Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A convenient workflow to spot photosensitizers revealed photo-activity in basidiomycetes†‡
Bianka Siewert *a,
Pamela Vrabl
*a,
Pamela Vrabl b,
Fabian Hammerlea,
Isabella Binggerbc and
Hermann Stuppner
b,
Fabian Hammerlea,
Isabella Binggerbc and
Hermann Stuppner a
a
aInstitute of Pharmacy/Pharmacognosy, Center for Molecular Biosciences Innsbruck (CMBI), Center for Chemistry and Biomedicine, University of Innsbruck, Innrain 80-82, Innsbruck, 6020 Austria. E-mail: Bianka.siewert@uibk.ac.at
bInstitute of Microbiology, University of Innsbruck, Technikerstraße 25d, Innsbruck, 6020 Austria
cManagement Center Innsbruck, Maximilianstraße 2, Innsbruck, 6020 Austria
First published on 5th February 2019
Abstract
Photodynamic therapy (PDT) is an alternative approach for the treatment of neoplastic diseases employing photosensitizers activated by light. In order to discover new natural photosensitizers, a convenient workflow was established. To validate the workflow, fungi were selected, because we hypothesized that fruiting bodies and mycelia are an overlooked source. The results proved the hypothesis, as exorbitant high photo-cytotoxicity values were detected. For example, the acetone extract of Cortinarius croceus was characterized by an EC50, 9.3 J cm−2 of 1 µg mL−1 against cells of a lung cancer cell-line (A549). In sum, a low-cost workflow for the detection and biological evaluation of photosensitizers is presented and discussed. Furthermore, this paper provides the first experimental evidence for phototoxic metabolites in basidiomycetes. This hints towards a new assignable function of fungal pigments, i.e. photochemical defense.
Introduction
In India's sacred book Atharva-Veda, dated back to around 3500 BC, the sun-worshipping prayer is recorded as a fundamental part of medicinal treatments.1 Today we know this prayer leads to the inevitable sun exposure needed to activate e.g. the ingredients of Psoralea corylifolia.1 Such light-combined therapies are, however, not restricted to India's ethnopharmaceutical knowledge. They are also found in many other ancient civilizations2 and are clinically implemented since the early nineties.3The major benefit of the so-called photodynamic therapy (PDT) is its selectivity: only illuminated cells are affected, cells or tissues left in the dark remain unharmed. This selectivity permits spatial controlled therapies and allows a higher therapeutic window while minimizing the threatening adverse-effects of classic therapies.4 Nevertheless, despite promising clinical results,5 PDT and antimicrobial photodynamic inhibition (aPDI)6 are not widely applied clinical therapies.7 Hampering factors are based on the light sources and the limited amount of approved photosensitizers. The revolution in lighting techniques, however, let limitations based on irradiation issues disappear.8,9 Optical fibers are nowadays used to transmit light. Such fibers can be implanted directly into the tumour permitting e.g. interstitial illuminations.5 Thus, tissue-penetration-depth issues based on the irradiation wavelength vanished and therefore the optical window of PDT broadened. Still missing, however, are new chemical entities. Therefore, this work focuses on establishing a tool to detect new lead-compounds for PDT and aPDI.
Nature might be the origin of new PS. Known classes of natural PS are of herbal origin and include perylenequinones with hypericin from St John's wort (Hypericum perforatum), furanocoumarins with psoralen from fool's parsley (Aethusa cynapium),10 phenalenones with lachnanthocarpone from paint-root (Lachnanthes tinctoria),11,12 and β-carboline alkaloids with harmane from the Chinese plant Evodia rutaecarpa.12 Moreover, anthraquinones from Argentinian shrubs (Heterophylla spp.) were identified as natural PSs, e.g. soranjidiol.13 From a chemical point of view, several fungal metabolites appear to be analogue structures or are precursors of known herbal PS. Austroventin, for example, is oxidized to hypericin when insects attack the fruiting bodies of Cortinarius austrovenetus (=Dermocybe austroveneta).14 Other webcap (Cortinarius) species are known to produce a vast number of anthraquinones, e.g. questin, emodin, or 7,7′-emodinphyscion.15 Anthraquinones belong to the top ten photosensitizers,16 but also alkaloids analogue to the PS harmane were isolated from fungi, e.g. infractin from C. infractus.17 Funalenone, a phenalenone similar to the one in paint-roots,18 is produced by several Aspergillus species like A. tubingensis19 or A. niger.11 Considering the structural similarities, we thus hypothesize that the kingdom fungi is an underestimated source for PS and decided to employ fungi as sample source to validate our workflow. Exemplary with selected fungi, we introduce the principles of our workflow, discuss the rationale behind the implemented assays, and provide an interpretation-guideline for the assessment of the source's photo-therapeutic potential.
Results and discussion
The designed workflow (Fig. 1) consists out of three assays. A chemical assay based on dimethyl-anthracene (DMA) indicates whether singlet oxygen (1O2) is produced due to the irradiation of an extract or not. Via an analytical assay based on the HPLC-DAD-MS technology insights are gained in the chemical profile of possible pigments responsible for the photo-effect. Finally, an in vitro photo-cytotoxicity assay is conducted to rank the biological relevance.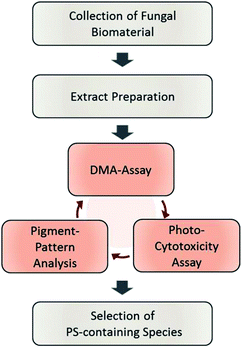 | ||
| Fig. 1 Flowchart of the steps involved in the established phototoxicity screening workflow. After sampling an adequate amount of the natural source, extracts of each are prepared. The prepared extracts are submitted to the DMA-assay, the photo-cytotoxicity-assay, and the pigment-pattern analysis. The order of these investigations is not fixed; the conclusion will be drawn from the combined results of all three sub-assays. For an interpretation guideline, see Fig. 4. | ||
The DMA-assay
Clinically most relevant are PDT Type II sensitizers producing 1O2 while being irradiated. For the detection of 1O2 several chemical probes are known.20,21 Out of them, DMA was chosen as inexpensive and selective agent.22 The selectivity is based on its detection mechanism, which is a photosensitized oxidation of the anthracene-core leading to a disruption of the conjugated π-system (see Fig. S2‡).This in turn, suppresses the typical absorption of DMA (λmax, EtOH = 377 nm) as well as emission. The extent of quenching, measured by the ΔOD377 nm can be correlated to the relative 1O2 production by using a reference PS via equation (eqn (1)) (detailed explanation in the ESI‡).
 | (1) |
The reference PS should be aligned to the colour of the investigated extracts and the available light source. For red-light absorbing blue pigments, one would use e.g. methylene blue (λirr = 632 nm, ϕ1O2, EtOH = 0.49)23 or hypericin (λirr = 590 nm, ϕ1O2, PB = 0.49)23 as reference for the production of 1O2. For green-light absorbing red pigments, Rose bengal (λirr = 555 nm, ϕ1O2, EtOH = 0.86)23 would be a good choice. In this study, the blue light absorbing natural photosensitizer berberine (λmax = 420 nm, ϕ1O2, DCM = 0.25, yellow pigment)23 was chosen in accordance to the colour of the fungal extracts (yellow to orange) and the blue irradiation panel (468 ± 27.3 nm). A PS of natural origin was preferred over a synthetic one, as in this way a plant extract containing the reference PS could be included allowing to rank the obtained results. Berberine, however, can not only produce 1O2 (Type II) it may also act itself as radical (Type I PDT).24 In the setting of the DMA assay, solely the 1O2 production capacity of berberine was utilized, which is guaranteed by the selective probe. The DMA-assay itself was conducted in 96 well-plates allowing a medium-size throughput. For each extract, the measurement of several experiments before and after irradiation was necessary. First, the DMA-absorbance (λabs = 377 nm) was measured to detect singlet oxygen. Second, the optical density of the pure extract at the irradiation wavelength (λabs = 468 nm) was recorded to correct the singlet oxygen production by the probability of absorption. Third, a co-incubation with a 1O2 quencher (i.e. ascorbic acid) was done to exclude false positive results. As the experiment is of chemical nature, and not of a more complex biological,21 one can conclude that 1O2 was produced if the consumption of DMA is quenched by adding a scavenger. Finally, a control experiment containing solely the extract and ascorbic acid was included to spot any unwanted side-reaction.
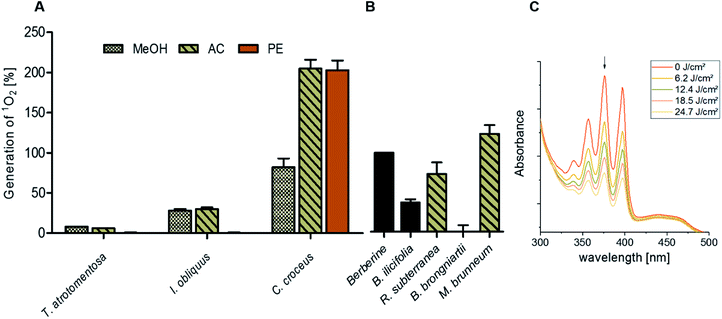
By submitting the extracts of the selected fungi to the DMA-assay intriguing insights were obtained: most of the basidiomycete extracts were able to produce 1O2 (Fig. 2). Especially all extracts of C. croceus seemed to contain PSs as shown by their significant ability to produce 1O2: the AC and the PE fractions, resulted in 205 ± 11% and 205 ± 12% 1O2 formation, respectively, and were thus more efficient than pure berberine (set to 100%). The methanol extract yielded 82 ± 11% singlet oxygen, which is still higher than the plant extract containing the known PS berberine (i.e. the root extract of B. ilicifolia yielding 37 ± 4% singlet oxygen). An activity similar to the Berberis extract was shown by the MeOH and AC extract of I. obliquus (i.e. 28 ± 2% and 30 ± 2%). The PE extract of T. atrotomentosa showed no activity, but the MeOH and AC fraction at least a minimal one (i.e. 6% each). Two of the three investigated ascomycetes showed a higher activity than the plant extract. In detail, R. subterranea produced 73 ± 15% singlet oxygen and for M. brunneum an activity of 124 ± 11% was found. In contrast, the extract of B. brongniartii showed no 1O2 production.
HPLC pigment profiling
A HPLC-DAD-MS analysis was implemented in the workflow to judge whether the extract is a promising sources to isolate natural PSs. Therefore, the extract was monitored with the standard wavelength 210, 254, and 280 nm as well as by the wavelength of light irradiation. The first set of wavelengths is intended to judge the complexity of the chemical matrix and thereby the ease of isolation. The latter is necessary to gain information about the number of pigments being potentially responsible for the singlet oxygen production.In this study, the fungal extracts were monitored at a wave light of 468 nm according to the emission maximum of the available irradiation setup. For a chemical profiling of the matrix and the pigments, the full electron absorption spectrum (210–800 nm) of each peak was additionally recorded. For all basidiomycetes, the majority of the pigments was found in the acetone fraction (Fig. S3‡). Based on the obtained mass, the UV-Vis spectra, and literature data, the main pigments of T. atrotomentosa and C. croceus could be identified as atromentin and 7,7′ biphyscion (refer to the ESI‡ for a detailed discussion).
For the ascomycetes, a suitable separation was obtained with a stationary phase containing phenyl-hexyl ligands leading to a selectivity for polar and aromatic compounds. The obtained spectra, however, revealed a systematic problem existing for samples with limited biomaterial, as e.g. those derived from Petri dishes: the concentration of the interesting metabolites was comparatively low, resulting in an intensity of the peaks close to the subjective LOQ. This effect is a direct consequence of the limited net mass of the biomaterial. With this limitation in mind, an interpretation of the HPLC-MS experiment was done and oosporein assigned for B. brongniartii. For a complete discussion, please refer to the ESI.‡
(Photo)-cytotoxicity
In order to validate the results of the DMA-assay in vitro, all investigated extracts were tested regarding their photo-cytotoxic activity against the lung cancer cell line A549 and the cervical carcinoma cell line HeLa. 24 hours after drug treatment, the cells were irradiated in drug-free medium. As endpoint-assay the SRB-assay25 was preferred over the common used MTT,26 as MTT (and analogues) led to false-positive results (data not shown) due to their general redox-activity.27,28The obtained results of the fungal extracts are displayed in detail in Table S2.‡ All photo-cytotoxic active basidiomycete extracts showed an activity in the DMA-assay. However, not all of the DMA-active extracts were active in the biological context, implying that the cellular membrane functioned as effective barrier for some PS. In detail, the extracts of T. atrotomentosa showed no phototoxic effect at all. Just a weak cytotoxic effect (approx. 40 µg mL−1) independent from light irradiation was observed for the PE extract. The extracts of I. obliquus were characterized by the absence of any significant light toxicity as well. The results for the Cortinarius species (Fig. 3a) were the most promising; a selectivity factor (see Fig. S9‡) of over 50 was obtained for the acetone fraction, which was non-toxic in the dark.
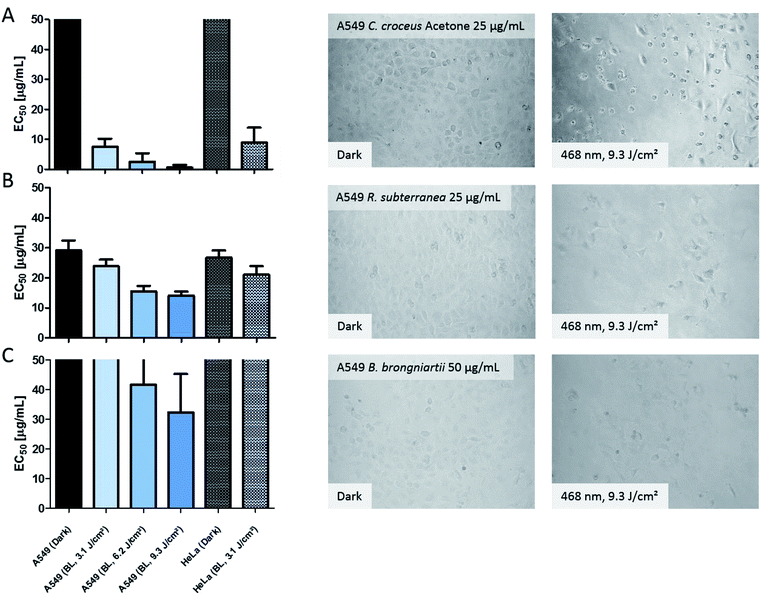 | ||
| Fig. 3 Photo-cytotoxicity results of the most promising extracts against A549 and HeLa cancer cells in the dark and under blue light irradiation. Bars: EC50 values with confidence interval (95%) if a reduction of the cell population was induced by the treatment. Top cutted bars indicate that the extracts were not active below 50 µg mL−1. Pictures: evaluation of the morphological changes induced by the extracts. The utilized concentration is indicated in the dark picture and was the same for the irradiated experiment. Line (A) acetone extract of C. croceus, line (B) R. subterranea, and line (C) B. brongniartii. The complete set of the microscopic investigation is depicted in the ESI.‡ | ||
The PE fraction showed also an impressive phototoxicity, it was, however, additionally cytotoxic in the dark. This might be due to membrane modulating fatty acids in the PE fraction leading to leaks in the cell membrane and therewith to a toxic effect detracting from the photo-activated effect of this fraction. Most likely, the high photo-cytotoxicity is based on 7,7′ biphyscion, an anthraquinone dimer, which was identified by HPLC-DAD-MS as main metabolite in the acetone and PE extracts.
The three ascomycete extracts were characterized by lower EC50 values (i.e. less activity) as compared to the basidiomycetes. This observation might be an artificial effect due to the nature of the acetone-only extract. Nevertheless, the extract of R. subterranea was characterized by a moderate activity in the dark (EC50, Dark = 29 µg mL−1) and a photo-enhanced effect due to the irradiation with blue light. Interesting observations were made for M. brunneum and B. brongniartii: while the M. brunneum-extract was active in the DMA assay, no activity was observed in the phototoxicity assay. For B. brongniartii, instead, the opposite was. The absence of any activity for M. brunneum indicated that the metabolites do not permeate the membrane or that the respective PSs are not stable in the medium. For the photo-cytotoxic B. brongniartii extract (Fig. 3C), singlet oxygen (Fig. 2) can be excluded as key player. Thus, one of the other PDT mechanisms29 was induced. Irradiation experiments with pure oosporein (B. brongniartii's main pigment) showed that a photochemical reaction was induced (data not shown), which might be the reason behind the observed photo-cytotoxicity.
Interpretation guide of the phototoxicity screening workflow
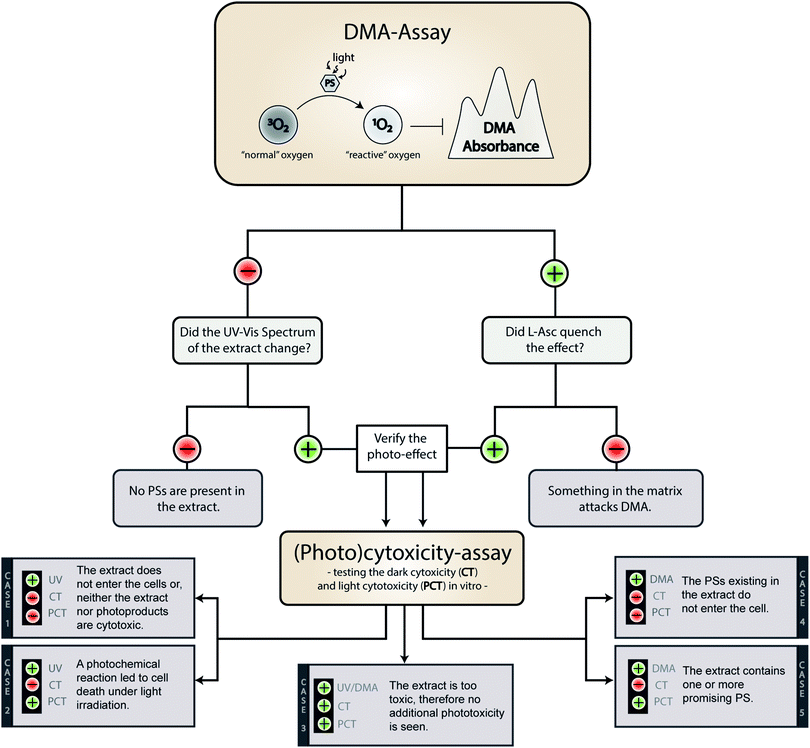
On a meta-level, the presented phototoxicity workflow leads to a multi-layered result (see Fig. 4) which can be used to rank extracts from different natural sources (e.g. plants, algae, or fungi) regarding their phototoxic potential. An extract active in the DMA-assay is ranked by a photo-cytotoxicity assay with three potential outcomes: (a) no activity in the dark (CT ⊖), but in the light (PCT ⊕), (b) active in the dark (CT ⊕) and in the light (PCT⊕), or (c) inactive in the dark (CT ⊖) as well as under irradiation (PCT ⊖). Combining all information, following conclusions can be drawn in order to rank the extracts potential.Case 1. The extract did not produce any 1O2, but showed a change in the UV-Vis spectra over the time of irradiation. Here the absence of any toxicity indicates a putative sun protecting action of the extract or simply the absence of any photobiologically relevant activity.
Case 2. The extract did not produce any 1O2, but was cytotoxic while being irradiated. In this case, one of the processes based on a photochemical mechanism was induced and the extract-matrix may contain a promising metabolite for the treatment of hypoxic tumours. In this study, the extract of B. brongniartii represents such a case and may therefore be an interesting candidate to develop oxygen independent PSs.
Case 3. The extract showed an activity on each level. This indicates that the extract might be too active and may lead to a systemic toxicity. Here it is recommended to carefully investigate the complexness of metabolites in the extract; an additional and/or synergistic effect of the ingredients might be present. The petrol ether extract of C. croceus fulfils these criteria, and as discussed above, matrix effects could be responsible. Thus, a detailed investigation of the extract should be done.
Case 4. The extract produced 1O2, but showed not photo-cytotoxicity. This might be a promising candidate for selective aPDI, in case it crosses bacterial or fungal membranes. The investigated extracts of M. brunneum showed this behaviour and should therefore be tested in more detail regarding their aPDI potential.
Case 5. The extract produced 1O2 and showed cytotoxicity solely under irradiation. Here a promising candidate for PDT-applications in mammalian might be present in the extract. In this preliminary study, the methanol extract of C. croceus achieved this promising behaviour. Therefore, it is subject of ongoing investigations.
Outlook. While this workflow selects fungi/plants containing unknown PDT-active metabolites, future research steps towards PDT-lead compounds are necessary. This steps should consider the following points: (1) the isolation and identification of the PS can be done via activity-guided isolation employing the DMA-assay. (2) The biological evaluation of the isolated metabolites should not only focus on the photo-cytotoxicity but also on the dark cytotoxicity. For the latter it is important to test the dark-effect against non-malignant cells in addition to the cancer cell line. These additional tests are important to gain preliminary information about potential side effects. (3) It might be that the isolated compound lacks its activity. In such a case, synergistic effects in the extracts were most likely responsible for the high ranking.
Conclusions
In general, for the first time a low-cost, medium-throughput workflow to screen natural extracts for PS was presented. The workflow is not limited to fungal extracts. Plant extracts or pure compounds can be evaluated as well. As in general light-enhanced pharmaceutical actions of natural products tend to be neglected, a systematic exploration holds an impressive potential. Furthermore, the bioactivity-guided isolation of the photosensitizers is possible in standard phytochemical laboratories employing one part of the workflow, i.e. the DMA-assay. This in turn is a substantial requirement for an efficient isolation of new PS, which are mandatory for alternative antitumor or antimicrobial strategies in order to overcome existing resistances.In detail, of the different fungal species exemplified, four appeared to produce PDT-type II PSs, while at least one seemed to contain another PS type. Neither one of the fungi was described as producer of PS before nor was one of the fungal pigments described as photoactive. For basidiomycetes, it is the first experimental evidence of photoactive metabolites. The existence of PS is of ecological interest as light-induced toxic effects belong to a yet unknown photochemical defence mechanism for these organisms. If such findings accumulate in the future, the discovery of photo activity will shed a new light on the physiological role of several fungal pigments, which otherwise – according to Spiteller30 – stays obscure.
Material and methods
Organisms and samples species
Petri dish cultures of B. brongniartii (BIPESCO 2) were grown on Sabouraud-2-Glucose agar medium at 25 °C for 9 days as described in the literature.31 Petri dish cultures of M. brunneum (BIPESCO 5) were cultivated on Sabouraud-4-Glucose agar medium at 25 °C for 12 days. R. subterranean were grown for approximately 4 weeks on PDA agar plates and kept at 4 °C until further use. Fruiting bodies of C. croceus and T. atrotomentosa were obtained in dry form from the herbarium in Innsbruck (kindly donated by U. Peintner and M. Kirchmair, University of Innsbruck, Austria) in 2017. Dried fruiting bodies of the orange black I. obliquus were kindly donated by P. L.'Archeveque (Forestia Inc, Canada) in 2017, and a voucher specimen (Herbarium nr: Chaga 2017) is stored in the herbarium of the Institute of Pharmacy/Pharmacognosy of the University of Innsbruck.Extraction of fruiting bodies (basidiomycetes)
The dried material of the basidiomycetes was milled to obtain finely granulated material for soxhlet extraction. In a first step, the fruiting bodies (exact amount see Table S1‡) were defatted in the dark with petrol ether (PE, 400 mL) for 6 h. Thereafter acetone (AC, 400 mL) and methanol (MeOH, 400 mL) were used under identical conditions. The yielded extracts were reduced under vacuum and stored in the dark. The amount of each obtained extract, the relative yield, and the colour are given in Table S1.‡ For all tests, an aliquot of the extract was solved in DMSO (final concentration of 10 mg mL−1) and filtrated through cotton wool.Extraction of Petri dish cultures (ascomycetes)
At least three individual colonies with the agar were cut in pieces, transferred into a centrifugation tube (50 mL) and subjected to an ultrasonic extraction (30 min) with acidified acetone (ca. 10 mL, 1 L acetone + 1 mL 2 N HCl). This different extraction method was chosen due to the limited biomaterial as compared to the fruiting bodies. After centrifugation (3000 rcf, 5 min), the supernatant was decanted and evaporated under a stream of air. The obtained yields and coloration of the extracts are listed in Table S1.‡ For analytical investigations, the extracts were solved in DMSO (final concentration 10 mg mL−1) and filtered through a membrane filter (Carl Roth, 0.45 µm, PTFE) before being subjected to any analysis.96 LED-setup for illumination
An irradiation setup was built by Leiden University with marginal modifications to the published setup by Hopkins et al.32 For the present setup, LEDs with an emission of 468 nm (±27.3 nm) were utilized. A power density of 20.6 ± 1.1 mW cm−2 was calculated. A chemical actinometer, i.e. ferrioxalate, was utilized32 to confirm the homogenous irradiation across the inner 60 wells (Fig. S1‡).Chemical analysis of singlet oxygen (1O2) production
An ethanolic DMA solution was prepared (0.35 mM) and transferred (190 µL per well) into of a 96-well plate. A DMSO solution (10 µL, 1 mg mL−1) of each extract was added to the respective wells. The absorption of the extract alone (50 µM, ethanol) was measured as well as the suppression of the DMA absorbance in the presence of L-ascorbic acid (5 mM, pH 7.0–7.4). An additional control experiment (extract and L-ascorbic acid) was performed. DMSO (5% in ethanol) was used as solvent control. Berberine (50 µg mL−1, 149 µM) was used as positive control. Excitation was performed with the 96 LED setup, whereby four irradiation steps (468 nm, 5 min each, 6.2 J cm−2 each) were chosen. The measurement of the absorption was performed with a microplate reader (Tecan, Spark M10) and done in triplicate. The singlet oxygen production after 20 min (24.8 J cm−2) was calculated relative to berberine using formula (eqn (2)). A detailed discussion of this formula can be found in the ESI.‡(Photo)-cytotoxicity-assay and cell culture maintenance
Cells of the non-small lung cancer cell line A549 (ATCC, Sigma-Aldrich) and the cervical cancer cell line HeLa (kindly donated by A. Trockenbacher (Management Center Innsbruck, Austria)) were maintained in T-flasks (25 cm2) and MEM-medium containing FCS (10%) and penicillin/streptomycin (1%). The (photo)-cytotoxicity was done as published before32 and is explained in more detail in the ESI.‡ The selectivity indices (S.I.) express the ratio of cells killed under irradiation and cells killed in the dark. It is calculated from the EC50 values as expressed in formula (eqn (2)). For each investigated concentration a biological triplicate of technical triplicates were done.| S.I. = EC50,Dark(EC50,Irradiated)−1 | (2) |
Author contributions
BS conceived the study and designed it with PV. PV cultured the ascomycete B. brongniartii and M. brunneum. BS extracted all fungal samples, performed together with FH and IB the assays, interpreted the results and designed all graphs with input from PV. BS and PV wrote the first draft of the manuscript. HS contributed to the material expenses and to the critical writing process. All authors contributed to the manuscript revision, read, and approved the submitted version.Conflicts of interest
There are no conflicts to declare.Abbreviation
| AC | Acetone |
| CT | Cytotoxicity |
| DAD | Diode-Array-Detector |
| DMA | 9,10 Dimethylanthracene |
| EC | Effective concentration |
| FCS | Fetal calf serum |
| MeOH | Methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid |
| PCT | Photo-cytotoxicity |
| PDT | Photodynamic therapy |
| aPDI | Antimicrobial photodynamic inhibition |
| PE | Petrolether |
| PS | Photosensitizer |
| SRB | Sulforhodamine B |
| SI | Selectivity index |
Acknowledgements
The authors thank H. Strasser, U. Peintner, M. Kirchmair (University of Innsbruck, Austria) and P.L.'Archeveque (Forestia Inc, Canada) for providing fungal strains or fruiting bodies as well as A. Trockenbacher (Management Center Innsbruck, Austria) for providing the HeLa cells to this work. We thank S. Rovoletta (University of Innsbruck, Austria) for the allocation of the B. ilicifolia extract and H. Strasser for an authentic sample of oosporein. We furthermore want to thank S. Bonnet and M. Meier (Leiden University, The Netherlands) for their support concerning the photophysical characterization of the irradiation setup. C. W. Schinagl and H. Strasser are thanked for their critical review of the manuscript. A funding from the University of Innsbruck (Nachwuchsförderung 2017, BS) and the FWF (Austrian Science Fund project T862, PV) supported this work.References
- M. A. Pathak and T. B. Fitzpatrick, J. Photochem. Photobiol., B, 1992, 14, 3–22 CrossRef CAS.
- L. M. Baltazar, A. Ray, D. A. Santos, P. S. Cisalpino, A. J. Friedman and J. D. Nosanchuk, Front. Microbiol., 2015, 6, 202 Search PubMed.
- M. R. Detty, S. L. Gibson and S. J. Wagner, J. Med. Chem., 2004, 47, 3897–3915 CrossRef CAS PubMed.
- I. O. Bacellar, T. M. Tsubone, C. Pavani and M. S. Baptista, Int. J. Mol. Sci., 2015, 16, 20523–20559 CrossRef CAS PubMed.
- G. Shafirstein, D. Bellnier, E. Oakley, S. Hamilton, M. Potasek, K. Beeson and E. Parilov, Cancers, 2017, 9, 12 CrossRef PubMed.
- M. R. Hamblin, Curr. Opin. Microbiol., 2016, 33, 67–73 CrossRef CAS.
- D. van Straten, V. Mashayekhi, H. S. de Bruijn, S. Oliveira and D. J. Robinson, Cancers, 2017, 9, 19 CrossRef PubMed.
- L. Brancaleon and H. Moseley, Lasers Med. Sci., 2002, 17, 173–186 CrossRef CAS PubMed.
- T. S. Mang, Photodiagn. Photodyn. Ther., 2004, 1, 43–48 CrossRef.
- H. Hönigsmann, A. Tanew, J. Brücke and B. Ortel, in Ciba Foundation Symposium 146 – Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use, John Wiley & Sons, Ltd., 2007, pp. 159–170, DOI:10.1002/9780470513842.ch11.
- M. F. Elsebai, M. Saleem, M. V. Tejesvi, M. Kajula, S. Mattila, M. Mehiri, A. Turpeinen and A. M. Pirttila, Nat. Prod. Rep., 2014, 31, 628–645 RSC.
- A. Lazzaro, M. Corominas, C. Marti, C. Flors, L. R. Izquierdo, T. A. Grillo, J. G. Luis and S. Nonell, Photochem. Photobiol. Sci., 2004, 3, 706–710 RSC.
- S. C. Núñez Montoya, L. R. Comini, M. Sarmiento, C. Becerra, I. Albesa, G. A. Argüello and J. L. Cabrera, J. Photochem. Photobiol., B, 2005, 78, 77–83 CrossRef.
- M. Gill and A. Gimenez, Phytochemistry, 1991, 30, 951–955 CrossRef CAS.
- N. N. Gessler, A. S. Egorova and T. A. Belozerskaya, Appl. Biochem. Microbiol., 2013, 49, 85–99 CrossRef CAS.
- M. Wainwright, Photosensitisers in Biomedicine, Wiley, 2009 Search PubMed.
- W. Steglich, L. Kopanski, M. Wolf, M. Moser and G. Tegtmeyer, Tetrahedron Lett., 1984, 25, 2341–2344 CrossRef CAS.
- R. Song, Y. Feng, D. Wang, Z. Xu, Z. Li and X. Shao, Sci. Rep., 2017, 7, 42058 CrossRef CAS PubMed.
- J. Zhan, G. M. K. B. Gunaherath, E. M. K. Wijeratne and A. A. L. Gunatilaka, Phytochemistry, 2007, 68, 368–372 CrossRef CAS PubMed.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, J. Biochem. Biophys. Methods, 2005, 65, 45–80 CrossRef CAS PubMed.
- B. Halliwell and J. Gutteridge, Free Radicals in Biology and Medicine, OUP, Oxford, 2007 Search PubMed.
- L. Schafzahl, N. Mahne, B. Schafzahl, M. Wilkening, C. Slugovc, S. M. Borisov and S. A. Freunberger, Angew. Chem., Int. Ed., 2017, 56, 15728–15732 CrossRef CAS PubMed.
- R. W. Redmond and J. N. Gamlin, Photochem. Photobiol., 1999, 70, 391–475 CrossRef CAS.
- J. J. Inbaraj, B. M. Kukielczak, P. Bilski, S. L. Sandvik and C. F. Chignell, Chem. Res. Toxicol., 2001, 14, 1529–1534 Search PubMed.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- E. Ulukaya, M. Colakogullari and E. J. Wood, Chemotherapy, 2004, 50, 43–50 CrossRef CAS.
- R. Bruggisser, K. v. Daeniken, G. Jundt, W. Schaffner and H. Tullberg-Reinert, Planta Med., 2002, 68, 445–448 CrossRef CAS PubMed.
- B. Q. Spring, I. Rizvi, N. Xu and T. Hasan, Photochem. Photobiol. Sci., 2015, 14, 1476–1491 RSC.
- P. Spiteller, Chem.–Eur. J., 2008, 14, 9100–9110 CrossRef CAS PubMed.
- D. Abendstein, B. Pernfuss and H. Strasser, Biocontrol. Sci. Technol., 2000, 10, 789–796 CrossRef.
- S. L. Hopkins, B. Siewert, S. H. C. Askes, P. Veldhuizen, R. Zwier, M. Heger and S. Bonnet, Photochem. Photobiol. Sci., 2016, 15, 644–653 RSC.
Footnotes |
| † Dedicated to Prof. Dr R. Csuk on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10181g |
| This journal is © The Royal Society of Chemistry 2019 |