NIS/CHP-mediated reaction of isocyanides with hydrazones: access to aminopyrazoles†
Tong-Hao
Zhu
,
Tian-Qi
Wei
,
Shun-Yi
Wang
* and
Shun-Jun
Ji
*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: shunyi@suda.edu.cn; shunjun@suda.edu.cn; Fax: +86-512-65880307; Tel: +86-512-65880307
First published on 19th January 2015
Abstract
A NIS/CHP-mediated [4 + 1]-cycloaddition of isocyanides with 1,2-diaza-1,3-dienes generated in situ from hydrazones under metal-free conditions has been developed. This protocol provides a new, atom efficient and step efficient way to construct aminopyrazoles in good to excellent yields via formation of new C–C/C–N bonds, utilizing a catalytic amount of NIS in the presence of CHP.
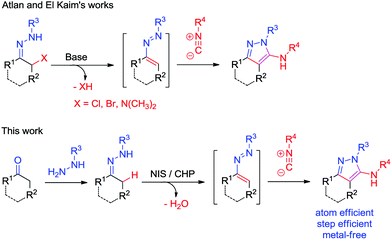
The design and development of new methods to construct heterocyclic systems attracts considerable ongoing interest due to their important activities and various useful applications.1 During the past few decades, 1,2-diaza-1,3-dienes (DDs) have attracted great attention.2,3 Direct C–H functionalization of sp3 C–H bonds has attracted great attention as it allows the efficient construction of new C–C/C–N/C–S bonds with atom economy and step economy.4–6 However, reactions via direct sp3 C–H functionalization of the α-position of hydrazones to construct DDs are rare.7,8
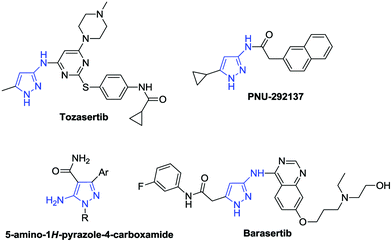
Aminopyrazoles are one of the most important heterocycles due to their wide bioactivities and are important building blocks in the synthesis of more important heterocycles (Fig. 1).9 The 3-aminopyrazole derivative Tozasertib has been shown to inhibit the activity of Aurora kinases.10 Danusertib, bearing a 1H-pyrazol-3-yl-amide scaffold, is a potential drug for the treatment of leukemias.11 5-Aminopyrazole-4-carboxamide can create inhibitors of calcium-dependent protein kinase-1.12 In view of the synthetic value of aminopyrazoles, it is desirable to develop new methods to construct them in an efficient way. Recently, hydrazones2,3,7,8,13–15 and isocyanides15–17,18 have attracted great attention as a result of their unique properties and high reactivities, and are utilized as important synthons for the synthesis of heterocycles, such as aminopyrazoles (Scheme 1).17 As a continuation of our works on isocyanide reactions18 and C–H functionalization under metal-free conditions,8 herein, we demonstrate a NIS/CHP-mediated [4 + 1]-cycloaddition of isocyanides with 1,2-diaza-1,3-dienes generated in situ from hydrazones via C–H functionalization under metal-free and halide substrate-free conditions. The corresponding aminopyrazoles could be obtained in good to excellent yields via formation of new C–C/C–N bonds, utilizing a catalytic amount of NIS in the presence of CHP.
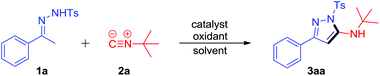
Initially, we examined the model reaction of N-tosylhydrazone 1a and tert-butyl isonitrile 2a under oxidative conditions in the presence of catalysts (for full details see the ESI†). Only trace new product was detected in the absence of catalyst or oxidant (Table 1, entries 1 and 10). To our delight, I2 could catalyze the reaction of 1a and 2a smoothly in the presence of 2.0 equiv. TBPB to furnish the desired product 3aa in 59% LC-yield (Table 1, entry 2), of which the structure was confirmed by NMR, IR and HRMS, as well as X-ray crystal structure (see the ESI†). Inspired by this result, we evaluated several other halide source catalysts such as KI, TBAB, TBAC, TBAI, and NIS. NIS led to the best result and 3aa could be obtained in 63% LC-yield (Table 1, entries 2–7). NIS (20 mol%) combined with 2 equiv. of DTBP and O2 could also promote the reaction well to furnish 3aa in 56% and 51% LC-yields, respectively (Table 1, entries 8 and 9). When NIS (20 mol%) and 2 equiv. of CHP were subjected to the reaction, the LC-yield of 3aa could be increased to 74% (Table 1, entry 11). Further examinations of the reaction temperature and the amount of NIS and CHP showed that 4 mol% NIS and 1 equiv. of CHP could promote the reaction efficiently at 100 °C, which afforded the desired product 3aa in 81% LC-yield (Table 1, entries 12–21). Therefore, the optimum reaction conditions are 4 mol% NIS and 1 equiv. of CHP as the oxidant in 1,4-dioxane at 100 °C for 12 h (entry 18, Table 1, 81% yield of 3aa).
| Entry | Catalyst (mol%) | Oxidant (equiv.) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: N-tosylhydrazone 1a (0.5 mmol), tert-butyl isonitrile 2a (1.2 equiv., 0.6 mmol), catalyst, and oxidant in 3 mL of solvent at 100 °C for 12 h. b Yields were determined by HPLC analysis with biphenyl as the internal standard. c TBPB = tert-butyl peroxybenzoate. d TBAC = tetrabutyl ammonium chloride. e TBAB = tetrabutyl ammonium bromide. f TBAI = tetrabutyl ammonium iodide. g NIS = N-iodosuccinimide. h DTBP = 2-(tert-butylperoxy)-2-methylpropane. i CHP = cumene hydroperoxide. j The system was reacted at 80 °C. k The system was reacted at 110 °C. l The system was reacted at room temperature. | |||
| 1 | — | TBPBc (2) | Trace |
| 2 | I2 (20) | TBPB (2) | 59 |
| 3 | KI (20) | TBPB (2) | 57 |
| 4 | TBACd (20) | TBPB (2) | Trace |
| 5 | TBABe (20) | TBPB (2) | Trace |
| 6 | TBAIf (20) | TBPB (2) | 43 |
| 7 | NISg (20) | TBPB (2) | 63 |
| 8 | NIS (20) | DTBPh (2) | 56 |
| 9 | NIS (20) | O2 | 51 |
| 10 | NIS (20) | Ar | Trace |
| 11 | NIS (20) | CHPi (2) | 74 |
| 12 | NIS (30) | CHP (2) | 71 |
| 13 | NIS (10) | CHP (2) | 75 |
| 14 | NIS (5) | CHP (2) | 76 |
| 15 | NIS (4) | CHP (2) | 80 |
| 16 | NIS (3) | CHP (2) | 70 |
| 17 | NIS (4) | CHP (3) | 79 |
| 18 | NIS (4) | CHP (1) | 81 |
| 19j | NIS (4) | CHP (1) | 79 |
| 20k | NIS (4) | CHP (1) | 75 |
| 21l | NIS (4) | CHP (1) | 31 |
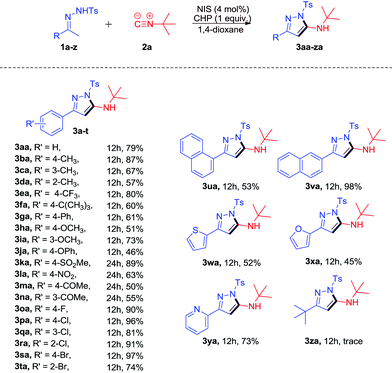
With the optimized reaction conditions in hand, the scope of this transformation was subsequently investigated and the results are summarized in Scheme 2. Most of the reactions proceeded smoothly to afford the desired products in moderate to excellent yields. Typical functional groups including electron-donating and electron-withdrawing groups, such as alkyl, methoxy, NO2, ketone, and halide, were tolerated under the same conditions. 4-Methyl-functionalized N-tosylhydrazone 1b led to the desired product 3ba in 87% yield. The reaction of 2-methyl-functionalized N-tosylhydrazone 1d afforded the desired product 3da in 57% yield due to steric effects. When halogen substituted N-tosylhydrazones 1o–t were subjected to the reaction, the corresponding products 3oa-ta were obtained in good to excellent yields (74%–97%). It should be noted that the more bulky substrates 1r and 1t could undergo the transformation to give the desired products 3ra and 3ta in 91% and 74% yields, respectively. When α-naphthyl N-tosylhydrazone 1u was applied to the reaction, 3ua could be obtained in 53% yield. To our delight, β-naphthyl N-tosylhydrazone 1v worked well to give 3va in 98% yield. In addition, the reaction with heteroaryl N-tosylhydrazones such as 1w–y could also proceed smoothly to give the desired products 3w–y in moderate to excellent yields (45%–73%). Unfortunately, the reaction of N′-(3,3-dimethylbutan-2-ylidene)-4-methylbenzenesulfono hydrazide 1z could only give the corresponding product 3za in trace yield.
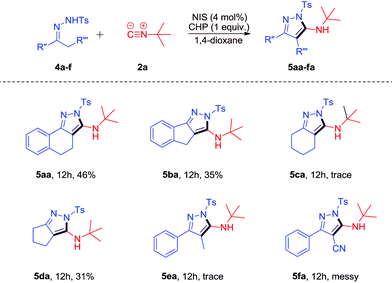
Encouraged by the above results, we applied other functionalized N-tosylhydrazones 4a–f to the [4 + 1]-cycloaddition reaction with 2a (Scheme 3). When rigid substrates 4a–d were applied to the reaction, the desired products 5aa-da could be observed in low yields. However, 4-methyl-N′-(1-phenylpropylidene)benzenesulfonohydrazide 4e could only lead to a trace amount of the desired product due to its lower reactivity. The reaction of N′-(2-cyano-1-phenylethylidene)-4-methylbenzenesulfonohydrazide 4f with 2a was messy due to the presence of the CN group.
We tried to subject 4-methyl-N′-(2-phenylethylidene)benzenesulfonohydrazide 4g to the reaction with 2a under the same conditions. However, the reaction was messy (eqn (1)).
 | (1) |
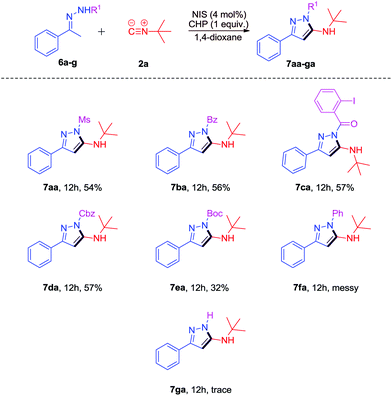
Then, we examined the substrate scope of some other N-substituted hydrazones 6a–g (Scheme 4). To our delight, the Ms, benzoyl, 2-iodobenzoyl, Cbz, and Boc substituted hydrazones 6a–e could also work well to give the desired products 7aa-ea in moderate yields (32%–57%). However, when 1-phenyl-2-(1-phenylethylidene)hydrazine 6f was used, the reaction was messy and no target product was detected. (1-Phenylethylidene)hydrazine 6g could not react under the same conditions.
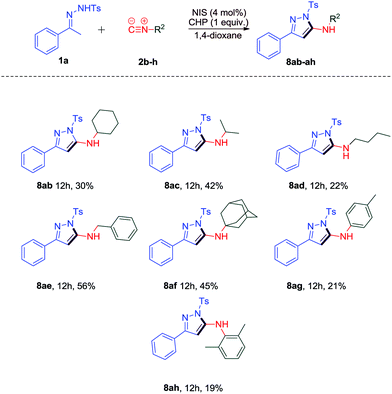
We also investigated the substrate scope of isocyanides (Scheme 5). The reaction of 1a with benzyl isocyanide 2e performed well under the optimal conditions and led to the desired product 8ae in 56% yield. When other isocyanides 2b–d and 2f were subjected to the reaction, it could furnish 2-aminopyrazole derivatives 8ab–ad and 8af in lower yields (22%–45%). Moreover, the reaction of aryl isonitriles 2g and 2h could result in the desired products 8ag and 8ah in 21% and 19% yields, respectively.
To further probe the applications of our protocol, we designed the reaction of tri-hydrazone 9a with 2a (Scheme 6). To our delight, the reaction worked well to furnish the desired product 10aa in 33% yield. In addition, the photophysical properties of 10aa were investigated using UV-Vis absorption photoluminescence measurements at room temperature in CH2Cl2 and low temperature PL spectra of 10aa were measured in 2-MeTHF at 77 K, which indicated that its triplet energy is 2.42 eV, calculated from the phosphorescence emission peak. This result showed that the tri-arm molecule 10aa is a promising potential green-light material.
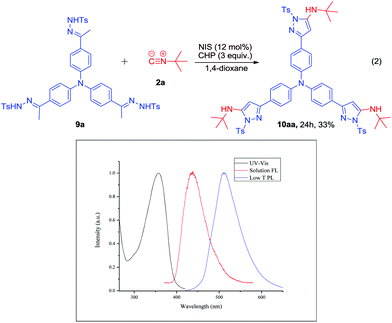 | ||
| Scheme 6 NIS-catalyzed oxidative coupling reaction of 2a with N-tosylhydrazone 9a, and the photophysical characterisation of the product, 10aa. | ||
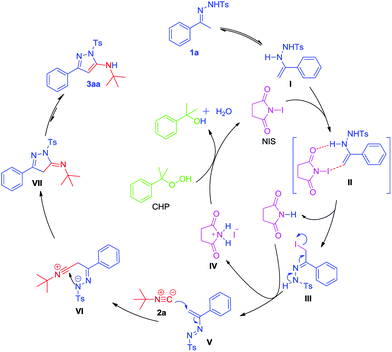
On the basis of the literature19,20 and the above results, a plausible catalytic cycle for this [4 + 1]-cyclization is proposed in Scheme 7. The tautomer of 1a reacts with NIS via transition state II to furnish the α-iodine-substituted intermediate III and pyrrolidine-2,5-dione. III further reacts with the pyrrolidine-2,5-dione to give 1,2-diaza-1,3-diene intermediate V and 2,5-dioxopyrrolidin-1-ium iodide IV. 1,4-Addition of tert-butyl isonitrile 2a to V leads to the intermediate VI. After subsequent cyclization of VI and further tautomerization, 3aa is formed. In addition, IV can be oxidized by CHP to regenerate NIS, which finishes the catalytic cycle.
In conclusion, a NIS/CHP-mediated [4 + 1]-cycloaddition of isocyanides with 1,2-diaza-1,3-dienes generated in situ from hydrazones via C–H functionalization under metal-free conditions has been developed. This protocol provides a new, low-cost, atom efficient and step efficient way to construct aminopyrazoles via formation of new C–C/C–N bonds, utilizing a catalytic amount of NIS in the presence of CHP. Further studies to understand the reaction mechanism are ongoing in our laboratory.
Experimental section
General procedures
N-Iodosuccinimide (NIS) (0.02 mmol) and hydrazones (0.5 mmol) were added to a test tube. 1,4-Dioxane (3.0 mL), cumene hydroperoxide (CHP) (0.5 mmol), and isocyanides (0.6 mmol) were added via syringe. The test tube was closed. The reaction mixture was stirred at 100 °C for 12 or 24 h. Then the reactions were cooled to room temperature. Removal of solvent followed by flash column chromatographic purification using petroleum and acetone afforded the products.Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (no. 21172162, 21372174), the Young National Natural Science Foundation of China (no. 21202111), the Ph.D. Programs Foundation of Ministry of Education of China (2013201130004), the Young Natural Science Foundation of Jiangsu Province (BK2012174), Key Laboratory of Organic Synthesis of Jiangsu Province (KJS1211), PAPD, and Soochow University for financial support.Notes and references
- (a) R. M. Nathan, C. J. Peter, M. Christophe and D. H. Bruce, J. Med. Chem., 2003, 46, 1066 CrossRef PubMed; (b) O. Allison and A. W. Gregory, J. Am. Chem. Soc., 2005, 127, 12178 CrossRef PubMed; (c) Y. Shan, J.-J. Zhou, H.-G. Zhao, X. Feng, Y.-F. Dong and B. Xia, Chem. Nat. Compd., 2010, 46, 66 CrossRef; (d) R. Brimblecombe, W. W. A. M. Duncan and G. J. Durant, J. Int. Med. Res, 1975, 3, 8 Search PubMed; (e) A. N. Akopian, L. Sivilotti and J. N. Wood, A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons, Proc. Jpn. Acad., Ser. B, 1996, 25 Search PubMed; (f) T. Narahashi, Proc. Jpn. Acad., Ser. B, 2008, 84, 147 CrossRef CAS.
- (a) O. A. Attanasi, L. D. Crescentini, G. Favi, P. Filippone, F. Mantellini, F. R. XPerrulli and S. Santeusanio, Eur. J. Org. Chem., 2009, 3109 CrossRef CAS; (b) O. A. Attanasi and L. Caglioti, Org. Prep. Proced. Int., 1986, 18, 299 CrossRef CAS.
- (a) O. A. Attanasi, L. De Crescentini, G. Favi, P. Filippone, S. Lillini, F. Mantellini and S. Santeusanio, Org. Lett., 2005, 7, 2469 CrossRef CAS PubMed; (b) O. A. Attanasi, S. Bartoccini, G. Favi, G. Giorgi, F. R. Perrulli and S. Santeusanio, Tetrahedron, 2012, 68, 6 CrossRef PubMed; (c) O. A. Attanasi, L. Bianchi, M. D'Auria, F. Mantellini and R. Racioppi, Curr. Org. Synth., 2013, 10, 631 CAS; (d) G. Giorgi, G. Favi and O. A. Attanasi, Org. Biomol. Chem., 2013, 11, 5006 RSC; (e) O. A. Attanasi, G. Favi, G. Giorgi, R. Majer, F. R. Perrulli and S. Santeusanio, Org. Biomol. Chem., 2014, 12, 4610 RSC.
- C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS PubMed.
- For selected reviews on C(sp3)–H bond functionalization, see: (a) C. Liu, H. Zhang, W. Shi and A.-W. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (b) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (c) S.-Y. Zhang, F.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2011, 40, 1937 RSC; (d) T. Newhouse and P. S. Baran, Angew. Chem., Int. Ed., 2011, 50, 3362 CrossRef CAS PubMed; (e) W. Shi, C. Liu and A.-W. Lei, Chem. Soc. Rev., 2011, 40, 2761 RSC; (f) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (g) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (h) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (i) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed; (j) G. Yan and A. J. Borah, Org. Chem. Front., 2014, 1, 838 RSC; (k) G. Qiu and J. Wu, Org. Chem. Front., 2015, 2, 169 RSC.
- For recent examples, see: (a) B. Wang, W. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952 RSC; (b) D. Affron, O. Davis and J. Bull, Org. Lett., 2014, 16, 4956 CrossRef CAS PubMed; (c) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef CAS PubMed; (d) M. Iyanaga, Y. Aihara and N. Chatani, J. Org. Chem., 2014, 79, 11933 CrossRef CAS PubMed; (e) Y. Wei, H. Tang, X. Cong, B. Rao, C. Wu and X. Zeng, Org. Lett., 2014, 16, 2248 CrossRef CAS PubMed; (f) J. He, S. Li, Y. Deng, H. Fu, B. Laforteza, J. Spangler, A. Homs and J.-Q. Yu, Science, 2014, 343, 1216 CrossRef CAS PubMed; (g) Y. Deng, W. Gong, J. He and J.-Q. Yu, Angew. Chem., Int. Ed., 2014, 53, 6692 CrossRef CAS PubMed; (h) S. Li, G. Chen, C.-G. Feng, W. Gong and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 5267 CrossRef CAS PubMed; (i) R.-Y. Zhu, J. He, X.-C. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 13194 CrossRef CAS PubMed; (j) K.-J. Xiao, D. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 8138 CrossRef CAS PubMed; (k) J.-X. Yan, H. Li, X.-W. Liu, J.-L. Shi, X. Wang and Z.-J. Shi, Angew. Chem., Int. Ed., 2014, 53, 4945 CrossRef CAS PubMed; (l) X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2014, 136, 1789 CrossRef CAS PubMed; (m) K. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2014, 53, 11950 CrossRef CAS PubMed; (n) T. Kang, Y. Kim, D. Lee, Z. Wang and S. Chang, J. Am. Chem. Soc., 2014, 136, 4141 CrossRef CAS PubMed; (o) Y. Zong and Y. Rao, Org. Lett., 2014, 16, 5278 CrossRef CAS PubMed; (p) Z. J. Li, Y. Zhang, L. Z. Zhang and Z. Q. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed; (q) Y. F. Zhu and Y. Y. Wei, Chem. Sci., 2014, 5, 2379 RSC; (r) B. L. Tran, B. J. Li, M. Driess and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 2555 CrossRef CAS PubMed; (s) B.-G. Du, B. Jin and P.-P. Sun, Org. Lett., 2014, 16, 3032 CrossRef CAS PubMed; (t) D. L. Priebbenow and C. Bolm, Org. Lett., 2014, 16, 1650 CrossRef CAS PubMed; (u) C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3372 CrossRef CAS PubMed; (v) J. Feng, M.-F. Lv, G.-P. Lu and C. Cai, Org. Biomol. Chem., 2015, 13, 677 RSC.
- (a) Z.-K. Chen, Q.-Q. Yan, Z.-X. Liu, Y.-M. Xu and Y.-H. Zhang, Angew. Chem., Int. Ed., 2013, 52, 13324 CrossRef CAS PubMed; (b) J. Schantl and P. Karpellus, Monatsh. Chem., 1978, 109, 1081 CrossRef CAS.
- Z.-J. Cai, X.-M. Lu, Y. Zi, C. Yang, L.-J. Shen, J. Li, S.-Y. Wang and S.-J. Ji, Org. Lett., 2014, 16, 5108 CrossRef CAS PubMed.
- (a) N. A. A. Elkanzi, Int. J. Res. Pharm. Biomed. Sci., 2013, 4, 17 CAS; (b) H. F. Anwar and M. H. Elnagdi, ARKIVOC, 2009, 198 CrossRef CAS; (c) H. Katrin, M.-B. Julia, N.-S. Luitgard, E. Matthias, M.-Z. Wolfgang, H. C. H. Anselm, S. Heinrich, S. Sharmistha, B. Gal and S. Thomas, J. Am. Chem. Soc., 2011, 133, 4348 CrossRef PubMed; (d) D. V. Tsyganov, D. L. Konyushkin, I. B. Karmanova, S. I. Firgang, Y. A. Strelenko, M. N. Semenova, A. S. Kiselyov and V. V. Semenov, J. Nat. Prod., 2013, 76, 1485 CrossRef CAS PubMed; (e) M. N. George, D. Constantin and T. O. Octavian, Int. J. Mol. Sci., 2013, 14, 21805 CrossRef PubMed; (f) K. Černovská, M. Kemter, H.-C. Gallmeier, P. Rzepecki, T. Schrader and B. König, Org. Biomol. Chem., 2004, 2, 1603 RSC.
- D. Bebbington, H. Binch, J.-D. Charrier, S. Everitt, D. Fraysse, J. Golec, D. Kay, R. Knegtel, C. Mak and F. Mazzei, et al. The discovery of the potent Aurora inhibitor MK-0457 (VX-680), Bioorg. Med. Chem. Lett., 2009, 19, 3586 CrossRef CAS PubMed.
- H. J. Meulenbeld, R. H. Mathijssen, J. Verweij, R. De Wit and M. J. De Jonge, Danusertib, an aurora kinase inhibitor, Expert Opin. Invest. Drugs, 2012, 3, 383 CrossRef PubMed.
- Z.-S. Zhang, K. K. Ojo, R. Vidadala, W.-L. Huang, J. A. Geiger, S. Scheele, R. Choi, M. C. Reid, K. R. Keyloun, K. Rivas, L. K. Siddaramaiah, K. M. Comess, K. P. Robinson, P. J. Merta, L. Kifle, W. G. J. Hol, M. Parsons, E. M. Merritt, D. J. Merritt, C. L. M. J. Verlinde, W. C. V. Voorhis and E.-K. Fan, ACS Med. Chem. Lett., 2014, 5, 40 CrossRef CAS PubMed.
- For recent reviews on hydrazone chemistry, see: (a) O. A. Attanasi, L. De Crescentini, P. Filippone, F. Mantellini and S. Santeusanio, ARKIVOC, 2002, 11, 274 Search PubMed; (b) M. Sugiura and S. Kobayashi, Angew. Chem., Int. Ed., 2005, 44, 5176 CrossRef CAS PubMed; (c) R. Lazny and A. Nodzewska, Chem. Rev., 2010, 110, 1386 CrossRef CAS PubMed; (d) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed.
- For recent examples on hydrazone chemistry, see: (a) O. A. Attanasi, G. Favi, A. Geronikaki, F. Mantellini, G. Moscatelli and A. Paparisva, Org. Lett., 2013, 15, 2624–2627 CrossRef CAS PubMed; (b) M.-K. Zhu, Y.-C. Chen and T. P. Loh, Chem. – Eur. J., 2013, 19, 5250 CrossRef CAS PubMed; (c) X.-Y. Duan, X.-L. Yang, R. Fang, X.-X. Peng, W. Yu and B. Han, J. Org. Chem., 2013, 78, 10692 CrossRef CAS PubMed; (d) X.-Q. Hu, J.-R. Chen, S. Gao, B. Feng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2013, 49, 7905–7907 RSC; (e) S. Gao, J.-R. Chen, X.-Q. Hu, H.-G. Cheng, L.-Q. Lu and W.-J. Xiao, Adv. Synth. Catal., 2013, 355, 3539 CrossRef CAS; (f) X.-F. Xu, P. Y. Zavalij, W.-H. Hu and M. P. Doyle, J. Org. Chem., 2013, 78, 1583 CrossRef CAS PubMed; (g) X. Zhu, Y.-F. Wang, W. Ren, F.-L. Zhang and S. Chiba, J. Org. Chem., 2013, 15, 3214 CAS; (h) G.-W. Zhang, Y. Zhao and H.-B. Ge, Angew. Chem., Int. Ed., 2013, 52, 2559 CrossRef CAS PubMed; (i) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, Y.-Q. Zou and W.-J. Xiao, Eur. J. Org. Chem., 2014, 3082 CrossRef CAS; (j) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS; (k) B. Chen, M. E. Scott, B. A. Adams, D. A. Hrovat, W. T. Borden and M. Lautens, Org. Lett., 2014, 16, 3930 CrossRef CAS PubMed; (l) X. Hong, H. B. Küçük, M. S. Maji, Y.-F. Yang, M. Rueping and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 13769 CrossRef CAS PubMed; (m) X.-H. Deng, J. T. Liang and N. S. Mani, Eur. J. Org. Chem., 2014, 410 CrossRef CAS; (n) X. Zhu and S. Chiba, Org. Biomol. Chem., 2014, 12, 4567 RSC; (o) X.-W. Li, X.-H. Liu, H.-J. Chen, W.-Q. Wu, C.-R. Qi and H.-F. Jiang, Angew. Chem., Int. Ed., 2014, 53, 14485 CrossRef CAS PubMed.
- For excellent reviews, see: (a) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (b) S. Lang, Chem. Soc. Rev., 2013, 42, 4867 RSC; (c) S. Chakrabarty, S. Choudhary, A. Doshi, F.-Q. Liu, R. Mohan, M. P. Ravindra, D. Shah, X. Yang and F. F. Fleming, Adv. Synth. Catal., 2014, 356, 2135 CrossRef CAS PubMed; (d) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS PubMed; (e) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed; (f) A. Domling, Chem. Rev., 2006, 106, 1 CrossRef PubMed; (g) A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef CAS PubMed.
- For recent selected examples, see: (a) J. Wang, S. Luo, J.-B. Huang, T.-T. Mao and Q. Zhu, Chem. – Eur. J., 2014, 20, 11220 CrossRef CAS PubMed; (b) J. Wang, S. Luo, J. Li and Q. Zhu, Org. Chem. Front., 2014, 1, 1285 RSC; (c) T.-B. Xiao, L.-Y. Li, G.-L. Lin, Q.-L. Wang, P. Zhang, Z.-W. Mao and L. Zhou, Green Chem., 2014, 16, 2418 RSC; (d) L.-J. Gu, C. Jin, J.-Y. Liu, H.-Y. Ding and B.-M. Fan, Chem. Commun., 2014, 50, 4643 RSC; (e) Z.-H. Xia, J.-B. Huang, Y.-M. He, J.-J. Zhao, J. Lei and Q. Zhu, Org. Lett., 2014, 16, 2546 CrossRef CAS PubMed; (f) B. Zhang and A. Studer, Org. Lett., 2014, 16, 3990 CrossRef CAS PubMed; (g) L. Wang, W.-X. Sha, Q. Dai, X.-M. Feng, W.-T. Wu, H.-B. Peng, B. Chen and J. Cheng, Org. Lett., 2014, 16, 2088 CrossRef CAS PubMed; (h) Z.-J. Li, F.-H. Fan, J. Yang and Z.-Q. Liu, Org. Lett., 2014, 16, 3396 CrossRef CAS PubMed; (i) Z.-Q. Zhu, T.-T. Wang, P. Bai and Z.-Z. Huang, Org. Biomol. Chem., 2014, 12, 5839 RSC; (j) W.-X. Sha, J.-T. Yu, Y. Jiang, H.-T. Yang and J. Cheng, Chem. Commun., 2014, 50, 9179 RSC; (k) Q. Dai, J.-T. Yu, X.-M. Feng, Y. Jiang, H.-T. Yang and J. Cheng, Adv. Synth. Catal., 2014, 356, 3341 CrossRef CAS; (l) J. Liu, C. Fan, H.-Y. Yin, C. Qin, G.-T. Zhang, X. Zhang, H. Yi and A.-W. Lei, Chem. Commun., 2014, 50, 2145 RSC; (m) H.-Y. Tu, Y.-R. Liu, J.-J. Chu, B.-L. Hu and X.-G. Zhang, J. Org. Chem., 2014, 79, 9907 CrossRef CAS PubMed; (n) X.-Q. Li, M.-W. Fang, P.-Z. Hu, G. Hong, Y.-C. Tang and X.-S. Xu, Adv. Synth. Catal., 2014, 365, 2103 CrossRef; (o) C.-D. Pan, J. Han, H.-L. Zhang and C.-J. Zhu, J. Org. Chem., 2014, 79, 5374 CrossRef CAS PubMed; (p) G. Wang, S.-Y. Chen and X.-Q. Yu, Tetrahedron Lett., 2014, 55, 5338 CrossRef CAS PubMed; (q) B. Zhang, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 250 CrossRef CAS PubMed; (r) Y.-Z. Gao, J. Wu, J. Xu, X.-R. Wang, G. Tang and Y.-F. Zhao, Asian J. Org. Chem., 2014, 3, 691 CrossRef CAS; (s) Y.-W. Li, G. Qiu, Q.-P. Ding and J. Wu, Tetrahedron, 2014, 70, 4652 CrossRef CAS PubMed; (t) H. Zhu, S.-J. Guo, J. Cheng and J.-T. Yu, Chem. Commun., 2014, 50, 10864 RSC; (u) H.-F. Jiang, H.-L. Gao, B.-F. Liu and W.-Q. Wu, RSC Adv., 2014, 4, 17222 RSC; (v) P. Mampuys, Y.-P. Zhu, T. Vlaar, E. Ruijter, R. V. A. Orru and B. U. W. Maes, Angew. Chem., Int. Ed., 2014, 53, 12849 CrossRef CAS PubMed.
- (a) V. Atlan, C. Buron and L. El Kaïm, Synlett, 2000, 489 CAS; (b) V. Atlan, L. El Kaïm, L. Grimaud, N. K. Jana and A. Majee, Synlett, 2002, 352 CrossRef CAS PubMed; (c) J. E. Ancel, L. El Kaïm, A. Gadras, L. Grimaud and N. K. Jana, Tetrahedron Lett., 2002, 43, 8319 CrossRef CAS; (d) X. Wang, F. Xu, Q. Xu, H. Mahmud, J. Houze, L. Zhu, M. Akerman, G. Tonn, L. Tang, B. E. McMaster, D. J. Dairaghi, T. J. Schall, T. L. Collins and J. C. Medina, Bioorg. Med. Chem. Lett., 2006, 16, 2800 CrossRef CAS PubMed.
- (a) X. Wang, S.-Y. Wang and S.-J. Ji, Org. Lett., 2013, 15, 1954 CrossRef CAS PubMed; (b) L.-L. Zhao, X.-P. Xu, S.-Y. Wang and S.-J. Ji, Chem. Commun., 2013, 49, 2569 RSC; (c) X. Wang, S.-Y. Wang and S.-J. Ji, Org. Lett., 2013, 15, 424 Search PubMed; (d) R. Wang, X.-P. Xu, H. Meng, S.-Y. Wang and S.-J. Ji, Tetrahedron, 2013, 69, 160 CrossRef PubMed; (e) R. Wang, S.-Y. Wang and S.-J. Ji, Tetrahedron, 2013, 69, 10836 CrossRef CAS PubMed; (f) T.-H. Zhu, X. Zhu, X.-P. Xu, T. Chen and S.-J. Ji, Tetrahedron Lett., 2011, 52, 2771 CrossRef CAS PubMed; (g) T.-H. Zhu, S.-Y. Wang, G.-N. Wang and S.-J. Ji, Chem. – Eur. J., 2013, 19, 5850 CrossRef CAS PubMed; (h) Z.-Y. Gu, T.-H. Zhu, J.-J. Cao, S.-Y. Wang and S.-J. Ji, ACS Catal., 2014, 4, 49 CrossRef CAS; (i) T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao, T.-Q. Wei and S.-J. Ji, Org. Lett., 2014, 16, 1260 CrossRef CAS PubMed; (j) T.-H. Zhu, X.-P. Xu, J.-J. Cao, T.-Q. Wei, S.-Y. Wang and S.-J. Ji, Adv. Synth. Catal., 2014, 356, 509 CrossRef CAS; (k) J.-J. Cao, T.-H. Zhu, S.-Y. Wang, Z.-Y. Gu, X. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 6439 RSC.
- T. Kano, M. Ueda and K. Maruoka, J. Am. Chem. Soc., 2008, 130, 3728 CrossRef CAS PubMed.
- (a) R. Soundararajan, S. Krishnamurthy, V. S. Srinivasan and T. R. Balasubramanian, J. Organomet. Chem., 1983, 255, 295 CrossRef CAS; (b) C. Djerassi and C. T. Lenk, J. Am. Chem. Soc., 1953, 75, 3493 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1009375. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00289j |
| This journal is © the Partner Organisations 2015 |