Cooking with active oxygen and solid alkali facilitates lignin degradation in bamboo pretreatment†
Ning
Ding
a,
Xiaoqiang
Song
a,
Yetao
Jiang
a,
Bin
Luo
a,
Xianhai
Zeng
 *ab,
Yong
Sun
ab,
Xing
Tang
*ab,
Yong
Sun
ab,
Xing
Tang
 ab,
Tingzhou
Lei
c and
Lu
Lin
*ab
ab,
Tingzhou
Lei
c and
Lu
Lin
*ab
aCollege of Energy, Xiamen University, Xiamen 361102, PR China. E-mail: xianhai.zeng@xmu.edu.cn; lulin@xmu.edu.cn; Fax: +86-592-2880701; Fax: +86-592-2880702; Tel: +86-592-2880701 Tel: +86-592-2880702
bFujian Engineering and Research Center of Clean and High-valued Technologies for Biomass, Xiamen Key Laboratory of High-valued Conversion Technology of Agricultural Biomass, Xiamen University, Xiamen 361102, PR China
cHenan Key Lab of Biomass Energy, Zhengzhou, Henan 450008, PR China
First published on 19th July 2018
Abstract
Cooking with active oxygen and solid alkali (CAOSA), developed by our group, is a promising alternative to both clean pulping and biomass pretreatment since it is a facile technique with advantages of environmental benignity and easy alkali recovery. Through comparative analysis using 2D-HSQC NMR, the superior delignification efficiency of the CAOSA process could be attributed to the more efficient cleavage of the benzene ring in lignin, which is in sharp contrast to the conventional pulping route. Abundant organic acids were detected in the product solution, which is also known as yellow liquor (YL). In addition, acid insoluble degradation products (AIDPs) containing aromatic compounds were present in the YL, which were separated and collected. The low AIDP content in the YL suggested the highly efficient decomposition of the aromatic rings. This delignification study not only presents a deeper investigation of the CAOSA process itself but also provides guidance for further research on biomass-lignin degradation.
Introduction
Lignocellulosic biomass is a low-cost, easily accessible, and renewable resource, which can potentially afford a variety of products such as light industrial materials and chemicals, in addition to energy.1,2 Nevertheless, industrial pretreatment of biomass is still a developing field. The traditional pulping industry requires an overhaul due to the large amount of waste that it produces and the consequent pollution of the environment.3 Even though different methods have been proposed and developed, problems plaguing current pulping techniques have not been completely eliminated.4Pollution, especially that caused by the production of wastewater with high BOD and COD values, remains the main problem restricting the production of chemical pulp. In addition, the huge consumption of energy and chemicals limits the production.5,6 Other constraints such as narrow substrate applicability, low reaction efficiency, and high capital investment also impede the development of the pulping industry.7,8 Even more regrettably, lignin, a potential precursor of aromatic products, has been poorly utilized by the current pulping industry due to its poor recovery and extremely complicated structure.9 Most of the lignin is incinerated as a kind of waste in black liquor.
Lignin is the second most abundant constituent of lignocellulosic biomass. Its unique structure, with a large number of aromatic rings, offers extensive application prospects in the biorefinery industry, fabrication of functional materials, etc.10 Meanwhile, the potential of lignin valorization for obtaining aromatic chemicals and fuel has also attracted the attention of researchers.11 Unfortunately, due to the great complexity of its structure, lignin has not been satisfactorily exploited in the pulping industry, with a major proportion of lignins being incinerated as a kind of waste in black liquor to recover heat energy.12
A novel and environmentally friendly pulping method has been developed and exploited by our group.1,13 In this process of cornstalk or bagasse cooking with active oxygen and solid alkali (CAOSA), a 50.5% yield of pulp was obtained, with a maximum delignification ratio of 95.3%.6,14 Taking its relatively light color and acceptable odor into consideration, the wastewater in our process was named yellow liquor (YL), to distinguish it from the traditional black liquor. YL could be easily precipitated with suitable precipitating agents for easy recovery of organic compounds. Furthermore, it cannot be denied that the solid alkali catalyst (MgO) could be easily recovered and reused in the heterogeneous catalytic reaction system. It has also been demonstrated that cellulosic pulp from this CAOSA process is well suited for the production of both fermentable sugars and value-added materials and chemicals.15
So far, there is no agreement among the researchers working in the area of pulping techniques or lignin decomposition regarding the mechanism of delignification in the wet-oxygen cooking process.16,17 In the current study, bamboo raw material was treated by CAOSA, and the resulting pulp and degradation products were analyzed to identify the chemical compounds present in the pulp and YL. From the perspective of structural changes in the lignin residue, which were detected by HSQC-NMR, the high delignification efficiency in CAOSA was explained in terms of the efficient cleavage of the aromatic rings during the cooking reaction. Lignin was decomposed into lower-molecular-weight fragments, which included acids, especially formic acid, acetic acid, glycolic acid and lactic acid. The most interesting aspect was the almost complete precipitation of the benzoic compounds upon acidification of the YL. β-O-4 units were detected in these acid-insoluble degradation products (AIDPs).
Experimental section
Materials
Bamboo chips were provided by HYA Group Co. Ltd. (Sanming, Fujian, China). Deuterated dimethyl sulfoxide (DMSO-d6) and deuterated water (D2O) with a purity of over 99.9% were obtained from Sigma-Aldrich (Shanghai, China). Other reagents were of analytical grade and were purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd.The process of cooking with active oxygen and solid alkali (CAOSA)
Raw material in the form of bamboo chips was milled to the size of 10 to 20 mesh and cooked in a ball-shaped digester (independently designed by our group and built by Yantai Keli Chemical Equipment Company) with oxygen and solid alkali, MgO. 1 kg of bamboo chips (dry weight) was cooked with 4 kg of water and 150 g MgO at an initial oxygen pressure of 2.0 MPa and temperature of 160 °C for 1/2/3/5/10 h. The reaction that was allowed to continue for 10 h proceeded to near completion.Cooling water was injected into the jacket of the reactor to quench the reaction, and the pressure was reduced through a blow-down valve. The muddy product, which was a mixture of pulp and YL, was filtered using filter cloth of 100 mesh to separate the YL and the solid pulp. The solid was further washed several times with clean water and stored as the pulp produced by the CAOSA method.
Quantitative determination of raw material and pulp
The weight of moisture in the raw material (RM) and pulp was calculated from the weight difference between 50 g of the solid before and after oven-drying at 105 °C for 4 h. In addition, the yield of the pulp could be calculated by subtracting the weight of the moisture from the wet weight of the pulp.The amount of ash, which mainly consisted of magnesium salt and solid alkali trapped in the pulp, was measured by thermogravimetric analysis (TGA). TGA was conducted on an STA 449 F5 Jupiter® thermogravimetric analyzer (NETZSCH Instruments) by heating the sample from 105 to 600 °C at a rate of 10 °C min−1, under air. The weight loss was obtained in this manner, and the residual mass was quantified as the ash content.
After removal of the excess alkali and magnesium salts by making the pH of the pulp neutral, the content of the pulp was determined according to the method recommended by NREL via a two-stage hydrolysis procedure (72% H2SO4/20 °C and 4% H2SO4/reflux).18,19 Concentrations of glucose and xylose were determined using high-performance liquid chromatography (HPLC, WATERS Alliance e4695) with a Bio-Rad Aminex HPX-87H column and refractive index detector. The column temperature was set at 60 °C; 5 mmol H2SO4 was used as the mobile phase with a flow rate of 0.6 mL min−1, and the detection time was 20 min. The external standard method was used to quantify the components.
Determination of the organic weight in the YL
The YL produced in the CAOSA process was first separated from the muddy product. A 50 g YL sample was loaded in a flask and the initial weight was determined. The sample was stored overnight at −20 °C. To prevent changes in the thermally unstable compounds present in the YL, a lyophilizer was used for drying off the sample, resulting in the formation of a yellow powder. The weight of the dried powder was determined, and the difference between the initial and final weights gave the water content of the sample. The ash content in the YL was obtained in a similar manner to the pulp. In addition, some low-molecular-weight (LMW) acids in the YL were identified by HPLC, as reported above.Preparation of milled wood lignin (MWL) from raw material and pulp
Raw material milled wood lignin (RMWL) and pulp milled wood lignin (PMWL) were prepared by the procedures described below.First, bamboo chips with sizes ranging from 40 to 100 mesh were Soxhlet extracted using a mixture of benzene and ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over 12 h, dried, and ball-milled for 48 h. The resulting powder was extracted with a mixture of dioxane and water (9
1) over 12 h, dried, and ball-milled for 48 h. The resulting powder was extracted with a mixture of dioxane and water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at indoor room temperature over 24 h. This extraction was repeated 3 times, and the combined extracts were dried using a rotary evaporator to obtain the solid product. The product was further dried in a vacuum oven with P2O5 and then dissolved in a mixture of acetic acid and water (9
1) at indoor room temperature over 24 h. This extraction was repeated 3 times, and the combined extracts were dried using a rotary evaporator to obtain the solid product. The product was further dried in a vacuum oven with P2O5 and then dissolved in a mixture of acetic acid and water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to remove carbohydrates. Finally, the solution was poured into water, leading to the formation of a precipitate, which was filtered and dried to obtain the RMWL. The PMWL was obtained using a method identical to that employed for the pulp but with a different cooking time.
1) to remove carbohydrates. Finally, the solution was poured into water, leading to the formation of a precipitate, which was filtered and dried to obtain the RMWL. The PMWL was obtained using a method identical to that employed for the pulp but with a different cooking time.
Acidification of the YL
A few drops of HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added to 10 g of YL to adjust the pH of the solution to about 2, as a consequence of which a flocculent precipitate appeared. The precipitate was separated by centrifugation and washed several times with dilute HCl. It was then frozen and lyophilized to get the dry AIDPs. The acidified yellow liquor (AYL) was subjected to a method identical to that employed for the YL to obtain the AYL powder.
1) were added to 10 g of YL to adjust the pH of the solution to about 2, as a consequence of which a flocculent precipitate appeared. The precipitate was separated by centrifugation and washed several times with dilute HCl. It was then frozen and lyophilized to get the dry AIDPs. The acidified yellow liquor (AYL) was subjected to a method identical to that employed for the YL to obtain the AYL powder.
NMR characterization
2D HSQC-NMR is a conventional method for analyzing structural changes in lignin. Several samples in this study were characterized by HSQC NMR, including RWML, PWML, YL, AYL and AIDPs, at different reaction times.20 DMSO-d6 was used as the NMR solvent for the analysis of RMWL, PMWL, and AIDPs, while D2O was used as the NMR solvent for YL and AYL. When DMSO-d6 was used, the chemical shifts were referenced to the residual DMSO peak at δC/δH, 40.0/2.50 ppm. All the 2D HSQC-NMR experiments were recorded on a Bruker AV 600 MHz NMR spectrometer (Germany).Results and discussion
Mass flow direction in the CAOSA process
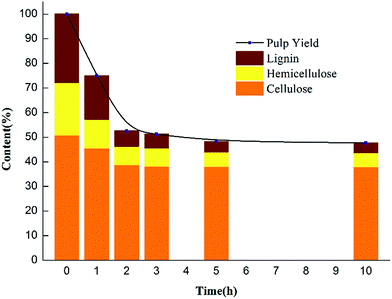
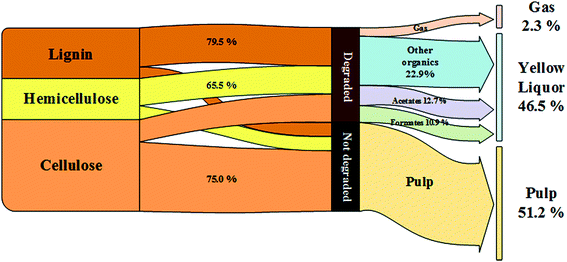
The pulp yield, an important index for evaluating the pulping process, reached 51.62% in the CAOSA process within a three-hour cooking time (Fig. 1), which is also a referable appropriate duration, and the total mass direction of biomass at such a residence time is shown in Fig. 2. With a further increase in the residence time, the pulp yield decreased gradually, implying that the composition of the pulp was still unstable and could be further deconstructed.The amount of ash, an inevitable constituent of the pulp produced by wet oxidation, was also lower than that obtained with current technologies, indicating lower alkali loss in the process (Table 1). Considering the delicate balance required between the energy consumed in maintaining the cooking temperature and the quality of the pulp product, an appropriate cooking time for industrial-scale production could be around 3 h. Nevertheless, attention should be paid to the specific nature of the biomass used as the raw material. With tractable matter, a higher pulp yield and delignification ratio could be achieved by the CAOSA technique.14
| Raw materials | 1 h pulp | 2 h pulp | 3 h pulp | 5 h pulp | 10 h pulp | ||
|---|---|---|---|---|---|---|---|
| Ash content/% | 0.59 | 6.23 | 6.50 | 4.51 | 4.58 | 4.75 | |
| Holocellulose | Cellulose/% | 50.51 | 55.16 | 63.18 | 69.96 | 74.52 | 75.15 |
| Hemicellulose/% | 21.29 | 14.60 | 13.03 | 13.77 | 11.72 | 11.14 | |
| Total/% | 71.81 | 69.76 | 76.22 | 83.73 | 86.24 | 86.29 | |
| Lignin | Acid-soluble/% | 3.12 | 2.25 | 1.37 | 1.47 | 0.75 | 0.68 |
| Acid-insoluble/% | 24.52 | 21.73 | 15.87 | 10.05 | 8.57 | 8.42 | |
| Total/% | 27.64 | 23.98 | 17.24 | 11.52 | 9.32 | 9.10 |
Structural changes of lignin in the pulp
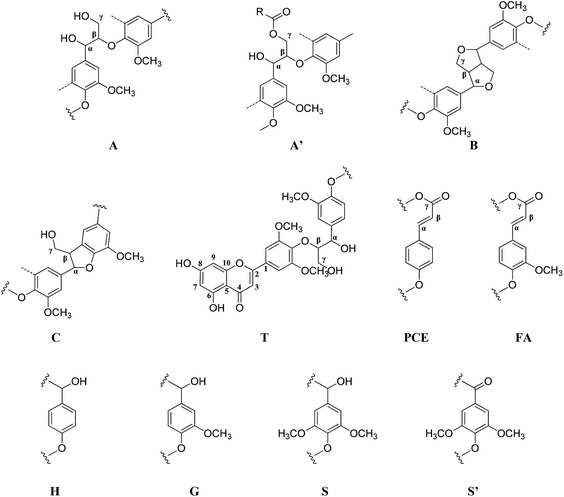
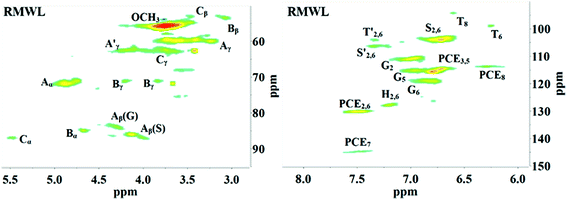
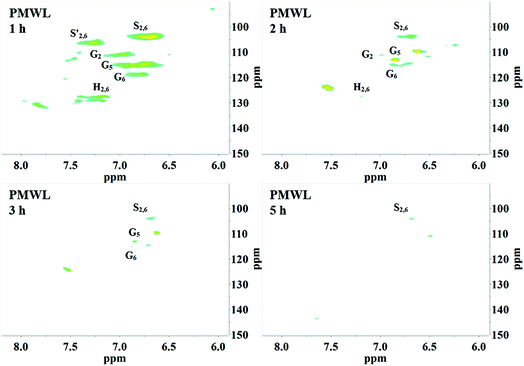
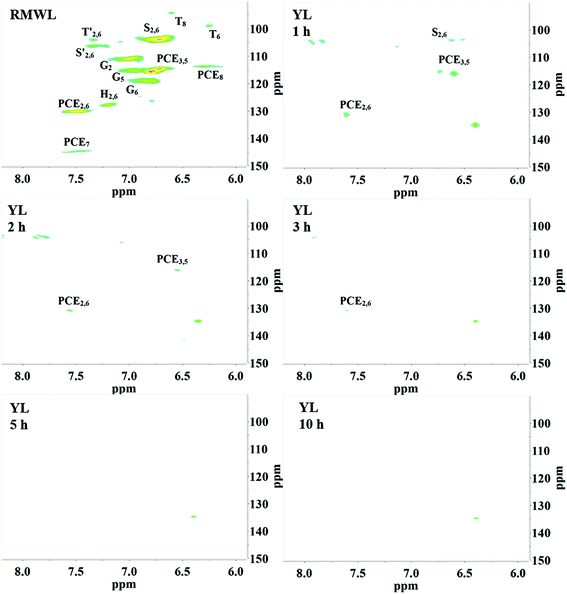
2D HSQC-NMR spectroscopy is widely used in the structural analysis of lignin and its derivatives. The relevant interpretation and characterization have been described in more detail previously.21–23 The typical structures of lignin were characterized (Fig. 3) and specific positions of these units were assigned (Table S2†) by comparison with the HSQC-NMR spectra of previously described lignin structures.For RMWL (Fig. 4), almost no difference could be found from other biomass samples, apart from structures such as T and PCE. Analysis of the spectra of PMWL and RMWL revealed a continuous decrease in the signals, which signified a continuous decrease in the amounts of the relevant compounds or structures.
There was no notable difference between the spectra of RMWL and the 1 h pulp (Fig. 4, 5 and S1†), except for the disappearance of peaks belonging to structure T and the emergence of peaks belonging to structure X, which represents the region of the saccharides. The results indicated that MgO-catalyzed oxygen delignification might occur only in the micro-framework instead of the molecular structure in the initial stages of cooking. Meanwhile, the micro-framework of biomass might be loosened at some parts, even disintegrating to result in a larger contact area with the reagents. Nevertheless, some conspicuous differences were noted as the reaction proceeded. It should be pointed out that a sharp cutoff was seen in the aromatic region since almost all the signal intensities decreased within 2 h (Fig. 5), implying the high rate of lignin degradation in biomass in the first 2 h. For the 10 h pulp sample, it was difficult to detect any PMWL signals, which could be attributed to the high efficiency of CAOSA in lignin degradation.
The T structure, which could be described as a tricin structure, was the most active species in lignin under such conditions. It decomposed first, leading to the fast removal of lignin from lignocellulose. The reactivity of the PCE structure, another remarkable constituent of PMWL, was relatively low due to the lower electron density distribution in the conjugated structure. All signals for the aromatic region faded with an increase in the reaction time, and few lignins remained in the pulp under severe oxidation conditions, similar to those used in the case of CAOSA. Some new groups, or C–H species in new chemical environments, were generated in the side chain region (Fig. S1†), as compared to the case of RMWL. Moreover, a majority of these groups with oxygen linkages, such as Cα or Cγ in the A structure, were oxidized to hydroxyl or aldehyde groups.24,25
Constituent changes in the YL
One of the important advantages of the CAOSA process is the waste production. The YL produced in this case was different from the waste produced by other techniques in terms of organic matter and other constituents. Because of the existence of excessive amounts of oxygen, the oxidation level of the dissolved fraction in the cooking solution was much higher than that in black liquor, and the former contained degradation products with a much smaller molecular weight.26 The qualitative study led to the conclusion that the benzene ring structure was severely ruptured, and LMW acids such as formic acid, acetic acid, and glycolic acid were formed.The total yield of the four main low molecular weight (LMW) acids tested by HPLC corresponded to around 50% of the organics in YL (Table 2), implying the potential to cogenerate formic acid/acetic acid, and even hydroxyl acids, in the CAOSA process. To achieve this goal, research on selectivity improvement of LMW acids should be further expanded.
| 1 h | 2 h | 3 h | 5 h | 10 h | |
|---|---|---|---|---|---|
| a Note: all the contents listed in the table are based on dry weight in YL. | |||||
| Formic acid/% | 9.76 | 10.82 | 10.92 | 12.73 | 12.05 |
| Acetic acid/% | 14.00 | 11.98 | 12.75 | 14.34 | 13.71 |
| Glycolic acid/% | 3.82 | 4.88 | 4.44 | 5.63 | 5.06 |
| 3-Hydracrylic acid/% | 2.45 | 3.87 | 3.65 | 4.86 | 4.85 |
| LMW acids/% | 30.04 | 31.56 | 31.76 | 37.55 | 35.68 |
| Other organics/% | 39.22 | 37.03 | 36.17 | 29.04 | 28.58 |
| Ash content/% | 30.69 | 31.41 | 32.12 | 33.41 | 35.77 |
Changes in the lignin structure in YL
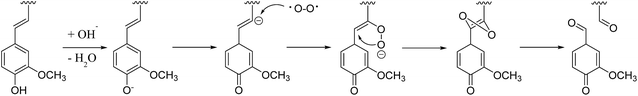
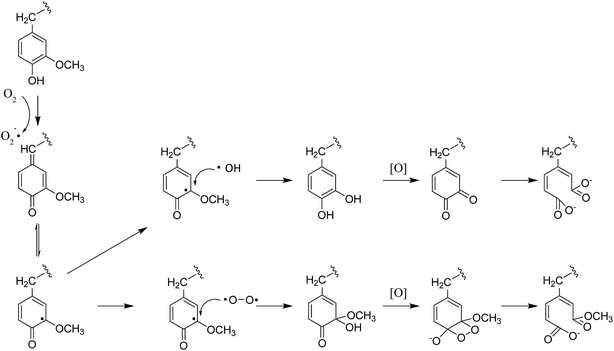
In the multiphasic system comprising solid-, liquid-, and gas-phase feedstocks, YL is the continuous phase for providing a contact medium for oxygen, biomass, and solid alkali. Thus the component changes in YL could provide much detailed information for studying the decomposition process. The HSQC NMR spectra of the aromatic region of the YL revealed that the number of signals and their intensities were much lower than those of RMWL or PMWL (Fig. 6). The rapid mass loss in the first cooking hour could be explained in terms of compounds that were peeled off from the bamboo bulk and subsequently dissolved and decomposed in the liquid phase instantly but not completely. Numerous structures in the bamboo bulk, such as the Aβ and B structures, disappeared once the cooking was started (Fig. S1†), indicating a possibility that after oxidation the fragments with these structures had better water solubility than native lignin. The disappearance of Aβ in YL before 3 h confirmed the decomposition of the β-O-4 ether bond (Fig. S2†). The fragment with a guaiacyl aromatic unit had even higher reactivity than that with a syringyl unit because of the higher decomposition rate. Cα in structure B is another reactive position, which indicated high cleavability of the α-O-4 ether bond to form an α-carbonyl group. By combining the information obtained from the pulp and YL, the cooking reaction could be described as a two-step process, in which an increasing number of compounds were initially transferred from the biomass raw material into the liquid phase and were further degraded with time.The fragments with an aromatic ring, which mainly originated from lignin, were deconstructed and decomposed. In addition, the benzene ring was cleaved continuously. As shown in Schemes 1 and 2, muconic acid is synthesized from the oxide degradation of the benzene ring, accompanied by the cleavage of the side chain between Cα and Cβ, as was also demonstrated by Yang et al.14 Thus, all the carboxylic acids generated from lignin or hemicellulose were strongly oxidized to organic acids, or even CO2.
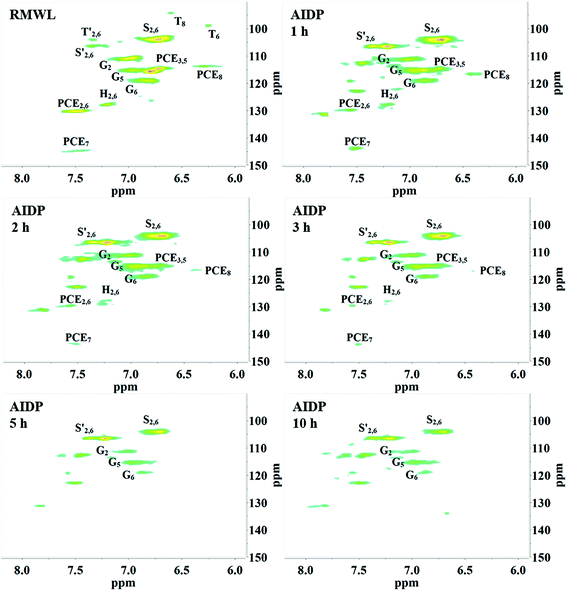
Interestingly, as the pH reached around 2.0, flocs were formed in the YL. The AIDPs were collected and characterized by 2D HSQC-NMR. Remarkably, the signal peaks in the AIDPs were similar to those in RMWL (Fig. 4, 7 and S3†). This observation indicated that the flocs might be the fragments with the original native lignin structure, which were soluble but stable in an alkaline oxidation system.
Moreover, the peaks indicated that the T structure in the AIDPs was fully degraded by the CAOSA process. Besides the signals for the methoxyl group and the carbons connected with oxygen directly, such as Aα and Aγ, which could be observed in PMWL, the Aβ structure remained in the YL precipitate, implying the existence of compounds with an β-O-4 ether bond. Given that a part of the β-O-4 unit could be preserved under such conditions, it might be possible to break the C–C bond in aromatic rings by selective oxidation to improve the yield of the biorefinery process. This could open up a new direction to the valorization of lignin.
After the AYL was lyophilized, hardly any signals attributed to the aromatic region were observed in the HSQC-NMR spectrum, implying an almost complete decomposition of aromatic compounds in the AYL (Fig. S4†). While little is known of the reason for precipitation as of now, it is probably a specific property of the compound, or a consequence of the residue being an acid-insoluble lignin. However, the method of acid precipitation was indeed found to be a promising way to recover aromatic compounds as well as to reduce the viscosity of the waste liquid in the pulping industry. Besides, the proportion of aromatic compounds in the total dried YL was less than 3% (w/w) and it decreased gradually with cooking time (Table 3), demonstrating superior delignification and breaking of the benzene ring.
| 1 h YL | 2 h YL | 3 h YL | 5 h YL | 10 h YL | |
|---|---|---|---|---|---|
| Dosage of YL/g | 50.00 | 50.00 | 49.98 | 49.99 | 49.89 |
| Initial pH | 8.06 | 8.09 | 8.10 | 8.04 | 7.90 |
| Final pH | 1.89 | 1.97 | 1.90 | 1.86 | 1.94 |
| Weight of AIDPs/g | 0.28 | 0.19 | 0.22 | 0.10 | 0.03 |
| Proportion in dry weight of YL/% | 3.05 | 1.52 | 1.74 | 0.82 | 0.24 |
Conclusion
As one of the bottlenecks in the biorefinery or pulping process, biomass pretreatment, especially the methods and mechanism of lignin degradation, would be worth analyzing. The remarkable performance of the improved CAOSA technology in the delignification process could be attributed to the destructuration and cleavage of the aromatic rings in lignin under severe oxidation conditions. Lignins were first peeled off from the lignocellulosic biomass and then broken down in the liquid phase. Few of the lignins were left in the biomass residue. In the YL, the dominant chemicals such as carboxylic acids and hydroxyl acids were the main degradation products. This study describes the development of a new CAOSA process for delignification and provides insights into the potential for application of lignin-derived products. However, further studies are needed for deciphering the details of the CAOSA ring opening of the aromatic rings in the biomass.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 21676223), the Fujian Provincial Development and Reform Commission, China (Grant No. 2015489), the Fundamental Research Funds for the Central Universities (Grant No. 20720160077, 20720160087, and 20720170062), the Natural Science Foundation of Fujian Province of China (Grant No. 2016J01077) and the Education Department of Fujian Province (Grant No. JZ160398).References
- Y. Jiang, X. Zeng, R. Luque, X. Tang, Y. Sun, T. Lei, S. Liu and L. Lin, ChemSusChem, 2017, 10, 3982–3993 CrossRef PubMed.
- M. Aresta, A. Dibenedetto and F. Dumeignil, Biorefinery: from biomass to chemicals and fuels, Walter de Gruyter, 2012 Search PubMed.
- S.-H. Yoon and A. Van Heiningen, Tappi J., 2008, 7, 22–27 Search PubMed.
- Y. H. Oh, I. Y. Eom, J. C. Joo, J. H. Yu, B. K. Song, S. H. Lee, S. H. Hong and J. P. Si, Korean J. Chem. Eng., 2015, 32, 1945–1959 CrossRef.
- C. S. Goh, K. T. Tan, K. T. Lee and S. Bhatia, Bioresour. Technol., 2010, 101, 4834–4841 CrossRef PubMed.
- Q. Yang, J. Shi, L. Lin, L. Peng and J. Zhuang, Bioresour. Technol., 2012, 123, 49–54 CrossRef PubMed.
- Y. Jiang, N. Ding, B. Luo, Z. Li, X. Tang, X. Zeng, Y. Sun, S. Liu, T. Lei and L. Lin, ChemCatChem, 2018, 9, 2544–2549 CrossRef.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CrossRef PubMed.
- Y. Jiang and L. Lin, Biotechnology & Business, 2017 Search PubMed.
- Y.-X. An, N. Li, H. Wu, W.-Y. Lou and M.-H. Zong, ACS Sustainable Chem. Eng., 2015, 3, 2951–2958 CrossRef.
- C. Peng, Q. Chen, H. Guo, G. Hu, C. Li, J. Wen, H. Wang, T. Zhang, Z. K. Zhao, R. Sun and H. Xie, ChemCatChem, 2017, 9, 1135–1143 CrossRef.
- X. Shen, P. Huang, J. Wen and R. Sun, Prog. Chem., 2017, 29, 162–180 Search PubMed.
- C. Pang, T. Xie, L. Lin, J. Zhuang, Y. Liu, J. Shi and Q. Yang, Bioresour. Technol., 2012, 103, 432–439 CrossRef PubMed.
- Q. Yang, J. Shi and L. Lin, Energy Fuels, 2012, 26, 6999–7004 CrossRef.
- T. Xie, L. Lin, C. Pang, J. Zhuang, J. Shi and Q. Yang, Carbohydr. Polym., 2013, 94, 807–813 CrossRef PubMed.
- P. C. Bruijnincx and B. M. Weckhuysen, Nat. Chem., 2014, 6, 1035–1036 CrossRef PubMed.
- S. D. Springer, J. He, M. Chui, R. D. Little, M. Foston and A. Butler, ACS Sustainable Chem. Eng., 2016, 4, 3212–3219 CrossRef.
- B. H. A. Sluiter, R. Ruiz, C. Scarlata, D. T. J. Sluiter and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass, National Renewable Energy Laboratory, Colorado, 2008 Search PubMed.
- S. Aziz and K. Sarkanen, Tappi J., 1989, 72, 169–175 Search PubMed.
- J.-L. Wen, S.-L. Sun, T.-Q. Yuan and R.-C. Sun, Green Chem., 2015, 17, 1589–1596 RSC.
- T. M. Liitiä, S. L. Maunu, B. Hortling, M. Toikka and I. Kilpeläinen, J. Agric. Food Chem., 2003, 51, 2136 CrossRef PubMed.
- F. Lu and J. Ralph, Plant J. Cell Mol. Biol., 2003, 35, 535–544 CrossRef.
- R. Samuel, M. Foston, N. Jaing, S. Cao, L. Allison, M. Studer, C. Wyman and A. J. Ragauskas, Fuel, 2011, 90, 2836–2842 CrossRef.
- J. Gierer, Wood Sci. Technol., 1985, 19, 289–312 Search PubMed.
- X. Zeng, F. Jin, J. Cao, G. Yin, Y. Zhang and J. Zhao, International Symposium on Aqua Science, 2010, vol. 1251, pp. 384–387 Search PubMed.
- Q. Yang, D. Huo, J. Shi, L. Lin, Q. Liu, Q. Hou, H. Zhang and C. Si, BioResources, 2015, 10, 7489–7500 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table of pulp yield with time and HSQC NMR spectra of the PMWL (side chain region), YL (side chain region), AIDPs (side chain region) and AYL (aromatic region). See DOI: 10.1039/c8se00181b |
| This journal is © The Royal Society of Chemistry 2018 |