Waste-minimised copper-catalysed azide–alkyne cycloaddition in Polarclean as a reusable and safe reaction medium†
Lorenzo
Luciani
a,
Emily
Goff
a,
Daniela
Lanari
b,
Stefano
Santoro
a and
Luigi
Vaccaro
 *a
*a
aLaboratory of Green Synthetic Organic Chemistry, Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy. E-mail: luigi.vaccaro@unipg.it
bDipartimento di Scienze Farmaceutiche, Università di Perugia, Via del Liceo, 1, 06123 Perugia, Italy
First published on 28th November 2017
Abstract
Herein we report the first example of a generally useful organic reaction, namely the copper-catalysed azide–alkyne cycloaddition, performed in a Polarclean/water mixture as a reaction medium. The process is very efficient, affording in 24 out of the 26 tested cases the desired triazole in quantitative yields. Product isolation is also very convenient, since the triazoles either precipitate or form a separate liquid phase, without the need to perform chromatographic separations. Moreover, since the metal catalyst is retained in the Polarclean/water phase, the catalyst/reaction medium can be easily reused for consecutive reaction runs, without an apparent loss in efficiency. This methodology is associated with very limited waste production, as evidenced by calculated E-factors in the range 2.6–3.7.
Introduction
It has been reported that the use of solvents accounts for the largest part of the waste generated in chemical productions.1 Moreover, life cycle assessments have concluded that most of the environmental footprints associated with the preparation of complex organic molecules, such as active pharmaceutical ingredients, are associated with the use of solvents.2 Thus, the choice of a particular reaction medium is expected to play a major role in determining the “greenness” of a chemical synthesis. This, in turn, justifies the great efforts devoted to the definition of novel chemical methodologies based on the use of alternative reaction conditions3 and to the replacement of common solvents with more sustainable substitutes.4 Most of the chemicals that find nowadays wide application as reaction media are derived from non-renewable fossil resources, which is obviously far from ideal from the point of view of sustainability. However, in recent years there has been growing interest in the development and in the application of bio-based solvents, i.e. chemicals derived from biomass resources used as reaction media.5 In this context, our research group pioneered the use of γ-valerolactone (GVL), a derivative of lignocellulosic biomass,6 as a promising substitute for dipolar aprotic solvents such as DMF, DMAc and NMP in transition-metal catalysed cross-coupling and C–H functionalisation reactions.7 In several cases the use of GVL resulted in additional benefits, aside from the expected advantages in terms of sustainability, such as a reduced metal leaching from the support when using a heterogeneous catalyst and consequently improved catalyst-recyclability.We have also been interested in the preparation of 1,2,3-triazoles and their chemistry,7c,8 where a central role is played by the widely-used copper-catalysed azide–alkyne cycloaddition (CuAAC).9 This process is well representative of these reactions that are typically conducted in a mixture of water and an organic solvent, such as alcohols (EtOH, MeOH, t-BuOH), tetrahydrofuran, dichloromethane or acetonitrile, and is generally catalysed by a Cu(I) species generated in situ from the reduction of a Cu(II) salt by ascorbic acid or sodium ascorbate.9
Very recently, within our research program aimed at the definition of new and safe reaction media, we have proposed the use of a furfuryl alcohol (FA)/water azeotrope, as an effective biomass-derived and easily recoverable reaction medium.8a The FA/water azeotrope proved to be crucial for the definition of a new protocol for CuAAC, very efficient both in terms of substrate scope and reactivity and of the minimization of the waste generated. This was evidenced by a calculated E-factor for a representative reaction as low as 4.3, when typical procedures have E-factors in the range of hundreds (Scheme 1).10
Looking for other chemicals that could find potential application as green reaction media, we reasoned that Rhodiasolv® Polarclean could be used as a substitute for commonly-employed polar solvents in organic reactions.11 Polarclean (Fig. 1), whose principal component is methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate, is commercially available and finds application as a solvent or co-solvent and crystal growth inhibitor in agrochemical formulations. It is miscible with water and has a boiling point of 278–282 °C and a melting point of −60 °C. Polarclean is industrially produced in a low carbon footprint process from methyleneglutarodinitrile (MDN), a by-product of Nylon 66 manufacturing, otherwise needed to be disposed.12
To the best of our knowledge Polarclean has found very little application as a reaction medium, limited to an example of metathesis polymerization,13 an attempt of its use, among many other solvents, in an olefin epoxidation reaction,14 and also for the fabrication of hollow fibre membranes.15
With the aim of investigating the potential applicability of Polarclean in useful organic transformations we decided to test it as a reaction medium in the above-mentioned CuAAC reaction. This would also allow for an easy and direct comparison of the process sustainability with known protocols and of course with our previously reported methodology based on the FA/water azeotrope.8a
Therefore, starting from this previous knowledge and also on the basis of our experience in the field, here we report the first example of an efficient waste-minimised CuAAC reaction performed in Polarclean as a reaction medium. To the best of our knowledge this is the first example of a successful use of Polarclean as a reaction medium in an organic chemical reaction.
Results and discussion
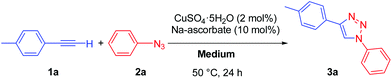
In CuAAC reactions the use of mixtures of water with a miscible organic solvent is justified to ensure an adequate solubility and mixing of the organic reagents and the required inorganic species (i.e. copper catalyst and additives, such as sodium ascorbate).We started our investigation by reacting representative 1-ethynyl-4-methylbenzene (1a) and azidobenzene (2a) and using 2 mol% of CuSO4·5H2O as a copper source together with 10 mol% of sodium ascorbate as a reductant (Table 1). First, we checked the solubility of the reaction mixture in Polarclean, which confirmed that inorganic reagents were not soluble. Still, the reaction proceeded at 50 °C for 24 h to give triazole 3a in 70% conversion, although the pure product could only be isolated by a classic procedure (aqueous work-up followed by column chromatography). When using a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Polarclean and water the reaction mixture was still partially heterogeneous and led only to a 50% conversion after 24 h at 50 °C.
1 mixture of Polarclean and water the reaction mixture was still partially heterogeneous and led only to a 50% conversion after 24 h at 50 °C.
Finally, optimal results were obtained when using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Polarclean and water (Table 1). This combination of a catalytic system and reaction medium proved extremely efficient in promoting the selective formation of the desired 1,4-disubstituted triazole 3a in 24 h and at a mild temperature (50 °C). Investigating further the substrate scope we found very satisfactory and consistent results. In fact, the protocol worked smoothly on all 26 tested combinations of aryl and alkyl acetylenes 1 with aryl and benzyl azides 2, affording the products in all cases in excellent yields (in 24 of the cases in quantitative yields) (Scheme 2). Importantly, the presence of halogens as substituents in aromatic rings of the azide and/or of the alkyne was in all cases well-tolerated, allowing for subsequent transformations of these functionalities. The protocol proved to be very general in terms of substrate scope, with product yields that are in most of the cases higher or comparable to those reported in the literature. We also tested our protocol in the preparation of six new triazoles (see the ESI† for the detailed list of products and for a comparison of product yields).
1 mixture of Polarclean and water (Table 1). This combination of a catalytic system and reaction medium proved extremely efficient in promoting the selective formation of the desired 1,4-disubstituted triazole 3a in 24 h and at a mild temperature (50 °C). Investigating further the substrate scope we found very satisfactory and consistent results. In fact, the protocol worked smoothly on all 26 tested combinations of aryl and alkyl acetylenes 1 with aryl and benzyl azides 2, affording the products in all cases in excellent yields (in 24 of the cases in quantitative yields) (Scheme 2). Importantly, the presence of halogens as substituents in aromatic rings of the azide and/or of the alkyne was in all cases well-tolerated, allowing for subsequent transformations of these functionalities. The protocol proved to be very general in terms of substrate scope, with product yields that are in most of the cases higher or comparable to those reported in the literature. We also tested our protocol in the preparation of six new triazoles (see the ESI† for the detailed list of products and for a comparison of product yields).
The reaction is operationally extremely easy, not requiring special conditions such as an inert atmosphere and allowing in general for an easy isolation of the pure products. Mixing of the reactants and the catalytic system (copper salt and reductant) in the Polarclean/water phase results in a clear homogeneous solution. Stirring the mixture at the reaction temperature (50 °C) results, with only one exception (product 3c), in the precipitation of the cycloaddition product 3. This can thus be isolated by simple filtration, and washing of the solid with water affords the pure product without the need for further purification. As mentioned in a single case among our tested reactions the product (3c) did not spontaneously precipitate upon formation, forming instead a separated liquid phase. This required a slightly modified isolation procedure, consisting of the separation of the two liquid phases and the addition of water to the oily product, which resulted in the immediate formation of a precipitate. Filtration and washing of the solid with water allowed isolating the pure product in still excellent yield (90%) (Scheme 2).
A fundamental aspect in the design of a truly sustainable methodology is the possibility to recycle the catalyst and the reaction medium, since this generally allows for a dramatic reduction in waste generation. To test to what extent our methodology allows for recycling, we repeated the representative reaction leading to the formation of product 3g on a larger scale (20 mmol), recovering and reusing the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Polarclean/water phase at the end of the process (Scheme 3). Initially we attempted the reuse of the medium by only adding fresh azide and alkyne reactants, without any further addition of catalysts or additives. However, this procedure resulted in slow conversion to the expected triazole. Reasoning that the poor reactivity could result from the oxidation of copper, rather than from its depletion from the Polarclean/water phase, we added additional 5 mol% of sodium ascorbate to the recovered reaction medium before performing a new run. This modified procedure allowed us to obtain quantitative yields (99%) of product 3g for at least five consecutive reaction runs (Scheme 3), proving the excellent recyclability of the catalyst/reaction medium system and supporting our hypothesis that copper is mostly retained in the Polarclean/water phase.
1 Polarclean/water phase at the end of the process (Scheme 3). Initially we attempted the reuse of the medium by only adding fresh azide and alkyne reactants, without any further addition of catalysts or additives. However, this procedure resulted in slow conversion to the expected triazole. Reasoning that the poor reactivity could result from the oxidation of copper, rather than from its depletion from the Polarclean/water phase, we added additional 5 mol% of sodium ascorbate to the recovered reaction medium before performing a new run. This modified procedure allowed us to obtain quantitative yields (99%) of product 3g for at least five consecutive reaction runs (Scheme 3), proving the excellent recyclability of the catalyst/reaction medium system and supporting our hypothesis that copper is mostly retained in the Polarclean/water phase.
In the context of green chemistry, a quantitative evaluation of the waste generated is essential to correctly evaluate the achieved advancement in terms of sustainability. We found that the cumulative E-factor calculated on the five consecutive reaction runs is as low as 3.7. The major contributors to this value are 4 mL of Polarclean/water eliminated as waste at the end of the fifth reaction run, and the 2 mL of water used after each reaction run to wash the solid product. However, it should be considered that the catalyst/medium system could likely be used for more than five runs, since even in the last one we observed full conversion to the desired product in 24 hours. Increasing the number of reaction runs, the E-factor could be further reduced to 2.6, which is the lowest value obtainable for each run considering full conversion and recovery of the catalyst/medium system (see the ESI† for all details about the recycling procedure and the calculation of the E-factors). Comparing these values with those reported in the literature for other CuAAC cycloaddition protocols, it can be clearly demonstrated that the procedure reported here is far superior in terms of reduced waste generation. Typical literature procedures, in fact, are associated with E-factors in the range of hundreds, without considering that usually the products are purified by column chromatography, which could easily result in an increase of the E-factors up to thousands.10 It is worth-mentioning that also our recently reported procedure based on the use of the recoverable FA/water azeotrope as a reaction medium8a is associated with a slightly higher E-factor (4.3), compared to the method reported here.
The Polarclean/water protocol features some evident advantages compared to our previously reported methodology. First, it allows for complete catalyst recycling, while in the FA/water protocol the copper salt had to be added in every reaction run. Second, the reaction medium is practically fully recovered at the end of the process by simple filtration of the solid products. When performing the reaction in FA/water, on the other hand, the azeotropic reaction medium had to be recovered by distillation, with an inevitable loss of material. This also contributes to the difference in waste generation between the FA/water and the Polarclean/water methodologies.
Conclusions
In conclusion, we have shown that a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Polarclean and water can be used as a reaction medium for the widely-used copper-catalysed alkyne–azide cycloaddition, providing the expected triazoles in excellent, and often quantitative, yields (26 different products prepared). In almost all the cases the product precipitates in the reaction medium and can thus be simply isolated by filtration, without the need for time-consuming and waste-generating chromatographic purifications. Moreover, the copper catalyst is retained in the Polarclean/water phase, which allows for an easy and convenient recycling of the catalyst/reaction medium system for several reaction runs, without an apparent loss of efficiency.
1 mixture of Polarclean and water can be used as a reaction medium for the widely-used copper-catalysed alkyne–azide cycloaddition, providing the expected triazoles in excellent, and often quantitative, yields (26 different products prepared). In almost all the cases the product precipitates in the reaction medium and can thus be simply isolated by filtration, without the need for time-consuming and waste-generating chromatographic purifications. Moreover, the copper catalyst is retained in the Polarclean/water phase, which allows for an easy and convenient recycling of the catalyst/reaction medium system for several reaction runs, without an apparent loss of efficiency.
To the best of our knowledge this is the first successful application of Polarclean as a reaction medium in an organic process of general interest. Moreover, this is the copper-catalysed alkyne–azide cycloaddition associated with the lowest generation of waste that we are aware of, as evidenced by the calculated E-factors.
Further experiments aimed at investigating the applicability of Polarclean as a reaction medium in other organic processes are currently ongoing in our laboratory, and will be published in due course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Università degli Studi di Perugia is acknowledged for financial support. S. S. gratefully acknowledges the MIUR for “Programma Giovani Ricercatori, Rita Levi Montalcini”, fellowship N. PGR123GHQY. Solvay is acknowledged for free samples of Rhodiasolv Polarclean.Notes and references
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- (a) C. Jiménez-González, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2004, 9, 114–121 CrossRef; (b) D. Cespi, E. S. Beach, T. E. Swarr, F. Passarini, I. Vassura, P. J. Dunn and P. T. Anastas, Green Chem., 2015, 17, 3390–3400 RSC.
- (a) Alternative Energy Sources for Green Chemistry, ed. G. Stefanidis and A. Stankiewicz, RSC Green Chemistry Series, Cambridge, 2016 Search PubMed; (b) Methods and Reagents for Green Chemistry: An Introduction, ed. P. Tundo, A. Perosa and F. Zecchini, John Wiley & Sons, Hoboken, 2007 Search PubMed.
- (a) F. M. Kerton and R. Marriott, Alternative Solvents for Green Chemistry, RSC Green Chemistry Series, Cambridge, 2nd edn, 2013 Search PubMed; (b) A. Jain, R. Vyas, A. Ameta and P. B. Punjabi, in Green Chemistry: Fundamentals and Applications, ed. S. C. Ameta and R. Ameta, CRC Press, 2013, pp. 161–198 Search PubMed; (c) C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC; (d) L. J. Diorazio, D. R. J. Hose and N. K. Adlington, Org. Process Res. Dev., 2016, 20, 760–773 CrossRef CAS; (e) D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC; (f) D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC; (g) D. Prat, O. Pardigon, H.-W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS; (h) P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC; (i) R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC; (j) J. H. Clark and S. J. Tavener, Org. Process Res. Dev., 2007, 11, 149–155 CrossRef CAS.
- (a) S. Santoro, F. Ferlin, L. Luciani, L. Ackermann and L. Vaccaro, Green Chem., 2017, 19, 1601–1612 RSC; (b) A. G. Corrêa, M. W. Paixão and R. S. Schwab, Curr. Org. Synth., 2015, 12, 675–695 CrossRef; (c) Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC; (d) I. T. Horváth, Green Chem., 2008, 10, 1024–1028 RSC.
- (a) P. Pongrácz, L. Kollár and L. T. Mika, Green Chem., 2016, 18, 842 RSC; (b) Z. Zhang, ChemSusChem, 2016, 9, 156–171 CrossRef CAS PubMed; (c) E. I. Gürbüz, J. M. R. Gallo, D. M. Alonso, S. G. Wettstein, W. Y. Lim and J. A. Dumesic, Angew. Chem., Int. Ed., 2013, 52, 1270–1274 CrossRef PubMed; (d) S. G. Wettstein, D. M. Alonso, Y. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199–8203 RSC; (e) Z.-Q. Duan and F. Hu, Green Chem., 2012, 14, 1581–1583 RSC; (f) L. Qi and I. T. Horváth, ACS Catal., 2012, 2, 2247–2249 CrossRef CAS; (g) I. T. Horváth, H. Mehdi, V. Fábos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238–242 RSC.
- (a) F. Ferlin, S. Santoro, L. Ackermann and L. Vaccaro, Green Chem., 2017, 19, 2510–2514 RSC; (b) D. Rasina, A. Kahler-Quesada, S. Ziarelli, S. Warratz, H. Cao, S. Santoro, L. Ackermann and L. Vaccaro, Green Chem., 2016, 18, 5025–5030 RSC; (c) X. Tian, F. Yang, D. Rasina, M. Bauer, S. Warratz, F. Ferlin, L. Vaccaro and L. Ackermann, Chem. Commun., 2016, 52, 9777–9780 RSC; (d) G. Strappaveccia, E. Ismalaj, C. Petrucci, D. Lanari, A. Marrocchi, M. Drees, A. Facchetti and L. Vaccaro, Green Chem., 2015, 17, 365–372 RSC; (e) G. Strappaveccia, L. Luciani, E. Bartollini, A. Marrocchi, F. Pizzo and L. Vaccaro, Green Chem., 2015, 17, 1071–1076 RSC; (f) E. Ismalaj, G. Strappaveccia, E. Ballerini, F. Elisei, O. Piermatti, D. Gelman and L. Vaccaro, ACS Sustainable Chem. Eng., 2014, 2, 2461–2464 CrossRef CAS.
- (a) D. Rasina, A. Lombi, S. Santoro, F. Ferlin and L. Vaccaro, Green Chem., 2016, 18, 6380–6386 RSC; (b) A. Marrocchi, A. Facchetti, D. Lanari, S. Santoro and L. Vaccaro, Chem. Sci., 2016, 7, 6298–6308 RSC; (c) G. D'Ambrosio, F. Fringuelli, F. Pizzo and L. Vaccaro, Green Chem., 2005, 7, 874–877 RSC; (d) D. Amantini, F. Fringuelli, O. Piermatti, F. Pizzo, E. Zunino and L. Vaccaro, J. Org. Chem., 2005, 70, 6526–6529 CrossRef CAS PubMed.
- (a) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210–216 CrossRef CAS PubMed; (b) V. O. Rodionov, V. V. Fokin and M. G. Finn, Angew. Chem., Int. Ed., 2005, 44, 2210–2215 CrossRef CAS PubMed; (c) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS; (d) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef . For recent applications, see:; (e) X. Zhang, S. Zhi, W. Wang, S. Liu, J. P. Jasinskid and W. Zhang, Green Chem., 2016, 18, 2642–2646 RSC; (f) S. Chassaing, V. Beneteau and P. Pale, Catal. Sci. Technol., 2016, 6, 923–957 RSC; (g) E. Haldon, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2015, 13, 9528–9550 RSC; (h) F. Alonso, Y. Moglie and G. Radivoy, Acc. Chem. Res., 2015, 48, 2516–2528 CrossRef CAS PubMed; (i) A. Qin, Y. Liu and B. Z. Tang, Macromol. Chem. Phys., 2015, 216, 818–828 CrossRef CAS; (j) H. Wu, H. Li, R. T. R. T. K. Kwok, E. Zhao, J. Z. Sun, A. Qin and B. Z. Tang, Sci. Rep., 2014, 4, 5107–5112 CrossRef PubMed; (k) M. Juríček, P. H. J. Kouwer and A. E. Rowen, Chem. Commun., 2011, 47, 8740–8749 RSC; (l) C. R. Becer, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900–4908 CrossRef CAS PubMed.
- Calculated as an average from procedures reported in ref. 9c and: (a) L. Rinaldi, K. Martina, F. Baricco, L. Rotolo and G. Cravotto, Molecules, 2015, 20, 2837–2849 CrossRef PubMed; (b) B.-Y. Lee, S. R. Park, H. B. Jeon and K. S. Kim, Tetrahedron Lett., 2006, 47, 5105–5109 CrossRef CAS ; see ref. 8a for details.
- (a) O. Jentzer and M. Guglieri, US Pat, US8735324, 2011 Search PubMed; (b) P. Baur, M. Steinbeck, I. Wetcholowsky, T. Auler, A. Daniels and R. Pontzen, US Pat, US9510589, 2011 Search PubMed; (c) O. Jentzer and M. Guglieri, US Pat, US9392785, 2014 Search PubMed; (d) H. B. Lopez and P. J. Porpiglia, US Pat, US2014031232, 2014 Search PubMed; (e) L. Iannotta, R. Pazhianur, K. M. Shanmuga, J. A. Latting and T. K. Woodall, US Pat, US2013145806, 2013 Search PubMed; (f) A. Randová, L. Bartovská, P. Morávek, P. Matějka, M. Novotná, S. Matějková, E. Drioli, A. Figoli, M. Lanč and K. Friess, J. Mol. Liq., 2016, 224, 1163–1171 CrossRef.
- http://www.rscspecialitychemicals.org.uk/docs/rsc-symposium/Sustainable-Solvents-Products-and-Process-Innovations_Thierry-Vidal_-RSC-Symposium-2012.pdf (accessed September 2017).
- T. Lebarbé, A. S. More, P. S. Sane, E. Grau, C. Alfos and H. Cramail, Macromol. Rapid Commun., 2014, 35, 479–483 CrossRef PubMed.
- A. Mouret, L. Leclercq, A. Mühlbauer and V. Nardello-Rataj, Green Chem., 2014, 16, 269–278 RSC.
- N. T. Hassankiadeh, Z. Cui, J. H. Kim, D. W. Shin, S. Y. Lee, A. Sanguineti, V. Arcella, Y. M. Lee and E. Drioli, J. Membr. Sci., 2015, 479, 204–212 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details, full characterization and NMR spectra of all compounds. See DOI: 10.1039/c7gc03022c |
| This journal is © The Royal Society of Chemistry 2018 |