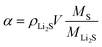Porous 3D graphene-based biochar materials with high areal sulfur loading for lithium–sulfur batteries†
Daoqing
Liu‡
 ab,
Qianwei
Li‡
c,
Jinbao
Hou
d and
Huazhang
Zhao
*ab
ab,
Qianwei
Li‡
c,
Jinbao
Hou
d and
Huazhang
Zhao
*ab
aDepartment of Environmental Engineering, Peking University, Beijing 100871, China. E-mail: zhaohuazhang@pku.edu.cn
bThe Key Laboratory of Water and Sediment Sciences, Ministry of Education, Beijing 100871, China
cState Key Laboratory of Heavy Oil Processing, Beijing Key Laboratory of Oil and Gas Pollution Control, China University of Petroleum, 18 Fuxue Road, Changping District, Beijing 102249, China
dCollege of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
First published on 10th August 2018
Abstract
The low electronic conductivity of sulfur and the high solubility of polysulfides seriously hinder the practical application of lithium–sulfur (Li–S) batteries. Therefore, the incorporation of sulfur into carbon-based materials is considered as a suitable solution. Here, a porous pomelo biochar/graphene composite (PBG) was prepared via a simple and green method combining hydrothermal carbonization and KOH activation. The obtained material was used as a host to encapsulate sulfur for the cathode of Li–S batteries, and the three-dimensional pore structure with enhanced conductivity is beneficial for the utilization of sulfur and absorption of soluble polysulfides. As a result, the PBG-S composite (63.3 wt% sulfur) delivered an initial discharge capacity of 1053 mA h g−1 at 0.1C (1C = 1675 mA g−1) and retained 418 mA h g−1 at 3C, even with a high sulfur loading of 4.0 mg cm−2. In addition, the performance of the composite was further improved by reducing the content of sulfur to an appropriate ratio in the PBG-S composite. The optimized PBG-S composite (48.6 wt% sulfur) exhibited a high initial discharge capacity of 1368 mA h g−1 at 0.1C and retained 638 mA h g−1 at 3C, and the discharge capacity remained as high as 664 mA h g−1 and 354 mA h g−1 even after the 200th and 600th cycles at 1C, respectively. The results indicated that PBG, with excellent electrochemical properties, is an ideal electrode material for lithium–sulfur batteries, and can also be prepared economically on an industrial scale.
Introduction
Lithium–sulfur (Li–S) batteries simultaneously exhibit high theoretical specific capacity (1675 mA h g−1) and energy density (2600 W h kg−1), and have received enormous attention recently and become a promising candidate for next-generation electrochemical energy storage systems.1–3 However, some drawbacks hinder the practical application of Li–S batteries, including the low electronic conductivity of sulfur and its discharge products, the “shuttle effect” induced by the high solubility of the polysulfides in the electrolyte,4 and the volume expansion during lithiation and delithiation processes. The low utilization of sulfur will lead to poor rate performance, while the loss of active materials will result in rapid capacity fading.5–7 Therefore, various strategies have been reported to tackle the problems mentioned above, such as designing new cathode nanostructures,8 functional modification of the separator,9 developing new electrolyte systems,10 and so on. Diverse porous carbon materials (e.g. activated carbon, carbon nanotubes, micro/mesoporous carbons, and graphene nanosheets) have been used as conducting and confining hosts for sulfur in Li–S batteries.Biomass is a sustainable natural resource that can be used to fabricate porous carbon materials.11–14 Porous carbon matrices prepared from bamboo biochar,15,16 olive stones,17 rice,18 soybean residue,19 lignosulfonate20 and silk cocoon21 have been sought to construct Li–S batteries with promising results.22 Biomass-derived porous carbons possess various morphologies, pore systems and conductive skeletons, which lead to different rate capabilities and cycling stabilities of the cathode. However, most of the biomass-derived porous carbons are amorphous with low conductivity.23 Therefore, it is a challenge to fabricate biomass-derived porous carbon with enhanced electrical conductivity, which not only provides abundant pores to adsorb polysulfides but also offers high conductivity to improve the utilization of sulfur. Graphene, as a 2D conductive material consisting of single layers of sp2 hybridized carbon atoms,24,25 has been utilized in many energy storage applications due to its unique properties.26–30 Therefore, the combination of biomass-derived porous carbon and graphene may indicate better performance due to the synergistic effect.31,32 It is reasonable to forecast that the composite of biomass-derived porous carbon and graphene is an ideal host for sulfur in commercial Li–S batteries with relatively low cost and good performance.33,34
Pomelos, as one of the popular fruits, consist of 44–45 wt% pomelo peel, which is considered as waste. Pomelo peel contains a foamy plant fiber, and a large amount of pectin, which could provide a wealth of oxygen-containing functional groups such as hydroxyl, carboxyl and amidogen, making it an economic resource for the production of biomass-derived porous carbons.35 Activated carbon originating from pomelo peel has been applied for wastewater treatment and as an electrode material for batteries.36
In this paper, a novel pomelo peel biochar integrated graphene composite (PBG) was applied as a cathode host for Li–S batteries. Pomelo peel was used as the precursor of biomass-derived porous carbon. 3D hierarchical porous carbon materials were obtained with the addition of GO sheets and then activated by KOH. The integrated graphene nanosheets provided a conductive network for porous carbon particles, which significantly enhanced the conductivity of the composite. When applied as a cathode host for lithium–sulfur batteries, the PBG-S composite exhibited excellent electrochemical performance, including high rate-performance and stable cyclability. The industrially scalable preparation method and excellent electrochemical properties of the graphene modified biomass carbon material (PBG) prove it to be an ideal and economical electrode material for lithium–sulfur batteries.
Experimental
Sample preparation
All of the reagents used were of analytical grade and were not subjected to any further treatment before use. Commercial activated carbon (AC) with product number C112242 was bought from Aladdin Industrial Corporation (Shanghai, China).Synthesis of PBG and PB
To prepare PBG, pomelo peel was used as a precursor of the carbon source. First, the pomelo peel was chopped into blocks and washed with distilled water. Then, the washed pomelo peel waste was sealed in a Teflon-lined autoclave with distilled water and heated at 180 °C for 24 h. After cooling to room temperature, the resulting sample was washed with distilled water and dried at 100 °C overnight. After that, the hydrothermally treated sample (5 g) was mixed with a GO dispersion (2 mg mL−1, 100 mL) and ammonia (25 wt%, 5 mL), and the resulting mixture was stirred for 2 h. Then, 15 g KOH was added slowly to the mixture and stirred for another 2 h. The mixture was poured into a PTFE plate and dried at 100 °C overnight. After that, the sample was activated and carbonized in a tube furnace at 800 °C for 1 h under an N2 atmosphere. Afterward, the resultant sample was thoroughly washed with the desired amount of HCl and distilled water, and then PBG was obtained by drying at 80 °C overnight.For comparison, the pure activated carbon material derived from pomelo peel (PB) was also synthesized without the addition of GO using the same hydrothermal and activation procedures as those for PBG.
Synthesis of AC-S, PB-S and PBG-S hybrids
The AC-S, PB-S and PBG-S hybrids were prepared by a melting diffusion method. 1 g sublimed sulfur and 0.5 g as-prepared samples (AC, PB and PBG) were mixed together. These mixtures were then sealed in Teflon containers and heated at 155 °C for 12 hours under an N2 atmosphere. After cooling to room temperature, the AC-S, PB-S and PBG-S composites were obtained.Sample characterization
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were recorded with a Merlin Compact (ZEISS, Germany) and JEM-2100F (JEOL, Japan), respectively. X-ray diffraction (XRD) measurements were conducted using a D/max-γB system (Cu Kα radiation, λ = 0.154178 nm, XRD, Rigaku, Japan). Raman spectra were measured with a Renishaw inVia Thermo Scientific DXRxi Raman imaging microscope employing a 532 nm laser beam. X-ray photoelectron spectroscopy (XPS) measurements were performed with a K-Alpha (Thermo Fisher Scientific company) which used an Al Kα monochromator. The pore structure characteristics of the porous carbon were measured with an ASAP2020 (Micromeritics, USA), and the SSA values and pore size distribution curves were obtained by Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) analyses of the isotherms, respectively. CO2 adsorption isotherms at 0 °C were measured using an Autosorb IQ (Quantachrome, USA), and the corresponding SSA values and pore size distribution curves were obtained by Density Functional Theory (DFT) analyses of the CO2 adsorption isotherms. Thermogravimetric (TG) analyses were carried out under an N2 atmosphere from room temperature to 500 °C on a Series Q600 instrument (TA Instruments, USA) to calculate the sulfur loading in the carbon/sulfur composites, and the heating rate was 10 °C min−1.Electrochemical measurements
The samples were mixed with carbon black and PTFE (dissolved in ethanol, 10 wt%) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The mixture was ground well in an agate mortar with the addition of ethanol, and was roll-pressed to produce a thin film. The film was punched into disks of 1.4 cm diameter with a loading of porous carbon–sulfur composite of ∼8.0 mg cm−2. The as-obtained film was heated at 60 °C for 12 h in a vacuum drying oven. 2025 coin cells using Li metal foil as the counter electrode and Celgard 2400 as the separator were assembled in an argon-filled glovebox with moisture and oxygen partial pressure under 1 ppm. The electrolyte was 1 M LiN (CF3SO2)2 (LiTFSI) dissolved in a mixture of 1,3-dioxolane (DOL) and dimethoxymethane (DME) (1
5. The mixture was ground well in an agate mortar with the addition of ethanol, and was roll-pressed to produce a thin film. The film was punched into disks of 1.4 cm diameter with a loading of porous carbon–sulfur composite of ∼8.0 mg cm−2. The as-obtained film was heated at 60 °C for 12 h in a vacuum drying oven. 2025 coin cells using Li metal foil as the counter electrode and Celgard 2400 as the separator were assembled in an argon-filled glovebox with moisture and oxygen partial pressure under 1 ppm. The electrolyte was 1 M LiN (CF3SO2)2 (LiTFSI) dissolved in a mixture of 1,3-dioxolane (DOL) and dimethoxymethane (DME) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume), and 0.1 M LiNO3 was added as an additive. LiTFSI and LiNO3 were dried at 110 °C in a vacuum drying oven overnight. DOL and DME were dried with 4Å molecular sieves for a week. Cyclic voltammetry (CV) was carried out on a CHI 660E electrochemical workstation (CH Instruments, Inc., Shanghai) in the potential range of 1.5–3.0 V (vs. Li/Li+) at different scan rates. Electrochemical impedance spectroscopy (EIS) was performed using a PARSTAT 2273 (Princeton Applied Research, USA) electrochemical system in the frequency range from 100 kHz to 10 mHz. Galvanostatic charge–discharge tests of the cells were conducted on a Neware battery-testing system (5 V, 5 mA & 50 mA) and the potential range was controlled between 1.7 and 2.8 V at room temperature.
1 by volume), and 0.1 M LiNO3 was added as an additive. LiTFSI and LiNO3 were dried at 110 °C in a vacuum drying oven overnight. DOL and DME were dried with 4Å molecular sieves for a week. Cyclic voltammetry (CV) was carried out on a CHI 660E electrochemical workstation (CH Instruments, Inc., Shanghai) in the potential range of 1.5–3.0 V (vs. Li/Li+) at different scan rates. Electrochemical impedance spectroscopy (EIS) was performed using a PARSTAT 2273 (Princeton Applied Research, USA) electrochemical system in the frequency range from 100 kHz to 10 mHz. Galvanostatic charge–discharge tests of the cells were conducted on a Neware battery-testing system (5 V, 5 mA & 50 mA) and the potential range was controlled between 1.7 and 2.8 V at room temperature.
Results and discussion
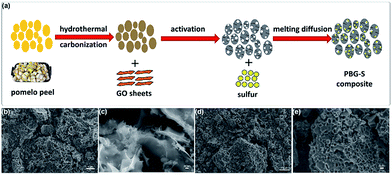
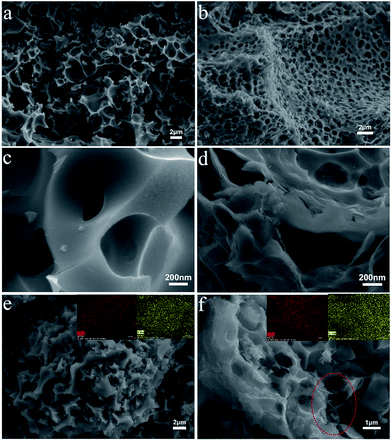
Here we introduced a simple and green strategy to synthesize a PBG-S composite as shown in Fig. 1a. First, the pomelo peel was carbonized in a Teflon-lined autoclave at 180 °C for 24 h via the hydrothermal approach. Second, the carbonized pomelo peel was mixed with GO sheets to get the hybrid precursor material. Third, a KOH chemical activation step was used to obtain PBG with the desired specific surface area (SSA) and conductivity. Finally, the as-prepared PBG was mixed with sublimed sulfur and heated under an N2 atmosphere, and the resultant composite was labeled as PBG-S. SEM images (Fig. 1c–e) of the PBG and PBG-S composites displayed a hierarchical carbon structure integrated with graphene.Fig. 2a–d show the SEM images of the PB and PBG materials. The 3D sponge-like morphology and porous structure were obtained after the carbonization and activation procedure. The carbon skeleton structure of PB displays a discontinuous granulated appearance, while PBG shows a relatively uniform pore structure with a honeycomb-like morphology. These SEM images indicated that the biomass-derived porous carbons integrated completely with graphene sheets in the PBG composite. The highly porous structure may facilitate electrolyte penetration and provide an ideal host to encapsulate sulfur. The microstructures of the PB-S and PBG-S composites, after grinding and melting diffusion of sulfur, are shown in Fig. 2e and f. Compared with Fig. 2a and b, the morphology of PB-S and PBG-S is similar to that of PB and PBG, and the submicron sulfur particles can hardly be seen on the surface compared with AC-S (Fig. S1†). The elemental maps (insets in Fig. 2e and f) show that sulfur is homogeneously distributed on the partial surface. These results confirm that sulfur was impregnated into the cavities of PB and PBG. TEM (Fig. 3a, c, and e) was further applied to substantiate the SEM result. Fig. 3a shows the TEM image of the PBG sample with an interconnected porous structure. Fig. 3c shows the edge of PBG at a higher magnification, which contains a highly curved or wrinkled surface. The high-resolution TEM images of PBG exhibit a sheet structure, and the corresponding hexagonal symmetry of diffraction spots crystal is characteristic of graphene sheets (Fig. 3e). Compared with PBG, the obtained PBG-S composite retains its original morphology with darker regions (Fig. 3b, d, and f).
 | ||
| Fig. 3 TEM images of PBG (a, c, and e), and TEM images and the corresponding elemental maps of PBG-S (b, d, and f). | ||
The EDS analysis (inset of Fig. 3f) shows that the S-rich map overlaps with the C-rich map. Furthermore, no reorganization of sulfur particles is observed on the surface of the PBG-S composite, indicating that sulfur is homogeneously dispersed in the pores of PBG, which is in agreement with the SEM images.
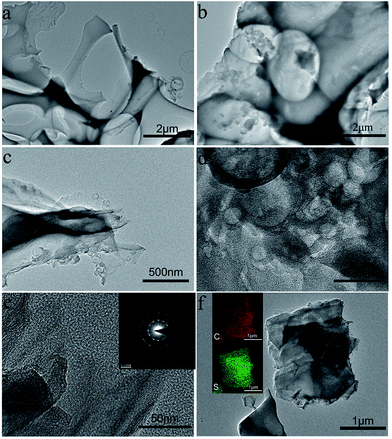
The N2 adsorption/desorption isotherms and the corresponding pore size distribution curves are shown in Fig. 4, and the porosity parameters derived from the isotherms are listed in Table 1. Commercial activated carbon AC and PB exhibit typical type I isotherms according to the IUPAC classification, indicating their microporous features.26 Comparatively, the isotherms of PBG show a combination of type I and type IV characteristics, suggesting a micro/mesoporous hybrid structure.37
 | ||
| Fig. 4 (a) N2 adsorption–desorption isotherms of AC, PB and PBG. (b) Pore size distribution curves of AC, PB and PBG. | ||
| Sample | S N2 [m2 g−1] | V t [cm3 g−1] | V BJH [cm3 g−1] | V BJH/Vtd | Pore sizee [nm] |
|---|---|---|---|---|---|
| a SSA calculated using the BET method from the N2 adsorption isotherm. b Single point adsorption pore volume at P/P0 = 0.97 from the N2 adsorption isotherm. c BJH adsorption cumulative volume of pores between 1.7 nm and 3.0 nm diameter. d Percentage of BJH adsorption cumulative volume based on single point adsorption pore volume from the N2 adsorption isotherm. e Adsorption average pore width (4V/A by BET) from the N2 adsorption isotherm. | |||||
| AC | 1085 | 0.5879 | 0.0777 | 0.132 | 2.16 |
| PB | 1988 | 0.9348 | 0.1634 | 0.174 | 1.88 |
| PBG | 1482 | 0.7606 | 0.1889 | 0.248 | 2.05 |
In addition, an H4-type hysteresis loop appeared in the isotherm of PBG, which indicates that PBG contains mesopores originating from the slits between graphene layers, and it is similar to many pure graphene materials.24 It can be seen that the mesopores centered at around 4 nm are correlated with the H4-type hysteresis loop. The BET SSA values of the AC, PB and PBG are 1085 m2 g−1, 1988 m2 g−1 and 1482 m2 g−1 (Table 1), respectively. The result indicates that the graphene layers in PBG actually reduce the surface area, and this is mainly due to the low SSA of restacked graphene nanosheets in the composite.
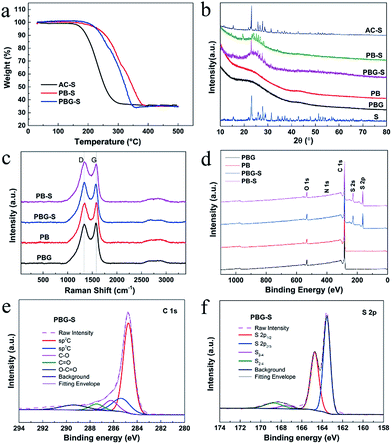
The sulfur loading content in the AC-S, PB-S and PBG-S composites was estimated using thermogravimetric (TG) curves recorded in a nitrogen flow as shown in Fig. 5a. It can be seen that obvious weight loss of AC-S, PB-S and PBG-S takes place between 200 and 400 °C, which is assigned to the evaporation of sulfur. The sulfur content of AC-S, PB-S and PBG-S is calculated to be 61.7 wt%, 64.4 wt% and 63.2 wt%, respectively, which are close to their theoretical proportions (66.6 wt%). In addition, the TG curves indicate that the speed of sulfur evaporation in PB-S and PBG-S is much lower than that in AC-S, which is possibly due to the strong confinement of sulfur within the abundant micro/mesopores of PB and PBG.
Fig. 5b shows the XRD patterns of the porous carbon PB, PBG, sublimed sulfur, and porous carbon-S composites PB-S, PBG-S, and AC-S. PBG shows a weak and broad (002) diffraction peak located at around 24°, corresponding to 0.37 nm interlayer spacing. However, the (002) diffraction peak of PB almost disappears, indicating an amorphous carbon structure. These differences indicated that the graphene nanosheets are integrated with pomelo peel-based biochar. The XRD pattern of the AC-S composite exhibits well-defined crystalline sulfur peaks, and is similar to that of sublimed sulfur. However, in the composites of PB-S and PBG-S, the intensity and number of characteristic peaks of sulfur decreased, indicating that most of the sulfur has entered the pores of PB and PBG, which is consistent with the results of SEM and TEM.
Fig. 5c shows the Raman spectra of PB, PBG, PB-S and PBG-S. The D-band at around 1350 cm−1 (corresponding to disordered graphitic carbon) and G-band at around 1590 cm−1 (corresponding to crystalline graphitic carbon) were present in the graphene-based materials. The intensity ratio of ID/IG is used to quantify the defect degree of carbon materials. The values of ID/IG for PB and PBG are calculated to be 2.67 and 2.41 according to the Lorentzian fitting results, and the difference is mainly due to the integrated graphene nanosheets in PBG. Sulfur peaks cannot be observed in the Raman spectra of PB-S and PBG-S, which further confirms that sulfur is embedded into the pores of PB and PBG.
XPS analysis reveals the surface chemical properties of PB, PBG, PB-S and PBG-S. The wide XPS spectra of PBG, PB, PBG-S, and PB-S are shown in Fig. 5d, and the surface elemental contents are listed in Table 2 and Table S1.† Peaks centered at around 284, 400, and 532 eV represent C 1s, N1s and O1s, respectively. The high-resolution C 1s spectrum in Fig. 5e consists of five components: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.7 eV), C–C (285.7 eV), C–O (286.7 eV), C
C (284.7 eV), C–C (285.7 eV), C–O (286.7 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.91 eV), and O–C
O (287.91 eV), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (290.3 eV). The presence of oxygen-containing functional groups in PBG-S is believed to play a key role in sulfur immobilization.38 N 1s shows a very weak signal and the content of N element is about 1 at% calculated from the XPS result. Two typical peaks of S 2s and 2p are detected in the wide XPS spectra, and the high-resolution spectrum of S 2p is shown in Fig. 5f. Two peaks located at 163.5 eV and 164.7 eV are assigned to S 2p3/2 and S 2p1/2 components, respectively. Furthermore, the additional peaks at higher binding energies of 169.3 and 168.1 eV arise from sulfur atoms located at the chain end of small S2–4 molecules.39 The XPS results confirm that sulfur has been dispersed in the pores of PBG to form small S2–4 composites. The pore size distribution obtained by the CO2 adsorption (Fig. S4 and Table S2†) shows that the micropores in PBG decreased dramatically after the melting diffusion of sulfur, and the small micropores (<0.8 nm) can effectively confine the small sulfur molecules (S2–4) according to previous reports.40 A summary of the surface elemental composition, carbon structure and electronic conductivity of AC, PB and PBG is listed in Table 1, and the method of testing electronic conductivity is according to a previous report.23 Compared with PB (7 S m−1) and AC (12 S m−1), the conductivity of PBG (143 S m−1) increases with the addition of GO nanosheets, suggesting that graphene layers play an important role in enhancing the electrical conductivity, which is consistent with the ratio of C/O and C 1s fitting results obtained using XPS (Fig. S3 and Table S1.†).
O (290.3 eV). The presence of oxygen-containing functional groups in PBG-S is believed to play a key role in sulfur immobilization.38 N 1s shows a very weak signal and the content of N element is about 1 at% calculated from the XPS result. Two typical peaks of S 2s and 2p are detected in the wide XPS spectra, and the high-resolution spectrum of S 2p is shown in Fig. 5f. Two peaks located at 163.5 eV and 164.7 eV are assigned to S 2p3/2 and S 2p1/2 components, respectively. Furthermore, the additional peaks at higher binding energies of 169.3 and 168.1 eV arise from sulfur atoms located at the chain end of small S2–4 molecules.39 The XPS results confirm that sulfur has been dispersed in the pores of PBG to form small S2–4 composites. The pore size distribution obtained by the CO2 adsorption (Fig. S4 and Table S2†) shows that the micropores in PBG decreased dramatically after the melting diffusion of sulfur, and the small micropores (<0.8 nm) can effectively confine the small sulfur molecules (S2–4) according to previous reports.40 A summary of the surface elemental composition, carbon structure and electronic conductivity of AC, PB and PBG is listed in Table 1, and the method of testing electronic conductivity is according to a previous report.23 Compared with PB (7 S m−1) and AC (12 S m−1), the conductivity of PBG (143 S m−1) increases with the addition of GO nanosheets, suggesting that graphene layers play an important role in enhancing the electrical conductivity, which is consistent with the ratio of C/O and C 1s fitting results obtained using XPS (Fig. S3 and Table S1.†).
| Sample | Surface chemical analysis (XPS, at%) | Carbon structure | Conductivity (S m−1) | ||
|---|---|---|---|---|---|
| C | O | N | I D/IG | ||
| PB | 90.78 | 8.38 | 0.74 | 2.67 | 7 |
| PBG | 92.45 | 6.74 | 0.81 | 2.41 | 143 |
| AC | 92.79 | 7.18 | 0.03 | 1.72 | 12 |
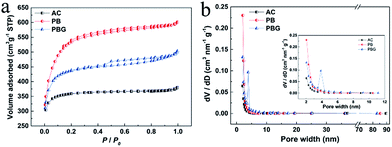
The electrochemical performance of the AC-S, PB-S and PBG-S composites as cathodes for Li–S batteries was investigated in a coin cell (CR2025) assembled with a lithium foil anode. Cyclic voltammetry (CV) curves were recorded to investigate the kinetic process of the PBG-S composite cathode in the first 4 cycles, and the potential range is 1.5–3.0 V and the scan rate is 0.2 mV s−1 (Fig. 6a). In the first cycle, there are two cathodic peaks located at 2.21 V and 1.83 V, corresponding to the reduction of S8 to polysulphides (Li2Sx, 2 < x ≤ 8) and conversion of polysulfides to the final discharge products Li2S2 and Li2S, respectively.41 The broad peak below 1.8 V could be attributed to the transformation from S2−4 to S2−.36,42 The anodic peak centered at 2.49 V is associated with the oxidation of Li2S2 and Li2S into the lithium polysulfides.43 In the subsequent scans, the spacing between the cathodic and anodic peaks decreases, indicating the enhanced reversibility of the sulfur cathode with cycling. As shown in Fig. S4,† several anodic peaks occurred from 2.2 to 3.0 V for the PB-S and AC-S composite cathodes. These peaks were located in a broad voltage range, suggesting poor charging efficiency and severe polarization due to the shuttle effect in Li–S batteries.44 The galvanostatic charge–discharge voltage profiles of the PBG-S cathode measured at 0.1C are shown in Fig. 6b. The discharge curves exhibit two typical plateaus consistent with the kinetic process of the electrochemical Li/S reaction described above. The PBG-S composite cathode demonstrates a high initial capacity of 1051 mA h g−1. The discharge capacity is around 900 mA h g−1 in the following several cycles, and there are two expected plateaus at around 2.3 V and 2.1 V corresponding to the CV curves, respectively. After 50 cycles, the discharge specific capacity is maintained at 572 mA h g−1.
 | ||
| Fig. 6 (a) First four cycles of CV curves of PBG-S at a scan rate of 0.2 mV s−1. (b) Charge/discharge profiles of the Li–S cell with the PBG-S composite cathode at 0.1C rate. | ||
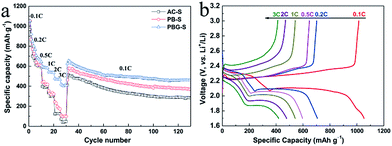
The rate capabilities and cycling performances of the AC-S, PB-S, and PBG-S composite cathodes are shown in Fig. 7a. PBG-S has the highest discharge capacity among the three samples, and PBG-S still delivers a capacity of 408 mA h g−1 when the rate is increased to 3C. Moreover, the discharge capacities of PB-S and AC-S are dramatically reduced to 41 mA h g−1 and 96 mA h g−1, respectively. When the rate is returned to 0.1C, the capacity of PBG-S recovers to 624 mA h g−1, which is significantly higher than the 426 mA h g−1 of PB-S and 410 mA h g−1 of AC-S. In the next 100 cycles at 0.1C rate, the capacity retention ratios of AC-S, PB-S and PBG-S are 56%, 69% and 73%, respectively. Above all, PBG-S exhibits enhanced rate capacity and cycling stability, which may benefit from the enhanced electrical conductivity due to integrated graphene nanosheets and hierarchical porous structure with good adsorption capacity for lithium polysulfides. The charge/discharge profiles of PBG-S cathode are recorded at different currents as shown in Fig. 7b, and the discharge capacity is determined to be 1053, 706, 595, 541, 469, 418 mA h g–1, respectively, which is achieved with a high sulfur loading of ∼4.0 mg cm–2.
The cycle performance of PBG-S (63.2 wt%) at a rate of 1C is shown in Fig. S4.† The long-term cycling stability of the electrode was also tested at higher rates of 1C after being activated at 0.25C for three cycles. After 50 cycles, the discharge capacity of the electrode dramatically decreased to 505 mA h g−1 at 1C, indicating a serious shuttle effect in Li–S batteries. After 600 cycles, the discharge capacity of the electrode remained at 299 mA h g−1 at 1C. The poor cycle performance is mainly due to the high sulfur content in the PBG-S (63.2 wt%) composite. Fig. S2† compares the morphology and elemental mapping results of the PBG-S cathode electrode before cycling and after 100 cycles at 1C. It is noteworthy that the sulfur signal became stronger in the cycled electrode, indicating that a serious shuttle effect occurred in the PBG-S (63.2 wt%) cathode electrode.
Electrochemical impedance spectroscopy (EIS) was carried out to examine the electrode kinetics. As shown in Fig. 8, the EIS spectra of the porous carbon–sulfur composites were measured before discharge and after 100 cycles. Before discharging, the EIS spectra are composed of a semicircle at the high and medium frequency region and an inclined line at the low frequency region, corresponding to the interfacial charge transfer and lithium polysulfide diffusion processes.44 After 100 cycles at 0.1C, the EIS spectra display two depressed semicircles and a short sloping line. The semicircle in the medium frequency area is associated with the solid-electrolyte-interface (SEI) film which is caused by the formation of Li2S (or Li2S2) on the carbon matrix in the cathode.45
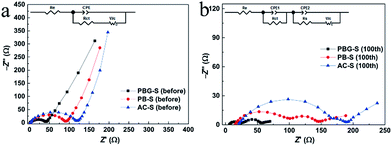 | ||
| Fig. 8 (a) EIS plots of the AC-S, PB-S and PBG-S composites before discharge and (b) after the 100th discharge at 0.1C. | ||
The resistance of the electrolyte (Re) and charge transfer resistance (Rct) can be inferred from the semicircle in the high frequency region, and Rs refers to the resistance in the SEI film, Wc refers to the Warburg impedance, and CPE refers to the constant phase element instead of capacitance.44,46 The fitting results of the EIS plots are shown in Table 3. It can be seen that the Re and Rct values of PBG-S (before discharge and after 100 cycles) are much smaller than those of AC-S and PBG-S, implying easy charge transportation in the electrochemical reactions of the PBG-S cathode as a result of the good conductivity.47
| Cycle number | Resistance | AC-S | PB-S | PBG-S |
|---|---|---|---|---|
| Before discharge | R e | 2.06 | 2.45 | 1.67 |
| R ct | 112.6 | 80.1 | 40.3 | |
| After 100 cycles | R e | 22.28 | 15.3 | 6.36 |
| R ct | 146.7 | 86.2 | 22.4 | |
| R s | 45.3 | 62.5 | 33.9 |
Therefore, the adequate mesopores induced by graphene nanosheets in PBG also result in better electrochemical kinetics. As concluded from above, the electrochemical properties of the cathode material are closely related to the conductivity and porosity parameters of the porous carbon. The utilization of the active material can be improved by the enhanced conductivity provided by the porous carbon host; therefore the capacity of PBG-S is higher than that of AC-S and PB-S. At the same time, the porous carbon with a hierarchical structure is more favorable for buffering the volume expansion of the active materials in the charge–discharge process, and the electrolyte can diffuse into the active materials more easily; consequently the charge transfer resistance becomes smaller and the rate performance of the materials can also be improved. In fact, the electrochemical performance of PBG-S is among the best reported for Li–S batteries based on biochar carbons, as shown in Table 4.
| Biochar | Initial capacity (mA h g−1) | Final capacity (mA h g−1) | Rate (mA g−1) | Areal S loading (mg cm−2) | S (wt%) | S BET (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Pomelo peel-graphene | 1053 | 459 (100th) | 167 | 4 | 63.2 | 1482 | This work |
| 1368 | 354 (600th) | 1675 | 4 | 48.6 | |||
| Cherry pits | 1148 | 400 (200th) | 1675 | 0.77 | 57 | 1662 | 48 |
| Bamboo carbon | 1295 | 756 (50th) | 160 | 1.4 | 50 | 792 | 16 |
| Bamboo leaves | 1487 | 707 (200th) | 1675 | 1.8 | 70.26 | 284 | 47 |
| Soybean residues | 1200 | 750 (100th) | 334 | 1.4 | 64 | 2690 | 19 |
| Soybean hulls | 1231 | 450 (200th) | 837 | 0.66 | 63 | 1232 | 49 |
| Olive stone | 930 | 670 (50th) | 100 | 0.76 | 80 | 587 | 17 |
| Silk cocoon | 1312 | 580 (100th) | 837 | 1 | 55.7 | 1540 | 21 |
| Mushroom | 1357 | 729 (100th) | 167 | 1.3 | 52 | 788 | 50 |
| Coir pith | 1350 | 609 (75th) | 167 | 0.75 | 50 | 1952 | 51 |
| Litchi shell | 1520 | 499 (200th) | 837 | 0.8 | 50 | 1438 | 52 |
| Corncob | 1100 | 720 (150th) | 558 | 1.2 | 40 | 2724 | 53 |
| Glucose | 1246 | 395 (100th) | 167 | 0.6 | 50 | 1246 | 54 |
| Apricot shell | 1277 | 710 (200th) | 334 | 0.8 | 53.5 | 2269 | 55 |
| Fish scales | 1400 | 1100 (20th) | 167 | ∼1 | 58.8 | 2441 | 56 |
Compared with the blank PBG carbon material with a BET surface area of 1482 m2 g−1, the BET surface area of the PBG-S composite (63.2 wt% sulfur) drastically decreases to 23 m2 g−1 (Fig. S6†). And the volume of the pores also decreases from 0.76 cm3 g −1 to 0.01 cm3 g−1. This also demonstrates that almost all the pores of porous carbon were filled with sulfur. If the sulfur loading is too high, the pore volume of activated carbon cannot afford the expansion of active material sulfur when it converts to low density Li2S, and this will result in serious capacity decay. The theoretical proportion of the maximum sulfur loading of activated carbon can be calculated using the following formula.
According to the formula, the theoretical sulfur loading of AC, PB and PBG should be 40.4 wt%, 51.8 wt% and 46.7 wt% (Table 5), respectively. Here, we apply a lower mass ratio of sulfur when using the melt-diffusion method (PBG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 5
S = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to get the carbon/sulfur composite, and the sulfur content in PBG-S is 48.6 wt%. The rate capabilities and cycling performances of PBG-S (48.6 wt%) composite cathodes are shown in Fig. 9a. The specific capacity of the PBG-S (48.6 wt%) cathode is significantly improved, and the initial discharge capacity reaches 1368.28 mA h g−1 at 0.1C, revealing an impressive 30% capacity increase compared to PBG-S (63.2 wt%). When the rate is increased to 3C, the PBG-S (48.6 wt%) cathode still delivers a capacity of 526 mA h g−1. When the rate is returned to 0.1C, the capacity of PBG-S recovers to 931 mA h g−1, and is maintained at 520 mA h g−1 after 470 cycles at 0.1C rate. The cycle performance of PBG-S (48.6 wt%) at a rate of 1C is shown in Fig. 9b. The long-term cycling stability of the electrode was also tested at a higher rate of 1C after being activated at 0.25C for three cycles. After 600 cycles, the discharge capacity of the electrode remained at 354 mA h g−1 at 1C, representing a fading rate of barely 0.107% with respect to the initial capacity per cycle. Moreover, in both tests, the average coulombic efficiency of the electrode is more than 95%. The cycle performance and specific capacity were greatly improved by optimizing the ratio of activated carbon and sulfur.
5) to get the carbon/sulfur composite, and the sulfur content in PBG-S is 48.6 wt%. The rate capabilities and cycling performances of PBG-S (48.6 wt%) composite cathodes are shown in Fig. 9a. The specific capacity of the PBG-S (48.6 wt%) cathode is significantly improved, and the initial discharge capacity reaches 1368.28 mA h g−1 at 0.1C, revealing an impressive 30% capacity increase compared to PBG-S (63.2 wt%). When the rate is increased to 3C, the PBG-S (48.6 wt%) cathode still delivers a capacity of 526 mA h g−1. When the rate is returned to 0.1C, the capacity of PBG-S recovers to 931 mA h g−1, and is maintained at 520 mA h g−1 after 470 cycles at 0.1C rate. The cycle performance of PBG-S (48.6 wt%) at a rate of 1C is shown in Fig. 9b. The long-term cycling stability of the electrode was also tested at a higher rate of 1C after being activated at 0.25C for three cycles. After 600 cycles, the discharge capacity of the electrode remained at 354 mA h g−1 at 1C, representing a fading rate of barely 0.107% with respect to the initial capacity per cycle. Moreover, in both tests, the average coulombic efficiency of the electrode is more than 95%. The cycle performance and specific capacity were greatly improved by optimizing the ratio of activated carbon and sulfur.
| Sample | α (g g−1) | Sulfur content (wt%) |
|---|---|---|
| AC | 0.6789 | 40.4 |
| PB | 1.079 | 51.8 |
| PBG | 0.8782 | 46.7 |
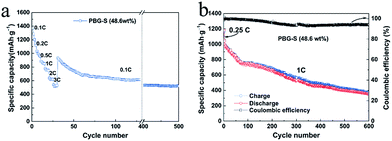 | ||
| Fig. 9 (a) Rate capabilities and (b) cycling performances of PBG-S composite cathodes with low sulfur content. | ||
Conclusions
This paper demonstrates a novel method of preparing a pomelo peel biochar integrated graphene composite (PBG) applied as a cathode host for Li–S batteries. The integrated graphene nanosheets provided a conductive network for porous carbon particles; thus the conductivity of the composite was enhanced. Due to the merits of a hierarchical porous structure and enhanced conductivity, the PBG-S composite (63.3 wt% sulfur) delivers an initial discharge capacity of 1053 mA h g−1 at 0.1C and retains 418 mA h g−1 at 3C, even with a high sulfur loading of ∼4.0 mg cm−2. By reducing the content of sulfur to an appropriate ratio, the optimized PBG-S composite (48.6 wt% sulfur) exhibits a high initial discharge capacity of 1368 mA h g−1 at 0.1C and retains 638 mA h g−1 at 3C, and the discharge capacity remains at 354 mA h g−1 even after the 600th cycle at 1C. The industrially scalable preparation method and excellent electrochemical properties of the graphene modified biomass carbon material (PBG) prove it to be a promising electrode material for lithium–sulfur batteries with low cost.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the China Postdoctoral Science Foundation (Grant No. 2018M630039), the Science Foundation of China University of Petroleum, Beijing (No. 2462017YJRC010), and the National Natural Science Foundation of China (No. 41701352) is acknowledged. The authors are grateful for the financial support is from Fund for Innovative Research Group of NSFC (grant No. 51721006), National Natural Science Foundation of China (grant No. 51578006).Notes and references
- L. Carbone, S. G. Greenbaum and J. Hassoun, Sustainable Energy Fuels, 2017, 1, 228–247 RSC.
- G. M. Zhou, L. C. Yin, D. W. Wang, L. Li, S. F. Pei, I. R. Gentle, F. Li and H. M. Cheng, ACS Nano, 2013, 7, 5367–5375 CrossRef PubMed.
- H. Bi, K. Yin, X. Xie, Y. Zhou, N. Wan, F. Xu, F. Banhart, L. Sun and R. S. Ruoff, Adv. Mater., 2012, 24, 5124–5129 CrossRef PubMed.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904–5908 CrossRef PubMed.
- M. K. Song, E. J. Cairns and Y. G. Zhang, Nanoscale, 2013, 5, 2186–2204 RSC.
- X. Y. Zhou, J. Xie, J. Yang, Y. L. Zou, J. J. Tang, S. C. Wang, L. L. Ma and Q. C. Liao, J. Power Sources, 2013, 243, 993–1000 CrossRef.
- L. F. Xiao, Y. L. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. M. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176–1181 CrossRef PubMed.
- K. Liao, P. Mao, N. Li, M. Han, J. Yi, P. He, Y. Sun and H. Zhou, J. Mater. Chem. A, 2016, 4, 5406–5409 RSC.
- J.-Y. Hwang, H. M. Kim, S. Shin and Y.-K. Sun, Adv. Funct. Mater., 2018, 28, 1704294 CrossRef.
- K. Jeddi, K. Sarikhani, N. T. Qazvini and P. Chen, J. Power Sources, 2014, 245, 656–662 CrossRef.
- G. Xu, J. Han, B. Ding, P. Nie, J. Pan, H. Dou, H. Li and X. Zhang, Green Chem., 2015, 17, 1668–1674 RSC.
- Q. W. Li, D. Q. Liu, Z. Jia, L. Csetenyi and G. M. Gadd, Curr. Biol., 2016, 26, 950–955 CrossRef PubMed.
- J. Wang, P. Nie, B. Ding, S. Dong, X. Hao, H. Dou and X. Zhang, J. Mater. Chem. A, 2017, 5, 2411–2428 RSC.
- G. Zhu, L. Ma, H. Lv, Y. Hu, T. Chen, R. Chen, J. Liang, X. Wang, Y. Wang, C. Yan, Z. Tie, Z. Jin and J. Liu, Nanoscale, 2017, 9, 1237–1243 RSC.
- J. J. Cheng, Y. Pan, J. A. Pan, H. J. Song and Z. S. Ma, RSC Adv., 2015, 5, 68–74 RSC.
- X. X. Gu, Y. Z. Wang, C. Lai, J. X. Qiu, S. Li, Y. L. Hou, W. Martens, N. Mahmood and S. Q. Zhang, Nano Res., 2015, 8, 129–139 CrossRef.
- N. Moreno, A. Caballero, L. Hernan and J. Morales, Carbon, 2014, 70, 241–248 CrossRef.
- Y. Zhong, X. Xia, S. Deng, J. Zhan, R. Fang, Y. Xia, X. Wang, Q. Zhang and J. Tu, Adv. Energy Mater., 2018, 8, 1701110 CrossRef.
- F. Chen, J. Yang, T. Bai, B. Long and X. Y. Zhou, Electrochim. Acta, 2016, 192, 99–109 CrossRef.
- L. Li, L. Huang, R. J. Linhardt, N. Koratkar and T. Simmons, Sustainable Energy Fuels, 2018, 2, 422–429 RSC.
- M. Xiang, Y. Wang, J. Wu, Y. Guo, H. Wu, Y. Zhang and H. Liu, Electrochim. Acta, 2017, 227, 7–16 CrossRef.
- M. K. Rybarczyk, H.-J. Peng, C. Tang, M. Lieder, Q. Zhang and M.-M. Titirici, Green Chem., 2016, 18, 5169–5179 RSC.
- L. Zhang, F. Zhang, X. Yang, G. K. Long, Y. P. Wu, T. F. Zhang, K. Leng, Y. Huang, Y. F. Ma, A. Yu and Y. S. Chen, Sci. Rep., 2013, 3, 1408 CrossRef PubMed.
- D. Q. Liu, Z. Jia and D. L. Wang, RSC Adv., 2016, 6, 36971–36977 RSC.
- D. Q. Liu, Z. Jia, J. X. Zhu and D. L. Wang, J. Mater. Chem. A, 2015, 3, 12653–12662 RSC.
- D. Q. Liu, Z. Jia and D. L. Wang, Carbon, 2016, 100, 664–677 CrossRef.
- D. Liu, Q. Li and H. Zhao, J. Mater. Chem. A, 2018, 6, 11471–11478 RSC.
- X. H. Xiong, G. H. Wang, Y. W. Lin, Y. Wang, X. Ou, F. H. Zheng, C. H. Yang, J. H. Wang and M. L. Liu, ACS Nano, 2016, 10, 10953–10959 CrossRef PubMed.
- Y. Xiang, Z. Chen, C. Chen, T. Wang and M. Zhang, J. Alloys Compd., 2017, 724, 406–412 CrossRef.
- Q. Baihua, J. Ge, D. Bo, L. Meihua, C. Weixiang and L. J. Yang, ChemElectroChem, 2015, 2, 1138–1143 CrossRef.
- M. X. Wang, Q. Liu, H. F. Sun, E. A. Stach, H. Y. Zhang, L. Stanciu and J. Xie, Carbon, 2012, 50, 3845–3853 CrossRef.
- L. B. Ma, W. J. Zhang, L. Wang, Y. Hu, G. Y. Zhu, Y. R. Wang, R. P. Chen, T. Chen, Z. X. Tie, J. Liu and Z. Jin, ACS Nano, 2018, 12, 4868–4876 CrossRef PubMed.
- T. Chen, Z. W. Zhang, B. R. Cheng, R. P. Chen, Y. Hu, L. B. Ma, G. Y. Zhu, J. Liu and Z. Jin, J. Am. Chem. Soc., 2017, 139, 12710–12715 CrossRef PubMed.
- T. Chen, B. R. Cheng, G. Y. Zhu, R. P. Chen, Y. Hu, L. B. Ma, H. L. Lv, Y. R. Wang, J. Liang, Z. X. Tie, Z. Jin and J. Liu, Nano Lett., 2017, 17, 437–444 CrossRef PubMed.
- J. Li, W. Liu, D. Xiao and X. Wang, Appl. Surf. Sci., 2017, 416, 918–924 CrossRef.
- J. Zhang, J. Y. Xiang, Z. M. Dong, Y. Liu, Y. S. Wu, C. M. Xu and G. H. Du, Electrochim. Acta, 2014, 116, 146–151 CrossRef.
- W. Sun, S. M. Lipka, C. Swartz, D. Williams and F. Q. Yang, Carbon, 2016, 103, 181–192 CrossRef.
- J. X. Song, T. Xu, M. L. Gordin, P. Y. Zhu, D. P. Lv, Y. B. Jiang, Y. S. Chen, Y. H. Duan and D. H. Wang, Adv. Funct. Mater., 2014, 24, 1243–1250 CrossRef.
- S. Z. Niu, G. M. Zhou, W. Lv, H. F. Shi, C. Luo, Y. B. He, B. H. Li, Q. H. Yang and F. Y. Kang, Carbon, 2016, 109, 1–6 CrossRef.
- Z. Li, L. X. Yuan, Z. Q. Yi, Y. M. Sun, Y. Liu, Y. Jiang, Y. Shen, Y. Xin, Z. L. Zhang and Y. H. Huang, Adv. Energy Mater., 2014, 4, 1301473 CrossRef.
- L. Ji, M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. Zhang, Energy Environ. Sci., 2011, 4, 5053–5059 RSC.
- J. Guo, Y. Xu and C. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef PubMed.
- Y. H. Qu, Z. A. Zhang, X. H. Zhang, G. D. Ren, Y. Q. Lai, Y. X. Liu and J. Li, Carbon, 2015, 84, 399–408 CrossRef.
- Y. S. Su and A. Manthiram, Electrochim. Acta, 2012, 77, 272–278 CrossRef.
- W. G. Wang, X. Wang, L. Y. Tian, Y. L. Wang and S. H. Ye, J. Mater. Chem. A, 2014, 2, 4316–4323 RSC.
- H. Shao, W. Wang, H. Zhang, A. Wang, X. Chen and Y. Huang, J. Power Sources, 2018, 378, 537–545 CrossRef.
- Y. Li, L. Wang, B. Gao, X. Li, Q. Cai, Q. Li, X. Peng, K. Huo and P. K. Chu, Electrochim. Acta, 2017, 229, 352–360 CrossRef.
- C. Hernandez-Rentero, R. Cordoba, N. Moreno, A. Caballero, J. Morales, M. Olivares-Marin and V. Gomez-Serrano, Nano Res., 2018, 11, 89–100 CrossRef.
- Y. Zhu, G. Xu, X. Zhang, S. Wang, C. Li and G. Wang, J. Alloys Compd., 2017, 695, 2246–2252 CrossRef.
- H. Wu, J. Mou, L. Zhou, Q. Zheng, N. Jiang and D. Lin, Electrochim. Acta, 2016, 212, 1021–1030 CrossRef.
- K. Balakumar, R. Sathish and N. Kalaiselvi, Electrochim. Acta, 2016, 209, 171–182 CrossRef.
- Z. J. Sun, S. J. Wang, L. L. Yan, M. Xiao, D. M. Han and Y. Z. Meng, J. Power Sources, 2016, 324, 547–555 CrossRef.
- Z. Geng, Q. Xiao, D. Wang, G. Yi, Z. Xu, B. Li and C. Zhang, Electrochim. Acta, 2016, 202, 131–139 CrossRef.
- F. Schipper, A. Vizintin, J. W. Ren, R. Dominko and T. P. Fellinger, Chemsuschem, 2015, 8, 3077–3083 CrossRef PubMed.
- K. Yang, Q. Gao, Y. Tan, W. Tian, L. Zhu and C. Yang, Microporous Mesoporous Mater., 2015, 204, 235–241 CrossRef.
- S. Zhao, C. Li, W. Wang, H. Zhang, M. Gao, X. Xiong, A. Wang, K. Yuan, Y. Huang and F. Wang, J. Mater. Chem. A, 2013, 1, 3334–3339 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the measurement of the electrical conductivity of the carbon products, C 1s XPS spectra of PB and PBG, and N2 adsorption–desorption isotherms of the PBG-S composite. See DOI: 10.1039/c8se00343b |
| ‡ Co-first author. |
| This journal is © The Royal Society of Chemistry 2018 |