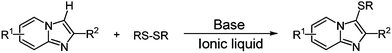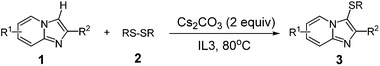Cs2CO3 promoted direct C–H bond sulfenylation of imidazo[1,2-a]pyridines and related heteroarenes in ionic liquid†
Zhaochang Gao,
Xun Zhu and
Ronghua Zhang*
Department of Chemical Engineering, Yancheng Institute of Industry Technology, Yancheng 224005, P. R. China. E-mail: rong_hua_zhang@163.com; Fax: +86 (0515) 88583630
First published on 25th April 2014
Abstract
An efficient and novel method was developed for the synthesis of 3-sulfenyl imidazo[1,2-a]pyridines in good to excellent yields via a Cs2CO3 promoted direct sulfenylation of imidazo[1,2-a]pyridines. The reaction proceeds smoothly with a wide range of structurally diverse heteroarenes and disulfides. This protocol is environmentally friendly because it is free of transition-metal catalysts and utilizes aryl-substituted imidazolium-based ionic liquid rather than volatile organic solvents.
Introduction
Sulfur-containing substances are an important class of compounds, which exist in a variety of natural products and synthetic drugs and play a significant role in medicinal chemistry for their biological activities.1 Sulfenyl aza-aromatics are especially valued due to their comprehensive therapeutic value against a variety of diseases, such as tubulin polymerization, human breast cancer and other tumors,2,3 and vascular4 and respiratory disorders.5Imidazopyridine and its derivatives exist in a variety of natural products and have attracted much attention due to their important biological activities and broad utilization in the pharmaceutical industry. Generally, the pharmacological profile is mainly dependent on the nature of the substitutional groups and introduction of sulfenyl groups on the aza-aromatic rings could impart markedly biological properties to the compounds, for example, 3-sulfenyl indoles, 3-sulfenyl pyrroles and 3-sulfenyl imidazopyridines are of considerable therapeutic value against a variety of diseases.6
Since the discovery of the important applications of sulfur-containing agent, many strategies for the construction of carbon–sulfur bond have been developed in recent years.
Research on copper-catalyzed Ullmann-type carbon–sulfur couplings have made great progresses in recent years.7 Chan–Evans–Lam-type reactions are also very efficient for C–S bond formation.8 Base-promoted procedure via reductive coupling of tosylhydrazones with thiols under metal-free conditions has been reported recently.9 As an important and attractive method, Friedel–Crafts reaction combined with selective C–H bond activation strategy has also been used for the selective C–S bond formation.10 In view of the synthetic strategies as indicated above, a number of procedures have been devised for sulfenylation of electron-rich aza-aromatics with transition-metal catalysts,11 new sulfur reagents12 and stoichiometric amounts of promoters.13
In recent years, rare earth metal catalysts were extensively used in organic synthesis.14 Cerium(III) chloride has emerged as a cheap, nontoxic, water-tolerant and very useful Lewis acid catalyst, which can promote the reactions of electron-rich aza-aromatics with several electrophiles to form carbon–sulfur, carbon–nitrogen, carbon–oxygen, and carbon–carbon bonds.15
In compliance to green chemistry principles, “ionic liquids” (ILs) constitute an attractive alternate in the field of organic synthesis, electrochemistry and separation process due to catalyst recycling, improve selectivity and ease in product isolation. Henceforth, imidazolium based ILs have been utilized widely in current organic chemistry.16
Although a number of synthetic protocols have been developed for the sulfenylation of electron-rich aza-aromatics, it is still desirable to explore new methods avoid using metal catalyst and complex sulfur-containing reagent under environmentally friendly reaction condition. Towards research in developing new methods for the carbon–heteroatom bond forming reactions, an efficient and simple base promoted direct C–H bond sulfenylation of imidazo[1,2-a]pyridines and related heteroarenes with disulfides in aryl-substituted imidazolium-based ionic liquid has been developed (Scheme 1).
Results and discussion
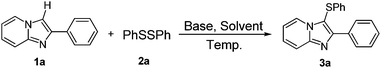
Inspired by the outcomes of recent reports on base promoted direct C–H bond sulfenylation in volatile organic solvents17 and the decisive role of ionic liquids in organic synthesis,18 we designed aryl-substituted 2-ethyl-imidazolium-based ionic liquid 1-benzyl-3-butyl 2-ethyl-imidazolium tetrafluoroborate ([BnEBIm]BF4) and 1-benzyl-3-octyl 2-ethyl-imidazolium tetrafluoroborate ([BnEOIm]BF4) and investigated their solvent effect for the present reaction (Scheme 2).16gWe started our studies by reacting phenyl disulfide with 2-phenylimidazo[1,2-a]pyridine in the presence of 2 equivalents of Cs2CO3 in DMSO at 80 °C under an air atmosphere. However, only 32% yield of product was detected after 15 hours reaction. Encouraged by this result, the reaction was optimized and the results are summarized in Table 1.
| Entry | Base | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), base (1 mmol), solvent (1 mL), 15 h, 80 °C in a 25 mL flask.b Yield of isolated product after column chromatography.c 1.5 equivalent of base was used.d 1.0 equivalent of base was used. | ||||
| 1 | Cs2CO3 | DMSO | 80 | 32 |
| 2 | K2CO3 | DMSO | 80 | 11 |
| 3 | KHCO3 | DMSO | 80 | 0 |
| 4 | KF | DMSO | 80 | 0 |
| 5 | NaOH | DMSO | 80 | 23 |
| 6 | tBuOK | DMSO | 80 | 15 |
| 7 | Cs2CO3 | EtOH | 80 | 36 |
| 8 | Cs2CO3 | CH3CN | 80 | 31 |
| 9 | Cs2CO3 | DMC | 80 | Trace |
| 10 | Cs2CO3 | Toluene | 80 | Trace |
| 11 | Cs2CO3 | IL1 | 80 | 56 |
| 12 | Cs2CO3 | IL2 | 80 | 68 |
| 13 | Cs2CO3 | IL3 | 80 | 89 |
| 14 | Cs2CO3 | IL4 | 80 | 91 |
| 15c | Cs2CO3 | IL3 | 80 | 77 |
| 16d | Cs2CO3 | IL3 | 80 | 53 |
| 17 | Cs2CO3 | IL3 | 100 | 89 |
| 18 | Cs2CO3 | IL3 | 50 | 20 |
| 19 | — | IL3 | 80 | 0 |
At first, several kinds of bases were tested and it was found that other bases such as K2CO3, KHCO3, KF, NaOH and tBuOK were less effective compared to Cs2CO3 due to their higher or lower pKa value (Table 1, entries 1–6). Subsequently, several solvents such as DMSO, EtOH, CH3CN, DMC, toluene and ionic liquids were tested for the reaction (Table 1, entries 7–14). Results show that ionic liquids can give moderate to excellent yield of the desired product and the highest yield was obtained in IL4, which may because the aryl and alkyl substituted groups of ionic liquids increased the interaction and solubility of reactants. However, when IL3 was used as the solvent, work-up manipulation is easier than IL4 because the melting point of IL4 is about 60 °C. Reducing the dosage of base from 2 equivalents to 1.5 and 1.0 equivalent led to decrease of the yield of 3a from 89% to 77% and 53% (Table 1, entries 15–16). When the reaction was carried out at 50 °C instead of 80 °C, the yield of 3a decreased from 89% to 20% (Table 1, entries 17–18). Blank experiment show that the reaction cannot proceed without the use of base (Table 1, entry 19).
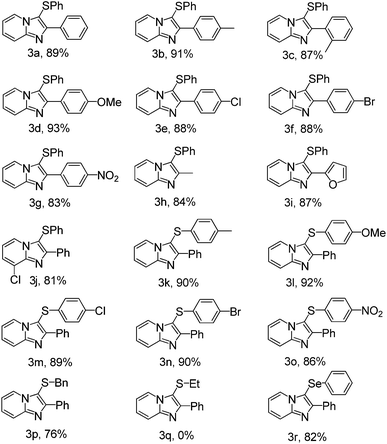
In order to extend the scope of this methodology, a variety of imidazo[1,2-a]pyridines were allowed to reacted with disulfides under optimized conditions. As can be seen from Table 2, the direct C–H bond sulfenylation reactions of imidazo[1,2-a]pyridines with disulfides generated the corresponding products in good to excellent yields.
Electron-donating and electron-withdrawing groups on the benzene rings of imidazo[1,2-a]pyridines were tolerated. Benzene rings of imidazo[1,2-a]pyridines with electron-donating groups (Table 2, entries 3b–d), such as CH3 and OCH3, gave a slightly higher yield than those bearing electron-withdrawing groups, such as Cl, Br and NO2 (Table 2, entries 3e–g). Benzene rings of imidazo[1,2-a]pyridines bearing CH3 group at the ortho-position of the benzene ring afforded the corresponding product in 87% yield, compared with the yield of 91%, when the CH3 group was located at the para-position of the benzene ring (Table 2, entries 3b–c). The result represented a slight ortho-position effect of imidazo[1,2-a]pyridines. Under the present reaction conditions, imidazo[1,2-a]pyridines bearing furan group can be easily converted to the desired product 3i in 87% yield (Table 2, entry 3i). To our delight, imidazo[1,2-a]pyridines bearing alkyl group can also be transformed to the desired product 3h in 84% yield (Table 2, entry 3h). On another hand, various disulfides used for the direct C–H bond sulfenylation reaction were also investigated. Disulfides bearing electron-donating groups on R (Table 2, entries 3k–l) gave better yields than those bearing electron-withdrawing groups (Table 2, entries 3m–o). It was worth noting that dibenzyl disulfide could also be converted to the corresponding product 3r in 82% under the presence reaction condition (Table 2, entry 3r). However, alkyl disulfides could not afford the desired product under the optimized reaction conditions (Table 2, entry 3q).
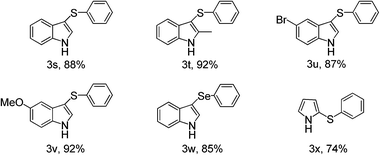
Other electron-rich heterocycles such as indoles and pyrroles were also tested under the present optimized reaction condition. To our delight, they can both transformed to the desired products in good to excellent yield after simple work-up manipulation steps and the results was listed in Table 3. Under the present reaction condition, diphenyl diselenide can also react with 1-H indole to form the desired product in 85%.
With respect to the reaction mechanism we assume that deprotonation of the heterocycle afforded an anion was a key reaction step. Then the anion reacted with disulfide by nucleophilic attack, resulting in C–S bond formation.17,18
Conclusions
In conclusion, we have developed an efficient and environmentally friendly Cs2CO3 promoted direct C–H bond sulfenylation of imidazopyridines, indoles, and pyrroles with disulfides in aryl-substituted imidazolium-based ionic liquid rather than volatile organic solvents. Further more, this protocol is free of transition-metal catalyst and ionic liquid designed above can be reused via an easy work-up manipulation. A wide range of heterocycles with both electron-releasing and electron-withdrawing groups were tested and the corresponding sulfenyl heterocycles were obtained in good to excellent yields.Experimental
All chemicals (AR grade) were obtained from commercial sources and were used without further purification. Petroleum ether (PE) refers to the fraction boiling in the 60–90 °C range. The progress of the reactions was monitored by TLC (silica gel, Polygram SILG/UV 254 plates). Column chromatography was performed on Silicycle silica gel (200–300 mesh). Melting points were obtained using a Yamato melting point apparatus Model MP-21 and are uncorrected. IR spectra were recorded on a Shimadzu spectrophotometer using KBr discs. 1H and 13C NMR spectra were obtained using a Bruker DRX 500 (500 MHz) spectrometer in CDCl3 or DMSO-d6 with TMS as the internal standard. All the products are known compounds and they were identified by comparison of their physical and spectral data with those reported in the literature.General procedure for the synthesis of aryl-substituted imidazolium-based ionic liquid
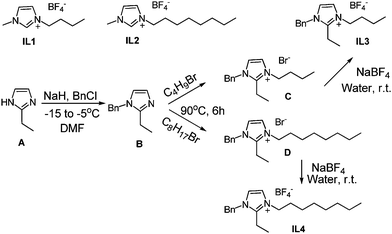
(1) Sodium hydride (60% suspension, 40 mmol) was placed in a two-necked flask and 2-ethyl imidazole (20 mmol) in DMF (20 mL) was added under nitrogen atmosphere at −15 °C. The reaction mixture was stirred for another 30 min at −15 °C, and then benzyl chloride (20 mmol) was added. The temperature of the reaction was raised to −5 °C and the reaction mixture was stirred for another 3 hours under N2. After completion, reaction was quenched with methanol (5 mL) and solvent was evaporated to give the crude product which was purified using silica gel column chromatography to obtain B in 92% yield.(2) Alkyl bromide (25 mmol) was added to B (20 mmol) in THF (20 mL) and the reaction was stirred for 4–6 hours at 80 °C. The reaction mixture was dried under reduced pressure and column chromatography over silica gel provided the desired compounds C and D in the yield of 91% and 90%.
(3) To the solutions of the imdazolium halides C and D (20 mmol) in water (20 mL) was added NaBF4 (21 mmol) and the reaction mixture was stirred for 1.5 h at room temperature. Reaction mixture was extracted with dichloromethane (3 × 10 mL) and purified by column chromatography to obtain ionic liquid IL3 and IL4 in the yield of 95% and 94%.
General procedure for sulfenylation of heterocycles with disulfides
A mixture of heterocycle (0.5 mmol), disulfide (0.5 mmol) and Cs2CO3 (1 mmol) were added in IL3 (1 mL) at 80 °C in a flask. The reaction was carried out under an air atmosphere for about 15 h until complete consumption of starting material as monitored by TLC. The solution was extracted with ethyl ether (3 × 10 mL). And then the organic layer was separated and concentrated under vacuum and the crude product was purified by column chromatography (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 15
EtOAc, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or recrystallization (PE
1) or recrystallization (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 5
EtOAc, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide the analytically pure product. The ionic liquid layer was washed with water (2 × 2 mL) and separated via simple work-up manipulation. And the ionic liquid can be easily recycled after drying under vacuum.
1) to provide the analytically pure product. The ionic liquid layer was washed with water (2 × 2 mL) and separated via simple work-up manipulation. And the ionic liquid can be easily recycled after drying under vacuum.
Acknowledgements
This project was funded by the joint innovation and research funding of Jiangsu province, joint research project (no. BY2013060).Notes and references
- (a) R. L. Sundberg, Indoles, Academic, London, 1996 Search PubMed; (b) A. R. Katritzky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, Pergamon, Oxford, 2000 Search PubMed; (c) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC; (d) A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS PubMed; (e) T. R. Garbe, M. Kobayashi, N. Shimizu, N. Takesue, M. Ozawa and H. Yukawa, J. Nat. Prod., 2000, 63, 596 CrossRef CAS PubMed; (f) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (g) B. Bao, Q. Sun, X. Yao, J. Hong, C. O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 711 CrossRef CAS PubMed.
- (a) M. C. Van Zandt, M. L. Jones, D. E. Gunn, L. S. Geraci, J. H. Jones, D. R. Sawicki, J. Sredy, J. L. Jacot, A. T. Dicioccio, T. Petrova, A. Mitschler and A. D. Podjarny, J. Med. Chem., 2005, 48, 3141 CrossRef CAS PubMed; (b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed.
- G. De Martino, M. C. Edler, G. La Regina, A. Coluccia, M. Barbera, D. Barrow, R. I. Nicholson, G. Chiosis, A. Brancale, E. Hamel and R. Silvestri, J. Med. Chem., 2006, 49, 947 CrossRef CAS PubMed.
- N. Zhou, W. Zeller, M. Krohn, H. Anderson, J. Zhang, E. Onua, A. S. Kiselyov, J. Ramirez, G. Halldorsdottir, T. Andrésson, M. E. Gurney and J. Singh, Bioorg. Med. Chem. Lett., 2009, 19, 123 CrossRef CAS PubMed.
- D. Ainge, M. Butters, E. Merifield, R. Ramakrishnan, R. N. Rayapati, P. R. Sharma and C. Thomson, PCT Int. Appl. WO 2011004182 A1, January, 13, 2011.
- (a) S. K. Guchhait, A. L. Chandgude and G. Priyadarshani, J. Org. Chem., 2012, 77, 4438 CrossRef CAS PubMed; (b) R. L. Yan, H. Han, C. Ma, Z. Y. Ren, X. A. Gao, G. S. Huang and Y. M. Liang, J. Org. Chem., 2012, 77, 2024 CrossRef CAS PubMed; (c) H. Cao, H. Y. Zhan, Y. G. Lin, X. L. Lin, Z. D. Du and H. F. Jiang, Org. Lett., 2012, 14, 1688 CrossRef CAS PubMed; (d) O. N. Burchak, L. Mugherli, M. Ostuni, J. J. Lacapère and M. Balakirev, J. Am. Chem. Soc., 2011, 133, 10058 CrossRef CAS PubMed; (e) P. Liu, C. L. Deng, X. S. Lei and G. Q. Lin, Eur. J. Org. Chem., 2011, 36, 7308 CrossRef; (f) S. Mishra and R. Ghosh, Synthesis, 2011, 3463 CAS; (g) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (h) H. Wang, Y. Wang, C. Peng, J. Zhang and Q. Zhu, J. Am. Chem. Soc., 2010, 132, 13217 CrossRef CAS PubMed; (i) E. Kianmehr, M. Ghanbari, M. N. Niri and R. Faramarzi, J. Comb. Chem., 2010, 12, 41 CrossRef CAS PubMed; (j) A. Herath, R. Dahl and N. D. P. Cosford, Org. Lett., 2010, 12, 412 CrossRef CAS PubMed.
- (a) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed; (b) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS PubMed; (c) L. Rout, T. K. Sen and T. Punnlyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583 CrossRef CAS PubMed.
- (a) P. S. Herradura, K. A. Pendola and R. K. Guy, Org. Lett., 2000, 2, 2019 CrossRef CAS PubMed; (b) D. Ma, Y. Zhang, J. Yao, S. Wu and F. Tao, J. Am. Chem. Soc., 1998, 120, 12459 CrossRef CAS; (c) N. Taniguchi, Synlett, 2006, 2006, 1351 CrossRef PubMed; (d) N. Taniguchi, J. Org. Chem., 2007, 72, 1241 CrossRef CAS PubMed.
- (a) Q. Ding, B. Cao, J. Yuan, X. Liu and Y. Peng, Org. Biomol. Chem., 2011, 9, 748 RSC; (b) Q. Ding, X. Liu, J. Yu, Q. Zhang, D. Wang, B. Cao and Y. Peng, Tetrahedron, 2012, 68, 3937 CrossRef CAS PubMed.
- (a) X. Zhao, E. Dimitrijević and V. M. Dong, J. Am. Chem. Soc., 2009, 131(10), 3466 CrossRef CAS PubMed; (b) O. Saidi, J. Marafie, A. E. W. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey and C. G. Frost, J. Am. Chem. Soc., 2011, 133(48), 19298 CrossRef CAS PubMed; (c) W. Ge, X. Zhu and Y. Wei, Eur. J. Org. Chem., 2013, 6015 CrossRef CAS.
- (a) M. Tudge, M. Tamiya, C. Savarin and G. R. Humphrey, Org. Lett., 2006, 8, 565 CrossRef CAS PubMed; (b) Z. Li, J. Q. Hong and X. J. Zhou, Tetrahedron, 2011, 67, 3690 CrossRef CAS PubMed; (c) Z. Li, L. Hong, R. Liu, J. Shen and X. Zhou, Tetrahedron Lett., 2011, 52, 1343 CrossRef CAS PubMed; (d) Y.-J. Guo, R.-Y. Tang, J.-H. Li, P. Zhong and X.-G. Zhang, Adv. Synth. Catal., 2009, 351, 2615 CrossRef CAS; (e) X.-L. Fang, R.-Y. Tang, P. Zhong and J.-H. Li, Synthesis, 2009, 24, 4183 Search PubMed.
- (a) K. Tomita, A. Terada and R. Tachikawa, Heterocycles, 1976, 4, 729 CrossRef CAS; (b) S. Jain, K. Shukla, A. Mukhopadhyay, S. N. Suryawanshi and D. S. Bhakuni, Synth. Commun., 1990, 20, 1315 CrossRef CAS; (c) M. Raban and L. Chern, J. Org. Chem., 1980, 45, 1688 CrossRef CAS; (d) P. F. Ranken and B. G. McKinnie, J. Org. Chem., 1989, 54, 2985 CrossRef CAS; (e) C. C. Browder, M. O. Mitchell, R. L. Smith and G. el-Sulayman, Tetrahedron Lett., 1993, 34, 6245 CrossRef CAS; (f) P. Hamel and P. Préville, J. Org. Chem., 1996, 61, 1573 CrossRef CAS PubMed; (g) P. Hamel, Tetrahedron Lett., 1997, 38, 8473 CrossRef CAS; (h) P. Hamel, J. Org. Chem., 2002, 67, 2854 CrossRef CAS PubMed; (i) M. Matsugi, K. Murata, K. Gotanda, H. Nambu, G. Anilkumar, K. Matsumoto and Y. Kita, J. Org. Chem., 2001, 66, 2434 CrossRef CAS PubMed; (j) M. Matsugi, K. Murata, H. Nambu and Y. Kita, Tetrahedron Lett., 2001, 42, 1077 CrossRef CAS; (k) K. M. Schlosser, A. P. Krasutsky, H. W. Hamilton, J. E. Reed and K. Sexton, Org. Lett., 2004, 6, 819 CrossRef CAS PubMed; (l) M. Tudge, M. Tamiya, C. Savarin and G. R. Humphrey, Org. Lett., 2006, 8, 565 CrossRef CAS PubMed; (m) G. Wu, J. Wu, J. Wu and L. Wu, Synth. Commun., 2008, 38, 1036 CrossRef CAS; (n) W. Ge and Y. Wei, Green Chem., 2012, 14(7), 2066 RSC; (o) F. L. Yang and S. K. Tian, Angew. Chem., Int. Ed., 2013, 52(18), 4929–4932 CrossRef CAS PubMed; (p) F. Xiao, H. Xie, S. Liu and G. J. Deng, Adv. Synth. Catal., 2014, 356(2–3), 364 CrossRef CAS; (q) C. D. Prasad, S. Kumar, M. Sattar, A. Adhikary and S. Kumar, Org. Biomol. Chem., 2013, 11(46), 8036 RSC.
- (a) S. Kisaki, Y. Tominaga, Y. Matsuda and G. Kobayashi, Chem. Pharm. Bull., 1974, 22, 2246 CrossRef CAS; (b) R. G. Woodbridge and G. Dougherty, J. Am. Chem. Soc., 1950, 72, 4320 CrossRef CAS; (c) G. Campiani, V. Nacci, S. Bechelli, S. M. Ciani, A. Garofalo, I. Fiorini, H. Wikström, P. de Boer, Y. Liao, P. G. Tepper, A. Cagnotto and T. Mennini, J. Med. Chem., 1998, 41, 3763–3772 CrossRef CAS PubMed; (d) X. Zhao, Z. Yu, T. Xu, P. Wu and H. Yu, Org. Lett., 2007, 9, 5263 CrossRef CAS PubMed; (e) D. H. R. Barton, X. Lusinchi and P. Milliet, Tetrahedron Lett., 1982, 23, 4949 CrossRef CAS; (f) Y. Bal'on, M. D. Shul'man, V. A. Smirnov, M. M. Yukhomenko and M. A. Yukhomenko, Zh. Org. Khim., 1990, 26, 1566 CAS; (g) D. Huang, J. Chen, W. Dan, J. Ding, M. Liu and H. Wu, Adv. Synth. Catal., 2012, 354, 2123 CrossRef CAS; (h) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730 CrossRef CAS PubMed.
- (a) R. W. Marshman, Aldrichimica Acta, 1995, 28, 77 CAS; (b) S. A. Soderquist, Aldrichimica Acta, 1991, 24, 15 Search PubMed.
- (a) G. Bartoli, E. Marcantoni, M. Marcolini and L. Sambri, Chem. Rev., 2010, 110(10), 6104 CrossRef CAS PubMed; (b) D. Dhara, L. K. S. Gayen, S. Khamarui, P. Pandit, S. Ghosh and D. K. Maiti, J. Org. Chem., 2012, 77(22), 10441 CrossRef CAS PubMed; (c) C. C. Silveira, S. R. Mendes, L. Wolf and G. M. Martins, Tetrahedron Lett., 2010, 51, 2014 CrossRef CAS PubMed; (d) E. Marcantoni, R. Cipolletti, L. Marsili, S. Menichetti, R. Properzi and C. Viglianisi, Eur. J. Org. Chem., 2012, 2013, 132 CrossRef.
- (a) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed; (b) P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108, 2238 CrossRef CAS PubMed; (c) R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792 CrossRef PubMed; (d) S. M. S. Chauhan, A. Kumar and K. A. Srinivas, Chem. Commun., 2003, 2348 RSC; (e) A. R. Hajipour, M. Mostafavi and A. E. Ruoho, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 1920 CrossRef CAS PubMed; (f) D. Singh, F. Z. Galetto, L. C. Soares, O. E. D. Rodrigues and A. L. Braga, Eur. J. Org. Chem., 2010, 2661 CrossRef CAS; (g) N. Kumara and R. J. Jain, Heterocycl. Chem., 2012, 49, 370 CrossRef.
- (a) P. Sang, Z. Chen, J. Zou and Y. Zhang, Green Chem., 2013, 15, 2096 RSC; (b) L.-H. Zou, J. Reball, J. Mottweiler and C. Bolm, Chem. Commun., 2012, 48, 11307 RSC.
- D. Giles, M. S. Prakash and K. V. Ramseshu, Eur. J. Chem., 2007, 4, 428 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4ra01240b |
| This journal is © The Royal Society of Chemistry 2014 |