Synthesis and characterization of UV upconversion material Y2SiO5:Pr3+, Li+/TiO2 with enhanced the photocatalytic properties under a xenon lamp†
Jianhong Wu,
Yanjie Song,
Boning Han‡
,
Jun Wei‡,
Zhiren Wei‡ and
Yanmin Yang*
The Midwest universities comprehensive strength promotion project, Hebei Key Lab of Optic-electronic Information and Materials, College of Physics Science and Technology, Hebei University, Baoding 071002, China. E-mail: mihuyym@163.com; 1240154584@qq.com; Fax: +86 159 3028 4830; Tel: +86 159 3028 4830
First published on 18th May 2015
Abstract
The upconversion luminescence agents Y2SiO5:Pr3+, Li+, that can be effectively excited by the blue light from a xenon lamp 150 W with grating as its light splitter, are fabricated by a hydrothermal method with mesoporous molecular sieves of MCM-48 as the suitable silica source. The obtained emission spectra of Y2SiO5:Pr3+, Li+ and Y2SiO5:Pr3+, Li+/TiO2 excited by a xenon lamp indicate that the UV upconversion luminescence of Pr3+ can be effectively transferred to TiO2. The photocatalytic performances of Y2SiO5:Pr3+, Li+/TiO2 are evaluated by the degradation of methylene blue upon xenon lamp irradiation. The degradation rate of the methylene blue with Y2SiO5:Pr3+, Li+/TiO2 is 14 times as high as that with only TiO2.
1. Introduction
With the growth of worldwide industry, severe environmental contaminations have become a major concern of our society. Photocatalysis, as an environmentally friendly technique in utilizing clean, safe, and renewable solar energy to eliminate toxic organic substances in air and water, has attracted considerable attention.1–5 TiO2 is known as the most widely investigated photocatalyst due to its high oxidative efficiency, high chemical stability, non-toxicity and low-cost.6–8 Presently, the photocatalytic degradation activity of TiO2 is still low, mainly due to the poor solar energy utilization. As is well known, TiO2 with a bandgap of 3.2 eV can be only excited by photons with light wavelengths shorter than about 400 nm in the UV wavelength range, which accounted for about 4% of the solar radiation energy.9 It is of interest to find a TiO2-based photocatalyst which is sensitive to visible light (∼48%) and near infrared-light (∼44%) in order to make more efficient use of solar energy in practical applications. Numerous methods had been adopted, such as surface modification,10,11 metal or nonmetal elements doping,12–15 and the combination with a visible light excited semiconductor (narrow band-gap semiconductor such as CdS, CdSe, CdSSe) and so on.16–20Recently, the upconversion luminescence agent that could transform the visible-light and near infrared-light into the ultraviolet light to satisfy the genuine requirement of TiO2 catalysts became the investigated subject.21–23 It is generally known that the upconversion emission power p is proportional to ϕn(n ≥ 2) (ϕ for the excitation density). So the upconversion emission power p would quickly decreased in magnitude as the excitation density ϕ decreased. When the excitation density ϕ decreased to a certain threshold value, the upconversion emission power p is close to zero. This threshold value is very high for general materials, about 1 mW cm−2.24,25 So almost all of the upconversion luminescence emissions can only be achieved under the laser excitation. Although laser can be used as the driving source for photocatalysis, it may not be practical in view of its small excitation area.26–29 In this scenario, only the point light source such as fluorescent lamp or xenon lamp, and also the area light source such as sunlight irradiation are meaningful for application. But those sources are polychromatic light and the excitation density of the effective light is relatively lower compared with laser, it is difficult to achieve upconversion luminescence. Now let's compare the excitation density of fluorescent lamp and fiber laser. The illumination area (light spot) is fixed at 1 cm2, the power of fiber laser and fluorescent lamp is 1 W and the distance from light sources to samples is fixed at 10 cm. So, the energy density of excitation light of fiber laser is 1/π(10 × 0.22)2 = 65.7 mW cm−2 (0.22 is the divergence angle provided by manufacturers) and the energy density of excitation light of fluorescent lamp is 1/4π(10)2/20 = 0.04 mW cm−2 (20 is the effective wavelength width of the visible-light). The excitation density of the fiber laser is 1642 times as high as the fluorescent lamp.
To make upconversion materials used for photocatalysis, it is necessary to reduce the threshold of the energy density of excitation light. Simultaneously, the excitation range of the activation center should be as wide as possible so as to improve the utilization rate of the excitation light. Rare earth ions have been commonly considered as the luminescence center in upconversion process, for example Er3+, Ho3+ and Tm3+. Er3+ ions can produce efficient ultraviolet (UV) emissions under the excitation of green or blue lasers.30,31 Recently, some reports demonstrated that TiO2 photocatalysis behavior was extremely enhanced by doping Er3+ ions under visible light irradiation.32–39 However, it still raises the question of whether UV upconversion emissions result in the enhanced photoactivities because no emission spectra have been given under a xenon lamp excitation. If upconversion emission cannot be achieved under 150 W xenon lamp excitation (the minimum power excitation source for spectrometers), it is hardly realistic for upconversion materials to be used for photocatalysis. Our previous works have reported UV upconversion emission of Er3+ upon 1 W 532 nm LED and 300 mW 460 nm LED irradiation.40 So, it is possible for Er3+ doped upconversion material to be used in many fields, such as biology application and photocatalysis. However, the narrow absorption range in visible light region prevents its practical use in the fields of biology and photocatalysis. Y2SiO5:Pr3+, Li+ can emit a wide UV radiation of 260–360 nm under blue, green even red light excitation.41 E. L. Cates et al. have systemically investigated this material and successfully used it in microbial inactivation with two “cool white” 13 W compact fluorescent bulbs radiation.42 All of those provide a possible for Y2SiO5:Pr3+, Li+ to be used for photocatalysis.
In this work, in order to make TiO2 fully absorbed, Y2SiO5:Pr3+, Li+ was synthesized using mesoporous silica (MCM-48) as the silica source material. The obtained data showed that Y2SiO5:Pr3+, Li+ can be effectively excited by 496 nm light obtained by a 150 W xenon lamp spectrometer (Hitachi F-4600). Afterwards, Y2SiO5:Pr3+, Li+/TiO2 composites were prepared through the ultrasonic dispersion and liquid boiling method. The photocatalytic property of the sample was examined in detail by decomposing the methylene blue. The results demonstrated that the visible-light photocatalytic activity of TiO2 could be significantly enhanced in the presence of Y2SiO5:Pr3+, Li+.
2. Experimental section
2.1 Synthesis of MCM-48 powders
The homogeneous precipitation method was used for the preparation of mesoporous silica MCM-48. At first, cetyltrimethylammonium bromide (C16H33(CH3)3NBr, 0.0143 mol), ammonia solution (NH3·H2O, 24 mL), ethanol (CH3CH2OH, 100 mL) as well as deionized water (H2O, 240 mL) were mixed by stirring magnetically until the homogenous solution was obtained. Then, tetraethylorthosilicate (TEOS, 7.8 mL) was added dropwise into the above mixed solution and further stirred until the transparent sol was successfully formed. After filtration and lavation, the separated deposit was calcined at 550 °C for 5 h, and then cooled down to room temperature naturally.2.2 Synthesis of Y2SiO5:Pr3+, Li+ microcrystals
The various concentrations of Y2SiO5:Pr3+, Li+ microcrystals were synthesized via a hydrothermal method. Briefly, 0.03592 mol Y(NO3)3·6H2O, 0.00048 mol Pr(NO3)3·6H2O, 0.0018 mol Li2CO3 and 0.02 mol MCM-48 were dissolved into deionized water by the ultrasonic dispersion with 100 W output power ultrasound. Then, 0.12 mol NaOH solution which should control the mole ratio of n(OH−)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Y3+ + Pr3+ + Li+) for 3
n(Y3+ + Pr3+ + Li+) for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was added dropwise into the above suspension. After thorough stirring, the mixture was transferred into a 100 mL teflon lined stainless steel autoclave. The autoclave was sealed and maintained in an oven at 110 °C for 20 h, and then cooled down slowly to room temperature. The product was centrifuged and washed by deionized water for several times. After drying in air at 60 °C for 20 h, the particles were heated in a muffle furnace at 700–1300 °C for 4 h. At last, the desired white Y2SiO5:Pr3+, Li+ particles were obtained.
1 was added dropwise into the above suspension. After thorough stirring, the mixture was transferred into a 100 mL teflon lined stainless steel autoclave. The autoclave was sealed and maintained in an oven at 110 °C for 20 h, and then cooled down slowly to room temperature. The product was centrifuged and washed by deionized water for several times. After drying in air at 60 °C for 20 h, the particles were heated in a muffle furnace at 700–1300 °C for 4 h. At last, the desired white Y2SiO5:Pr3+, Li+ particles were obtained.
2.3 Preparation of Y2SiO5:Pr3+, Li+/TiO2 composites
Y2SiO5:Pr3+, Li+/TiO2 catalysts were prepared through the ultrasonic dispersion and liquid boiling methods in terms of the literature.43 In a typical procedure, Y2SiO5:Pr3+, Li+ and TiO2 powders (mole ratio for 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were added into a 200 mL beaker filled with 30 mL deionized water, adequately dispersed by ultrasound for 30 min. The suspended liquid was heated up to the boiling point and kept for 30 min. After filtration and lavation, the separated deposit was put into a crucible and heated in a muffle furnace. The temperature was controlled at 550 °C for 90 min.
2) were added into a 200 mL beaker filled with 30 mL deionized water, adequately dispersed by ultrasound for 30 min. The suspended liquid was heated up to the boiling point and kept for 30 min. After filtration and lavation, the separated deposit was put into a crucible and heated in a muffle furnace. The temperature was controlled at 550 °C for 90 min.
2.4 The evaluation of the photocatalytic performance
The photocatalytic performances of the obtained products were evaluated by the degradation of methylene blue (MB) in aqueous solution. In the experiment, 1.15 g of Y2SiO5:Pr3+, Li+/TiO2 composite was dispersed into a teflon lined stainless steel reactor containing 80 mL 10 mg L−1 MB aqueous solution. Prior to irradiation, the reactor was kept in dark for 12 h for establishing an adsorption–desorption equilibrium of MB on the surface of photocatalysts. Then the suspensions were irradiated using a 500 W xenon lamp. A cut off filter (λ > 400 nm) was fitted to the aforementioned light source to get the applicable light irradiation. After each irradiation, 6.0 mL of the MB aqueous solution was taken out for the absorbance measurements. The dye concentration was calibrated using the absorption peak at 664 nm of MB.2.5 Characterizations
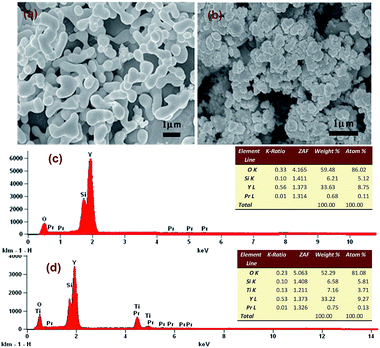
Phase identification was performed at ambient temperature by X-ray diffraction (XRD, Bruker Optics, Ettlingen, Germany) with Cu Kα radiation (40 kV, 40 mA). The emission (PL) spectra were measured by a spectrophotometer (Hitachi F-4600) equipped with a 150 W Xenon lamp source and double excitation monochromators. The morphology was characterized by SEM (JSM-7500FJEOL, Japan) with the operating voltage of 30.0 kV. Energy dispersive spectroscopy (EDS) was obtained on a JSM-7500F microscope coupled with an EDS spectrometer. The absorption spectra were recorded with a UV-NIR spectrophotometer (Hitachi U-4100). Fourier transformation infrared spectroscopy (FTIR, Tensor 27, Bruker, Germany) was employed to check the crystal and groups of samples. Similar photocatalysis experiments were carried out under the 500 W xenon lamp (CEL-LAX500) of sunlight to check the photocatalytic activity of the Y2SiO5:Pr3+, Li+/TiO2 catalysts.3. Results and discussion
3.1 Structural and morphological characterizations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ, λ = 0.154 nm, n = 1), the mesoporous channels calculated value of diameter d was 3.31 nm. The scanning electron image indicated that the average particle size of the as-prepared sample was about 400 nm.
θ = nλ, λ = 0.154 nm, n = 1), the mesoporous channels calculated value of diameter d was 3.31 nm. The scanning electron image indicated that the average particle size of the as-prepared sample was about 400 nm.
 | ||
| Fig. 1 XRD pattern and SEM image of the as-prepared MCM-48 powder and the XRD pattern of the standard data of MCM-48 powder. | ||
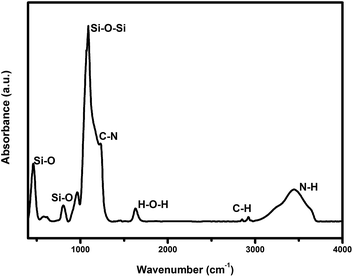
Fourier transformation infrared spectroscopy (FTIR) was carried out to investigate the composition of the sample, as shown in Fig. 2. The absorption band at 462.9, 811.9, 960.5 cm−1 could be attributed to the bending vibration, the stretching vibration as well as the asymmetric vibration of Si–O bond, respectively. Similarly, the Si–O–Si bond appeared at 1091 cm−1. The absorption bands at 1260–1620 cm−1 were attributed to the bending vibration of O–H bond of H2O, which was adsorbed on the internal holes from the surface of the mesoporous silica (MCM-48) in some ways. The other absorption bands, such as 1468, 1491, 2855, 2924 cm−1 and 3458 cm−1 were mainly derived from the vibration absorption of the quaternary ammonium surfactant (CTAB). It was shown that some organic residues still existed in the material.
From the obtained data, it can be concluded that the calcinations temperature at 1100 °C with the concentration of Li+ above 7% allowed obtaining more suitable β phase structure.
| (a) | ||
|---|---|---|
| Temperature °C | Phase(0% Li+ co-doped) | Phase(9% Li+ co-doped) |
| 700 | Un-crystallization | Un-crystallization |
| 800 | Un-crystallization | Un-crystallization |
| 850 | Un-crystallization | α Phase |
| 900 | α Phase | α Phase |
| 950 | α Phase | α Phase |
| 1000 | α Phase | β Phase |
| 1100 | α Phase | β Phase |
| 1200 | β Phase | β Phase |
| 1300 | β Phase | β Phase |
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Li+ concentration% | 0 | 5 | 7 | 8 | 9 | 10 | 11 | 15 |
| Phase 1100 °C | α | α | β | β | β | β | β | β |
3.2 Optical properties
3.3 Photocatalytic activity and mechanism
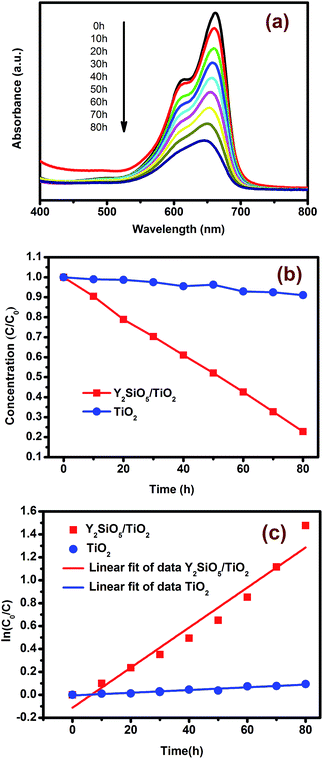
From the previous section, it can be seen that Y2SiO5:Pr3+, Li+ can be efficiently excited by a xenon lamp 150 W and UV upconversion emission can be efficiently transferred to TiO2. In this section, the decolorization of MB was carried out to verify the photocatalytic activity of Y2SiO5:Pr3+, Li+/TiO2 catalysts under visible-light by the 500 W xenon lamp. Fig. 6(a) showed the absorption spectra of MB catalyzed by the Y2SiO5:Pr3+, Li+/TiO2 composites as a function of irradiation time. With the increase of irradiation time, the strong absorption band at 664 nm decreased steadily. This result clearly demonstrated that the obtained composites could be used to decompose MB by visible light, as predicted. The changes in concentration of C/C0 with different exposure times were plotted in Fig. 6(b), where C0 was the original concentration of MB and C was the concentration of MB irradiated with a 500 W xenon lamp for time t. The degradation efficiency could be evaluated through the change of the MB concentrations before and after irradiation. Fig. 6(b) indicated that 80% of MB was decomposed after 80 h irradiation by Y2SiO5:Pr3+, Li+/TiO2 composites, which was far higher than that by pure TiO2. First-order fitting of the ln(C/C0) versus time for the photodegradation was usually used to study the reaction kinetics. Based on the photodegradation rate of MB with time, the photodegradation reaction kinetics lines were drawn out, as shown in Fig. 6(c). According to the reaction kinetics linear equation and correlation indexes: −ln(C/C0) = 0.01747t − 0.11227, R = 0.97520, −ln(C/C0) = 0.00119t − 0.00555, R = 0.96991, for Y2SiO5:Pr3+, Li+/TiO2 composites and pure TiO2, respectively, it clearly presented that the rate constant for Y2SiO5:Pr3+, Li+/TiO2 composites (0.01747) was about 14 times as high as that for pure TiO2 (0.00119). All of these results implied that the degradation reaction of MB in the visible light follows the first-order kinetic law.To explain the above phenomenon, the photocatalysis mechanism of Y2SiO5:Pr3+, Li+/TiO2 catalyst was discussed. In Y2SiO5:Pr3+, Li+/TiO2 composite, Pr3+ ion only exists in Y2SiO5 host. Fig. 7 showed the energy transfer upconversion (ETU) mechanism between neighboring Pr3+ ions and TiO2. The population of 3PJ states via blue photon absorption and the subsequent energy transfer between ions could promote an electron to the 4f5d energy band, resulting in the emission of UVB- and UVC-range photons which corresponding to 4f5d → 3HJ/3FJ.45,46 Then TiO2 was excited by the ultraviolet-light photon. The activated TiO2 produced reductive electrons (e−) and oxidative holes (h+) in the conduction band (CB) and the valence band (VB), respectively, and these electron–hole pairs migrated from the inner region to the surfaces to take part in surface reactions. Eventually, hydroxyl radical (˙OH), O22− and H+ were formed.47 The product can mineralize the organic chemicals and it could also destroy the chromophore position. Obviously, from the above photocatalysis mechanism, the photocatalytic activity of Y2SiO5:Pr3+, Li+/TiO2 composite depended strongly on the contact area between Y2SiO5:Pr3+, Li+ and TiO2 particles. To enhance the TiO2 photocatalysis behavior, it is necessary to enlarge the contact area between Y2SiO5:Pr3+, Li+ and TiO2 particles.
4. Conclusions
The upconversion luminescence agents that can be effectively excited by the ordinary illumination sources (fluorescent lamp or xenon lamp) have broad applying prospect in biology, medicine, environment and soon. In this paper, Pr3+ doped Y2SiO5 upconversion nanomaterials can be effectively excited by the blue light from a xenon lamp 150 W with grating as its light splitter. The obtained data indicated that UV upconversion emission can be effectively transferred to TiO2. All of these performances ensure the Y2SiO5:Pr3+, Li+/TiO2 composites to be a good visible light catalyst.Acknowledgements
This work was supported by National Science Foundation of China (no. 11474083), Hebei Province Department of Education Fund (ZD2014069).Notes and references
- S. Shen, J. Chen, X. Wang, L. Zhao and L. Guo, J. Power Sources, 2011, 196, 10112–10119 CrossRef CAS PubMed.
- G. Kim, S. H. Lee and W. Choi, Appl. Catal., B, 2015, 162, 463–469 CrossRef CAS PubMed.
- D. X. Xu, Z. W. Lian, M. L. Fu, B. Yuan, J. W. Shi and H. J. Cui, Appl. Catal., B, 2013, 142–143, 377–386 CrossRef CAS PubMed.
- Z. Zhang and W. Wang, Dalton Trans., 2013, 12072–12074 RSC.
- Y. He, Y. Wang, L. Zhang, B. Teng and M. Fan, Appl. Catal., B, 2015, 168–169, 1–8 CAS.
- Z. Gao, Z. Cui, S. Zhu, Y. Liang, Z. Li and X. Yang, J. Power Sources, 2015, 283, 397–407 CrossRef CAS PubMed.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS PubMed.
- A. Fujishima and X. Zhang, C. R. Chim., 2006, 9, 750–760 CrossRef CAS PubMed.
- Y. Chen, S. Mishra, G. Ledoux, E. Jeanneau, M. Daniel, J. Zhang and S. Daniele, Chem.–Asian J., 2014, 9, 2415–2421 CrossRef CAS PubMed.
- Q. Yin, R. Qiao, L. Zhu, Z. Li, M. Li and W. Wu, Mater. Lett., 2014, 135, 135–138 CrossRef CAS PubMed.
- L. W. Zhang, H. B. Fu and Y. F. Zhu, Adv. Funct. Mater., 2008, 18, 2180–2189 CrossRef CAS PubMed.
- Y. H. Ng, S. Ikeda, M. Matsumura and R. Amal, Energy Environ. Sci., 2012, 5, 9307–9318 CAS.
- J. Reszczyńska, T. Grzyb, J. W. Sobczak, W. Lisowski, M. Gazda, B. Ohtani and A. Zaleska, Appl. Catal., B, 2015, 163, 40–49 CrossRef PubMed.
- D. Hou, R. Goei, X. Wang, P. Wang and T. T. Lim, Appl. Catal., B, 2012, 126, 121–133 CrossRef CAS PubMed.
- A. Zaleska, P. Górska, J. W. Sobczak and J. Hupka, Appl. Catal., B, 2007, 76, 1–8 CrossRef CAS PubMed.
- X. Guo, C. Chen, W. Song, X. Wang, W. Di and W. Qin, J. Mol. Catal. A: Chem., 2014, 387, 1–6 CrossRef CAS PubMed.
- S. Guo, D. Li, Y. Zhang, Y. Zhang and X. Zhou, Electrochim. Acta, 2014, 121, 352–360 CrossRef CAS PubMed.
- C. Li, F. Wang, J. Zhu and J. C. Yu, Appl. Catal., B, 2010, 100, 433–439 CrossRef CAS PubMed.
- H. Wang, G. Wang, Y. Ling, M. Lepert, C. Wang, J. Z. Zhang and Y. Li, Nanoscale, 2012, 4, 1463–1466 RSC.
- Y. Zhu, Z. Chen, T. Gao, Q. Huang, F. Niu, L. Qin, P. Tang, Y. Huang, Z. Sha and Y. Wang, Appl. Catal., B, 2015, 163, 16–22 CrossRef CAS PubMed.
- Y. Li, K. Pan, G. Wang, B. Jiang, C. Tian, W. Zhou, Y. Qu, S. Liu, L. Feng and H. Fu, Dalton Trans., 2013, 7971–7979 RSC.
- X. Guo, W. Song, C. Chen, W. Di and W. Qin, Phys. Chem. Chem. Phys., 2013, 15, 14681–14688 RSC.
- J. Méndez-Ramos, P. Acosta-Mora, J. C. Ruiz-Morales, T. Hernández, M. E. Borges and P. Esparza, RSC Adv., 2013, 3, 23028 RSC.
- Y. Yang, C. Mi, F. Jiao, X. Su, X. Li, L. Liu, J. Zhang, F. Yu, Y. Liu, Y. Mai and J. Ballato, J. Am. Ceram. Soc., 2014, 97, 1769–1775 CrossRef CAS PubMed.
- Y. Yang, C. Mi, F. Yu, X. Su, C. Guo, G. Li, J. Zhang, L. Liu, Y. Liu and X. Li, Ceram. Int., 2014, 40, 9875–9880 CrossRef CAS PubMed.
- S. Huang, Z. Lou, Z. Qi, N. Zhu and H. Yuan, Appl. Catal., B, 2015, 168–169, 313–321 CrossRef CAS PubMed.
- X. Guo, W. Di, C. Chen, C. Liu, X. Wang and W. Qin, Dalton Trans., 2014, 1048–1054 RSC.
- W. Qin, D. Zhang, D. Zhao, L. Wang and K. Zheng, Chem. Commun., 2010, 46, 2304–2306 RSC.
- Y. Tang, W. Di, X. Zhai, R. Yang and W. Qin, ACS Catal., 2013, 3, 405–412 CrossRef CAS.
- F. Qin, Y. Zheng, Y. Yu, C. Zheng, H. Liang, Z. Zhang and L. Xu, J. Lumin., 2009, 129, 1137–1139 CrossRef CAS PubMed.
- H. Guo, Y. Li, D. Wang, W. Zhang, M. Yin, L. Lou and S. Xia, J. Alloys Compd., 2004, 376, 23–27 CrossRef CAS PubMed.
- J. Wang, R. Li, Z. Zhang, W. Sun, R. Xu, Y. Xie, Z. Xing and X. Zhang, Appl. Catal., A, 2008, 334, 227–233 CrossRef CAS PubMed.
- S. Obregon and G. Colon, Chem. Commun., 2012, 48, 7865–7867 RSC.
- L. Yin, Y. Li, J. Wang, Y. Zhai, J. Wang, Y. Kong, B. Wang and X. Zhang, Environ. Prog. Sustainable Energy, 2013, 32, 697–704 CrossRef CAS PubMed.
- S. L. Guangjian Feng, Z. Xiu, Y. Zhang, J. Yu, Y. Chen, P. Wang and X. Yu, J. Phys. Chem. C, 2008, 112, 13692–13699 Search PubMed.
- S. Li, Y. Guo, L. Zhang, J. Wang, Y. Li, Y. Li and B. Wang, J. Power Sources, 2014, 252, 21–27 CrossRef CAS PubMed.
- Y. Yang, C. Zhang, Y. Xu, H. Wang, X. Li and C. Wang, Mater. Lett., 2010, 64, 147–150 CrossRef CAS PubMed.
- Y. Zheng and W. Wang, J. Solid State Chem., 2014, 210, 206–212 CrossRef CAS PubMed.
- S. Obregón, A. Kubacka, M. Fernández-García and G. Colón, J. Catal., 2013, 299, 298–306 CrossRef PubMed.
- Y. Yang, C. Mi, X. Su, F. Jiao, L. Liu, J. Zhang, F. Yu, X. Li, Y. Liu and Y. Mai, Opt. Lett., 2014, 39, 2000 CrossRef CAS PubMed.
- E. L. Cates and J.-H. Kim, Opt. Mater., 2013, 35, 2347–2351 CrossRef CAS PubMed.
- E. L. Cates, M. Cho and J.-H. Kim, Environ. Sci. Technol., 2011, 45, 3680–3686 CrossRef CAS PubMed.
- B. Mitu, S. Vizireanu, R. Birjega, M. Dinescu, S. Somacescu, P. Osiceanu, V. Pârvulescu and G. Dinescu, Thin Solid Films, 2007, 515, 6484–6488 CrossRef CAS PubMed.
- E. L. Cates, A. P. Wilkinson and J.-H. Kim, J. Phys. Chem. C, 2012, 116, 12772–12778 CAS.
- S. L. Cates, E. L. Cates, M. Cho and J. H. Kim, Environ. Sci. Technol., 2014, 48, 2290–2297 CAS.
- C. L. Sun, J. F. Li, C. H. Hu, H. M. Jiang and Z. K. Jiang, Eur. Phys. J. D, 2006, 39, 303–306 CrossRef CAS.
- Q. Kang, Q. Z. Lu, S. H. Liu, L. X. Yang, L. F. Wen, S. L. Luo and Q. Y. Cai, Biomaterials, 2010, 31, 3317–3326 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06416c |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |