kim, J. Ind. Eng. Chem., 2019, 79, 473–481 CrossRef CAS.
- C. Zhang, J. Bai, L. Ma, Y. Lv, F. Wang, X. Zhang, X. Yuan and S. Hu, Diamond Relat. Mater., 2018, 87, 215–222 CrossRef CAS.
- M. Zhou, G. Dong, F. Yu and Y. Huang, Appl. Catal., B, 2019, 256, 117825 CrossRef CAS.
- Y. Wang, Y. Li, X. Bai, Q. Cai, C. Liu, Y. Zuo, S. Kang and L. Cui, Catal. Commun., 2016, 84, 179–182 CrossRef CAS.
- Y. Wang, Y. Wang, Y. Li, H. Shi, Y. Xu, H. Qin, X. Li, Y. Zuo, S. Kang and L. Cui, Catal. Commun., 2015, 72, 24–28 CrossRef CAS.
- H. Che, C. K. Ngaw, P. Hu, J. Wang, Y. Li, X. Wang and W. Teng, J. Alloys Compd., 2020, 849, 156440 CrossRef CAS.
- J. Li, B. Zhang, Q. Song, X. Xu and W. Hou, Ceram. Int., 2020, 46, 14178–14187 CrossRef CAS.
- R. Zhang, A. Zhang, Y. Cao, S. Wang, F. Dong and Y. Zhou, Chem. Eng. J., 2020, 401, 126028 CrossRef CAS.
- Y. Wang, Y. Zhang, S. Zhao, Z. Huang, W. Chen, Y. Zhou, X. Lv and S. Yuan, Appl. Catal., B, 2019, 248, 44–53 CrossRef CAS.
- N. Wang, J. Wang, J. Hu, X. Lu, J. Sun, F. Shi, Z. Liu, Z. Lei and R. Jiang, ACS Appl. Energy Mater., 2018, 1, 2866–2873 CrossRef CAS.
- H. Li, Y. Xia, Z. Liang, G. Ba and W. Hou, ACS Appl. Energy Mater., 2020, 3, 377–386 CrossRef CAS.
- Z. Liang, Y. Xia, G. Ba, H. Li, Q. Deng and W. Hou, Catal. Sci. Technol., 2019, 9, 6627–6637 RSC.
- R. Ding, S. Cao, H. Chen, F. Jiang and X. Wang, Colloids Surf., A, 2019, 563, 263–270 CrossRef CAS.
- W. Iqbal, B. Yang, X. Zhao, M. Rauf, I. M. A. Mohamed, J. Zhang and Y. Mao, Catal. Sci. Technol., 2020, 10, 549–559 RSC.
- X. Hu, W. Zhang, Y. Yong, Y. Xu, X. Wang and X. Yao, Appl. Surf. Sci., 2020, 510, 145413 CrossRef CAS.
- Y. Huang, Q. Yan, H. Yan, Y. Tang, S. Chen, Z. Yu, C. Tian and B. Jiang, ChemCatChem, 2017, 9, 4083–4089 CrossRef CAS.
- Y. Guo, T. Chen, Q. Liu, Z. Zhang and X. Fang, J. Phys. Chem. C, 2016, 120, 25328–25337 CrossRef CAS.
- Q. Han, C. Hu, F. Zhao, Z. Zhang, N. Chen and L. Qu, J. Mater. Chem. A, 2015, 3, 4612–4619 RSC.
- T. S. Bui, P. Bansal, B. K. Lee, T. Mahvelati-Shamsabadi and T. Soltani, Appl. Surf. Sci., 2020, 506, 144184 CrossRef CAS.
- D. Long, W. Chen, S. Zheng, X. Rao and Y. Zhang, Ind. Eng. Chem. Res., 2020, 59, 4549–4556 CrossRef CAS.
- S. K. Kuila, D. K. Gorai, B. Gupta, A. K. Gupta, C. S. Tiwary and T. K. Kundu, Chemosphere, 2021, 268, 128780 CrossRef CAS PubMed.
- T. Pan, D. Chen, J. Fang, K. Wu, W. Feng, X. Zhu and Z. Fang, Mater. Res. Bull., 2020, 125, 110812 CrossRef CAS.
- K. Wu, D. Chen, S. Lu, J. Fang, X. Zhu, F. Yang, T. Pan and Z. Fang, J. Hazard. Mater., 2020, 382, 121027 CrossRef CAS PubMed.
- Y. Xie, S. Peng, Y. Feng and D. Wu, Chemosphere, 2020, 239, 124612 CrossRef CAS PubMed.
- N. Masunga, B. B. Mamba and K. K. Kefeni, Colloids Surf., A, 2020, 602, 125107 CrossRef CAS.
- M. Wang, P. Guo, Y. Zhang, C. Lv, T. Liu, T. Chai, Y. Xie, Y. Wang and T. Zhu, J. Hazard. Mater., 2018, 349, 224–233 CrossRef CAS PubMed.
- J. Zhou, H. Luo, R. Ding, X. Cao, X. Zhou, Q. Chen and F. Jiang, Colloids Surf., A, 2020, 585, 123853 CrossRef CAS.
- G. Li, B. Wang, J. Zhang, R. Wang and H. Liu, Chem. Eng. J., 2020, 391, 123500 CrossRef CAS.
- G. Zeng, M. Duan, Y. Xu, F. Ge and W. Wang, Spectrochim. Acta, Part A, 2020, 241, 118649 CrossRef CAS PubMed.
- C. Li, Y. Wang, C. Li, S. Xu, X. Hou and P. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 20770–20777 CrossRef CAS PubMed.
- C. Gervais, E. Framery, C. Duriez, J. Maquet, M. Vaultier and F. Babonneau, J. Eur. Ceram. Soc., 2005, 25, 129–135 CrossRef CAS.
- D. J. Heldebrant, A. Karkamkar, N. J. Hess, M. Bowden, S. Rassat, F. Zheng, K. Rappe and T. Autrey, Chem. Mater., 2008, 20, 5332–5336 CrossRef CAS.
- J. Luo, X. Kang, Z. Fang and P. Wang, Dalton Trans., 2011, 40, 6469–6474 RSC.
- Z. Huang, F. Li, B. Chen and G. Yuan, Catal. Sci. Technol., 2014, 4, 4258–4264 RSC.
- Y. Wang, M. K. Bayazit, S. J. A. Moniz, Q. S. Ruan, C. C. Lau, N. Martsinovich and J. Tang, Energy Environ. Sci., 2017, 10, 1643–1651 RSC.
- E. Han, Y. Li, Q. Wang, W. Huang, L. Luo, W. Hu and G. Huang, J. Mater. Sci. Technol., 2019, 35, 2288–2296 CrossRef.
- E. B. Azimi, A. Badiei and J. B. Ghasemi, Appl. Surf. Sci., 2019, 469, 236–245 CrossRef.
- S. Tang, Z. Fu, Y. Li and Y. Li, Appl. Catal., A, 2020, 590, 117342 CrossRef CAS.
- J. Su, Y. Zhao and J. Xi, Electrochim. Acta, 2018, 286, 22–28 CrossRef CAS.
- B. Amanulla, K. N. Perumal and S. K. Ramaraj, Sens. Actuators, B, 2019, 281, 281–287 CrossRef CAS.
- Q. Zhang, Y. Liu, Y. Nie, Y. Liu and Q. Ma, Anal. Chem., 2019, 91, 13780–13786 CrossRef CAS PubMed.
- H. Han, G. Ding, T. Wu, D. Yang, T. Jiang and B. Han, Molecules, 2015, 20, 12686–12697 CrossRef CAS PubMed.
- L. Muniandy, F. Adam, A. R. Mohamed, A. Iqbal and N. R. A. Rahman, Appl. Surf. Sci., 2017, 398, 43–55 CrossRef CAS.
- A. Liu, R. M. Wentzcovitch and M. L. Cohen, Phys. Rev. B: Condens. Matter, 1989, 39, 1760–1765 CrossRef CAS PubMed.
- T. Kokulnathan and T. J. Wang, Composites, Part B, 2019, 174, 106914 CrossRef CAS.
- S. Sarkar, N. Kamboj, M. Das, T. Purkait, A. Biswas and R. S. Dey, Inorg. Chem., 2020, 59, 1332–1339 CrossRef CAS PubMed.
- K. Mori, T. Murakami and H. Yamashita, ACS Appl. Nano Mater., 2020, 3, 10209–10217 CrossRef CAS.
- J. Deng, Q. Zhang, X. Lv, D. Zhang, H. Xu, D. Ma and J. Zhong, ACS Energy Lett., 2020, 5, 975–993 CrossRef CAS.
- M. Li, B. Wang, M. Yang, Q. Li, D. G. Calatayud, S. Zhang, H. Wang, L. Wang and B. Mao, Sep. Purif. Technol., 2020, 239, 116515 CrossRef CAS.
- Z. Luo, M. Zhou and X. Wang, Appl. Catal., B, 2018, 238, 664–671 CrossRef CAS.
- D. H. Park, K. S. Lakhi, K. Ramadass, M. K. Kim, S. N. Talapaneni, S. Joseph, U. Ravon, K. Al-Bahily and A. Vinu, Chemistry, 2017, 23, 10753–10757 CrossRef CAS PubMed.
- G. P. Mane, S. N. Talapaneni, K. S. Lakhi, H. Ilbeygi, U. Ravon, K. Al-Bahily, T. Mori, D. H. Park and A. Vinu, Angew. Chem., Int. Ed., 2017, 56, 8481–8485 CrossRef CAS PubMed.
- Y. Zhang, T. Mori, L. Niu and J. Ye, Energy Environ. Sci., 2011, 4, 4517–4521 RSC.
- C. Dong and L. Vayssieres, Chemistry, 2018, 24, 18356–18373 CrossRef CAS PubMed.
- J. Zhong, H. Zhang, X. Sun and S. T. Lee, Adv. Mater., 2014, 26, 7786–7806 CrossRef CAS PubMed.
- K. Kvande, D. K. Pappas, E. Borfecchia and K. A. Lomachenko, ChemCatChem, 2020, 12, 2385–2405 CrossRef CAS.
- Y. Zhang, S. Jiang, W. Song, P. Zhou, H. Ji, W. Ma, W. Hao, C. Chen and J. Zhao, Energy Environ. Sci., 2015, 8, 1231–1236 RSC.
- H. Chang, Y. Fu, W. Lee, Y. Lu, Y. Huang, J. Chen, C. Chen, W. Chou, J. Chen, J. F. Lee, S. Shen and C. Dong, Nanotechnology, 2018, 29, 064002 CrossRef PubMed.
- F. Hu, H. Wang, Y. Zhang, X. Shen, G. Zhang, Y. Pan, J. T. Miller, K. Wang, S. Zhu, X. Yang, C. Wang, X. Wu, Y. Xiong and Z. Peng, Small, 2019, 15, 1901020 CrossRef PubMed.
- L. Zhang, J. Ran, S. Qiao and M. Jaroniec, Chem. Soc. Rev., 2019, 48, 5184–5206 RSC.
- K. Jiang, S. Siahrostami, A. J. Akey, Y. Li, Z. Lu, J. Lattimer, Y. Hu, C. Stokes, M. Gangishetty, G. Chen, Y. Zhou, W. Hill, W. Cai, D. Bell, K. Chan, J. K. Norskov, Y. Cui and H. Wang, Chem, 2017, 3, 950–960 CAS.
- C. Asokan, L. DeRita and P. Christopher, Chin. J. Catal., 2017, 38, 1473–1480 CrossRef CAS.
- T. G. Lach, M. J. Olszta, S. D. Taylor, K. H. Yano, D. J. Edwards, T. S. Byun, P. H. Chou and D. K. Schreiber, J. Nucl. Mater., 2021, 549, 12 CrossRef.
- I. M. Hill, S. Hanspal, Z. D. Young and R. J. Davis, J. Phys. Chem. C, 2015, 119, 9186–9197 CrossRef CAS.
- H. Zhang, X. Han, H. Yu, Y. Zou and X. Dong, Sep. Purif. Technol., 2019, 226, 128–137 CrossRef CAS.
- S. Le, T. Jiang, Q. Zhao, X. Liu, Y. Li, B. Fang and M. Gong, RSC Adv., 2016, 6, 38811–38819 RSC.
- R. Li, J. Huang, M. Cai, J. Huang, Z. Xie, Q. Zhang, Y. Liu, H. Liu, W. Lv and G. Liu, J. Hazard. Mater., 2020, 384, 121435 CrossRef CAS PubMed.
- P. Raizada, A. Sudhaik, P. Singh, P. Shandilya, P. Thakur and H. B. Jung, Arabian J. Chem., 2020, 13, 3196–3209 CrossRef CAS.
- G. Dong, P. Qiu, F. Meng, Y. Wang, B. He, Y. Yu, X. Liu and Z. Li, Chem. Eng. J., 2020, 384, 123330 CrossRef CAS.
- P. Raizada, A. Sudhaik, P. Singh, P. Shandilya, A. K. Saini, V. K. Gupta, J. H. Lim, H. Jung and A. Hosseini-Bandegharaei, Sep. Purif. Technol., 2019, 212, 887–900 CrossRef CAS.
- P. Babu, S. Mohanty, B. Naik and K. Parida, ACS Appl. Energy Mater., 2018, 1, 5936–5947 CrossRef.
- W. M. A. El Rouby, A. E. A. Aboubakr, M. D. Khan, A. A. Farghali, P. Millet and N. Revaprasadu, Sol. Energy, 2020, 211, 478–487 CrossRef CAS.
- B. Liu, L. Ye, R. Wang, J. Yang, Y. Zhang, R. Guan, L. Tian and X. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 4001–4009 CrossRef CAS PubMed.
- A. Mohammad, M. E. Khan and M. H. Cho, J. Alloys Compd., 2020, 816, 152522 CrossRef CAS.
- R. Zhu, Y. Zhang, J. Wang, C. Yue, W. Fang, J. Dang, H. Zhao and Z. Li, Anal. Bioanal. Chem., 2019, 411, 7137–7146 CrossRef CAS PubMed.
- A. Sakthivel, A. Chandrasekaran, S. Jayakumar, P. Manickam and S. Alwarappan, J. Electrochem. Soc., 2019, 166, B1461–B1469 CrossRef CAS.
- T. Kokulnathan and S. M. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 7893–7905 CrossRef CAS PubMed.
- R. Zhu, Y. Zhang, X. Fang, X. Cui, J. Wang, C. Yue, W. Fang, H. Zhao and Z. Li, J. Mater. Chem. B, 2019, 7, 2320–2329 RSC.
- J. Zou, D. Mao, A. Arramel, N. Li and J. Jiang, Appl. Surf. Sci., 2020, 506, 144672 CrossRef CAS.
- A. Hassani, P. Eghbali and O. Metin, Environ. Sci. Pollut. Res. Int., 2018, 25, 32140–32155 CrossRef CAS PubMed.
- N. Ertugay and F. N. Acar, Appl. Surf. Sci., 2014, 318, 121–126 CrossRef CAS.
- S. Kamal, G. T. Pan, S. Chong and T. C. K. Yang, Processes, 2020, 8, 104 CrossRef CAS.
- X. Ren, Q. Wu, H. Xu, D. Shao, X. Tan, W. Shi, C. Chen, J. Li, Z. Chai, T. Hayat and X. Wang, Environ. Sci. Technol., 2016, 50, 9361–9369 CrossRef CAS PubMed.
- H. Xu, Z. Qu, C. Zong, W. Huang, F. Quan and N. Yan, Environ. Sci. Technol., 2015, 49, 6823–6830 CrossRef CAS PubMed.
- J. S. Taurozzi, M. Y. Redko, K. M. Manes, J. E. Jackson and V. V. Tarabara, Sep. Purif. Technol., 2013, 116, 415–425 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |