Studying localized corrosion using liquid cell transmission electron microscopy†
See Wee
Chee
*a,
Sarah H.
Pratt
b,
Khalid
Hattar
b,
David
Duquette
a,
Frances M.
Ross
c and
Robert
Hull
a
aDepartment of Materials Science and Engineering, Rensselaer Polytechnic Institute, Troy, NY 12180, USA. E-mail: see.wee.chee@gmail.com
bSandia National Laboratories, Albuquerque, NM 87123, USA
cIBM T. J. Watson Research Center, Yorktown Heights, NY 10598, USA
First published on 7th November 2014
Abstract
Localized corrosion of Cu and Al thin films exposed to aqueous NaCl solutions was studied using liquid cell transmission electron microscopy (LCTEM). We demonstrate that potentiostatic control can be used to initiate pitting and that local compositional changes, due to focused ion beam implantation of Au+ ions, can modify the corrosion susceptibility of Al films.
A liquid platform for performing electrochemical experiments in situ within the transmission electron microscope (TEM) was first demonstrated by Williamson et al.1 and was used for direct observations of the electrodeposition of Cu from solution. A key feature was the use of standard semiconductor microfabrication techniques to construct the microfluidic cell. Since then, liquid cell electron microscopy has seen rapid development and application, particularly in the areas of electrochemistry2–6 and nanoparticle dynamics.7 Liquid cell TEM (LCTEM) work by sandwiching a fluid layer between electron-transparent and impermeable membranes (e.g. silicon nitride) fabricated on chips, which are separated by a spacer typically a few hundred nanometers in height. Hermetic sealing of the liquid cell isolates fluids from the vacuum of the TEM column. Liquid dynamics within the cell may be manipulated by an external pump if flow channels are integrated into the TEM holder. Electrical connections can be made to the sample via wiring that pass through the holder and make contact with thin film electrodes patterned within the cell. Recent reports demonstrated real-time electrochemical studies of the solid–electrolyte interphases6 in lithium ion battery electrodes and of lithiation processes.3–5
The corrosion of metals and alloys is another branch of electrochemistry where LCTEM can have significant impact. Developing a complete understanding of localized corrosion remains one of the fundamental challenges in corrosion science. Metals exhibiting passivity, such as aluminum alloys and stainless steels, commonly suffer from localized corrosion, especially pitting corrosion. Pitting is caused by local breakdown of the protective oxide film leading to accelerated dissolution of the underlying metal.8 However, with existing experimental techniques, it can be challenging to probe the early stages of pitting.9 Difficulties include the small dimensions of pit initiation events, the rare and stochastic nature of these events, the rapid and dynamic nature of pit growth immediately after initiation, and the chemical characterization of the surface film within aqueous environments at length scales comparable with the pit dimensions. LCTEM, coupled with potentiostatic control, has tantalizing potential to provide insight into pit initiation events. One can resolve nanometer size features in LCTEM, even when achievable resolution is degraded by imaging through the liquid layer.10,11 Elemental characterization of corrosion products is also possible. Recent studies have demonstrated the feasibility of chemical analysis using energy dispersive X-ray spectroscopy12,13 (EDX) and electron energy loss spectroscopy2,14 (EELS) in liquid samples.
In this paper, we show images obtained during pitting corrosion of metal films induced by aqueous sodium chloride (NaCl) solutions. Two materials were chosen, Cu because of its relatively high resistance to corrosion, leading to widespread use in water systems, and Al, because it is a model system for pitting corrosion that includes an extensive literature of ex situ observations, which can be compared with in situ results. We show that LCTEM experiments produce corrosion damage similar to those seen ex situ, and identify different morphologies in Al. We then present results aimed at controlling the onset of pitting. We first show the initiation of corrosion by applying a potential to an Al film. Second, we show that compositional changes can be used to modify corrosion susceptibility. Compositional modulations were introduced by patterning an Al film with a focused ion beam of Au ions. Finally, based on these results, we discuss the experimental considerations in performing corrosion studies using LCTEM in the ESI.† For example, the sample must meet the thickness requirement for TEM imaging, in addition to fulfilling the design considerations of a standard electrochemical experiment. The microfluidic cell imposes limitations in terms of sample geometry and electrochemical cell design. Use of corrosive fluids can damage the holder and even the microscope. In spite of the experimental challenges, we conclude that LCTEM can provide detailed and unique information during the onset of corrosion processes.
Fig. 1 illustrates corrosion morphologies observed in Cu and Al films after exposure to aqueous NaCl solutions. Experimental details are presented in the ESI.† Images from a 50 nm thick Cu film under flow of a 6 M NaCl solution are presented in the first row (Fig. 1a–c). After two hours of liquid flow, pits with sizes of a few hundred nanometers and in different stages of development are visible in the copper film. The growth of an existing pit can also be tracked in real time. A representative movie is provided in Movie S1 (ESI†). The images and movies suggest that corrosion occurs via the dissolution of individual grains for Cu. While it is known that Cu will pit under certain conditions, the mechanism of pitting remains poorly understood.15 The susceptibility of Cu to pitting can be influenced by HCO3−, SO42−, and Cl− ions.16 For Cl− ions, pitting behavior shows a complex dependence on the concentration because it controls both oxide film formation and pit initiation via the formation of CuCl.17
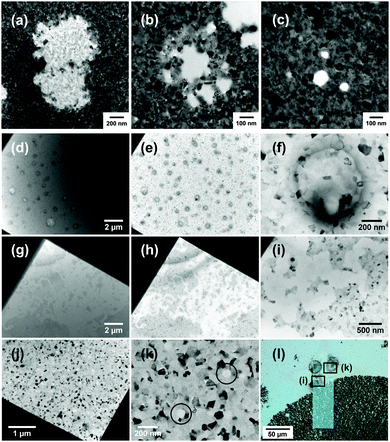 | ||
| Fig. 1 (a–c) Pits at different stages of development observed in 50 nm Cu films after flow of 6 M NaCl solution for 2 hours. (d–f) Blisters formed in a 100 nm Al film after exposure to 0.01 M NaCl solution for 15 hours. (g–i) Meandering corrosion tracks formed in a 100 nm Al film after exposure to 1 M NaCl solution for 4 hours. In (j) and (k), single pits observed in the opposite corner of the windowed area, where in the higher magnification image (k), pits are highlighted with black circles. Images (a–d and g) are obtained with liquid in the cell and (e, f, and h–k) were taken after liquid had been de-wetted with ethanol–water mixtures according to the method given in ref. 23. (l) Light microscope image of the window area in the chip with Al film exposed to 1 M NaCl, taken after the experiment. The locations of (i) and (k) are highlighted. It can be seen from (l) that (i) was an extension of a propagating fractal-like corrosion pattern, whereas (k) is taken from a region of intact film. The two circular features are contamination arising from de-wetting of liquid. | ||
In the experiments shown here, the dissolution rate appear to accelerate when a condensed beam and high electron dose is used. It is clearly important to quantify the role of radiolysis by the electron beam on any corrosion reaction under investigation. Radiolysis of aqueous NaCl solutions by energetic particles is known to create species such as HClO˙−,18 which can turn the solutions oxidizing via formation of hypochlorite.19 Hence, the dose rates used in TEM can strongly alter the solution chemistry.20–22 Simulations (see ESI†) show that under standard imaging conditions used in the current experiments, the concentration of HClO˙− remains low. If the dose rate is increased, it may be necessary to consider the effects of HClO˙− and other radiolysis products when interpreting data.
In contrast, Al is known to only pit in the presence of halide ions such as Cl−. This process can be tracked in the liquid cell, and has been reported in ref. 23 and 24. Fig. 1(d–l) shows representative results. Two prototypical structures found in Al thin film corrosion are reproduced in the in situ TEM experiments. Blisters (Fig. 1d–f), observed after exposure to 0.01 M NaCl solutions, and meandering corrosion tracks (Fig. 1g–i) observed in 1 M solutions. Single pits were also found in the film exposed to 1 M NaCl solution (Fig. 1j and k). Post-mortem images taken with a light microscope (Fig. 1l) show that (Fig. 1i) was an extension of a propagating fractal-like corrosion pattern, whereas (Fig. 1k) belonged to a region of intact film that had begun to pit. Also, note the relatively long times (at least a few hours) required to induce visible corrosion, with the time needed increasing for lower NaCl concentrations. To limit electron dose to the sample during these time periods, development of the film microstructure was observed in snapshots, i.e. at intervals and using the minimum practical electron doses for image recording without condensing the beam (1–2 s exposure at low magnifications and up to 20 s at high magnifications). The liquid layer can also be selectively de-wetted by forming a gas bubble with the electron beam to obtain higher resolution images and then the liquid is re-introduced. The procedure is described in ref. 23. This technique enables optimal imaging resolution of the corrosion structures at reduced dose, minimizing beam effects and radiolysis.
The morphologies shown in Fig. 1 using LCTEM are similar to corrosion structures observed in previous ex situ experiments. Blister formation is known to occur in the early stages of pit formation.25 Chloride ions are thought to penetrate the oxide film and cause metal dissolution beneath the oxide–metal interface. This reaction generates hydrogen gas that causes the oxide film to delaminate from the metal and thus forming the blister. As the amount of hydrogen gas increases, the increasing pressure eventually ruptures the blister and exposes the pit to the bulk solution. The formation of fractal-like corrosion patterns in Al thin films has also been studied.26 It starts with the perforation of the film by a growing pit. When the pit reaches the inert substrate, propagation has to continue laterally in a two dimensional manner. Morphology of the resulting structures is known depend on the concentration of dissolved ions and can range from round pits to fractal patterns.26
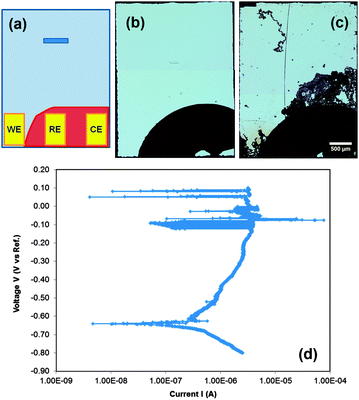
The corrosion structures (blisters, fractal patterns, pits) in Fig. 1 were obtained by exposing the metallic thin films under open circuit conditions (free corrosion). Pit formation can also be induced by anodic polarization using a potentiostat. Fig. 2 shows an ex situ bench top example performed in a basic corrosion cell. The cell was fabricated by depositing Al film over the surface of a blank chip, except in the area where it touches the contacts in the holder. This area was selectively masked so that only one metal lead touches the film (see diagram in Fig. 2(a)). In this case, the reference and working electrodes are made of Ti metal. Depositing a blanket film on blank chips avoids galvanic reactions with the pre-patterned Au electrodes found in electrochemical cells supplied by the manufacturers (discussed further in the ESI†).
The liquid cell was then sealed, a flowing solution of 0.01 M NaCl introduced, and electrochemical experiments carried out on the laboratory bench. The polarization curve in Fig. 2(d) shows the expected behavior for Al in chloride solutions. Fig. 2(b and c) compares light microscopy images of the chip before and after the test. Other than a long scratch made during splitting of the cell, it can be seen that corrosion occurred in the form of fractal corrosion networks. These structures are similar to those seen in Fig. 1(g–i) at higher solution concentrations without the application of potential. The region of Al under the contact point was also often found to be severely corroded. This corrosion at the contact eventually breaks the electrical connection, preventing extended biasing of the samples. Even so, the results demonstrate that this geometry is feasible for electrochemical studies and suggest powerful future opportunities for imaging corrosion under potentiostatic control with improved designs of the corrosion cell.
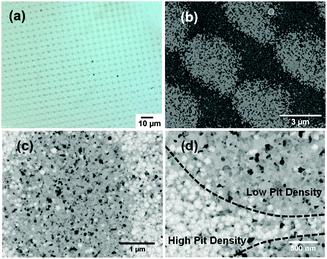
Real world corrosion is often initiated at particular sites on a surface where the composition changes or where two dissimilar materials come into contact in an electrolyte. It is therefore of great interest to examine corrosion in thin films patterned with different metals. In liquid cell chips, physical masking is challenging to implement because of the small area accessible to the electron beam. However, focused ion beams can be used to pattern features with dimensions in the tens of nanometers to micrometers, such that several features can be observed in the electron transparent window. In Fig. 3 we show a 100 nm thick Al film that has been implanted with Au+ ions in an array of spots (5 μm spot to spot separation) using a mass-separated focused ion beam column. Under the conditions used, Au+ ions have a range of about 19 nm (according to SRIM27 simulations) and the implanted ions add less than 1% Au to the entire 450 × 450 μm implanted area. After immersing the sample in 0.1 M NaCl solution for 3 hours, it is clear that corrosion is modulated by the patterning. In Fig. 3(a) and (b), the implanted pattern is reproduced in the corroded film and darker regions indicate a greater amount of corrosion. In Fig. 3(c) and (d), individual pits are resolved, showing that the density of pits within and around the implanted regions is lower than in the surrounding area. Since Au is noble relative to Al, the ion implantation appears to have modified the surface chemistry of the Al film and its susceptibility to pitting. This suggests that useful information can be expected from monitoring the microstructural evolution of corrosion in patterned films in situ.
We have shown that corrosion experiments carried out in situ using liquid cell TEM (LCTEM) can provide information on the onset and development of corrosion morphologies with high spatial and temporal resolution. Corrosion features are visible and can be followed during the evolution of the process. Promising results were achieved using a focused ion beam to modify the surface chemistry and hence pitting sites. Furthermore, polarization curves obtained for Al thin films suggest that LCTEM can provide information on corrosion under an applied bias, given suitable film and electrode geometry. However, it is clear that additional optimization will be beneficial. Further development of these techniques should greatly expand our understanding of fundamental corrosion processes.
S.W.C, D.D., F.M.R. and R.H. acknowledge Dr Joseph Grogan and Dr Jeung-Hun Park for discussions on beam effects, Mr Nicholas Scheidner for modelling the radiolysis species and Ms Ainsley Pinkowitz for her assistance with electrochemistry experiments. Funding at Rensselaer Polytechnic Institute is provided by the National Science Foundation, grant number DMR-1309509. K.H. and S.H.P acknowledge Ms Aubrianna Kinghorn and Dr Bernadette Hernandez-Sanchez for their assistance and the Division of Materials Science and Engineering, the U.S. Department of Energy's Office of Basic Energy Sciences along with Office of Energy and Renewable Energy Water Power Program for partial support. Sandia National Laboratories is a multi-program laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy's National Nuclear Security Administration under contract DE-AC04-94AL85000.
Notes and references
- M. J. Williamson, R. M. Tromp, P. M. Vereecken, R. Hull and F. M. Ross, Nat. Mater., 2003, 2, 532–536 CrossRef CAS PubMed.
- M. E. Holtz, Y. Yu, J. Gao, H. D. Abruna and D. A. Muller, Microsc. Microanal., 2013, 19, 1027–1035 CrossRef CAS PubMed.
- M. Gu, L. R. Parent, B. L. Mehdi, R. R. Unocic, M. T. McDowell, R. L. Sacci, W. Xu, J. G. Connell, P. Xu, P. Abellan, X. Chen, Y. Zhang, D. E. Perea, J. E. Evans, L. J. Lauhon, J.-G. Zhang, J. Liu, N. D. Browning, Y. Cui, I. Arslan and C.-M. Wang, Nano Lett., 2013, 13, 6106–6112 CrossRef CAS PubMed.
- M. E. Holtz, Y. Yu, D. Gunceler, J. Gao, R. Sundararaman, K. A. Schwarz, T. A. Arias, H. D. Abruña and D. A. Muller, Nano Lett., 2014, 14, 1453–1459 CrossRef CAS PubMed.
- B. Layla Mehdi, M. Gu, L. R. Parent, W. Xu, E. N. Nasybulin, X. Chen, R. R. Unocic, P. Xu, D. A. Welch, P. Abellan, J.-G. Zhang, J. Liu, C.-M. Wang, I. Arslan, J. Evans and N. D. Browning, Microsc. Microanal., 2014, 20, 484–492 CrossRef CAS PubMed.
- R. L. Sacci, N. J. Dudney, K. L. More, L. R. Parent, I. Arslan, N. D. Browning and R. R. Unocic, Chem. Commun., 2014, 50, 2104–2107 RSC.
- H.-G. Liao, K. Niu and H. Zheng, Chem. Commun., 2013, 49, 11720–11727 RSC.
- G. Frankel, J. Electrochem. Soc., 1998, 145, 2186 CrossRef CAS PubMed.
- G. Frankel and N. Sridhar, Mater. Today, 2008, 11, 38–44 CrossRef CAS.
- K. L. Klein, I. M. Anderson and N. de Jonge, J. Microsc., 2011, 242, 117–123 CrossRef CAS PubMed.
- N. de Jonge and F. M. Ross, Nat. Nanotechnol., 2011, 6, 695–704 CrossRef CAS PubMed.
- N. J. Zaluzec, M. G. Burke, S. J. Haigh and M. A. Kulzick, Microsc. Microanal., 2014, 20, 323–329 CrossRef CAS PubMed.
- E. A. Lewis, S. J. Haigh, T. J. A. Slater, Z. He, M. A. Kulzick, M. G. Burke and N. J. Zaluzec, Chem. Commun., 2014, 50, 10019–10022 RSC.
- K. L. Jungjohann, J. E. Evans, J. A. Aguiar, I. Arslan and N. D. Browning, Microsc. Microanal., 2012, 18, 621–627 CrossRef CAS PubMed.
- M. Edwards, J. Ferguson and S. Reiber, J. - Am. Water Works Assoc., 1994, 86, 74–90 CAS.
- H. Cong, H. T. Michels and J. R. Scully, J. Electrochem. Soc., 2009, 156, C16 CrossRef CAS PubMed.
- A. El Warraky, H. A. El Shayeb and E. M. Sherif, Anti-Corros. Methods Mater., 2004, 51, 52–61 CrossRef CAS.
- E. Atinault, V. De Waele, U. Schmidhammer, M. Fattahi and M. Mostafavi, Chem. Phys. Lett., 2008, 460, 461–465 CrossRef CAS PubMed.
- M. Kelm, E. Bohnert and I. Pashalidis, Res. Chem. Intermed., 2001, 27, 503–507 CrossRef CAS PubMed.
- P. Abellan, T. J. Woehl, L. R. Parent, N. D. Browning, J. E. Evans and I. Arslan, Chem. Commun., 2014, 50, 4873–4880 RSC.
- J. M. Grogan, N. M. Schneider, F. M. Ross and H. H. Bau, Nano Lett., 2013, 14, 359–364 CrossRef PubMed.
- N. M. Schneider, M. M. Norton, B. J. Mendel, J. M. Grogan, F. M. Ross and H. H. Bau, J. Phys. Chem. C, 2014, 118, 22373–22382 CAS.
- S. W. Chee, F. M. Ross, D. Duquette and R. Hull, MRS Proceedings, 2013, vol. 1525, p. mrsf12-1525-ss11-03 Search PubMed.
- S. W. Chee, D. J. Duquette, F. M. Ross and R. Hull, Microsc. Microanal., 2014, 20, 462–468 CrossRef CAS PubMed.
- E. McCafferty, Corros. Sci., 2003, 45, 1421–1438 CrossRef CAS.
- L. Balazs and J. Gouyet, Physica A, 1995, 217, 319–338 CrossRef CAS.
- J. F. Ziegler, M. D. Ziegler and J. P. Biersack, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, 268, 1818–1823 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Movie S1 showing pit growth in Cu films, details of experimental methods, discussion on design considerations for in situ corrosion studies and supplementary figures. See DOI: 10.1039/c4cc06443g |
| This journal is © The Royal Society of Chemistry 2015 |