Thieno[2,3,a]carbazole donor-based organic dyes for high efficiency dye-sensitized solar cells†
Jian
Zhao
a,
Kazuaki
Oniwa
a,
Ashraful
Islam
*b,
Chuanjiang
Qin
b,
Naoki
Asao
a,
Liyuan
Han
b,
Yoshinori
Yamamoto
ac and
Tienan
Jin
*a
aWPI-Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan. E-mail: tjin@m.tohoku.ac.jp
bPhotovoltaic Materials Unit, National Institute for Materials Science, Tsukuba 305-0047, Japan. E-mail: ISLAM.Ashraful@nims.go.jp
cState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116023, China
First published on 20th January 2015
Abstract
A new series of donor–π-acceptor organic K-dyes based on the thieno[2,3,a]carbazole moiety as a new electron donor, trithiophene as a π-linker and cyanoacrylic acid as an electron acceptor were designed and synthesized for achieving high performances in dye-sensitized solar cells (DSCs). The substituent effect and the molecular planarity for photophysical properties and DSC performances of K-dyes have been investigated, and the highest power conversion efficiency of 7.4% has been achieved. EIS and IMVS were employed to study the effect of the molecular structure on the charge transfer process and electron lifetime.
Introduction
As one of the most promising next-generation photovoltaic technologies, dye-sensitized solar cells (DSCs) have attracted increasing attention in the past few decades due to their versatile, energy-saving, and environmentally friendly nature.1 Considerable efforts have been made for developing new TiO2 photosensitizers to enhance the photon-to-current efficiency (η) of DSCs. At present, the state-of-the-art DSCs based on ruthenium(II)-polypyridyl complex photosensitizers have an overall power conversion efficiency (η) approaching 12% under standard (Global Air Mass 1.5) illumination.2 However, the rarity and high cost of the ruthenium metal may limit their large scale applications. Metal-free organic dyes have attracted considerable attention in recent years due to their high molar extinction coefficients, flexible structural modifications, and low costs, though their conversion efficiencies are still insufficient for practical utilization.3 New molecular designs and the study of the structure–performance relationship toward increasing both the short-circuit current (Jsc) and open-circuit voltage (Voc) are a key point for achieving high DSC efficiencies.4The planar π-conjugated donor–π-linker-acceptor (D–π-A) dyes facilitate intramolecular charge transfer (ICT) from the donor to the acceptor that increases the molar extinction coefficient (ε) and broadens the ICT absorption spectra,5 though they often cause dye-aggregation and intermolecular charge transfer to reduce Voc.3d,6 On the other hand, the twisted organic dyes are favourable for suppression of dye-aggregation and charge recombination to increase the Voc, but they also hamper the efficient ICT, and thus reduce the Jsc.7 Recently, some D–π-A dyes with fused π-systems, such as dithienothiophene, cyclopentadithiophene, and dithienosilole with bulky substituents as a π-linker, have been developed to expand the absorption spectra, suppress charge recombination, and increase both Jsc and Voc simultaneously depending on the appropriate donor moieties.8 A new molecular design using a circular chain embraced D–π-A dye with planar π-conjugation has been developed by Han et al. to show high Jsc and Voc.9 Recently, we have succeeded in construction of a thienocarbazole framework by an efficient gold-catalysed cascade cyclization of aniline-tethered arenyl diynes.10 In our continuous efforts to enhance the DSC performance,11 we were interested in studying the new organic dyes based on the thienocarbazole as a donor which contains electron-donating carbazole and thienyl moieties. It was also expected that a linkage between a donor and a π-bridge with the thienyl moiety of thienocarbazole may increase the molecular planarity and hence enhance the ICT due to the efficient π-conjugation of the D–π-A molecule. In this study, we designed and synthesized a new series of D–π-A K-dyes based on thieno[2,3-a]carbazole as a new electron donor, trithiophene as a π-linker, and cyanoacrylic acid as an electron acceptor (Scheme 1). The planar molecule K-3 and K-4 dyes having bulky substituents on donor units showed the highest incident photon-to-current efficiency (IPCE) of 89% and enhanced ICT absorption. The K-5 photosensitizer having hexyl chains on the trithiophene π-linker has a twisted π-conjugated backbone, which showed a longer electron lifetime and the highest power conversion efficiency of 7.42% with a high Jsc and Voc.
Results and discussion
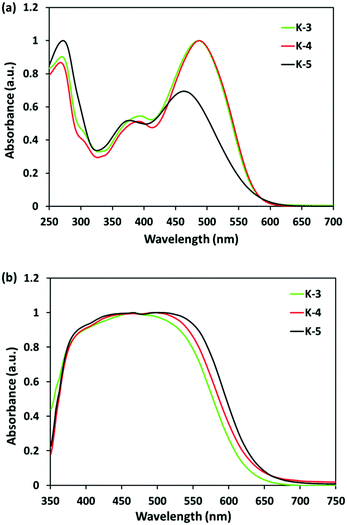
Three new K-dyes were designed by connecting the thienyl moiety of thieno[2,3-a]carbazole with the thiophene ring of trithiophene and introducing various substituents to the donor or the π-linker to systematically investigate the DSC performance versus the molecular structure. The synthesis routes of the K-dyes are shown in Scheme 1. Thieno[2,3-a]carbazole units 1a–c having varied substituents on the nitrogen atom were synthesized by our gold-catalysed cascade cyclization of aniline-substituted arenyl diynes.10 Sequential lithiation and boronation of the α-position of the thienyl moiety in 1a–c followed by the Suzuki–Miyaura coupling of the resulting boronates with the π-linker intermediates 2a and 2b afforded the corresponding aldehydes 3a–c in high yields. The resulting aldehydes 3a–c reacted with cyanoacetic acid in the typical Knoevenagel condensation to afford the desired K-dyes in high yields.The photophysical properties of K-dyes in CHCl3 solution and on the TiO2 film are summarized in Table 1. In solution, K-dyes exhibit two distinct absorption bands from 350 nm to 650 nm as shown in Fig. 1a. The absorption band in the range of 350–400 nm corresponds to the π–π* electron transition of the conjugated molecules and the band in the visible region can be assigned to the ICT from the thienocarbazole unit to the cyanoacrylic acid acceptor moiety. The ICT absorption bands of the K-3 and K-4 dyes are almost similar, which are red-shifted by about 20 nm compared with that of K-5. The K-5 dye exhibits a lower ε value relative to those of K-3 and K-4. These properties imply that the K-3 and K-4 dyes possess a higher planarity of the π-conjugated backbone in comparison with that of the K-5 dye having three hexyl chains on the π-linkage, which increase the probability of the ICT transition from the donor to the acceptor and the ε values. The K-dyes on the transparent TiO2 film show broad absorption spectra and the absorption onsets are red-shifted compared to those observed in solution, which indicate the existence of the intermolecular π–π interaction in K-dyes on the TiO2 film. The onsets of K-dyes extended up to 700 nm, which are desirable for harvesting the solar spectrum and may lead to a large photocurrent.
| Dye |
λ
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (nm) (ε × 104 M−1 cm−1) a (nm) (ε × 104 M−1 cm−1) |
λ
onset![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (nm) on TiO2 b (nm) on TiO2 |
IPc (eV) |
E
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (eV) d (eV) |
S
+/*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (eV) e (eV) |
|---|---|---|---|---|---|
| a Absorption maxima, measured in chloroform (2 × 10−5 M) at room temperature. b Absorption measured on a transparent 4 μm TiO2 film. c The ionization potential (IP) of absorbed dyes on the nanocrystalline TiO2 film was determined by using a photoemission yield spectrometer (Riken Keiki, AC-3E). d E 0–0 was determined from the onset of the absorption on TiO2. e The excited-state oxidation potential, S+/* levels were calculated from the expression S+/* = IP + E0–0. | |||||
| K-3 | 486 (4.2) | 670 | −5.70 | 1.93 | −3.77 |
| K-4 | 488 (4.3) | 700 | −5.66 | 1.87 | −3.79 |
| K-5 | 464 (3.4) | 700 | −5.45 | 1.87 | −3.58 |
The ionization potential (IP) of K-dyes bound to the nanocrystalline TiO2 film was measured using a photoemission yield spectrometer (Riken Keiki, AC-3E) (Table 1, Fig. S1 in ESI†). The IP values which correspond to ground-state oxidation potentials (S+/0) were found to be −5.70 eV for K-3, −5.66 eV for K-4, and −5.45 eV for K-5, which were sufficiently lower than the redox potential of I−/I3− (−4.20 eV),12 ensuring the favorable thermodynamic ground states for efficient regeneration of the dye through the reaction of the oxidized dye with iodide. The onset of the optical energy gap (E0–0) of K-dyes was in the range of 1.93–1.87 eV. The excited-state oxidation potentials (S+/*) were calculated to be −3.77 eV for K-3, −3.79 eV for K-4, and −3.58 eV for K-5, which lie above the conduction band edge (−4.2 eV) of the nanocrystalline TiO2, ensuring efficient electron injection into the conduction band edge of TiO2 from the adsorbed K-dyes. The electrochemical properties of K-dyes were estimated by electrochemical cyclic voltammetry (CV) in CH2Cl2 (Fig. S2 in ESI†). All the dyes exhibit irreversible oxidation processes and the highest occupied molecular orbital (HOMO) energy levels of K-dyes are in accordance with the ground-state oxidation potential (S+/0). The HOMO of K-5 dye is measured to be −5.55 eV, which is higher than that of K-3 (−5.67 eV) and K-4 (−5.59 eV) dyes. It is noted that the K-5 dye having three hexyl groups on the π-linker shows up-shifted ground-state and excited state potentials compared with that of the K-3 and K-4 dyes due to the electron donating properties of the hexyl group.
To gain further insight into the molecular structures and the frontier orbitals of K-dyes, we optimized their ground-state geometries and calculated their molecular orbitals using the hybrid DFT energy functional B3LYP13,14 and the basis set of DGDZVP (density Gauss double-ζ with polarization functions)15,16 (Fig. S3 in ESI†). In all these dyes, the HOMO is delocalized over the thieno[2,3-a]carbazole donor and the oligothiophene π-linkage unit, whereas the LUMO is localized on the cyanoacrylic acid moiety and the adjacent π-linkage. These HOMO and LUMO distributions are appropriate for efficient charge transfer and electron injection into the TiO2 conduction band. The dihedral angles between the thienocarbazole moiety and the neighbouring thiophene ring for the K-3 and K-4 dyes are about 1°, indicating that these two dyes are rigid and almost coplanar despite the bulky group on the donor moiety. In contrast, the K-5 dye exhibits a twisted configuration with a dihedral angle of 47° between the thienocarbazole and the neighbouring hexylthiophene ring due to the steric repulsion between the large sulphur atom and the hexyl group. The DFT calculations and the absorption properties indicate that the K-3 and K-4 dyes having a coplanar structure facilitate the ICT and increase the ε value as mentioned above.
The photovoltaic characteristics of DSCs based on K-dyes were evaluated under AM 1.5 G irradiation at 100 mW cm−2. 4-tert-Butylpyridine (TBP) was used to obtain a higher Voc, which is known to up-shift the conduction band of the TiO2. The IPCEs based on K-dyes are plotted in Fig. 2a. The IPCE spectra of K-dyes are consistent with their UV-visible absorption spectra observed for TiO2 films. The IPCE spectra broaden toward the red region in the order of K-5 > K-4 ≈ K-3. The results of the desorption experiment showed that the amount of uploaded K-5 dye as high as 8.50 × 10−8 mol cm−2 was slightly lower than that of the adsorbed K-3 dye of 9.69 × 10−8 mol cm−2 and the K-4 dye of 9.82 × 10−8 mol cm−2 (Table 2). All the dye samples show a high molar absorption coefficient at the low energy absorption maxima, 3.4 − 4.2 × 104 M cm−2 (Table 1). The light-harvesting efficiency (LHE) values of K-dyes are close to unity because of their high molar absorption coefficient and high dye upload concentrations on TiO2 films.17 It should be noted that, in comparison with a similar onset of IPCE spectra of K-dyes, the maximum quantum efficiency values of K-dyes in the plateau region decreased from 89% (K-3) to 75% (K-5) in the plateau region reducing the planarity of the π-conjugated backbone. These results implied that the DSCs based on the planar dyes are favorable for efficient electron injection quantum yield.
 | ||
| Fig. 2 (a) IPCEs of the nanocrystalline TiO2 film sensitized by K-dyes. (b) I–V characteristics of K-dyes. | ||
| Dye | J sc [mA cm−2] | V oc [V] | FF | η [%] | Adsorbed dye [10−8 mol cm−2] |
|---|---|---|---|---|---|
| a 0.2 M iodine was used instead of 0.05 M. | |||||
| K-3 | 13.2 | 0.63 | 0.70 | 5.9 | 9.69 |
| K-4 | 13.1 | 0.68 | 0.73 | 6.5 | 9.82 |
| K-5 | 13.9 | 0.74 | 0.68 | 7.0 | 8.50 |
| K-5 | 13.8 | 0.73 | 0.73 | 7.4 | — |
The solar cell performances for the DSCs based on the K-dyes are listed in Table 2, and the corresponding I–V curves are shown in Fig. 2b. The K-3 and K-4 dyes with trithiophene as a π-bridge showed the Jsc of 13.2 mA cm−2 and 13.1 mA cm−2, respectively. The substituents on the donor moiety do not influence the Jsc value, but the Voc of the K-4 cell (0.68 V) is higher than that of the K-3 cell (0.63 V), implying the more efficient blocking effect of the former for charge recombination. The lower Voc of the K-3 cell can be rationalized by the increased intermolecular π–π interaction of the K-3 dye which may cause a self-quenching process. The improved Jsc of 13.9 mA cm−2 and Voc of 0.74 V, and the decreased fill factor (FF) of 0.68 were obtained in the K-5 cell, which produced the highest η of 7.0% under the same conditions. We ascribed the higher Jsc of the K-5 cell to its widest IPCE response and higher excited-state oxidation potential, and the higher Voc to the introduction of hexyl groups on the π-bridge which may sufficiently reduce the charge recombination of the electrons in the TiO2 conduction band with the electrolyte.
The typical EIS was employed to study the influence of electron recombination and interfacial charge transfer processes on Voc.18 In the EIS Nyquist plots (Fig. 3a), the first semicircles in the left frequency range corresponding to the series resistance and electrolyte resistance are almost the same. The second semicircle in the middle-frequency range represents the charge-transfer resistance at the TiO2/dye/electrolyte interface. The middle frequency semicircles were in the following order of increased charge recombination resistance K-5 > K-4 > K-3, which was in the order of the actual photovoltage obtained from the corresponding solar devices. The EIS Bode plots of the DSCs based on K-dyes were also investigated (Fig. 3b). The characteristic frequency response in the range of 1–500 Hz represents the charge recombination rate at the TiO2/dye/electrolyte interface. The injected electron lifetime in TiO2 can be determined by using the relationship, τ = 1/(2πf), where τ is the lifetime of electrons in TiO2 and f is the mid-frequency (1–500 Hz) peak in the Bode plots. The frequency peaks of the DSCs in the range of 1–500 Hz based on K-3, K-4, and K-5 were at 62 Hz, 47 Hz and 38 Hz, corresponding to electron lifetimes in TiO2 of 2.6 ms, 3.4 ms, and 4.2 ms, respectively, thus resulting in a lower Voc for K-3 and K-4 compared to K-5. This trend is well-matched with the increasing sequence of Voc in the K-cells (Table 2).
To further clarify the substituent effect on the Voc difference observed for the K-DSCs, we measured the charge extraction and τe in the conduction band of TiO2 (ECB) by means of CEM and IMVS. As shown in Fig. 4a, the measurement of the ECB of all devices with the CEM shows that the Voc increases linearly with the logarithm of the electron density, exhibiting a similar slope. At the given value of electron density, the Voc for both K-5 and K-4 cells is slightly higher than that of the K-3 cell, indicating a negative shift of the ECB. In general, this negative shift of ECB improves the Voc. The ECBs of K-5 and K-4 cells are almost similar, but the τe of K-5 is higher than that of the K-4. At a fixed voltage, the τe of the DSCs based on K-5 is longer than the τe values for K-3 and K-4 (Fig. 4b). This trend is in accordance with the τevs. Voc results. The EIS and IMVS results suggest that the K-5 dye having hexyl chains on the π-linker shows an enhanced blocking ability to prevent the contact between the I3− in the electrolyte and the TiO2 electrode, compared to the K-3 and K-4 dyes having bulky groups on the donor moiety, and hence it more sufficiently suppresses the charge recombination.
 | ||
| Fig. 4 (a) EIS Nyquist plots for DSCs sensitized with K-dyes. (b) Electron lifetime (τe) as a function of voltage for DSCs based on K-dyes. | ||
Finally, the iodine concentration was increased from 0.02 M to 0.2 M for DSC based on the K-5 dye. It was found that the FF was improved from 0.68 to 0.73 without decreasing the Jsc and Voc, yielding a high η of 7.42%.
Conclusion
In conclusion, we have designed and synthesized a series of new K-dyes based on thieno[2,3,a]carbazole as a donor. DFT calculations indicate that the K-3 and K-4 dyes having bulky substituents on the donor moiety show a planar π-conjugated backbone, while the K-5 dye having hexyl chains on the π-linker has a twisted structure. The K-3 and K-4 dyes showed red-shifted ICT absorption bands, increased ε values, and higher maximal IPCE values compared with that of K-5 dyes. Among the K-dyes, DSCs based on the K-5 dye produced the highest efficiency due to the increased Jsc and Voc, which we ascribed to the reduced intermolecular π–π interaction and the enhanced charge recombination resistance of the K-5 cell. Taking into consideration both IPCE and Voc, our results indicate that the better planarity in the π-conjugated system is beneficial for the maximal IPCE value in the plateau region, though it also causes aggregated intermolecular π–π interaction and dye aggregation, which can be avoided via introducing the bulk substituents on the π-linkers. Our study in this article suggests that the new molecular designs by improving the molecular planarity while reducing the dye aggregation should be one of the efficient ways to achieve high DSC performances.Acknowledgements
This work was supported by World Premier International Research Center Initiative (WPI), MEXT (Japan). This work was partly supported by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency. A.I. acknowledges the support of the JSPS KAKENHI grant no. 26288113.Notes and references
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef; (b) A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49 CrossRef CAS; (c) A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 2000, 33, 269 CrossRef CAS PubMed.
- (a) Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS; (b) L. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. F. Zhang, X. D. Yang and M. Yanagida, Energy Environ. Sci., 2012, 5, 6057 RSC.
- For recent selected reviews: (a) N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338 CrossRef CAS PubMed; (b) A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS PubMed; (c) A. Hagfeldt, G. Boschloo, L. C. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed; (d) Z. J. Ning, N. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170 RSC; (e) J. N. Clifford, E. Martinez-Ferrero, A. Viterisi and E. Palomares, Chem. Soc. Rev., 2011, 40, 1635 RSC; (f) Y. S. Chen, H. H. Chou, Y. C. Chen, C. Y. Hsu and J. T. Lin, J. Mater. Chem., 2012, 22, 8734 RSC; (g) M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453 RSC; (h) Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039 RSC.
- (a) S. Kim, J. K. Lee, S. O. Kang, J. Ko, J. H. Yum, S. Fantacci, F. DeAngelis, D. DiCenso, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 16701 CrossRef CAS PubMed; (b) N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS PubMed; (c) S. Ito, H. Miura, S. Uchida, M. Takada, K. Sumioka, P. Liska, P. Comte, P. Pechy and M. Grätzel, Chem. Commun., 2008, 5194 RSC; (d) D. P. Hagberg, J.-H. Yum, H. Lee, F. De Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259 CrossRef CAS PubMed; (e) G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 2198 RSC; (f) J. Mao, N. He, Z. Ning, Q. Zhang, F. Guo, L. Chen, W. Wu, J. Hua and H. Tian, Angew. Chem., Int. Ed., 2012, 51, 9873 CrossRef CAS PubMed; (g) B. Liu, B. Wang, R. Wang, L. Gao, S. Huo, Q. Liu, X. Li and W. Zhu, J. Mater. Chem. A, 2014, 2, 804 RSC; (h) S. Qu, C. Qin, A. Islam, Y. Wu, W. Zhu, J. Hua, H. Tian and L. Han, Chem. Commun., 2009, 6972 Search PubMed.
- Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903 CrossRef CAS.
- (a) S. Kim, H. Choi, D. Kim, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 9206 CrossRef CAS PubMed; (b) R. Chen, X. Yang, H. Tian and L. Sun, J. Photochem. Photobiol., A, 2007, 189, 295 CrossRef CAS PubMed; (c) G. Li, K. Jiang, Y. Li, S. Li and L. Yang, J. Phys. Chem. C, 2008, 112, 11591 CrossRef CAS; (d) K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597 CrossRef CAS; (e) S. E. Koops, P. R. F. Barnes, B. C. O'Regan and J. R. Durrant, J. Phys. Chem. C, 2010, 114, 8054 CrossRef CAS.
- (a) G. L. Zhang, H. Bala, Y. M. Cheng, D. Shi, X. J. Lv, Q. J. Yu and P. Wang, Chem. Commun., 2009, 2198 RSC; (b) L. Y. Lin, C. H. Tsai, K. T. Wong, T. W. Huang, L. Hsieh, S. H. Liu, H. W. Lin, C. C. Wu, S. H. Chou, S. H. Chen and A. I. Tsai, J. Org. Chem., 2010, 75, 4778 CrossRef CAS PubMed.
- (a) R. Li, J. Liu, N. Cai, M. Zhang and P. Wang, J. Phys. Chem. B, 2010, 114, 4461 CrossRef CAS PubMed; (b) J.-H. Chen, C.-H. Tsai, S.-A. Wang, Y.-Y. Lin, T.-W. Huang, S.-F. Chiu, C.-C. Wu and K.-T. Wong, J. Org. Chem., 2011, 76, 8977 CrossRef CAS PubMed; (c) X. Zhu, H. Tsuji, A. Yella, A. Chauvin, M. Grätzel and E. Nakamura, Chem. Commun., 2013, 49, 582 RSC.
- J. Liu, Y. Numata, C. Qin, A. Islam, X. Yang and L. Han, Chem. Commun., 2013, 49, 7587 RSC.
- G. Ferrara, T. Jin, K. Oniwa, J. Zhao, A. M. Asiri and Y. Yamamoto, Tetrahedron Lett., 2012, 53, 914 CrossRef CAS PubMed.
- (a) M. Akhtaruzzaman, A. Islam, F. Yang, N. Asao, E. Kwon, S. P. Singh, L. Han and Y. Yamamoto, Chem. Commun., 2011, 47, 12400 RSC; (b) M. Akhtaruzzaman, Y. Seya, N. Asao, A. Islam, E. Kwon, A. El-Shafei, L. Han and Y. Yamamoto, J. Mater. Chem., 2012, 22, 10771 RSC; (c) G. Ferrara, T. Jin, M. Akhtaruzzaman, A. Islam, L. Han, H. Jiang and Y. Yamamoto, Tetrahedron Lett., 2012, 53, 1946 CrossRef CAS PubMed; (d) F. Yang, M. Akhtaruzzaman, A. Islam, T. Jin, A. El-Shafei, C. Qin, L. Han, K. A. Alamry, S. A. Kosa, M. K. Hussien, A. M. Asiri and Y. Yamamoto, J. Mater. Chem., 2012, 22, 22550 RSC; (e) M. Akhtaruzzaman, Menggenbateer, A. Islam, A. El-Shafei, N. Asao, T. Jin, L. Han, K. A. Alamry, S. A. Kosa, A. M. Asiri and Y. Yamamoto, Tetrahedron, 2013, 69, 3444 CrossRef CAS PubMed; (f) J. Zhao, T. Jin, A. Islam, E. Kwon, M. Akhtaruzzaman, N. Asao, L. Han, K. A. Alamry, S. A. Kosa, A. M. Asiri and Y. Yamamoto, Tetrahedron, 2014, 70, 6211 CrossRef CAS PubMed.
- G. Oskam, B. V. Bergeron, G. J. Meyer and P. C. Searson, J. Phys. Chem. B, 2001, 105, 6867 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785 CrossRef CAS.
- N. Godbout, D. R. Salahub, J. Andzelm and E. Wimmer, Can. J. Chem., 1992, 70, 560 CrossRef CAS.
- C. Sosa, J. Andzelm, B. C. Elkin, E. Wimmer, K. D. Dobbs and D. A. Dixon, J. Phys. Chem., 1992, 96, 6630 CrossRef CAS.
- A. Islam, H. Sugihara, K. Hara, L. P. Singh, R. Katoh, M. Yanagida, Y. Takahashi, S. Murata and H. Arakawa, J. Photochem. Photobiol., A, 2001, 145, 135 CrossRef CAS.
- (a) H. Tian, I. Bora, X. Jiang, E. Gabrielsson, K. M. Karlsson, A. Hagfeldt and L. Sun, J. Mater. Chem., 2011, 21, 12462 RSC; (b) C. Qin, A. Islam and L. Han, J. Mater. Chem., 2012, 22, 19236 RSC; (c) S. Haid, M. Marszalek, A. Mishra, M. Wielopolski, J. Teuscher, J.-E. Moser, R. Humphry-Baker, S. M. Zakeeruddin, M. Grätzel and P. Bäuerle, Adv. Funct. Mater., 2012, 22, 1291 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00285g |
| This journal is © the Partner Organisations 2015 |