Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An overview of recent developments in the analytical detection of new psychoactive substances (NPSs)
Jamie P.
Smith
,
Oliver B.
Sutcliffe
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Science and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, UK. E-mail: c.banks@mmu.ac.uk; Fax: +44 (0)1612476831; Tel: +44 (0)1612471196
First published on 2nd June 2015
Abstract
New psychoactive substances (NPSs), sometimes referred to as “legal highs” in more colloquial environments/the media, are a class of compounds that have been recently made available for abuse (not necessarily recently discovered) which provide similar effects to the traditional well studied illegal drugs but are not always controlled under existing local, regional or international drug legislation. Following an unprecedented increase in the number of NPSs in the last 5 years (with 101 substances discovered for the first time in 2014 alone) its, occasionally fatal, consequences have been extensively reported in the media. Such NPSs are typically marketed as ‘not for human consumption’ and are instead labelled and sold as plant food, bath salts as well as a whole host of other equally nondescript aliases in order to bypass legislative controls. NPSs are a new multi-disciplinary research field with the main emphasis in terms of forensic identification due to their adverse health effects, which can range from minimal to life threatening and even fatalities. In this mini-review we overview this recent emerging research area of NPSs and the analytical approaches reported to provide detection strategies as well as detailing recent reports towards providing point-of-care/in-the-field NPS (“legal high”) sensors.
Introduction
New Psychoactive Substance (NPS) is an umbrella term to refer to substances which mimic the effects of common illicit materials (for example, methamphetamine and cannabis) however they are not controlled by drug legislation such as the Misuse of Drugs Act1 in the United Kingdom and other similar controls internationally. Designed, in some cases deliberately, to evade international control, NPSs may pose a significant danger to the health and safety of the public. As with controlled substances, NPSs are understood to have potentially negative short-term side effects such as paranoia, psychosis and seizures however these may not always be fully understood on account of the materials often being fairly new and understudied, as such their long term health risks are also not always clearly understood.2 The United Nations Office on Drugs and Crime (UNODC) and European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) standardised the term “New Psychoactive Substance(s)” and detailed the following sub-categories: synthetic cannabinoids, synthetic cathinones, ketamine, phenethylamines, piperazines, plant-based substances: Khat, Kratom, Salvia divinorum and Miscellaneous: aminoindanes, phencyclidine, tryptamines.Given the nature of NPSs underhanded production, purposely designed to evade international drug legislation, they are intrinsically marketed and sold as “legal highs”. Easily available at ‘head shops’ (a commercial outlet selling cannabis and tobacco paraphernalia), market stalls and the internet; vendors of NPSs are often operating on the edge of legality by being both vague and creative in their description of the products contents and its purported uses. NPSs may be sold as research chemicals, plant food, bath salts, exotic incenses etc. together with slightly more telling descriptors such as: party pills, herbal highs and smoking blends although these names can often be mercurial, for example, mephedrone (a synthetic cathinone) pre-control was plant food whereas after becoming a controlled substance it was referred to as a ‘research chemical’.
Although given these nondescript aliases, NPSs products often have brand names; examples of “legal high” brand names are ‘Benzo Fury’, ‘Afghan Incense’, ‘NRG-1’ and ‘NRG-2’. The name or description given to a NPSs or “legal high” product may not always pertain to what is the actual psychoactive substance present, for example mephedrone was detected in products sold as naphyrone or NRG-1 in the UK even after its ban,3 another survey found 70% of NRG-1 and NRG-2 products examined contained mixtures of substituted cathinones and not, at the time uncontrolled, naphyrone.4 Clearly there are no assurances to the customer of these NPS products that the contents are the same as advertised, furthermore they may be unwittingly violating drug legislation as the contents within are controlled substances.
Abuse of NPSs has been reported to be increasing since ca. 2009 and has continued to be an ever growing market5 emerging at an unprecedented rate something also reflected in the online marketplace with the number of online vendors in the UK increasing by more than 300% between 2010 and 2011.6 New materials made available for abuse appear rapidly and, at times, can gain a ‘foothold’ in the market – such as mephedrone. In 2014, 101 new substances were reported for the first time to the EU early warning system (EWS) run by the EMCDDA up from 81 in 2013 which is also an increase from the 74 substances notified in 2012.7 Of the findings of the EWS synthetic cannabinoids are the most frequently discovered with 102 detected between 2005 and 2013. A graphical representation of NPSs notified to the EWS between 2005–2014 is shown in Fig. 1.7
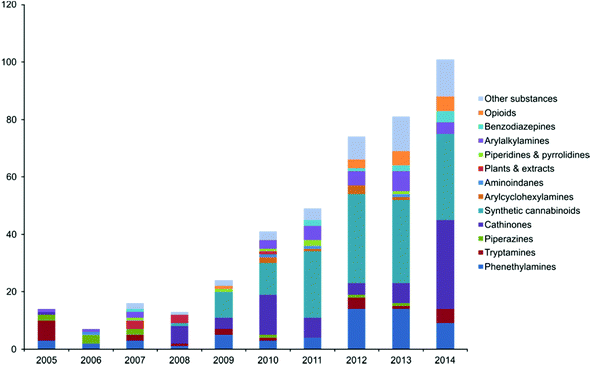 | ||
| Fig. 1 A graphical representation of new psychoactive substances notified to the EWS between 2005–2014. Reproduced from ref. 7 with permission of the EMCDDA. | ||
The media has reported on numerous deaths related to “legal highs” and given the wide variety of NPS and the ever-changing composition of existing products, a completely new field of research has emerged in the continual development of analytical techniques along with presumptive tests and in-the-field sensors. To date, there are reviews on the chemistry, pharmacology and toxicology of NPSs but no comprehensive review of the current techniques for the analysis of these substances has, to-date, been compiled.
In this mini-review a thorough overview of this new analytical field of NPSs is provided which covers: synthetic cannabinoids (most frequently discovered NPS by the EWS), synthetic cathinones; particularly mephedrone (amidst reports by the Crime Survey for England and Wales [CSEW] detailing mephedrone as the most prevalent of abused NPSs) and in lesser detail pieces of interesting research of the other NPSs notified to the EWS (visible in Fig. 1).7
Synthetic cathinones
Synthetic cathinones are an amphetamine-like cheap alternative to Ecstasy derived from cathinone; an organic stimulant found in Khat – a plant native to East Africa and the Middle-East and they possess pharmacological similarity to the phenethylamine class of psychoactives (e.g. amphetamine and methamphetamine). The effects of synthetic cathinones on the body are reported to have both cardiovascular and neurological side-effects; believed to block the reuptake of norepinephrine, dopamine and serotonin8 whilst there are also reports that they also induce the release of more dopamine9 suggesting synthetic cathinones act like both methamphetamine and cocaine synchronously.8–12Internationally there has been a tightening of the legislation regarding synthetic cathinone derivatives, for example, cathinones are illegal in the UK as well as Germany, The United States, Canada and many others.13,14 The European Monitoring Centre for Drugs and Drug Addiction's (EMCDDA) Early Warning System (EWS) has reported 74 new synthetic cathinones between 2005 and 2014, with 30 new substances discovered in the year 2014 alone (Fig. 1). Clearly, the epidemic initiated by synthetic cathinones is showing no signs of cessation within the near future hence the development of methods for their detection and quantification is timely and urgently required. Mephedrone in particular, since it's availability for abuse, is popular amongst users of “legal high” products and despite its classification in 2009 reports from the Crime Survey for England and Wales (CSEW) reveal mephedrone was still being abused in England and Wales in 2014.
Popularly known as ‘bath salts’, ‘research chemicals’ or ‘plant food’, synthetic cathinones are sold under, often mercurial, non-descript brand names such as ‘Energy’ (NRG), Blizzard and Ivory Snow containing warning labels such as ‘not for human consumption’ or ‘not tested for hazards or toxicity’ in an attempt to bypass legislative controls. The active component in a “legal high” product can vary wildly, even within the same brand name;3,10,15 there are no clear assurances to the customer of these NPS products that the contents are the same as advertised (see introduction).
The list of case reports concerning synthetic cathinone-induced intoxication is extensive and ever increasing. In the United States the number of calls to emergency centres, as a result of synthetic cathinone abuse, increased from 303 to 6100 between 2010 and 2011. A plethora of case reports are reported in the literature and media spanning a sizeable age range, including both of the sexes and include fatalities as well as the curious report of the murder of a goat whilst dressed in lingerie.16 For instance, a female aged 15 had symptoms of nausea, vomiting, altered mental status, euvolaemic hypo-osmotic hyponatremia with encephalopathy and increased intracranial pressure – mephedrone metabolites were found in her urine.17 A male aged 31 after admitting to taking three 1500 mg packets of “bath salts” and was reported to have hallucinations, paranoia, agitation; elevated serum CPK level, hyperkalemia, dehydration, rhabdomyolysis and acute renal failure.
Considering all the synthetic cathinones discovered, there can be no assertions to which are the being abused but what is evident from the literature is that the most prominent synthetic cathinones found within “legal high” products globally are mephedrone (4′-methylmethcathinone; 4-MMC) and 3′,4′-methylenedioxypyrovalerone (MDPV).15 Mephedrone is more prevalent in Europe and MDPV in the United States;15 a list of the most prevalent cathinone derivatives18 abused worldwide can be found in Table 1 although the focus of the review will apply generally towards the detection and quantification of mephedrone.
| Usual names | Chemical name |
|---|---|
| Amfepramone or diethylpropion | 2-Diethylamino-1-phenyl-1-propanone |
| Benzedrone or 4′-methyl-N-benzylcathinone or 4-MBC | 1-(4-Methylphenyl)-2-benzylamino-1-propanone |
| BMDB | 2-Benzylamino-1-(3,4-methylenedioxyphenyl)-1-butanone |
| BMDP or 3,4-MDBC | 2-Benzylamino-1-(3,4-methylenedioxyphenyl)-1-propanone |
| Brephedrone or 3′-bromomethcathinone or 4-BMC | 1-(4-Bromophenyl)-2-(methylamino)-1-propanone |
| Buphedrone | 2-(Methylamino)-1-phenyl-1-butanone |
| Bupropion | 1-(3-Chlorophenyl)-2-(tert-butylamino)-1-propanone |
| Butylone or bk-MBDB | 2-(Methylamino)-1-(3,4-methylenedioxyphenyl)-1-butanone |
| Cathinone | 2-Amino-1-phenyl-1-propanone |
| Dibutylone or methylbutylone or bk-DMBDB | 2-(Dimethylamino)-1-(3,4-methylenedioxyphenyl)-1-butanone |
| Dimethylone or bk-MDDMA | 1-(1,3-Benzodioxol-5-yl)-2-(dimethylamino)-1-butanone |
| 3′,4′-dimethylmethcathinone or 3,4-DMMC | 1-(3,4-Dimethylphenyl)-2-(methylamino)-1-propanone |
| Ephedrone or methcathinone | 2-(Methylamino)-1-(4-ethylphenyl)-1-propanone |
| Ethylbuphedrone or NEB | 2-(Ethylamino)-1-phenyl-1-butanone |
| Ethylcathinone or ethcathinone or ethylpropion | 2-(Ethylamino)-1-phenyl-1-propanone |
| 4′-ethylmethcathinone or 4-EMC | 2-(Methylamino)-1-phenyl-1-propanone |
| Ethylone or bk-MDEA | 2-(Ethylamino)-1-(3,4-methylenedioxyphenyl)-1-propanone |
| Eutylone ou bk-EBDB | 1-(1,3-Benzodioxol-5-yl)-2-(ethylamino)-1-butanone |
| Flephedrone or 4′-fluoromethcathinone or 4-FMC | 2-(Methylamino)-1-(4-fluorophenyl)-1-propanone |
| Fluorocathinone or 4-FC | 2-Amino-1-(4-fluorophenyl)-1-propanone |
| Fluoromethcathinone or 3-FMC | 2-(Methylamino)-1-(3-fluorophenyl)-1-propanone |
| Isoethcathinone | 2-(Ethylamino)-1-phenyl-2-propanone |
| Isopentedrone | 2-(Methylamino)-1-phenyl-2-pentanone |
| MDMPP | 1-(3,4-Methylenedioxyphenyl)-2-methyl-2-pyrrolidinyl-1-propanone |
| MDPBP | 1-(3,4-Methylenedioxyphenyl)-2-(1-pyrrolidinyl)-1-butanone |
| MDPPP | 1-(3,4-Methylenedioxyphenyl)-2-(1-pyrrolidinyl)-1-propanone |
| MDPV or MDPK | 1-(3,4-Methylenedioxyphenol)-2-pyrrolidinyl-1-pentanone |
| Mephedrone or 4′-methylmethcathinone or 4-MMC | 2-(Methylamino)-1-(4-methylphenyl)-1-propanone |
| Metamfepramone or dimethylcathinone or dimethylpropion | 2-Dimethylamino-1-phenyl-1-propanone |
| Methedrone or 4′-methoxymethcathinone or bk-PMMA | 1-(4-Methoxyphenyl)-2-(methylamino)-1-propanone |
| Methylbuphedrone or 4-Me-MABP or bk-N-methyl-4-MAB | 2-(Methyllamino)-1-(4-methylphenyl)-1-butanone |
| 4′-methyl-N-ethylcathinone or 4-MEC | 2-(Ethylamino)-1-(4-methylphenyl)-1-propanone |
| 3′-methylmethcathinone or 3-MMC | 2-(Methylamino)-1-(3-methylphenyl)-1-propanone |
| Methylone or MDMC or bk-MDMA | 2-Methylamino-1-[3,4-methylenedioxyphenyl]-1-propanone |
| MOPPP | 4′-Methoxy-α-pyrrolidinovalerophenone |
| MPBP | 1-(4-Methylphenyl)-2-(1-pyrrolidinyl)-1-butanone |
| MPHP | 4′-Methyl-α-pyrrolidinovalerophenone |
| MPPP | 4′-Methyl-α-pyrrolidinovalerophenone |
| Naphyrone | 1-Naphthalen-2-yl-2-pyrrolidin-1-yl-1-pentanone |
| Propylbutylone or bk-PBDB | 2-(Propylamino)-1-(3,4-methylenedioxyphenyl)-1-butanone |
| Pentedrone or ethyl-methcathinone | 2-(Methylamino)-1-phenyl-1-pentanone |
| Pentylone | 2-(Methylamino)-1-(3,4-methylenedioxyphenyl)-1-pentanone |
| PBP | 1-Phenyl-2-(1-pyrrolidinyl)-1-butanone |
| PEP | 1-Phenyl-2-(1-pyrrolidinyl)-1-heptanone |
| PPP | 1-Phenyl-2-(1-pyrrolidinyl)-1-propanone |
| PVP | 1-Phenyl-2-(1-pyrrolidinyl)-1-pentanone |
| Pyrovalerone | 11-(4-Methylphenyl)-2-(1-pyrrolidinyl)-1-pentanone |
| 2′,4′,5′-trimethoxymethcathinone or 2,4,5-TMMC | 2-(Methylamino)-1-(2,4,5-trimethylphenyl)-1-propanone |
Studying the patterns of NPS abuse can be difficult as it is frequently based upon self-reported user surveys.19 This is potentially problematic as in many instances users are, due to poorly labelled products (see earlier), not in fact aware of the substances they are taking. In light of this, numerous groups are making advances towards screening the current NPSs being abused. A number of revered groups using a range of chromatographic techniques including high performance-liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), with LC-MS methods seemingly the preferred and established technique of choice, have published exhaustively upon the laboratory-based analysis of synthetic cathinones,3,20–40 phase I and II metabolites41,42 and more recently, in light of the often nonenantioselective NPS synthesis, chiral separation of racemic mixtures.43
In 2014 Archer et al.19 analysed urine samples collected from a night club over one weekend. The manuscript with its real and imaginative title, “Taking the Pissoir – a novel and reliable way of knowing what drugs are being used in nightclubs”, reported the detection of classical recreational drugs and NPSs such as: mephedrone, 3′-trifluoromethylphenylpiperazine and 2-aminoindane using various chromatographic and mass spectrometric methods.19 Furthermore parent drug/metabolites were also detected for amphetamine, cocaine, ketamine, 3′,4′-methylenedioxymethamphetamine (MDMA), mephedrone and 3-trifluoromethylphenylpiperazine (3-TFMPP); this is important as it indicates drugs were being used and not simply discarded into the urinal.19 In the same year, Leffler et al.44 (located in the United States) analysed 14 separate street samples wherein 10 synthetic cathinones were identified employing a variety of techniques, including gas chromatography with mass spectrometric detection (GC-MS) and flame ionization (GC-FID).44 HPLC direct infusion tandem mass spectrometry (MS/MS) was also used to identify compounds which were not available as reference materials. Out of the synthetic cathinones detected: 3′,4′-methylenedioxypyrovalerone (MDPV), 3′,4′-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP), 4′-fluoromethcathinone (4-FMC), butylone, mephedrone, naphyrone, 4′-methylethcathinone (4-MEC), ethcathinone, α-pyrrolidinopentiophenone (α-PVP), and 3′-methyl-α-pyrrolidinopropiophenone (3-MPPP). MDPV was the most prevalent, found in five of the 14 samples and ranging from 11% to 73% (w/w) between samples.44
Earlier reports in Denmark, Pedersen et al.35 presented an automated solid-phase extraction (SPE) and ultra-high-performance liquid chromatography (UHPLC) with time of flight-mass spectrometry (TOF-MS) screening method for 256 illicit compounds in blood and 95 of these compounds were validated with regard to matrix effects, extraction recovery, and process efficiency with the limit of detection (LOD) ranging from 0.001 to 0.1 mg kg−1.35 Application of the technique to the analysis of 1335 forensic traffic cases revealed 992 cases (74%) were positive for one or more traffic-relevant drugs above the Danish legal limits. Commonly abused drugs such as amphetamine, cocaine, and frequent types of benzodiazepines were the major findings. Nineteen less frequently encountered drugs were detected: buprenorphine, butylone, cathine, fentanyl, lysergic acid diethylamide, m-chlorophenylpiperazine, MDPV, mephedrone, 4′-methylamphetamine, p-fluoroamphetamine, and p-methoxy-N-methylamphetamine.35
Even as early as 2011, there have been numerous attempts at constructing screening methods for substituted cathinones in a number of different matrices, Bell and co-workers29 reported a rapid multi-analyte direct urinalysis LC-MS/MS screening method being able to detect eight analytes including; 4′-methylmethcathinone (mephedrone), 3′,4′-methylenedioxymethcathinone (bk-MDMA, ‘methylone’), 4′-methoxymethcathinone (bk-PMMA, ‘methedrone’) and 3′,4′-methylenedioxypyrovalerone (MDPV).29 Using a dilution of 1 part urine to 4 mobile phase to reduce matrix effects and although not all compounds were completely chromatographically resolved, there was sufficient specificity to allow target analyte identification. All the analytes were readily detected at a concentration of 500 ng mL−1 offering an attractive method for the routine screen of NPSs.29 The global impact of synthetic cathinones is compounded when substances such as mephedrone and MDPV have been detected following sewage-based epidemiology in Chinese ‘megacities’.45
In terms of quantification, Santali et al. provided the first fully validated HPLC method for the quantification of mephedrone22 where limits of detection and quantification of 0.1 and 0.3 μg mL−1 respectively were reported. Khreit et al. further refined this method enabling the detection of both mephedrone and two novel derivatives, 4′-methyl-N-ethylcathinone (4-MEC) and 4′-methyl-N-benzylcathinone (4-MBC), in seized samples of “NRG-2”. In this case the limits of detection and quantification were reported as 0.03 and 0.08 for 4-MEC and 0.05 and 0.14 μg mL−1 for 4-MBC both in their pure form and in the presence of common adulterants such as caffeine and benzocaine.3,23 There has also been work using chromatographic methods on the detection of cathinone based “legal highs” in biological matrices24,37 in which Beyer et al. were able to detect and quantify 25 designer cathinones in a validated LC-MS-MS method.37
Other work26 has seen an attempt to screen chronic abuse of mephedrone through GC-MS analysis of hair. The hair was first decontaminated in methylene chloride and incubated overnight in a pH 7 buffer in the presence of deuterated MDMA at 40 degrees Celsius. The work saw 67 hair specimens tested for mephedrone with 13 yielding positive results of concentrations ranging from 0.2–313.2 ng mg−1.26 The work showed that like other stimulant drugs, mephedrone is well incorporated into hair and the analytical method reported appears sensitive enough to reveal occasional to regular use of mephedrone.26
Recently direct analysis in real time mass spectrometry (DART-MS) has been utilised to quantify and characterise the multitude of new and emerging NPSs.46 Solid synthetic cathinone samples (2-FMC, 2-MEC, 2-FEC and 2-EEC) were sampled directly without pre-treatment and positive ion mass spectra were acquired using a DART-SVP™ ion source interfaced to an AccuTOF mass spectrometer. Further advancements in this methodology by the same authors47 has seen the application of a time-of-flight (TOF) mass analyzer along with in-source collision-induced dissociation (CID) spectra to provide data for presumptive analysis of various synthetic cathinones in a similar fashion to GC-MS analysis.47 The authors scope for this work is to provide a rapid screening method to quickly respond to the rapid evolution of designer drugs and the consequent testing backlogs that develop.46,47 Ion mobility spectrometry (IMS) has also been applied to the screening of an array of NPSs within the literature with acceptable results.48,49
Smith et al.50,51 provided an alternative to chromatography and proposed a novel sensing protocol based upon the electrochemical methods. Of note is the reduction of the cathinone substitutes; mephedrone and 4-MEC with a scope to provide an on-the-spot analytical screening tool with cyclic voltammetry.50 Analysed in pH 4.3 acetate buffer, limits of detection were found to correspond to 11.80 μg mL−1 for 4-MMC and 11.60 μg mL−1 for 4-MEC.50 This work demonstrated for the first time a rapid, accurate, and sensitive method for the quantification of synthetic cathinone components found in seized street “legal high” samples (NRG-2) via the use of an electrochemical protocol utilizing graphite screen-printed electrodes (GSPEs) which was also independently verified with HPLC.50
Interesting developments in the detection of synthetic cathinone derivatives is the use of surface enhanced Raman-spectroscopy (SERS) have also been reported.52,53 In this novel approach, the usually required thin metallic surface (typically gold or silver) was provided by galvanising a British two pence coin with silver. Note that a pre-1992 two pence coin (97% Copper) is required as post-1992 two pence coins are composed of copper-plated steel and have an undefined composition.52Fig. 2 shows the concept when dendritic structures are evident on the two pence surface, demonstrating proof-of-concept for SERS detection of mephedrone, MDMA and aminoindane 5′,6′-methylenedioxy-2-aminoindane (MDAI).52 Further developments saw the researchers working towards a new optimization strategy for the SERS detection of mephedrone using a portable Raman system employing a fractional factorial design approach to significantly reduce the statistical experiments whilst maintaining statistical integrity.53 Furthermore, four optimised SERS protocols for which the reproducibility of the SERS signal and the limit of detection of mephedrone were established with an estimated limit of detection of 1.6 μg mL−1.53
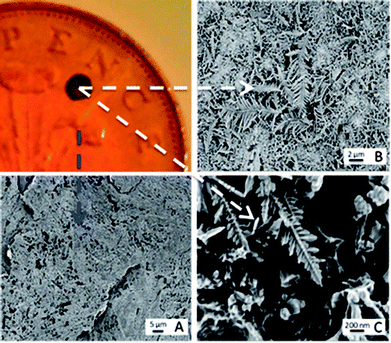 | ||
| Fig. 2 Characterisation of galvanic displacement. The optical image (top left) shows a clean British 2p coin, with silver deposited onto its surface. (A) Shows an SEM of the rough surface of the two pence after cleaning. The SEM in (B) shows the silver dendritic structures that are formed on the coins surface once 10 μL of AgNO3 was left to mature for 20 s at room temperature (23 °C). The fern like structures are magnified in (C) and show that secondary crystalline domains grow perpendicular from a primary silver backbone.52 Reproduced from ref. 52 with permission of The Royal Society of Chemistry. | ||
Another alternative to the well-established chromatographic methods, NPS detection has been reported with the use of immunochemistry, Paillet-Loilier et al.18 noted the use of this technique to test the cross-reactivity of some synthetic cathinones using the semi-quantitative AxSYM amphetamine/methamphetamine II assay in tandem with Fluorescence Polarization Immunoassay (FPIA). Evaluating the responses from aqueous solutions of 14 substituted cathinones at 1 mg L−1, 10 mg L−1 and 100 mg L−1, the authors observe pentedrone, pentylone, α-pyrrolidinovalerophenone (PVP), and 3′,4′-methylenedioxypyrovalerone (MDPV) did not react with the protocol. Some synthetic cathinones, however, reacted in the assay at 10 mg L−1: ethylone, mephedrone, methylone, methedrone, and 4′-methyl-N-ethylcathinone (MEC) scrutiny of this reveals that each of these that did react had the least substitutions on the ethylamine chain suggesting the method has limitations to larger molecules.18 Commercially available enzyme-linked immunosorbent assays have been used to analyse eight synthetic cathinone derivatives amongst 30 designer drugs.54 The test demonstrated cross-reactivity at concentrations at low as 0.15 mg L−1 when tested against the Randox Mephedrone/Methcathinone ELISA kit (RANDOX Toxicology, Crumlin, UK), a protocol recently developed for forensic specific cathinone screening in urine and blood specimens.54
Presumptive testing of cathinone derivatives was carried out by Nic Daeid and colleagues40 as per United Nations recommended guidelines. Various presumptive tests were investigated, however results suggested the Zimmerman test, which relies on the presence of a carbonyl group in close proximity to a methyl group on the same molecule and reaction with 2′,4′-dinitrobenzene to form a Meisenheimer reddish-purple colour, was the most consistently effective test method. A small amount of each test sample was placed into a well of a spotting tile and 2 drops of 1% 2′,4′-dinitrobenzene in methanol followed by 2 drops of 15% potassium hydroxide in water were added. Any colour change or other noticeable effect occurring immediately on addition of the reagents was noted and observations were made again after 5 minutes; Specific colour changes were observed in all cases apart from bupropion. Nic Daeid et al. have also reported using stable isotopic fractionation/profiling (isotope ratio mass spectrometry; IRMS), to provide a potentially quantifiable link between the precursor (4′-methylpropiophenone) and the illicit drug product (4′-methylmethcathinone) for a particular manufacturer and synthetic route of mephedrone.55
Synthetic cannabinoids
Synthetic cannabinoids emerged as a recreational product ca. 2008 in the form of aminoalkylindoles such as JWH-018. They were originally investigated by Professor Huffman56 as therapeutic compounds, however they were subsequently abandoned due to the unwanted psychoactive side effects. Despite many classes synthetic cannabinoids becoming controlled under drug legislation, there are still many which remain legal whilst still posing threat to the population. As with synthetic cathinone derivatives, there is often limited to no information on the packaging of the products and the active ingredients present can vary greatly between products of the same name.57–61 These compounds were first introduced into products known as ‘K2’ and ‘Spice’ with the latter having a market range of: Spice Silver, Spice Gold and Spice Diamond.† The products, advertised as incense or smoking mixtures, are typically sold consisting of a few grams of finely cut green/brown plant material as to perhaps replicate the appearance of cannabis whilst being infused with the active synthetic cannabinoid component(s). There are instances of retailers selling the active components as research chemicals (similarly to synthetic cathinones) which arrive as a crystalline powder of high purity.61There are various case reports to support the literature and media claims that synthetic cannabinoids have psychoactive effects akin to that of cannabis. Indeed, the components of Spice and related herbal products have been identified as aminoalkylindoles originally synthesised by Huffman and Atwood et al. and have demonstrated that JWH-018 is a potent and effective CB1 receptor agonist.62
Interesting case reports with regards to the effects of the Spice epidemic include a report by Schneir et al.,63 who published case studies on two women admitted to a San Diego (USA) emergency department after smoking Spice “Banana Cream Nuke” – disorientated, feeling unusual and “as if they did not know where they were”.63 Another report describes three cases of the effects of Spice,64 all having a negative urine drug screen whilst exhibiting agitation, paranoia and tachycardia. Follow up analysis revealed the urine to contain metabolites of JWH-018 and JWH-073.64 More recent reports also highlight similar observations in adolescents and young adults after intoxication with synthetic cannabinoids.65 Vardakou et al.66 have given an overview of other case reports66 and the psychoactive properties of Spice products and “legal highs”.
Laboratory analysis revealed the active components of first generation Spice and related products to be, the previously mentioned, aminoalkylindoles such as JWH-018 and also cyclohexylphenols such as CP-47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497. As their popularity rose through sales in so-called ‘head shops’ as well as on the internet, the substances were legislated as illegal in most countries worldwide;67 the range of active synthetic cannabinoid components of first generation Spice products can be observed in Scheme 1. Note: the aminoalkylindoles (see Scheme 1) are given the notation of JWH after the academic who first synthesised these compound, Professor J.W. Huffman.
497. As their popularity rose through sales in so-called ‘head shops’ as well as on the internet, the substances were legislated as illegal in most countries worldwide;67 the range of active synthetic cannabinoid components of first generation Spice products can be observed in Scheme 1. Note: the aminoalkylindoles (see Scheme 1) are given the notation of JWH after the academic who first synthesised these compound, Professor J.W. Huffman.
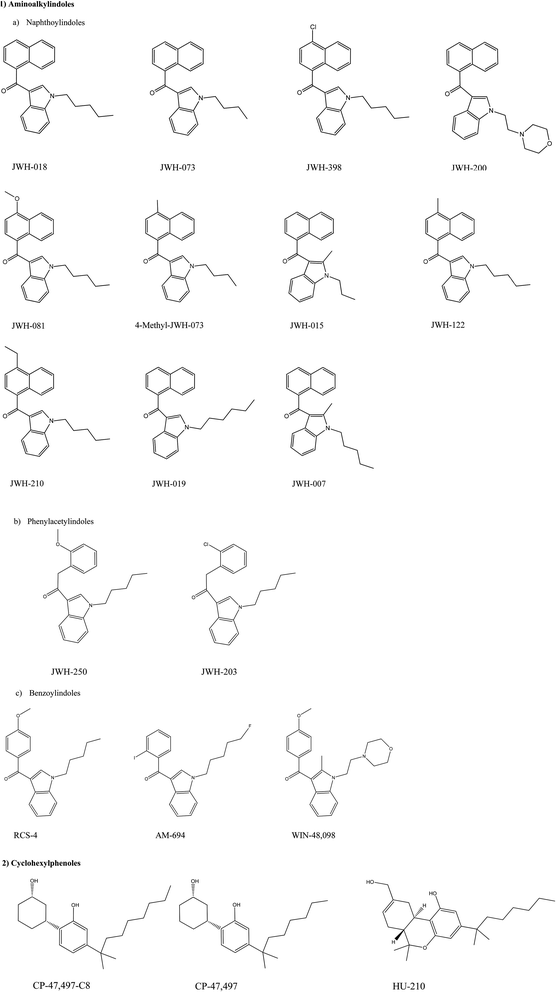 | ||
| Scheme 1 Chemical structures of synthetic cannabinoids found in herbal products such as the spice range,67 scheme reproduced from ref. 67 with permission from UNODC. | ||
Further confirmation of this came at the end of 2008 when the German company THC Pharma reported JWH-018 was an active ingredient in Spice products.68 Following on from this Auwater et al.69 and Uchiyama et al.70 identified and characterized the CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 (see Scheme 1) as its isomer – a synthetic by-product in Spice Silver, Gold and Diamond as well as in products named ‘Yuctan Fire’ and ‘Sence’ which is reported to have 5 to 10 times more analgesic potency that tetrahydrocannibol.71
497-C8 (see Scheme 1) as its isomer – a synthetic by-product in Spice Silver, Gold and Diamond as well as in products named ‘Yuctan Fire’ and ‘Sence’ which is reported to have 5 to 10 times more analgesic potency that tetrahydrocannibol.71
An interesting paper from the point of view of the medical staff that have had to deal with the Spice usage patients has a light-hearted title of: “Spice” girls: Synthetic cannabinoid intoxication.63 The authors noted that a urine drugs-of-abuse immunoassay was negative for amphetamines, barbiturates, benzodiazepines, benzoylecgonine (cocaine metabolite), methadone and opiates, oxycodone, phencyclidine, propoxyphene and tetrahydrocannabinoids. The residue of the patient's Spice product “Banana Cream Nuke” was found to contain the synthetic cannabinoids JWH-018 and JWH-073 through gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography with ultraviolet detection (HPLC-UV). The report highlighted the need for drugs-of-abuse screenings to be able to detect the JWH class of compounds, particularly within a clinical setting.
In Germany Lindigkeit et al. analysed Spice Gold with a GC-MS method wherein the herbal mixtures were ground and put through a two hour Soxhlet extraction with petroleum ether.58 Analysis revealed the samples contained CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 and JWH-018 until German health authorities on the 22nd January 2009 prohibited the sale of the active components found in Spice – from this point JWH-018 was absent from Spice, however it wasn't long until a new analogue, JWH-073, was found to be contained in Spice products.58 Because the manufacturers of such products can readily change the active components in Spice, a rapid method of detecting prohibited compounds in the complex mixtures is highly sought after.
497-C8 and JWH-018 until German health authorities on the 22nd January 2009 prohibited the sale of the active components found in Spice – from this point JWH-018 was absent from Spice, however it wasn't long until a new analogue, JWH-073, was found to be contained in Spice products.58 Because the manufacturers of such products can readily change the active components in Spice, a rapid method of detecting prohibited compounds in the complex mixtures is highly sought after.
To this end, Emanuel and co-workers68 reported for the first time the components of Spice “Gold Spirit” using GC-MS (following a simple liquid extraction) alongside the analysis of Spice “Gold” and “Diamond”; at the time the three most popular Spice products used. Results indicated that Spice “Gold” contained CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 along with ethyl vanillin, α-tocopherol and γ-tocopherol whereas Spice “Diamond” contained caffeine, α-tocopherol, γ-tocopherol, palmitic acid along with CP 47
497-C8 along with ethyl vanillin, α-tocopherol and γ-tocopherol whereas Spice “Diamond” contained caffeine, α-tocopherol, γ-tocopherol, palmitic acid along with CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 and JWH-018. As for Spice “Gold Spirit”, JWH-018 and α-tocopherol were found to be present.68
497-C8 and JWH-018. As for Spice “Gold Spirit”, JWH-018 and α-tocopherol were found to be present.68
Other work has of course followed on the analysis of Spice and related herbal products for instance Uchiyama and co-workers59 who analysed 46 different herbal products with 44 having synthetic cannabinoids as determined via GC-MS and LC-MS. Two major cannabinoids were found; [2-hydroxy-4-(2-methylnonan-2-yl)phenyl]cyclohexan-1-ol (cannabicyclohexanol) and JWH-018 and the analysis of the herbal product (amount of NPS per gram) were found to range from 1.1 to 16.9 mg g−1 and 2.0 to 35.9 mg g−1 respectively.59
In addition to the identification of the chemical components contained within the Spice product range there is a need to understand the effects of the synthetic cannabinoids on the human metabolism. Sobolevsky72 reported for the first the time, urinary metabolites of JWH-018; clearly highly useful for the analysis of patients admitted to emergency departments and for the development of point-of-care tests (see the story of the “Spice girls” earlier in this mini-review). Using LC-MS and GC-MS, two main monohydroxylated metabolites were identified which are almost completely glucuroconjugated with minor metabolites such as N-despentyl hydroxy-, carboxy-, dihydroxy-, and reduced di- and trihydroxy-metabolites.72 It should be noted the parent compound (JWH-018) was reported to not be detected in urine.72 The authors observed that there are two main metabolites that are valuable for detection of JWH-018 in post-administration urine and LC-MS is a more useful technique as minor metabolites can also be analysed to support analytical findings.72 Different analytical approaches on Spice and related products have been reported73–78 with literature reporting the presence of new cannabimimetic compounds.60,79,80 Following this pioneering work, there has been a pursuit of studying synthetic cannabinoids in urine.81–87 Further work by Moran et al.88 has extended the work of Sobolevsky72 and validated an LC-MS/MS method for the quantitation of the human urine metabolites of JWH-018 and JWH-073. The work highlighted 6 metabolites for each molecule with the primary metabolites being distinguishable between JWH-018 and JWH-073. The authors have also extended this using a solid-phase extraction approach.89 One criticism of the above work exploring the metabolites in urine is the limited population studies – clearly larger studies will be needed to further understand the pharmacology of synthetic cannabinoids. Other research has been devised to quantify cannabinoids in serum and blood.90–94
A different strategy has been to analyse cannabinoids in hair to show long term past consumption.95 To this end, Hutter et al.96 reported the hair testing of 22 synthetic cannabinoids in human hair. The methodology involves a simple ultrasonication of the hair sample in ethanol and has a limit of quantification (LOQ) of 0.5 pg mg−1.96 Perhaps more interestingly, synthetic cannabinoids have even been found in the urine of US athletes (although its use to enhance performance is questionable.). Urine samples were collected from 5956 athletes and analysed via high performance-liquid chromatography-tandem mass spectrometry (HPLC-MS) for the presence of JWH-018, JWH-073 and their metabolites.97 In 4.5% of the samples, metabolites of both synthetic cannabinoid compounds were detected; metabolites of JWH-018 and JWH-073 (50%), JWH-018 (49%), and only JWH-073 (1%) were detected in positive samples.
The focus of the research above has focused on laboratory based instrumentation, rightly so in order to unambiguously quantify NPSs but as highlighted in the case of the “Spice girls”, synthetic cannabinoids do not react using tradition THC immunoassay tests. To this end Arnston et al.98 have designed two enzyme linked immunosorbent assays for detection of JWH-018 and JWH-250 in urine. The assay of JWH-018 has significant cross reactivity with several synthetic cannabinoids and their metabolites contrary to the JWH-250 assay which exhibits limited cross-reactivity. To start, assays are calibrated at 5 ng mL−1 with the 5-OH metabolite of JWH-018 and the 4-OH metabolite of JWH-250. To validate the method, 114 and 84 samples of urine for JWH-018 and JWH-250 respectively were used and confirmed by using liquid chromatograph tandem mass spectrometry (LC-MS/MS) testing for metabolites of JWH-018, JWH-019, JWH-073, JWH-250 and AM-2201. Accuracy was deemed to be greater than 98% with 95% sensitivity and specificity for both assays.
Another approach of interest is a presumptive test marketed by “Narcotic Testing Supplies & Equipment Store”.99 The test works by inserting a small quantity of a suspected sample into a plastic ampoule containing 25 μL reagent and 150 mg of specially treated absorbing crystals (sodium 36%, potassium iodide 98% and 0.2% ethanol) stirring and comparing the colour of the liquid to a pre-determined colour chart clearly visible from Fig. 3 however the specificity of such a screening test is questionable.99
 | ||
| Fig. 3 Visual representation of synthetic cannabinoid presumptive test, reproduced from ref. 99 with permission of Narcotic Testing Supplies & Equipment Store. | ||
As components of Spice and related substances become banned, they are replaced with a compound which exhibits similar psychoactive properties yet negating the effectiveness of the newly introduced ban, see the paper: “Spice: A Never ending story?” for example.58 As such there is an urgent need for a faster laboratory method; Emanuel et al. reported the use of solid probe mass spectrometry alleviating the need for any sample pre-treatment such as liquid–liquid extraction.68 Since α-tocopherol is always present in the Spice herbs range, the authors demonstrated that once α-tocopherol was subtracted from the obtained spectra, the fragmentation patterns of CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 and JWH-018 become ‘visible’.68 This screening methodology is useful for the rapid analysis of the prohibited substances within the Spice product range (as well as related substances) with a positive response nullifying the need for any pre-treatment step (such as liquid–liquid extraction) allowing a full quantification via GC-MS or similar approaches i.e. LC-MS. Work from Lesiak et al.100 has also attempted to rapidly detect synthetic cannabinoids without the need for sample preparation with the use of direct analysis in real time mass spectrometry (DART-MS)100 being able to screen for AM-2201, JWH-122, JWH-203, JWH-210 and RCS-4.
497-C8 and JWH-018 become ‘visible’.68 This screening methodology is useful for the rapid analysis of the prohibited substances within the Spice product range (as well as related substances) with a positive response nullifying the need for any pre-treatment step (such as liquid–liquid extraction) allowing a full quantification via GC-MS or similar approaches i.e. LC-MS. Work from Lesiak et al.100 has also attempted to rapidly detect synthetic cannabinoids without the need for sample preparation with the use of direct analysis in real time mass spectrometry (DART-MS)100 being able to screen for AM-2201, JWH-122, JWH-203, JWH-210 and RCS-4.
To highlight the ever moving field of “legal highs” with respect to synthetic cannabinoids, in October 2012 new variants were reportedly found where the structures were a modification of compounds from the 3-naphthoylindole series57–60,69,70,80,101–107 identified from regular seizures made by police in Russia and Belarus.101 Shevyrin et al. have reported on the analytical characterisation of these new class of synthetic cannabinoids using GC-HRMS, UHPLC-HRMS, NMR and FT-IR101 providing robust and reliable confirmatory analytical approaches. Reports from South Korea also highlight the ever-changing market detailing the different synthetic cannabinoids which have been identified by their National Forensic Service between 2009–June 2013.108 The authors note that whilst initially it was largely naphthoylindoles (e.g. JWH-018, JWH-073), phenylacetylindoles (e.g. JWH-203, JWH-250), benzoylindoles (e.g. RCS-2, RCS-4) and CP-47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497 derivatives abused; after legislative bans were introduced, gradually over time, the molecules identified became new, typically halogenated, substances such as cyclopropylindoles (e.g. UR-144, XLR-11) and adamantylindoles (e.g. APICA, APINACA)108 which are represented in Scheme 2.
497 derivatives abused; after legislative bans were introduced, gradually over time, the molecules identified became new, typically halogenated, substances such as cyclopropylindoles (e.g. UR-144, XLR-11) and adamantylindoles (e.g. APICA, APINACA)108 which are represented in Scheme 2.
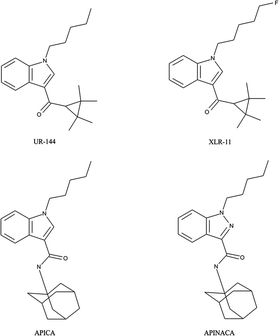 | ||
| Scheme 2 Chemical structures of synthetic cathinones discovered after legislative bans were introduced: cyclopropylindoles e.g. UR-144, XLR-11 and adamantylindoles (APICA and APINACA). | ||
Following the influx of new compounds, groups worldwide moved towards their detection. Scheidweiler et al.109 developed and validated a liquid chromatography-tandem mass spectrometric (LC-MS/MS) method for simultaneously quantifying JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, JWH-398, RCS-4, AM-2201, MAM-2201, UR-144, CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C7, CP 47
497-C7, CP 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497-C8 and their metabolites, and JWH-203, AM-694, RCS-8, XLR-11 and HU-210 parent compounds in urine.109 Previously there were no extensive synthetic quantitative methods reported in the literature until this work which presented the novel LC-MS/MS protocol quantifying 20 synthetic cannabinoids and 21 metabolites, and semi-quantifying 12 alkyl–hydroxy-metabolites.109
497-C8 and their metabolites, and JWH-203, AM-694, RCS-8, XLR-11 and HU-210 parent compounds in urine.109 Previously there were no extensive synthetic quantitative methods reported in the literature until this work which presented the novel LC-MS/MS protocol quantifying 20 synthetic cannabinoids and 21 metabolites, and semi-quantifying 12 alkyl–hydroxy-metabolites.109
Continuing from this, another approach towards the detection of the new generation of synthetic cannabinoid agonist, Mohr et al.110 applied Enzyme-Linked Immunosorbent Assay (ELISA) towards one of the most prevalent synthetic cannabinoids in urine, UR-144, and XLR-11. Once again testing in urine, the method was validated against liquid chromatography-tandem mass spectrometry with 90 positive and negative control samples for UR-144, XLR-11 and its metabolites.
Miscellaneous
As reported in the introduction, the new psychoactive substance epidemic is an ever growing market with a vast array of new materials discovered each year.7 To cover every known substance is beyond the scope of this review however; in this section, interesting pieces of research from around the world will be covered.Piperazines
N-Benzylpiperazine (BZP), the structure of which is shown in Scheme 3, is known to be a central nervous system stimulant with its effects reported to be similar to amphetamine in that it also triggers the release of dopamine and norepinephrine whilst inhibiting the uptake of dopamine, norepinephrine and serotonin.111 Although BZP is structurally similar to amphetamine it is reported to have only one-tenth the potency.111 Marketed as a ‘party pill’ before legal restrictions BZP was viewed as a safe alternative to amphetamines such as MDMA,112 however recently it has varying degrees of legislative control internationally.113 Its appearance in “legal high” samples is still reported114,115 however after being made illegal the prevalence of its use has declined; for example in New Zealand after being made a prohibited substance in 2008, the use of BZP amongst the general population dropped from 15.3% in 2006 to 3.2% in 2009.116In the UK, the first deaths associated with BZP and 3-TFMPP were three separate fatalities wherein one of both of the drugs were confirmed to be present although not determined to be the direct mechanism of death.117 Dickson et al.118 reported that BZP, 3′-TFMPP and MCPP are present in ecstasy tablets since the former, in some nations, is a legal alternative to MDMA. The authors analysed 251 MDMA positive urine samples using GC-MS via a liquid–liquid extraction and pentafluoropropionic anhydride (PFPA) derivatisation as sample pre-treatment to screen for 33 drugs potentially present.118 In 36% of the sample, drugs other than MDMA were found to be present; BZP, 3-TFMPP and MCPP were detected in 15%, 7% and 1% of the samples respectively.118
A wide array of analytical approaches have been reported by many different authors such as LC-MS,24,119 capillary electrophoresis,120 HPLC-fluorescence,121 LC with diode array,122,123 GC-MS124–126 and chemiluminescence.127 Arbo and co-workers128 provided a thorough overview of piperazine compounds as drugs of abuse with the full range of analytical techniques and matrices applied, readers are directed to this paper.128
It is clear, something that is generally the case with all “legal highs”, confirmatory laboratory based analysis is well developed. Lesser developed, however, are approaches that could adapted for used in-the-field or within a clinical setting where a near-instantaneous response is required. To this end, currently there are no immunoassays for the detection of piperazines derivatives128 and cross-reactivity of these compounds in fluorescence polarization immunoassay using AxSYM®, amphetamine/methamphetamine assay has been reported.129
Recently Philp et al.130 have reported on the development and validation of a specific colour test using 1′,2′-naphthoquinone-4-sulphonate (NQS) forming an intense bright orange-red complex with BZP at room temperature. The authors reported that common cutting agents such as glucose and caffeine did not affect the test. 3-TFMPP, MCPP, pCPP, MeOPP and piperazines produced an orange-red colour change where the apparent brilliance of the BZP-NQS complex made it apparently to be distinguishable from the other colour changes with the potential cross-reactants.
Aminoindanes
Aminoindanes are a group of synthetic compounds characterised by the presence of a phenethylamine skeleton, they are currently not controlled globally131 and have more recently been found to be contained in “legal high” products sold as powders akin to synthetic cathinones.132,133 2-Aminoindane has a basic ring structure that is similar to amphetamine (and therefore by proxy, substituted cathinones also) that can be chemically modified and the following derivatives (Scheme 4); 5′,6′-methylenedioxy-2-aminoindane (MDAI), 5′,6′-methylenedioxy-N-methyl-2-aminoindane (MDMAI), 5′-iodo-2-aminoindane (5-IAI), and 5′-methoxy-6′-methyl-2-aminoindane (MMAI) have all reportedly been found in “legal highs”.132A number of aminoindane compounds have been thoroughly characterized by Casale and Hays134 who provided analytical protocols in the form of NMR, MS and IR for 5-IAI, 4-IAI, their synthetic intermediates and impurities in order to assist forensic analysts.134 There is other work that reports a LC-MS/MS screening method for 26 analytes,34 including MDAI, and such an approach is designed to provide screening, within a clinical toxicology setting, for the potential misuse of “legal highs” via analysis of urine.34
Particularly of note, work by Elie and co-workers reports that microcrystalline identification of MDAI, mephedrone and N-benzylpiperazine (BZP) is possible.114 In this protocol the illicit compound is dissolved into methanol and diluted with water to produce a content of 10% (v/v) with mercury chloride (10 g L−1 + 10% methanol) used as the microcrystalline agent.114 This approach involves dropping 10 μL of the drug solution with 10 μL of the reagent solution onto a glass slide; the resulting structures were optically imaged following assisted nucleation (gently swirling a plastic pipette tip in the freshly mixed drop).114Fig. 4 shows the observed crystal structure which is compared to the crystal structure of illicit drugs. The MDAI free base (Fig. 4bi) was found to form flat serrated blades of various dimensions which become irregular with increasing sizes. Smaller crystals are observed to be single blades whereas larger crystals develop two dimensional bunch structures – after drying larger blade crystals are evident. It was noted that crystals grew within 60 s following assisted nucleation indicating the potential for a fast presumptive test strategy.114 The uniqueness of these tests were determined through comparisons of MDAI structure with a range of illicit drugs, indicating that potentially this approach is feasible to identify the MDAI structure in a real sample containing other illicit drugs. To this end the authors114 purchased “legal high” samples and utilised their microcrystalline presumptive test approach which when collaborated with FTIR/GC-MS.
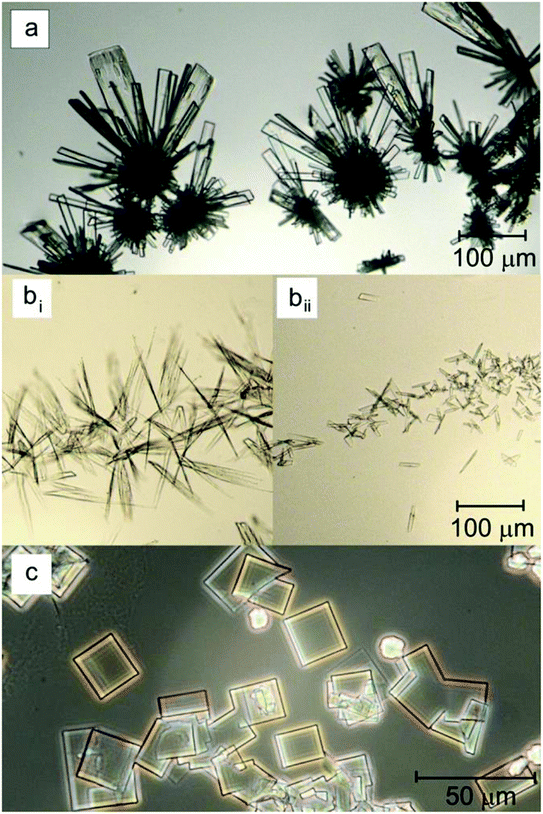 | ||
| Fig. 4 Microcrystals formed with mercury chloride and (a) mephedrone (c = 10 g L−1), (bi) MDAI freebase (c = 1 g L−1), (bii) MDAI hydrochloride (c = 1 g L−1) and (c) BZP (c = 1 g L−1).114 Reproduced from ref. 114 with permission of Elsevier. | ||
Salvinorin A (Saliva divinorum)
Salvia divinorum is a hallucinogenic psychoactive herb local to Oaxaca in Central Mexico and for centuries has been used by cultures indigenous to the region.135,136 This rare member of the mint family is also known as ‘magic mint’ and more colloquially: ‘ska Maria’, ‘ska Pastora’, ‘hierba de Maria’, ‘hojas de la Pastora’ all names which pertain to the belief that S. divinorum is the reincarnation of the Virgin Mary.137 The use of this plant as a psychoactive substance has spread globally, its major constituent – salvinorin A (SA) is a known selective opioid antagonist and to this end emphasis in the literature has been put on detecting SA.135 A dosage between 200–500 μg of SA has been found to induce profound hallucinations with feelings of physical or mental displacement as well as experiencing extraordinary illusions.138 Recently studies have postured SAs effects involve the endocannabinoid system.139To analyse intact S. divinorum leaves for the presence of SA there has been the employing of both thin layer chromatography using desorption electrospray ionization mass spectrometry (TLC-DESI-MS)140 and thin layer chromatography teamed with gas chromatography/mass spectrometry (TLC-GS/MS).141 By utilizing these techniques, the authors of both techniques were able to confirm the presence of salvinorin A in a submitted plant material suspected to be Salvia divinorum.140,141
Pichini and co-workers142 attempted the detection of Salvinorin A in different biological matrices opposed to the solid leaf matter. Utilising a gas chromatography mass spectrometric protocol, it was applied to detecting SA in plasma, urine, saliva and sweat.142 Following validation with 17-alpha-methyltestosterone as an internal standard the method was applied to the analysis of urine, saliva and sweat from two consumers after smoking 75 mg plant leaves. Salvinorin A was detected in urine (2.4 and 10.9 ng mL−1) and saliva (11.1 and 25.0 ng mL−1), but not in sweat patches from consumers.142 The quantification of SA in plasma and cerebrospinal fluid (from a rhesus monkey) has also been attempted and successfully completed using a negative ion liquid chromatography-mass spectrometry atmospheric pressure chemical ionization (LC-MS/APCI).143 Using the United States Food and Drug Administration (FDA) guidelines the authors of the method concluded the technique had a lower limit of quantification (LLOQ) of 2 ng mL−1 for 0.5 mL of plasma samples over the linear range 2–1000 ng mL−1.143
Mitragynine (Kratom)
Mitragynine is an indole alkaloid derived from the plant Mitragyna speciosa which is indigenous to Thailand and other Southeast Asian countries. This is a common “legal high” and is known commonly as Kratom which is also the chemical's Thai name. The leaves of the M. speciosa were historically used as an opium substitute as well as being used traditionally by villages in southern Thailand as a medicine for diarrhoea, muscle pain and hypertension in addition to also being used by agricultural workers and labourers to relieve tiredness and improve efficiency.144 Its study remains pertinent as reports of a fatality associated with Kratom are as recent as 2013.145Interestingly, mitragynine is the major constituent of Kratom reported to be 66.2% based on the crude base from the young leaves.146,147 Levels of mitragynine in adults plants from Thailand have been reported to be approximately over 60% whereas in Malaysia only over 10%. Paynantheine and the mitragynine diastereomer speciogynine were the second most abundant alkaloids and the mitragynine diastereomer speciogynine was the third abundant alkaloid in both plants.148
The pharmacology of mitragynine has been extensively studied and has been reported to have analgesic activity on the opioid system.144,149–151 Unlike the case of other NPSs reported in this review where they have emerged and analytical techniques have had to be developed/invented for their quantification, mitragynine, due to its historical use analytical methods already exist and are generally applied to facilitate pharmacological studies. To this end, Janchawee144 reported the first analytical methodology utilising HPLC-UV. A linear range of 0.1–10 μg mL−1 was reported with a LOD of 0.03 μg mL−1 and LOQ of 0.1 μg mL−1. Their protocol was applied to determine the pharmacokinetic characteristic of mitragynine in the serum of rats following oral administration.
As the leaves of Kratom became sold as “legal highs” in many other countries Kikura-Hanajiri and colleagues146 reported the detection of mitragynine and 7-OH-mitragynine (oxidative derivatives of mitragynine)152 in 13 “legal high” products using LC-ESI-MS. The authors found that 11 of the 13 products were found to contain mitragynine and 7-OH-mitragynine with their content found to range from 1 to 6% and in the latter 0.01 to 0.04%.146 Other researchers have directed research to study the methods of mitragynine in biological matrices using LC-MS153–155 and UHPLC-UV.156,157
From inspection of the literature, it is evident that there are multiple ways for the detection and quantification of Kratom ingestion/consumption with detection levels as low as 0.02 μg mL−1.158 For example Arndt and co-workers reported upon a case of a drug and rehabilitation centre reporting an analysis for Krypton (another name for Kratom) in the urine of a former opiate addicted woman.159 The immunological drug screenings were performed with test strips and a cloned enzyme donor immunoassay wherein alkaloids and tramadol metabolites were analysed by LC-MS/MS. The immunoassays yielded negative responses for amphetamines, barbiturates, benzodiazepines, benzoylecgonine, buprenorphine, ethylgluconoride, methadone, opiates, oxycodone and THC-COOH just as the test strips were negative from tramadol and its metabolites. The LC-MS/MS detected the alkaloids typically found in Kratom mitragynine, speciociliatine, speciogynine, mitraciliatine and paynantheine – detection of these alkaloids served sufficient proof of Kratom abuse and after confrontation with data the patient admitted to several infusions of the plant.159
Conclusion and future challenges
The work described in this review demonstrates the range of new analytical methods and techniques applied to the detection and quantification of NPSs, which have recently emerged on the recreational drugs market. Given the rapidly evolving nature of the recreational drugs market, in terms of the number of new substances being identified (101 new substances, in Europe, in 2014); the ease at which these substances are available through on-line vendors or “head shops”; the freely-available information regarding NPS production and/or pharmacology and the lack of globalised drug/precursor control legislation – makes the current analytical, forensic and legal challenges clearly apparent. These issues coupled with the limited availability and range of certified primary reference standards; fully validated, simple and cheap laboratory-based analytical methods and selective and sensitive in-field testing technology highlights the growing gap in knowledge and necessitates economic investment and focused research in this underfunded area.Future advances can be expected in the following areas: (i) Design and development of miniaturised in-field detection systems for NPSs in bulk samples or adulterated products (such as alcoholic drinks); (ii) Rapid, non-evasive bioanalytical methods for detection of the principle metabolites of common NPSs; (iii) simple, selective and validated laboratory-based chromatographic methods for the discrimination of new psychoactive substances, their isomers and their principle metabolites in biological matrices and; (iv) impurity profiling and/or source identification of common NPSs.
Clearly, the “war on drugs” is showing no sign of relenting in the near future and the principle challenge facing law enforcement agencies is to be ‘one-step-ahead’ of the clandestine drug manufacturers. By working collectively, analytical chemists, policy makers, law enforcement and forensic practitioners can suitably identify potential classes of molecules that may become the next generation of NPSs and develop advanced methods/technologies for the simultaneous detection/quantification of these substances thereby legislating against potentially dangerous compounds before they pose a serious threat to human health.
References
- B. P. G. Ltd, Br. Med. J., 1971, 4, 60–61 Search PubMed.
- L. A. Johnson, R. L. Johnson and R.-B. Portier, J. Emerg. Med., 2013, 44, 1108–1115 CrossRef PubMed.
- S. D. Brandt, H. R. Sumnall, F. Measham and J. Cole, Drug Test. Anal., 2010, 2, 377–382 CrossRef CAS PubMed.
- O. Corazza, S. Assi and G. Trincas, Ital. J. Addict., 2011, 1, 25–30 Search PubMed.
- European Monitoring Centre for Drugs and Drug Addiction, Europol, EU drug markets report: a strategic analysis, http://www.emcdda.europa.eu/publications/joint-publications/drug-markets, Accessed April, 2015.
- European Monitoring Centre for Drugs and Drug Addiction, Annual report 2011: the state of the drugs problem in Europe, New drugs and emerging trends, http://www.emcdda.europa.eu/online/annual-report/2011/new-drugs-and-trends/5, Accessed April, 2015.
- New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015), 2015.
- N. V. Cozzi, M. K. Sievert, A. T. Shulgin, P. Jacob 3rd and A. E. Ruoho, Eur. J. Pharmacol., 1999, 381, 63–69 CrossRef CAS.
- J. Kehr, F. Ichinose, S. Yoshitake, M. Goiny, T. Sievertsson, F. Nyberg and T. Yoshitake, Br. J. Pharmacol., 2011, 164, 1949–1958 CrossRef CAS PubMed.
- M. H. Baumann, M. A. Ayestas, J. S. Partilla, J. R. Sink, A. T. Shulgin, P. F. Daley, S. D. Brandt, R. B. Rothman, A. E. Ruoho and N. V. Cozzi, Neuropsychopharmacology, 2012, 37, 1192–1203 CrossRef CAS PubMed.
- N. V. Cozzi and K. E. Foley, Pharmacol. Toxicol., 2003, 93, 219–225 CAS.
- N. V. Cozzi, M. K. Sievert, A. T. Shulgin, P. Jacob and A. E. Ruoho, Eur. J. Pharmacol., 1999, 381, 63–69 CrossRef CAS.
- K. Morris, Lancet, 2010, 375, 1333–1334 CrossRef.
- J. A. Fass, A. D. Fass and A. S. Garcia, Ann. Pharmacother., 2012, 46, 436–441 CrossRef PubMed.
- J. B. Zawilska and J. Wojcieszak, Forensic Sci. Int., 2013, 231, 42–53 CrossRef CAS PubMed.
- The Charleston Gazette, Man high on bath salts kills neighbor's goat, police say, http://www.wvgazette.com/News/201105020871, Accessed April, 2015.
- E. M. Sammler, P. L. Foley, G. D. Lauder, S. J. Wilson, A. R. Goudie and J. I. O'Riordan, Lancet, 2010, 376, 742–742 CrossRef.
- M. Paillet-Loilier, A. Cesbron, R. Le Boisselier, J. Bourgine and D. Debruyne, Subst. Abuse Rehabil., 2014, 5, 37–52 Search PubMed.
- J. R. H. Archer, P. I. Dargan, S. Hudson, S. Davies, M. Puchnarewicz, A. T. Kicman, J. Ramsey, F. Measham, M. Wood, A. Johnston and D. M. Wood, J. Subst. Use, 2014, 19, 103–107 CrossRef.
- J. Beyer, F. T. Peters, T. Kraemer and H. H. Maurer, J. Mass Spectrom., 2007, 42, 150–160 CrossRef CAS PubMed.
- H. Torrance and G. Cooper, Forensic Sci. Int., 2010, 202, E62–E63 CrossRef CAS PubMed.
- E. Y. Santali, A. K. Cadogan, N. N. Daeid, K. A. Savage and O. B. Sutcliffe, J. Pharm. Biomed. Anal., 2011, 56, 246–255 CrossRef CAS PubMed.
- O. I. G. Khreit, C. Irving, E. Schmidt, J. A. Parkinson, N. Nic Daeid and O. B. Sutcliffe, J. Pharm. Biomed. Anal., 2012, 61, 122–135 CrossRef CAS PubMed.
- P. M. O'Byrne, P. V. Kavanagh, S. M. McNamara and S. M. Stokes, J. Anal. Toxicol., 2013, 37, 64–73 CrossRef PubMed.
- M. J. Swortwood, D. M. Boland and A. P. DeCaprio, Anal. Bioanal. Chem., 2013, 405, 1383–1397 CrossRef CAS PubMed.
- A. Ambrosi, S. Y. Chee, B. Khezri, R. D. Webster, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2012, 51, 500–503 CrossRef CAS PubMed.
- B. D. Paul and K. A. Cole, J. Anal. Toxicol., 2001, 25, 525–530 CrossRef CAS PubMed.
- S. D. Brandt, S. Freeman, H. R. Sumnall, F. Measham and J. Cole, Drug Test. Anal., 2011, 3, 569–575 CrossRef CAS PubMed.
- C. Bell, C. George, A. T. Kicman and A. Traynor, Drug Test. Anal., 2011, 3, 496–504 CrossRef CAS PubMed.
- P. Jankovics, A. Varadi, L. Tolgyesi, S. Lohner, J. Nemeth-Palotas and H. Koszegi-Szalai, Forensic Sci. Int., 2011, 210, 213–220 CrossRef CAS PubMed.
- L. K. Sorensen, J. Chromatogr., B: Biomed. Appl., 2011, 879, 727–736 CrossRef CAS PubMed.
- S. V. R. C. Rambabu, G. Ramu and A. Biksham Babu, Rasayan J. Chem., 2010, 3, 796–799 Search PubMed.
- G. Frison, M. Gregio, L. Zamengo, F. Zancanaro, S. Frasson and R. Sciarrone, Rapid Commun. Mass Spectrom., 2011, 25, 387–390 CrossRef CAS PubMed.
- Y. Al-Saffar, N. N. Stephanson and O. Beck, J. Chromatogr., B: Biomed. Appl., 2013, 930, 112–120 CrossRef CAS PubMed.
- A. J. Pedersen, P. W. Dalsgaard, A. J. Rode, B. S. Rasmussen, I. B. Muller, S. S. Johansen and K. Linnet, J. Sep. Sci., 2013, 36, 2081–2089 CrossRef CAS PubMed.
- E. M. Mwenesongole, L. Gautam, S. W. Hall, J. W. Waterhouse and M. D. Cole, Anal. Methods, 2013, 5, 3248–3254 RSC.
- D. Ammann, J. M. McLaren, D. Gerostamoulos and J. Beyer, J. Anal. Toxicol., 2012, 36, 381–389 CrossRef CAS PubMed.
- S. Strano-Rossi, L. Anzillotti, E. Castrignano, F. S. Romolo and M. Chiarotti, J. Chromatogr., A, 2012, 1258, 37–42 CrossRef CAS PubMed.
- M. Mayer, A. Benko, A. Huszar, K. Sipos, A. Lajtai, A. Lakatos and Z. Porpaczy, J. Chromatogr. Sci., 2013, 51, 861–866 CAS.
- N. Nic Daeid, K. A. Savage, D. Ramsay, C. Holland and O. B. Sutcliffe, Sci. justice: J. Forensic Sci. Soc., 2013, 54, 22–31 CrossRef PubMed.
- E. L. Menzies, S. C. Hudson, P. I. Dargan, M. C. Parkin, D. M. Wood and A. T. Kicman, Drug Test. Anal., 2014, 6, 506–515 CrossRef CAS PubMed.
- O. I. G. Khreit, M. H. Grant, T. Zhang, C. Henderson, D. G. Watson and O. B. Sutcliffe, J. Pharm. Biomed. Anal., 2013, 72, 177–185 CrossRef CAS PubMed.
- M. Taschwer, Y. Seidl, S. Mohr and M. G. Schmid, Chirality, 2014, 26, 411–418 CrossRef CAS PubMed.
- A. M. Leffler, P. B. Smith, A. de Armas and F. L. Dorman, Forensic Sci. Int., 2014, 234, 50–56 CrossRef CAS PubMed.
- U. Khan, A. L. N. van Nuijs, J. Li, W. Maho, P. Du, K. Li, L. Hou, J. Zhang, X. Meng, X. Li and A. Covaci, Sci. Total Environ., 2014, 487, 710–721 CrossRef CAS PubMed.
- A. D. Lesiak, R. A. Musah, R. B. Cody, M. A. Domin, A. J. Dane and J. R. E. Shepard, Analyst, 2013, 138, 3424–3432 RSC.
- R. A. Musah, R. B. Cody, M. A. Domin, A. D. Lesiak, A. J. Dane and J. R. E. Shepard, Forensic Sci. Int., 2014, 244, 42–49 CrossRef CAS PubMed.
- M. Joshi, B. Cetroni, A. Camacho, C. Krueger and A. J. Midey, Forensic Sci. Int., 2014, 244, 196–206 CrossRef CAS PubMed.
- S. Armenta, S. Garrigues, M. de la Guardia, J. Brassier, M. Alcala, M. Blanco, C. Perez-Alfonso and N. Galipienso, Drug Test. Anal., 2015, 7, 280–289 CrossRef CAS PubMed.
- J. P. Smith, J. P. Metters, O. I. G. Khreit, O. B. Sutcliffe and C. E. Banks, Anal. Chem., 2014, 86, 9985–9992 CrossRef CAS PubMed.
- J. P. Smith, J. P. Metters, C. Irving, O. B. Sutcliffe and C. E. Banks, Analyst, 2014, 139, 389–400 RSC.
- S. Mabbott, A. Eckmann, C. Casiraghi and R. Goodacre, Analyst, 2013, 138, 118–122 RSC.
- S. Mabbott, E. Correa, D. P. Cowcher, J. W. Allwood and R. Goodacre, Anal. Chem., 2013, 85, 923–931 CrossRef CAS PubMed.
- M. J. Swortwood, W. L. Hearn and A. P. DeCaprio, Drug Test. Anal, 2014, 6, 716–727 CrossRef CAS PubMed.
- N. Nic Daeid, W. Meier-Augenstein, H. F. Kemp and O. B. Sutcliffe, Anal. Chem., 2012, 84, 8691–8696 CrossRef CAS PubMed.
- J. W. Huffman, D. Dai, B. R. Martin and D. R. Compton, Bioorg. Med. Chem. Lett., 1994, 4, 563–566 CrossRef.
- S. Dresen, N. Ferreiros, M. Puetz, F. Westphal, R. Zimmermann and V. Auwaerter, J. Mass Spectrom., 2010, 45, 1186–1194 CrossRef CAS PubMed.
- R. Lindigkeit, A. Boehme, I. Eiserloh, M. Luebbecke, M. Wiggermann, L. Ernst and T. Beuerle, Forensic Sci. Int., 2009, 191, 58–63 CrossRef CAS PubMed.
- N. Uchiyama, R. Kikura-Hanajiri, J. Ogata and Y. Goda, Forensic Sci. Int., 2010, 198, 31–38 CrossRef CAS PubMed.
- J. i. Nakajima, M. Takahashi, T. Seto, C. Kanai, J. Suzuki, M. Yoshida and T. Hamano, Forensic Toxicol., 2011, 29, 95–110 CrossRef CAS PubMed.
- V. Auwärter, P. I. Dargan and D. M. Wood, in Novel Psychoactive Substances, ed. P. I. D. M. Wood, Academic Press, Boston, 2013, pp. 317–343 Search PubMed.
- B. K. Atwood, J. Huffman, A. Straiker and K. Mackie, Br. J. Pharmacol., 2010, 160, 585–593 CrossRef CAS PubMed.
- A. B. Schneir, J. Cullen and B. T. Ly, J. Emerg. Med., 2011, 40, 296–299 CrossRef PubMed.
- J. Simmons, L. Cookman, C. Kang and C. Skinner, Clin. Toxicol., 2011, 49, 431–433 CrossRef CAS PubMed.
- J. Cohen, S. Morrison, J. Greenberg and M. Saidinejad, Pediatrics, 2012, 129, E1064–E1067 CrossRef PubMed.
- I. Vardakou, C. Pistos and C. Spiliopoulou, Toxicol. Lett., 2010, 197, 157–162 CrossRef CAS PubMed.
- New York: United Nations Office on Drugs and Crime (UNODC), World Drug Report, UNODC, 2009.
- C. E. J. Emanuel, B. Ellison and C. E. Banks, Anal. Methods, 2010, 2, 614–616 RSC.
- V. Auwarter, S. Dresen, W. Weinmann, M. Muller, M. Putz and N. Ferreiros, J. Mass Spectrom., 2009, 44, 832–837 CrossRef PubMed.
- N. Uchiyama, R. Kikura-Hanajiri, N. Kawahara, Y. Haishima and Y. Goda, Chem. Pharm. Bull., 2009, 57, 439–441 CrossRef CAS.
- B. K. Koe, G. M. Milne, A. Weissman, M. R. Johnson and L. S. Melvin, Eur. J. Pharmacol., 1985, 109, 201–212 CrossRef CAS.
- T. Sobolevsky, I. Prasolov and G. Rodchenkov, Forensic Sci. Int., 2010, 200, 141–147 CrossRef CAS PubMed.
- H. J. Penn, L. J. Langman, D. Unold, J. Shields and J. H. Nichols, Clin. Biochem., 2011, 44, 1163–1165 CrossRef CAS PubMed.
- H. Koskela, U. Hakala, L. Loiske, P. Vanninen and I. Szilvay, Anal. Methods, 2011, 3, 2307–2312 RSC.
- R. A. Musah, M. A. Domin, M. A. Walling and J. R. E. Shepard, Rapid Commun. Mass Spectrom., 2012, 26, 1109–1114 CrossRef CAS PubMed.
- G. Merola, Z. Aturki, G. D'Orazio, R. Gottardo, T. Macchia, F. Tagliaro and S. Fanali, J. Pharm. Biomed. Anal., 2012, 71, 45–53 CrossRef CAS PubMed.
- A. O. Cox, R. C. Daw, M. D. Mason, M. Grabenauer, P. G. Pande, K. H. Davis, J. L. Wiley, P. R. Stout, B. F. Thomas and J. W. Huffman, J. Anal. Toxicol., 2012, 36, 293–302 CrossRef CAS PubMed.
- R. Gottardo, A. Chiarini, I. Dal Pra, C. Seri, C. Rimondo, G. Serpelloni, U. Armato and F. Tagliaro, J. Mass Spectrom., 2012, 47, 141–146 CrossRef CAS PubMed.
- A. Gregori, F. Damiano, M. Bonavia, V. Mileo, F. Varani and M. Monfreda, Sci. Jus., 2013, 53, 286–292 CrossRef CAS PubMed.
- F. Westphal, F. D. Soennichsen and S. Thiemt, Forensic Sci. Int., 2012, 215, 8–13 CrossRef CAS PubMed.
- S. Beuck, I. Moeller, A. Thomas, A. Klose, N. Schloerer, W. Schaenzer and M. Thevis, Anal. Bioanal. Chem., 2011, 401, 493–505 CrossRef CAS PubMed.
- A. Wohlfarth, K. B. Scheidweiler, X. H. Chen, H. F. Liu and M. A. Huestis, Anal. Chem., 2013, 85, 3730–3738 CrossRef CAS PubMed.
- D. P. Lovett, E. G. Yanes, T. W. Herbelin, T. A. Knoerzer and J. A. Levisky, Forensic Sci. Int., 2013, 226, 81–87 CrossRef CAS PubMed.
- M. J. Jin, J. Lee, M. K. In and H. H. Yoo, J. Forensic Sci., 2013, 58, 195–199 CrossRef CAS PubMed.
- A. D. de Jager, J. V. Warner, M. Henman, W. Ferguson and A. Hall, J. Chromatogr., B: Biomed. Appl., 2012, 897, 22–31 CrossRef CAS PubMed.
- U. Kim, M. J. Jin, J. Lee, S. B. Han, M. K. In and H. H. Yoo, J. Pharm. Biomed. Anal., 2012, 64–65, 26–34 CrossRef CAS PubMed.
- E. G. Yanes and D. P. Lovett, J. Chromatogr., B: Biomed. Appl., 2012, 909, 42–50 CrossRef CAS PubMed.
- C. L. Moran, V.-H. Le, K. C. Chimalakonda, A. L. Smedley, F. D. Lackey, S. N. Owen, P. D. Kennedy, G. W. Endres, F. L. Ciske, J. B. Kramer, A. M. Kornilov, L. D. Bratton, P. J. Dobrowolski, W. D. Wessinger, W. E. Fantegrossi, P. L. Prather, L. P. James, A. Radominska-Pandya and J. H. Moran, Anal. Chem., 2011, 83, 4228–4236 CrossRef CAS PubMed.
- K. C. Chimalakonda, C. L. Moran, P. D. Kennedy, G. W. Endres, A. Uzieblo, P. J. Dobrowolski, E. K. Fifer, J. Lapoint, L. S. Nelson, R. S. Hoffman, L. P. James, A. Radominska-Pandya and J. H. Moran, Anal. Chem., 2011, 83, 6381–6388 CrossRef CAS PubMed.
- M. A. Neukamm, T. E. Muerdter, C. Knabbe, H.-D. Wehner and F. Wehner, Blutalkohol, 2009, 46, 373–379 CAS.
- S. Dresen, S. Kneisel, W. Weinmann, R. Zimmermann and V. Auwaerter, J. Mass Spectrom., 2011, 46, 163–171 CrossRef CAS PubMed.
- J. Teske, J.-P. Weller, A. Fieguth, T. Rothaemel, Y. Schulz and H. D. Troeger, J. Chromatogr., B: Biomed. Appl., 2010, 878, 2659–2663 CrossRef CAS PubMed.
- M. Dziadosz, J.-P. Weller, M. Klintschar and J. Teske, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2013, 929, 84–89 CrossRef CAS PubMed.
- S. Kneisel and V. Auwarter, J. Mass Spectrom., 2012, 47, 825–835 CrossRef CAS PubMed.
- A. Salomone, E. Gerace, F. D'Urso, D. Di Corcia and M. Vincenti, J. Mass Spectrom., 2012, 47, 604–610 CrossRef CAS PubMed.
- M. Hutter, S. Kneisel, V. Auwarter and M. A. Neukamm, J. Chromatogr., B: Biomed. Appl., 2012, 903, 95–101 CrossRef CAS PubMed.
- R. Heltsley, M. K. Shelby, D. J. Crouch, D. L. Black, T. A. Robert, L. Marshall, C. L. Bender, A. Z. DePriest and M. A. Colello, J. Anal. Toxicol., 2012, 36, 588–593 CrossRef CAS PubMed.
- A. Arntson, B. Ofsa, D. Lancaster, J. R. Simon, M. McMullin and B. Logan, J. Anal. Toxicol., 2013, 37, 284–290 CrossRef CAS PubMed.
- Narcotic Testing Supplies & Equipment Store, Synthetic Cannabinoids test (K2, Spice), http://shop.narcotictests.com/products/narcotic-field-tests/synthetic-cannabinoids-test-k2-spice/details, Accessed April 2015.
- A. D. Lesiak, R. A. Musah, M. A. Domin and J. R. E. Shepard, J. Forensic Sci., 2014, 59, 337–343 CrossRef CAS PubMed.
- V. Shevyrin, V. Melkozerov, A. Nevero, O. Eltsov and Y. Shafran, Forensic Sci. Int., 2013, 232, 1–10 CrossRef CAS PubMed.
- N. Uchiyama, R. Kikura-Hanajiri, N. Kawahara and Y. Goda, Forensic Toxicol., 2009, 27, 61–66 CrossRef CAS.
- N. Uchiyama, M. Kawamura, R. Kikura-Hanajiri and Y. Goda, Forensic Toxicol., 2011, 29, 25–37 CrossRef CAS PubMed.
- S. Kneisel, P. Bisel, V. Brecht, S. Broecker, M. Mueller and V. Auwaerter, Forensic Toxicol., 2012, 30, 126–134 CrossRef CAS.
- J. i. Nakajima, M. Takahashi, R. Nonaka, T. Seto, J. Suzuki, M. Yoshida, C. Kanai and T. Hamano, Forensic Toxicol., 2011, 29, 132–141 CrossRef CAS PubMed.
- L. Ernst, H.-M. Schiebel, C. Theuring, R. Lindigkeit and T. Beuerle, Forensic Sci. Int., 2011, 208, E31–E35 CrossRef CAS PubMed.
- S. Hudson and J. Ramsey, Drug Test. Anal., 2011, 3, 466–478 CrossRef CAS PubMed.
- H. Chung, H. Choi, S. Heo, E. Kim and J. Lee, Forensic Toxicol., 2014, 32, 82–88 CrossRef CAS PubMed.
- K. B. Scheidweiler and M. A. Huestis, J. Chromatogr., A, 2014, 1327, 105–117 CrossRef CAS PubMed.
- A. L. Mohr, B. Ofsa, A. M. Keil, J. R. Simon, M. McMullin and B. K. Logan, J. Anal. Toxicol., 2014, 38, 427–431 CrossRef CAS PubMed.
- M. Wikstrom, P. Holmgren and J. Ahlner, J. Anal. Toxicol., 2004, 28, 67–70 CrossRef.
- M. S. Monteiro, M. D. Bastos, P. G. de Pinho and M. Carvalho, Arch. Toxicol., 2013, 87, 929–947 CrossRef CAS PubMed.
- B. M. Z. Cohen and R. Butler, Int. J. Drug Policy, 2011, 22, 95–101 CrossRef PubMed.
- L. Elie, M. Baron, R. Croxton and M. Elie, Forensic Sci. Int., 2012, 214, 182–188 CrossRef CAS PubMed.
- D. M. Wood, J. Button, S. Lidder, J. Ramsey, D. W. Holt and P. I. Dargan, J. Med. Toxicol., 2008, 4, 254–257 CrossRef.
- C. Wilkins and P. Sweetsur, Drug Alcohol Depend., 2013, 127, 72–80 CrossRef PubMed.
- S. Elliott and C. Smith, J. Anal. Toxicol., 2008, 32, 172–177 CrossRef CAS PubMed.
- A. J. Dickson, S. P. Vorce, J. M. Holler and T. P. Lyons, J. Anal. Toxicol., 2010, 34, 464–469 CrossRef CAS PubMed.
- C. Montesano, M. Sergi, M. Moro, S. Napoletano, F. S. Romolo, M. Del Carlo, D. Compagnone and R. Curini, J. Mass Spectrom., 2013, 48, 49–59 CrossRef CAS PubMed.
- S. C. Bishop, B. R. McCord, S. R. Gratz, J. R. Loeliger and M. R. Witkowski, J. Forensic Sci., 2005, 50, 326–335 CrossRef CAS.
- M. Wada, K. Yamahara, R. Ikeda, R. Kikura-Hanajiri, N. Kuroda and K. Nakashima, Biomed. Chromatogr., 2012, 26, 21–25 CrossRef CAS PubMed.
- I. E. D. Moreno, B. M. da Fonseca, M. Barroso, S. Costa, J. A. Queiroz and E. Gallardo, J. Pharm. Biomed. Anal., 2012, 61, 93–99 CrossRef CAS PubMed.
- M. Takahashi, M. Nagashima, J. Suzuki, T. Seto, I. Yasuda and T. Yoshida, Talanta, 2009, 77, 1245–1272 CrossRef CAS PubMed.
- M. Monteiro, M. Carvalho, M. L. Bastos and P. G. de Pinho, Toxicol. Lett., 2013, 221, S185–S186 CrossRef PubMed.
- K. M. Abdel-Hay, J. DeRuiter and C. R. Clark, Rapid Commun. Mass Spectrom., 2013, 27, 2551–2558 CrossRef CAS PubMed.
- K. M. Abdel-Hay, C. R. Clark and J. DeRuiter, Forensic Sci. Int., 2013, 233, 113–120 CrossRef CAS PubMed.
- R. J. Waite, G. J. Barbante, N. W. Barnett, E. M. Zammit and P. S. Francis, Talanta, 2013, 116, 1067–1072 CrossRef CAS PubMed.
- M. D. Arbo, M. L. Bastos and H. F. Carmo, Drug Alcohol Depend., 2012, 122, 174–185 CrossRef CAS PubMed.
- D. de Boer, I. J. Bosman, E. Hidvegi, C. Manzoni, A. A. Benko, L. dos Reys and R. A. A. Maes, Forensic Sci. Int., 2001, 121, 47–56 CrossRef CAS.
- M. Philip, R. Shimmon, N. Stojanovska, M. Tahtouh and S. L. Fu, Anal. Methods, 2013, 5, 5402–5410 RSC.
- United Nations Office on Drugs and Crime, Details for Aminoindanes, https://www.unodc.org/LSS/SubstanceGroup/Details/8fd64573-c567-4734-a258-76d1d95dca25#_ftn1, Accessed April, 2015.
- P. D. Sainsbury, A. T. Kicman, R. P. Archer, L. A. King and R. A. Braithwaite, Drug Test. Anal., 2011, 3, 479–482 CrossRef CAS PubMed.
- C. T. Gallagher, S. Assi, J. L. Stair, S. Fergus, O. Corazza, J. M. Corkery and F. Schifano, Hum. Psychopharmacol. Clin. Exp., 2012, 27, 106–112 CrossRef CAS PubMed.
- J. Casale and P. Hays, Microgram J., 2012, 9, 18–26 CAS.
- O. Grundmann, S. M. Phipps, I. Zadezensky and V. Butterweck, Planta Med., 2007, 73, 1039–1046 CrossRef CAS PubMed.
- J. W. Gruber, D. J. Siebert, A. H. Der Marderosian and R. S. Hock, Phytochem. Anal., 1999, 10, 22–25 CrossRef CAS.
- C. Medana, C. Massolino, M. Pazzi and C. Baiocchi, Rapid Commun. Mass Spectrom., 2005, 20, 131–136 CrossRef PubMed.
- M. K. Paudel, O. Shirota, K. Sasaki-Tabata, H. Tanaka, S. Sekita and S. Morimoto, J. Nat. Prod., 2013, 76, 1654–1660 CrossRef CAS PubMed.
- R. Capasso, F. Borrelli, M. G. Cascio, G. Aviello, K. Huben, J. K. Zjawiony, P. Marini, B. Romano, V. Di Marzo, F. Capasso and A. A. Izzo, Br. J. Pharmacol., 2008, 155, 681–689 CrossRef CAS PubMed.
- J. H. Kennedy and J. M. Wiseman, Rapid Commun. Mass Spectrom., 2010, 24, 1305–1311 CrossRef CAS PubMed.
- J. D. Jermain and H. K. Evans, J. Forensic Sci., 2009, 54, 612–616 CrossRef CAS PubMed.
- S. Pichini, S. Abanades, M. Farre, M. Pellegrini, E. Marchei, R. Pacifici, L. Torre Rde and P. Zuccaro, Rapid Commun. Mass Spectrom., 2005, 19, 1649–1656 CrossRef CAS PubMed.
- M. S. Schmidt, T. E. Prisinzano, K. Tidgewell, W. Harding, E. R. Butelman, M. J. Kreek and D. J. Murry, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2005, 818, 221–225 CrossRef CAS PubMed.
- B. Janchawee, N. Keawpradub, S. Chittrakarn, S. Prasettho, P. Wararatananurak and K. Sawangjareon, Biomed. Chromatogr., 2007, 21, 176–183 CrossRef CAS PubMed.
- M. F. Neerman, R. E. Frost and J. Deking, J. Forensic Sci., 2013, 58(Suppl 1), S278–S279 CrossRef PubMed.
- R. Kikura-Hanajiri, M. Kawamura, T. Maruyama, M. Kitajima, H. Takayama and Y. Goda, Forensic Toxicol., 2009, 27, 67–74 CrossRef CAS PubMed.
- D. Ponglux, S. Wongseripipatana, H. Takayama, M. Kikuchi, M. Kurihara, M. Kitajima, N. Aimi and S. Sakai, Planta Med., 1994, 60, 580–581 CrossRef CAS PubMed.
- H. Takayama, Chem. Pharm. Bull., 2004, 52, 916–928 CrossRef CAS.
- K. Matsumoto, M. Mizowaki, T. Suchitra, Y. Murakami, H. Takayama, S. Sakai, N. Aimi and H. Watanabe, Eur. J. Pharmacol., 1996, 317, 75–81 CrossRef CAS.
- M. Tohda, S. Thongpraditchote, K. Matsumoto, Y. Murakami, S. Sakai, N. Aimi, H. Takayama, P. Tongroach and H. Watanabe, Biol. Pharm. Bull., 1997, 20, 338–340 CAS.
- S. Thongpradichote, K. Matsumoto, M. Tohda, H. Takayama, N. Aimi, S. Sakai and H. Watanabe, Life Sci., 1998, 62, 1371–1378 CrossRef CAS.
- H. Takayama, H. Ishikawa, M. Kurihara, M. Kitajima, N. Aimi, D. Ponglux, F. Koyama, K. Matsumoto, T. Moriyama, L. T. Yamamoto, K. Watanabe, T. Murayama and S. Horie, J. Med. Chem., 2002, 45, 1949–1956 CrossRef CAS PubMed.
- A. A. Philipp, D. K. Wissenbach, S. W. Zoerntlein, O. N. Klein, J. Kanogsunthornrat and H. H. Maurer, J. Mass Spectrom., 2009, 44, 1249–1261 CrossRef CAS PubMed.
- N. V. de Moraes, R. A. C. Moretti, E. B. Furr, C. R. McCurdy and V. L. Lanchote, J. Chromatogr., B: Biomed. Appl., 2009, 877, 2593–2597 CrossRef PubMed.
- S. J. Lu, B. N. Tran, J. L. Nelsen and K. M. Aldous, J. Chromatogr., B: Biomed. Appl., 2009, 877, 2499–2505 CrossRef CAS PubMed.
- S. Parthasarathy, S. Ramanathan, S. Ismail, M. I. Adenan, S. M. Mansor and V. Murugaiyah, Anal. Bioanal. Chem., 2010, 397, 2023–2030 CrossRef CAS PubMed.
- A. A. Philipp, D. K. Wissenbach, A. A. Weber, J. Zapp and H. H. Maurer, J. Mass Spectrom., 2010, 45, 1344–1357 CrossRef CAS PubMed.
- R. Kronstrand, M. Roman, G. Thelander and A. Eriksson, J. Anal. Toxicol., 2011, 35, 242–247 CrossRef CAS PubMed.
- T. Arndt, U. Claussen, B. Guessregen, S. Schroefel, B. Stuerzer, A. Werle and G. Wolf, Forensic Sci. Int., 2011, 208, 47–52 CrossRef CAS PubMed.
Footnote |
| † Ingredients listed on the packaging of products are as follows – Spice Gold: bay bean, blue lotus, Lion's Tail, Indian Warrior, Dwarf Skullcap, Maconha brava, Pink Lotus, Marshmallow, Red Clover, Rose, Siberian motherwort, Vanilla and honey. Spice Gold Spirit: Leonurus, Cardiaca, Pedicularis, Canadensis, Scutellaria, Latero flora, Athaea officinalis, Rosa damascene, Vanilla planifolia. Spice Diamond: Bay bean, Blue lotus, Lion's tail, Indian Warrior, Dwarf Skullcap, Maconha brava, Pink Lotus, Marshmallow, Red Clover, Rose, Siberian motherwort, vanilla, honey, aroma. Note the lack of any real ingredients (chemical) and no mention of any aminoalkylindole (JWH compounds) or cyclohexylphenyls (CP compounds) |
| This journal is © The Royal Society of Chemistry 2015 |