 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improvements in Cd stable isotope analysis achieved through use of liquid–liquid extraction to remove organic residues from Cd separates obtained by extraction chromatography†
Katy
Murphy
*,
Mark
Rehkämper
,
Katharina
Kreissig
,
Barry
Coles
and
Tina
van de Flierdt
Department of Earth Science and Engineering, Imperial College London, London SW7 2AZ, UK. E-mail: k.murphy12@imperial.ac.uk; Tel: +44 (0)2075947140
First published on 7th August 2015
Abstract
Organic compounds released from resins that are commonly employed for trace element separations are known to have a detrimental impact on the quality of isotopic analyses by MC-ICP-MS. A recent study highlighted that such effects can be particularly problematic for Cd stable isotope measurements (M. Gault-Ringold and C. H. Stirling, J. Anal. At. Spectrom., 2012, 27, 449–459). In this case, the final stage of sample purification commonly applies extraction chromatography with Eichrom TRU resin, which employs particles coated with octylphenyl-N,N-di-isobutyl carbamoylphosphine oxide (CMPO) dissolved in tri-n-butyl phosphate (TBP). During chromatography, it appears that some of these compounds are eluted alongside Cd and cannot be removed by evaporation due to their high boiling points. When aliquots of the zero-ε reference material were processed through the purification procedure, refluxed in concentrated HNO3 and analyzed at minimum dilution (in 1 ml 0.1 M HNO3), they yielded Cd isotopic compositions (ε114/110Cd = 4.6 ± 3.4, 2SD, n = 4) that differed significantly from the expected value, despite the use of a double spike technique to correct for instrumental mass fractionation. This result was accompanied by a 35% reduction in instrumental sensitivity for Cd. With increasing dilution of the organic resin residue, both of these effects are reduced and they are insignificant when the eluted Cd is dissolved in ≥3 ml 0.1 M HNO3. Our results, furthermore, indicate that the isotopic artefacts are most likely related to anomalous mass bias behavior. Previous studies have shown that perchloric acid can be effective at avoiding such effects (Gault-Ringold and Stirling, 2012; K. C. Crocket, M. Lambelet, T. van de Flierdt, M. Rehkämper and L. F. Robinson, Chem. Geol., 2014, 374–375, 128–140), presumably by oxidizing the resin-derived organics, but there are numerous disadvantages to its use. Here we show that liquid–liquid extraction with n-heptane removes the organic compounds, dramatically improving quality of the Cd isotope data for samples that are analyzed at or close to minimum dilution factors. This technique is quick, simple and may be of use prior to analysis of other isotope systems where similar resins are employed.
1. Introduction
Recently, interest in the measurement of Cd isotopes has grown due to advances in mass spectrometry and the realization that there are resolvable isotopic differences in seawater, meteorites and other samples of geological, biological and anthropogenic origin. As natural terrestrial samples display only small isotopic differences, Cd isotopic compositions are most commonly reported using an ε notation as follows: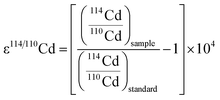 | (1) |
The dominant method for the determination of Cd isotope compositions applies multiple collector inductively coupled plasma mass spectrometry (MC-ICP-MS), with instrumental mass bias correction by the double spike technique.8,9 Prior to the measurements, Cd is separated from the sample matrix by a first stage of anion exchange chromatography, followed by extraction chromatography with Eichrom TRU resin for the removal of Sn.8–15
Various Eichrom resins, as well as similar homemade resin materials, are commonly employed for extraction chromatography in geochemical laboratories for the purification of numerous trace metals. Amongst users, it is common knowledge that the organic extraction agent (present as a coating on the resin particles) to some extent always elutes alongside the analyte element. This organic material can have a detrimental impact on isotopic analyses by TIMS (thermal ionization mass spectrometry) if it hinders the ionization process.16 For MC-ICP-MS, the presence of the organic residue appears to be relatively unproblematic for many analyses. In such cases, any effects caused by the organic material are presumably sufficiently small to be of no concern and this is supported by minimizing the amount of residue present, through pre-leaching the coated particles by storage in water or dilute acids and/or cleaning of the resin within the columns by acid elution directly prior to use.11,17
In some cases, however, problems can occur despite such precautions. In particular, Gault-Ringold and Stirling13 reported that the Cd isotope data for selected samples displayed a reproducibility and accuracy that was poor compared to that achievable for unprocessed standard solutions and concluded that this was caused by the organic residue that eluted alongside Cd from the TRU resin that was used in their chromatographic procedure. They furthermore suggested that this problem can be avoided if sufficiently large samples are processed on a single column. In this case, the final Cd sample solutions for isotopic analysis are diluted to a relatively large volume such that the eluted organic material is not present in concentrated form.13 In practice, however, this is often not possible. The concentration of Cd in seawater can be as low as 0.1 pg g−1 (or 1 pmol l−1), whilst other samples, such as meteorites, mineral separates or ferromanganese crusts, may only be available in small quantities.18
In cases where resin pre-rinsing or sample dilution is unable to circumvent the analytical problems that can be caused by organic resin residues, the material must be separated from the target element after extraction chromatography. To improve the quality of Cd and Nd isotope analyses following use of Eichrom resins, it was suggested that the organic material is best removed by oxidation of the residue with reagents such as HNO3, HNO3 with added H2O2, or HClO4.13,19,20 Of these, HClO4 was found to be the most effective oxidant,13,20 but there are many disadvantages to its use, such as various safety considerations, a high boiling temperature, and the availability of sufficiently pure supplies. Oxidation with HNO3 + H2O2, can also be successful but is more time consuming (24 hours).13 In the current study, we have developed an improved alternative method for the removal of organic resin residues from Cd sample solutions for subsequent isotopic analysis by MC-ICP-MS. The procedure avoids use of oxidising agents entirely, as the organic compounds are separated by liquid–liquid extraction with heptane, and may be applicable for improving the sample preparation procedures of other elements prior to isotopic measurements.
2. Method
2.1 Materials and reagents
All sample preparation work was carried out in class 10 (ISO 4) laminar flow hoods in the class 1000 (ISO 6) MAGIC clean room laboratory at the Department of Earth Science and Engineering, Imperial College London. Some acids were purified by sub-boiling distillation in either Teflon (12 M HCl) or quartz (6 M HCl, 16 M HNO3) stills, whilst Optima grade 9 M HBr was purchased from Fisher Scientific. The HBr–HNO3 mixtures were prepared on the day of use and all water was of 18.2 MΩ quality from a Milli-Q Academic dispensing system. The 111Cd–113Cd double spike, with 113Cd/111Cd = 0.5829, in 2 M HCl was prepared from solutions of enriched single isotopes purchased from Oak Ridge National Laboratory (USA) and characterized by MC-ICP-MS using external normalization relative to admixed Ag.8Heptane was pre-cleaned by carrying out a liquid–liquid extraction with 6 M HCl. Specifically, approximately 40 ml of Alfa Aesar 99% n-heptane was shaken by hand for 30 seconds with approximately 20 ml 6 M HCl in a 90 ml Savillex beaker and then left to stand for approximately 3 minutes. Once phase separation had been verified, the beaker was shaken again and allowed to stand for a further three minutes. The heptane was then transferred to a clean 90 ml Savillex beaker, being careful not to take up any of the underlying acid. This process was repeated twice for further purification.
2.2 Samples
Two different samples were used. The first, a pure solution of NIST SRM 3108 Cd (hereafter also referred to as NIST Cd), was employed so that complications arising from matrix effects due to the presence of other elements were avoided. Direct comparison between processed samples and unprocessed NIST Cd was enabled by using NIST Cd as the ε114/110Cd = 0 reference material to bracket the sample analyses. A second sample, the powdered ferromanganese nodule reference material Nod-A-1 from the USGS, was chosen as a natural material that is readily available. The Cd isotope composition of this material has been characterized previously as ε114/110Cd = 2.3 ± 0.6 (2SE, n = 2 (ref. 11)) and ε114/110Cd = 1.3 ± 0.2 (2SE, n = 2 (ref. 21)), possibly reflecting minor sample heterogeneity.2.3 Sample preparation
Aliquots of Nod-A-1 were digested and purified following the procedure described by Horner et al.11 In brief, this involved digestion of the sample powder with 6 M HCl, addition of double spike to obtain an optimum ratio of spike Cd/natural Cd of S/N ≈ 1,9 anion exchange chromatography with 200 μl Bio-Rad AG1-X8 resin (200–400 mesh size; for separation of Cd from the matrix) and then extraction chromatography with 200 μl Eichrom TRU resin (100–150 mesh size; primarily to remove any remaining Sn). The Eichrom TRU resin was pre-cleaned by shaking with 18.2 MΩ water and removing the foam that was produced. This was repeated 5–10 times (until no more foam appeared) and then stored in 18.2 MΩ water. Once settled in the columns the resin was cleaned with 9 ml 6 M HCl.11 As the NIST Cd solution is sufficiently pure to make the first stage of column chemistry redundant, samples using this solution were only processed through the second stage of the separation procedure before any attempts were made to remove the organic resin residue.2.4 Organic resin residue removal
At this point, the samples were further treated using one of four different protocols and therefore we differentiate between untreated, refluxed, and extracted samples, and samples that were both extracted and refluxed or refluxed and extracted (note the different order). Unless otherwise specified, each individual sample processed separately through the column chemistry contained approximately 30 ng of natural Cd plus 30 ng Cd from the double spike for a total of 60 ng Cd. Following the treatments described in Table 1, most samples were dissolved in 1 ml 0.1 M HNO3 to produce a 60 ng ml−1 solution for analysis by MC-ICP-MS. This approach highlights the problem caused by organic compounds eluted from the TRU resin, as it simulates the scenario where the Cd content of a sample suffices only for a single analysis (a ‘one shot’ sample), so that dilution of the organics is not possible.| Sample treatment protocol | Elution dried down | Dried with 1 drop 16 M HNO3 | Refluxeda | Dried down | Dissolved in 1 ml 0.1 M HNO3 | Extraction procedureb | Refluxeda | Dried down | Dissolved in enough 0.1 M HNO3 to produce a 60 ng ml−1 solution |
|---|---|---|---|---|---|---|---|---|---|
| a Refluxed in 0.5 ml 16 M HNO3 at 140 °C for 5 days. b Extraction procedure is as follows: 1.2 ml pre-cleaned n-heptane added to beaker. Shaken for 30 seconds, stood for 3 minutes, repeated once. Organic upper layer carefully removed with a pipette. 1.2 ml pre-cleaned n-heptane added. Shaken for 30 seconds, stood for 3 minutes, repeated once. Organic upper layer carefully removed with a pipette. Residual heptane allowed to evaporate at ambient temperature in a laminar flow hood for 30–60 minutes. c 1 ml 0.1 M HNO3. d Most refluxed samples contained 60 ng total Cd, but some had up to 240 ng. All samples, however, had S/N ≈ 1 and were dissolved in the appropriate amount of 0.1 M HNO3 to produce solutions with a total Cd concentration of 60 ng ml−1 for analysis. In one exceptional case, two samples, each with 30 ng total Cd dissolved in 0.5 ml 0.1 M HNO3, were combined to produce a single 1 ml solution with 60 ng ml−1 Cd. This approach enabled us to generate Cd solutions that featured variable dilution factors for the eluted organic resin compounds. | |||||||||
| Untreated | ✓ | ✓✓ | ✓c | ||||||
| Refluxed | ✓ | ✓ | ✓ | ✓ | ✓d | ||||
| Extracted | ✓ | ✓ | ✓c | ||||||
| Extracted and refluxed | ✓ | ✓ | ✓ | ✓c | |||||
| Refluxed and extracted | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓c | |
2.5 Mass spectrometry
All samples were analyzed on a Nu Plasma HR MC-ICP-MS (Nu Instruments Ltd, Wrexam, UK) at the MAGIC Laboratories. For sample introduction, either an Aridus I or Aridus II desolvating nebulizer system was used, fitted with a PFA nebulizer (CETAC Technologies) operating at a solution flow rate of about 120 μl min−1. The data acquisition procedures were similar to those outlined by Xue et al.9Solutions of spiked NIST Cd with S/N ≈ 1 and Cd concentrations similar to those of samples were repeatedly analyzed as bracketing standards throughout each measurement session, with between three and eight standards run between each sample. This allowed the stability of the instrument to be monitored, and any change in mass bias to be observed. Each analysis typically consumed about 30 ng natural Cd and 30 ng spike Cd, and a sensitivity of between 200 and 300 V (μg ml−1)−1 was normally achieved during the course of the study. All runs were bracketed by approximately four minutes of rinsing with 0.1 M HNO3.
The ε114/110Cd values of the samples were determined offline, by using the online collected raw ion beam intensities and electronic baselines as input to an Excel spreadsheet. The spreadsheet applies previously outlined iterative methods to correct the measured 112Cd/111Cd, 113Cd/111Cd, and 114Cd/111Cd isotope ratios for isobaric interferences and solve the double spike equations.8,9,22 The general power law is applied using a mass dependence of n = −0.1 to correct for instrumental mass fractionation.23
Calculations analogous to eqn (1) were performed to obtain the ε114/113Cd values of samples, relative to the results that were obtained for bracketing analyses of spiked solutions of NIST SRM 3108 Cd. In the majority of cases, the three or four analyses of the standard solution on either side of the sample were chosen. On occasions where the instrument was particularly stable, many more (up to 68) analyses of the standard solution were included.
Finally, the ε114/113Cd data were translated into ε114/110Cd using the relationship
 | (2) |
The exponent, β, was determined assuming the kinetic law
 | (3) |
Samples were excluded from the data set where either the internal (within-run) 2SE or the external (bracketing standards) 2SD uncertainties exceeded ±1 ε114/110Cd, except for occasional cases where the internal 2SE (typically better than ±0.8ε) was not unusually high for the measurement session. As unstable instrument behavior is problematic, Table 2 also highlights the number of sample measurements that were discarded relative to the total number of attempted analyses for the different sample treatments that were investigated. Such data selection was necessary to enable a reasonable evaluation of the results, as is further explained in the discussion below. The complete data set, including all discarded measurements, is provided in the ESI.†
| Sample | Source | Treatment after purification | Mean ε114/110Cd | 2SD | Number analyses included | Number analyses discardeda |
|---|---|---|---|---|---|---|
| a Analyses were excluded when either the internal precision (2SE) or the external precision (2SD) exceeded ±1 ε114/110Cd, unless the internal 2SE was not unusual for that particular instrument session (see text for details). b NIST Cd samples underwent just the second stage of the separation chemistry. c Nod-A-1 samples underwent both stages of the separation chemistry. d Reported uncertainty is 2SE, not 2SD. e Analysed by thermal ionisation mass spectrometry (TIMS). | ||||||
| NIST SRM 3108 Cdb | This study | None | −0.1 | 0.8 | 6 | 1 |
| This study | Refluxed | 4.6 | 3.4 | 4 | 4 | |
| This study | Extracted | 0.0 | 0.5 | 6 | 2 | |
| This study | Extracted and refluxed | 0.0 | 0.7 | 9 | 1 | |
| This study | Refluxed and extracted | 3.0 | 0.8 | 3 | 0 | |
| USGS SRM Nod-A-1c | This study | None | 2.5 | 3.4 | 2 | 1 |
| This study | Refluxed | 4.3 | 5.9 | 2 | 0 | |
| This study | Extracted (preferred value) | 1.7 | 0.5 | 6 | 0 | |
| This study | Extracted and refluxed | 2.1 | 0.7 | 2 | 0 | |
| This study | Refluxed and extracted | 3.6 | 1.2 | 2 | 0 | |
| Horner et al.11 | None | 2.3 | 0.6d | 2 | ||
| Schmitt et al.21 | Nonee | 1.3 | 0.2 | 2 | ||
| BAM-I012 | This study | No purification procedure | −13.2 | 0.7 | 15 | 0 |
| Abouchami et al.1 | No purification procedure | −13.3 | 0.4 | |||
Changes in the instrumental mass bias of Cd during measurements sessions were determined by monitoring changes in the 111Cd/114Cd ratio of the spike-sample mixtures and quantified in terms of the fractionation coefficient (sometimes also called beta factor, mass bias factor or fractionation factor), fKin which is applied in the exponential mass fractionation law:
 | (4) |
Typical procedural blanks contained <20 pg Cd. As the blank constitutes just 0.03% or less of the total Cd in a sample, no blank corrections were applied to the isotope compositions. Repeat measurements of the secondary Cd isotope standard BAM-I012 gave ε114/110Cd = −13.2 ± 0.7 (2SD, n = 15), which is in excellent agreement with the consensus literature value of −13.3 ± 0.4.1
In the following discussion, the quoted uncertainties for mean values refer to twice the standard deviation (2SD) of the individual sample results included in this average. The uncertainty of individual results refers to twice the standard deviation (2SD) obtained for bracketing runs of the NIST Cd standard.
3. Results and discussion
3.1 Isotope ratios and sensitivity
Analyses of NIST Cd samples all gave the correct isotope composition and a good overall reproducibility (ε114/110Cd = −0.1 ± 0.8, n = 6; Table 2). There was also no disruption to the isotope composition of following standards, but the observation of sudden shifts in fKin of up to 0.07 (Fig. 1) suggests that there are mass bias changes between samples and subsequent standard analyses.
The results for the Nod-A-1 samples also provide a mean that is in accord with reference data (Table 2) but the overall precision is unsatisfactory (ε114/110Cd = 2.5 ± 3.4, n = 2). Further details of these analyses can be found in the ESI.†
We also observed that many runs of refluxed NIST Cd clearly disturbed the instrumental running conditions, so that subsequent measurements of the bracketing NIST Cd standard yielded isotope compositions that were substantially offset from the runs immediately preceding the sample analysis. Such effects are most likely due to the presence of organic resin residue in the refluxed NIST Cd samples. In detail, the isotopic offset between the bracketing standard measurements was large enough, such that they displayed 2SD precisions exceeding ±1ε for about 50% of the refluxed NIST Cd samples (Table 2). In these cases, it was not possible to robustly calculate the ε114/110Cd values of the samples relative to the bracketing standards, and these measurements were, therefore, not included in calculation of the mean given in Table 2. The rational for this approach is that such analyses would also be recognized as problematic and discarded when ‘unknowns’ are analyzed.
Accompanying these unexpected Cd isotope compositions, we also observed that the fractionation coefficient fKin for the refluxed NIST Cd samples frequently (for 5 out of 8 sample analyses) differed substantially from the bracketing standards (Fig. 1). This was also the case for the refluxed sample of Nod-A-1 shown in Fig. 2, indicating that the mass bias behavior is different for refluxed samples and pure, unprocessed NIST Cd solutions.
The analyses of refluxed samples also revealed that erroneous Cd isotope compositions were consistently accompanied by a reduced instrumental sensitivity for Cd (Fig. 3). Refluxed NIST Cd samples with a total Cd content of 60 ng, had been processed by extraction chromatography (Table 2), refluxing with 16 M HNO3 and dilution with 0.1 M HNO3 to a Cd concentration of about 60 ng ml−1. Such samples produced an instrumental sensitivity for Cd that was up to almost 40% lower, compared to the Cd sensitivity recorded by the unprocessed bracketing standards (Fig. 3). When larger Cd samples were refluxed and diluted to larger volumes, thereby producing more favourable (larger) ratios of Cd to organic residue, the sensitivity improved and the measured isotope compositions were closer to the expected value of ε114/110Cd = 0. Results that were unbiased by the presence of organic resin residue were achieved when samples with at least 180 ng total Cd were processed and diluted to a Cd concentration of 60 ng ml−1 using at least 3 ml 0.1 M HNO3 (i.e. a dilution factor of three; Fig. 3).
The total blank for the procedure including the extraction was only about 10 pg Cd, and as this was less than 0.02% of the total Cd for most samples, no correction was applied to ε114/110Cd data. The low blank was not unexpected given that cleaned n-heptane was found to have undetectable levels of Cd.
Our results, therefore, indicate that the extraction method can provide near complete removal of the organic material from the aqueous phase. Our method is, furthermore, easy to implement, as no unusual reagents or instrumentation is required, and was found to be reliable on a routine basis. In addition, it is quick in its use, as a batch of 10 samples can be readily extracted in about 30 minutes.
3.2 Causes of poor analytical results
Most analyses of untreated and refluxed samples yielded inaccurate data combined with poor reproducibility. These effects are inferred to result from contamination of the Cd sample solutions by organic compounds, which are released from the Eichrom TRU resin that is used for purification. Eichrom TRU resin applies octylphenyl-N,N-di-isobutyl carbamoylphosphine oxide (CMPO) dissolved in tri-n-butyl phosphate (TBP)24 as active ingredients. These long chain organic compounds have boiling points of more than 480 °C (ref. 25) and hence they are not removed by evaporation from Teflon beakers, and oxidation with concentrated nitric acid is insufficient.Our findings are in general accord with the study of Gault-Ringold and Stirling.13 These workers found large shifts in the Cd isotope composition of bracketing standards after samples containing organic resin residue had been analysed. Gault-Ringold and Stirling13 also applied Cd purification by extraction chromatography with TRU resin and the mass spectrometric methods, including double spiking, were similar to those used here. To circumvent this problem, the authors suggested that samples should be oxidized with HClO4/HNO3 or H2O2/HNO3 mixtures and they show that samples treated in this manner yield accurate and precise results. This method appears to be successful because the treatment with such media likely oxidizes the majority of the organic material present, possibly largely to CO2 and H2O. Crocket et al.,20 who use Eichrom RE (CMPO dissolved in TBP but at a different ratio to that of TRU resin) and Ln resins to separate neodymium in fossil corals, found that organic compounds from the RE resin had a detrimental effect on Nd yields and that oxidation of the sample aliquot after the RE chemistry using HClO4 solved the problem. However, this approach can be further improved. Oxidation of the organic residue may be incomplete in some cases, and this is difficult to monitor. At the very least, even complete oxidation of CMPO and TBP will leave behind a small residue of H3PO4, which cannot be effectively removed from Teflon beakers by evaporation due to its high boiling point of 213 °C.
Gault-Ringold and Stirling13 concluded that the analytical artefacts stemming from the use of TRU resins were caused either by polyatomic interferences (e.g., organophosphorus compounds) or anomalous mass bias behavior, such as mass independent or non-exponential mass dependent isotope fractionation. The results of modelling that was carried out to study the impact of spectral interferences show (i) that differences in ε114/110Cd of approximately 3 to 6 ε-units can be produced by a 100 to 200 ppm increase in ion beam intensity at either mass 111 or 114, and (ii) that changes in Cd isotope compositions will exhibit a linear correlation with the magnitude of the interferences (see ESI†). Our data therefore indicate that the observed analytical artefacts are not due to spectral interferences, because the Cd isotope compositions of refluxed NIST Cd samples do not decrease linearly with dilution factor (Fig. 3).
The results obtained for such refluxed samples, however, cast further light on the processes responsible for the poor analytical results. The residual organic material, when only partially oxidised, has a clear impact on the instrumental sensitivity that is achieved for Cd (Fig. 3). A simple explanation of this would be that organic material alters the behaviour of the membrane of the desolvation unit that is used for sample introduction. However, as the reduction in sensitivity is accompanied by changes in the isotopic composition, we suggest that this is not the case and that a better explanation may be that the introduction of organic residue changes the conditions within the plasma and/or the plasma interface. Given that the organic matrix material has such a significant effect on sensitivity, it is conceivable that it can also impact the mass bias behavior to a sufficient extent to produce inaccurate results, despite use of the double spike technique. Indeed, unusual mass bias conditions (as quantified by fKin) are observed for about half of the bracketing NIST Cd standards that were analyzed after refluxed samples. Hence, we speculate that the poor data quality is most likely related to anomalies in the absolute mass bias and mass bias behavior that are caused by the presence of organic compounds. We demonstrate this to be feasible by modelling the effects of changing the mass dependence of the instrumental mass fractionation. To this end, we use the general power law (GPL)
 | (5) |
Under normal circumstances, our data reduction applies n ≈ −0.1,23 but other values may be more appropriate for samples that are contaminated with particular organic compounds. To test this, we recalculated the instrumental mass fractionation-corrected (or assumed true) isotope compositions of refluxed NIST Cd samples using a range of n values, relative to analyses of the bracketing standard, which were corrected with n = −0.1. For all refluxed NIST Cd samples, there is a linear relationship with negative gradient between n and ε114/110Cd (see ESI†). This implies that the inaccurate ε114/110Cd data for refluxed NIST Cd samples can, in principle, be corrected to accurate results simply by adjusting the n to a more appropriate value. In detail, the modelling shows that n values of between 0.2 and 0.6 are needed for such a correction (see ESI†). This finding has two main implications. Firstly, our results indicate that the presence of organic compounds generates a mass dependence of mass fractionation that is intermediate between the kinetic/exponential and power laws (n ≈ 0.2 to 0.6 for refluxed NIST Cd samples) whilst it is normally intermediate between the kinetic/exponential and equilibrium laws (e.g., n ≈ −0.1 for unprocessed NIST Cd). This suggests that the organic material induces a significant change in the processes that are responsible for the instrumental mass bias. Secondly, the range of calculated n values implies that the induced changes in mass bias and mass dependence are either not constant, or that the samples differ in their matrix content. Neither of these explanations can be ruled out at present. As changes in the mass dependence of isotope fractionation can produce both positive or negative artefacts, this process may also explain the deviations towards lighter isotope compositions seen by Gault-Ringold and Stirling.13
The sources of instrumental mass bias in MC-ICP-MS are not well understood, but the majority of the relevant processes are generally thought to occur either in the plasma or the interface region of the instruments. In the plasma, the radial spread of ions is mass dependent so the portion of the sample that passes through the aperture in the sample cone has a heavier isotope composition than the original sample.26,27 In addition the ionisation environment of the plasma can be altered by the presence of matrix, so the distribution of isotopes in the plasma can also change. This has the potential to have an impact on both instrumental sensitivity and isotope ratios, as under normal running conditions the plasma sampling depth (i.e. distance between the load coil and sampler cone) is kept constant.26 Between the sample and skimmer cones, ions gain kinetic energy by supersonic expansion. Lighter ions do not gain as much kinetic energy as heavier ions, and are therefore more likely to be deflected away from the beam axis when collisions with argon neutrals occur.27 Specifically for Cd, Kivel et al.28 showed that the Cd exiting the skimmer cone has a heavier isotope composition than the original sample, and that the outer part of the ion beam before the first lens features a lighter Cd isotope composition than the central region. Therefore, we speculate that the presence of organic compounds, which are produced by partial oxidation of organic material leached from Eichrom TRU resin, may change the distribution of isotopes in the plasma and thus alter both the extent and mass dependence of the instrumental mass bias.
4. Conclusion
This study provides further evidence that organic resin residues can have a significant detrimental impact on the reproducibility and accuracy of Cd isotope analyses by MC-ICP-MS. These organic residues are eluted from Eichrom TRU resin during Cd purification by extraction chromatography and do not evaporate, even when heated to high temperatures. When left untreated, the organic residue can cause the Cd isotope composition of samples to be isotopically heavier than expected, and this effect is even more severe (with analytical artefacts of up to ∼6.6 ε114/110Cd) when samples are refluxed with concentrated nitric acid prior to analysis. In such cases, the instrumental sensitivity for Cd is also reduced by up to ∼40%. Our results indicate that spectral interferences are unlikely to be the cause of these analytical artefacts. Rather, they are probably related to changes in the extent and mass dependence of the instrumental mass bias, which are induced by the presence of the resin-derived organic material. These analytical problems for Cd isotope measurements are circumvented by performing a quick and easy liquid–liquid extraction step using heptane, which removes resin-derived organic compounds from sample solutions. Given the efficiency of this method it should be particularly appropriate for analyses of samples with low Cd contents.It is common knowledge that traces of organic material are leached from the resins that are commonly used in isotope geochemistry for sample purification by ion exchange and extraction chromatography. In many cases, this leaching is not problematic and has no impact on data quality. However, as ever-more ambitious analytical targets are addressed, more analytical problems related to resin-derived organics may become apparent and knock-on effects may arise when the problematic resin is not used in the final purification stage. Although not yet tested on organic compounds eluted from resins other than Eichrom TRU, the principles of our liquid–liquid extraction technique should be applicable to and may solve problems associated with other resin types and isotopic analyses of other elements.
Acknowledgements
Funding for this research was provided by the Natural Environment Research Council (grant number NE/J021636/1). We thank the three anonymous reviewers for their constructive comments which helped improve the manuscript.References
- W. Abouchami, S. J. G. Galer, T. J. Horner, M. Rehkämper, F. Wombacher, Z. Xue, M. Lambelet, M. Gault-Ringold, C. H. Stirling, M. Schönbächler, A. E. Shiel, D. Weis and P. F. Holdship, Geostand. Geoanal. Res., 2013, 37, 5–17 CrossRef CAS.
- T. M. Conway and S. G. John, Geochim. Cosmochim. Acta, 2015, 148, 269–283 CrossRef CAS.
- S. Ripperger, M. Rehkämper, D. Porcelli and A. N. Halliday, Earth Planet. Sci. Lett., 2007, 261, 670–684 CrossRef CAS.
- C. Cloquet, J. Carignan, G. Libourel, T. Sterckeman and E. Perdrix, Environ. Sci. Technol., 2006, 40, 2525–2530 CrossRef CAS PubMed.
- B. Gao, Y. Liu, K. Sun, X. R. Liang, P. Peng, G. Sheng and J. Fu, Anal. Chim. Acta, 2008, 612, 114–120 CrossRef CAS PubMed.
- A. E. Shiel, D. Weis and K. J. Orians, Sci. Total Environ., 2010, 408, 2357–2368 CrossRef PubMed.
- F. Wombacher, M. Rehkämper, K. Mezger, A. Bischoff and C. Munker, Geochim. Cosmochim. Acta, 2008, 72, 646–667 CrossRef.
- S. Ripperger and M. Rehkämper, Geochim. Cosmochim. Acta, 2007, 71, 631–642 CrossRef CAS.
- Z. C. Xue, M. Rehkämper, M. Schönbächler, P. J. Statham and B. J. Coles, Anal. Bioanal. Chem., 2012, 402, 883–893 CrossRef CAS PubMed.
- F. Wombacher, M. Rehkämper, K. Mezger and C. Munker, Geochim. Cosmochim. Acta, 2003, 67, 4639–4654 CrossRef CAS.
- T. J. Horner, M. Schönbächler, M. Rehkämper, S. G. Nielsen, H. Williams, A. N. Halliday, Z. Xue and J. R. Hein, Geochem., Geophys., Geosyst., 2010, 11(4) CrossRef CAS.
- M. Gault-Ringold, T. Adu, C. H. Stirling, R. D. Frew and K. A. Hunter, Earth Planet. Sci. Lett., 2012, 341, 94–103 CrossRef.
- M. Gault-Ringold and C. H. Stirling, J. Anal. At. Spectrom., 2012, 27, 449–459 RSC.
- S.-C. Yang, D.-C. Lee and T.-Y. Ho, Geochim. Cosmochim. Acta, 2012, 98, 66–77 CrossRef CAS.
- M. Lambelet, M. Rehkämper, T. van de Flierdt, Z. Xue, K. Kreissig, B. Coles, D. Porcelli and P. Andersson, Earth Planet. Sci. Lett., 2013, 361, 64–73 CrossRef CAS.
- I. Croudace, P. Warwick, R. Taylor and S. Dee, Anal. Chim. Acta, 1998, 371, 217–225 CrossRef CAS.
- R. S. Pappas, B. G. Ting and D. C. Paschal, J. Anal. At. Spectrom., 2004, 19, 762–766 RSC.
- M. Rehkämper, F. Wombacher, T. J. Horner and Z. Xue, in Handbook of Environmental Isotope Geochemistry, ed. M. Baskaran, Springer-Verlag, Berlin, Heidelberg, 2011, ch. 8, pp. 125–153, DOI:10.1007/978-3-642-10637-8_8.
- A. E. Shiel, J. Barling, K. J. Orians and D. Weis, Anal. Chim. Acta, 2009, 633, 29–37 CrossRef PubMed.
- K. C. Crocket, M. Lambelet, T. van de Flierdt, M. Rehkämper and L. F. Robinson, Chem. Geol., 2014, 374–375, 128–140 CrossRef CAS.
- A. D. Schmitt, S. J. G. Galer and W. Abouchami, Earth Planet. Sci. Lett., 2009, 277, 262–272 CrossRef.
- C. Siebert, T. F. Nagler and J. D. Kramers, Geochem., Geophys., Geosyst., 2001, 2(7) CrossRef CAS.
- F. Wombacher and M. Rehkämper, J. Anal. At. Spectrom., 2003, 18, 1371–1375 RSC.
- Eichrom Technologies LLC, http://www.eichrom.com/eichrom/products, accessed June 2014.
- ChemSpider, Royal Society of Chemistry, CSID:139303, http://www.chemspider.com/Chemical-Structure.139303.html, accessed Mar 14, 2015.
- J. Barling and D. Weis, J. Anal. At. Spectrom., 2012, 27, 653–662 RSC.
- G. H. Fontaine, B. Hattendorf, B. Bourdon and D. Gunther, J. Anal. At. Spectrom., 2009, 24, 637–648 RSC.
- N. Kivel, I. Günther-Leopold, F. Vanhaecke and D. Günther, Spectrochim. Acta, Part B, 2012, 76, 126–132 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ja00115c |
| This journal is © The Royal Society of Chemistry 2016 |



