Oxidant-induced dopamine polymerization for multifunctional coatings†
Qiang
Wei
,
Fulong
Zhang
,
Jie
Li
,
Beijia
Li
and
Changsheng
Zhao
*
College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, 610065, China. E-mail: zhaochsh70@scu.edu.cn; zhaochsh70@163.com; Fax: +86-28-85405402; Tel: +86-28-85400453
First published on 7th September 2010
Abstract
Polydopamine-coatings can be prepared in acidic, neutral and alkaline aqueous media by oxidant-induced polymerization, which is material-independent and multifunctional for surface modification.
Modification of materials’ surfaces plays an important role in modern chemical, biological, and materials sciences.1 Widespread methods for surface modification, such as self-assembled monolayers, organosilane chemistry, Langmuir–Blodgett deposition, layer-by-layer assembly, and genetically engineered surface-binding peptides, work well on particular materials’ surfaces compatible with the specific strategy for surface conjugation,2 but lack efficacy on broad ranges of materials’ surfaces.
To solve the problem, pH-induced polymerization of dopamine (DA) and its derivant have been introduced for surface modification. Dopamine, which was inspired by the composition of adhesive proteins in mussels,3 was reported to form thin, surface-adherent polymer films onto virtually all material surfaces, in alkaline aqueous media.4 Only by a simple immersion of substrate in a dilute aqueous solution of dopamine, buffered to a alkaline pH, could result in spontaneous deposition of a thin polydopamine film. It has been widely used to prepare a variety of ad-layers, including self-assembled monolayers through deposition of long-chain molecular building blocks,5 multilayer films,6 surface modification of the inorganic materials,7 nanocapsules for drug delivery8 and bioinert and bioactive surfaces via grafting of macromolecules.9 However, many alkaline corrosive and pH sensitive materials, such as cellulose, polyester, phenolic resin, protein, pH sensitive gel, pH sensitive filter membrane and so on,10 are not suitable for modification by dopamine in an alkaline aqueous media.
The dopamine polymerization mechanism involves the oxidation of catechol in dopamine to quinone by alkaline pH-induced oxidation.4a Why could not induce dopamine polymerization by an oxidant in neutral or acidic aqueous media? In fact, it has been mentioned to oxidize catechol in 3,4-dihydroxyphenylalanine to quinone by oxidants in some literature.11 In another study,12 ammonium persulfate was used to induce dopamine polymerization to prepare dopamine nanowire; however, the ammonium persulfate was only regarded as an initiator. This inspired us to further investigate the oxidant-induced-polymerization of DA to expand the application of polydopamine film. Herein, we present a general and easy surface modification strategy of oxidant-induced dopamine polymerization for multifunctional coatings. Oxidant was used to induce dopamine polymerization to modify several substrates, including metal, ceramic, and polymer, as indicated in Scheme 1. And a variety of secondary immobilization reactions using the oxidant-induced modified surfaces as the bases can also led to various functional coatings as the pH-induced modified surface.
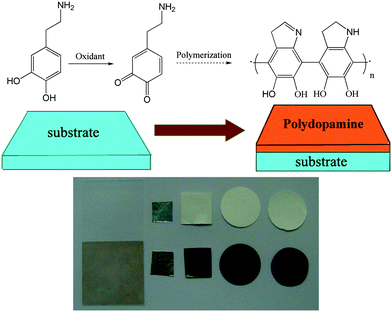 | ||
| Scheme 1 Multifunctional coating formation by oxidant-induced dopamine polymerization (left to right: glass, Al, polyethersulfone, nylon and cellulose). | ||
Ammonium persulfate (AP) was firstly chosen as the oxidant. Substrates were modified by simple immersion in a solution of 2 mg ml−1 dopamine·HCl (the same concentration as reported in the literature of ‘pH-induced’4a) mixed with required amount of AP. After several hours of incubation, the colour of the substrates changed to brown, as shown in Scheme 1. The brown colour of the coated film was due to the catechol oxidation followed by polymerization of DA, which was analogous to the pH-induced dopamine polymerization.
The reactivity of dopamine was measured at room temperature using UV-vis spectroscopy by following the formation of quinone with time. Fig. 1a shows the UV-vis spectra of DA during the AP-mediated oxidation reaction. After AP addition (the molar ratio of AP to DA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the pH value was about 4 in this system), an absorption peak around 350 nm developed due to the formation of the quinone. The data was in agreement with the proposed reaction process of the oxidation of the catechol groups into quinone and the subsequent polymerization.11b A broad peaking around 480 nm developed, which is a characteristic electronic transition of the chrome formation of the oxidized state of DA after intramolecular cyclization. The basic pathway probably responsible for the oxidant-induced polymerization of DA was deduced from the reported pH-induced polymerization pathway:4a dopamine was oxidized to dopaminequinone; then the intramolecular cyclization of the dopaminequinone via 1,4-Michael addition led to the leucodopaminechrome; and then leucodopaminechrome was oxidized to dopaminechrome; and then the dopaminechrome could be polymerized to form polydopamine. The absorption intensity at 350 nm of DA by both oxidant-induced and pH-induced was shown in Fig. 1b. The absorption intensity for oxidant-induced polymerization increased with time, and the results could be comparable with the pH-induced polymerization. The colour of the DA solution had a gradual change from colourless to dark brown (Fig. 1e). Some precipitates could be observed after centrifugation for 10 min, which was resulted from the AP-induced polymerization of DA (photographs not shown). Different molar ratios of DA to AP in the polymerization process were investigated, as shown in Fig. 1c and 1d. The absorption intensity increased linearly with the increase of the molar ratios of AP to DA, after either 2 h or 24 h running time. The added AP is beneficial to the DA polymerization.
2, and the pH value was about 4 in this system), an absorption peak around 350 nm developed due to the formation of the quinone. The data was in agreement with the proposed reaction process of the oxidation of the catechol groups into quinone and the subsequent polymerization.11b A broad peaking around 480 nm developed, which is a characteristic electronic transition of the chrome formation of the oxidized state of DA after intramolecular cyclization. The basic pathway probably responsible for the oxidant-induced polymerization of DA was deduced from the reported pH-induced polymerization pathway:4a dopamine was oxidized to dopaminequinone; then the intramolecular cyclization of the dopaminequinone via 1,4-Michael addition led to the leucodopaminechrome; and then leucodopaminechrome was oxidized to dopaminechrome; and then the dopaminechrome could be polymerized to form polydopamine. The absorption intensity at 350 nm of DA by both oxidant-induced and pH-induced was shown in Fig. 1b. The absorption intensity for oxidant-induced polymerization increased with time, and the results could be comparable with the pH-induced polymerization. The colour of the DA solution had a gradual change from colourless to dark brown (Fig. 1e). Some precipitates could be observed after centrifugation for 10 min, which was resulted from the AP-induced polymerization of DA (photographs not shown). Different molar ratios of DA to AP in the polymerization process were investigated, as shown in Fig. 1c and 1d. The absorption intensity increased linearly with the increase of the molar ratios of AP to DA, after either 2 h or 24 h running time. The added AP is beneficial to the DA polymerization.
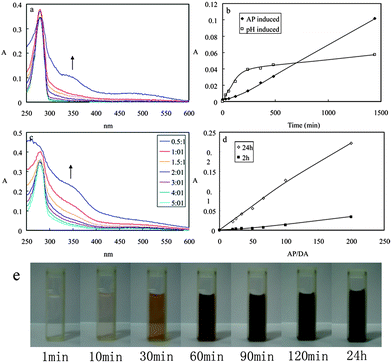 | ||
| Fig. 1 (a) UV-vis spectra of DA during the AP-mediated oxidation reaction. (b) Absorption intensity at 350 nm of DA by both oxidant-induced and pH-induced. (c) UV-vis spectra of DA after 24 h running time by mixed with different amount of AP. (d) Absorption intensity at 350 nm of DA after 2 h and 24 h running time when mixed with different amounts of AP. (e) Colour change of DA with the running time. | ||
Other oxidants were also used to induce DA polymerization. Both the strong oxidant sodium periodate and the weak oxidant potassium chlorate could induce DA polymerization. The absorption intensity was larger, when stronger oxidants was added (Fig. S1, ESI†).
The oxidant-induced DA polymerization in weak acidic, neutral and weak alkaline aqueous media was investigated with the same AP concentration, respectively (Fig. S2†). At pH 5.5 and pH 7.0, the DA can hardly polymerize without AP, but can polymerize when AP added. At pH 8.5, the DA can polymerize either with or without AP, but the reaction speed of the system with AP is much faster than that without AP. The absorption intensity at 350 nm of DA is larger at higher pH value (Fig. S2d†). This indicated that a high pH condition was beneficial to the DA polymerization when AP was added, and the added AP was helpful to speed up the reaction of DA.
Several materials, including metal (Al), ceramic (glass) and polymer (polyethersulfone (PES), cellulose and nylon), were coated with PDA, by simple immersion in the solution of DA mixed with AP, at pH 7.0, for several hours. The thickness of the PDA film was about 70 nm on the PES (analyzed by AFM), after 2 h of incubation. It would cost more than 10 h incubation to reach this thickness by pH-induced polymerization. XPS analysis (KRATOS XSAM800, Britain) had been employed to quantitatively determine the chemical composition of the coated layer. The XPS spectrum of the PDA-coated substrates showed no evident substrate signal. Instead, peaks from the atomic composition of PDA were observed (Fig. 2a and Fig. S3, ESI†). The N/C molar ratio for the PDA layers ranged from 0.112 to 0.129 (Fig. 2b), which approached to the theoretical value of pure DA (0.125) and the same to the reported pH-induced PDA layers on these substrates.4a Compared to the initial nylon substrate, the C1s and O1s XPS spectra signal altered evident after PDA coated (Fig. S4, ESI†). In the case of PDA-coated nylon substrate, the C1s spectra signal at 284.8 eV was attributed to the carbon in C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H, the signal at 286.0 eV was attributed to the carbon ether bond C–O, the signal at 287.4 eV was attributed to the carbon in C–N, and the signal at 288.6 eV was attributed to the carbon in C
C and C–H, the signal at 286.0 eV was attributed to the carbon ether bond C–O, the signal at 287.4 eV was attributed to the carbon in C–N, and the signal at 288.6 eV was attributed to the carbon in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Both C–O and C
O. Both C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were existed, which confirmed that both catechol and quinone groups were contained in the PDA molecules. The O1s spectra signal (Fig. S4c and S4d, ESI†) showed the same result. These suggested that PDA layers were coated onto the substrates.
O were existed, which confirmed that both catechol and quinone groups were contained in the PDA molecules. The O1s spectra signal (Fig. S4c and S4d, ESI†) showed the same result. These suggested that PDA layers were coated onto the substrates.
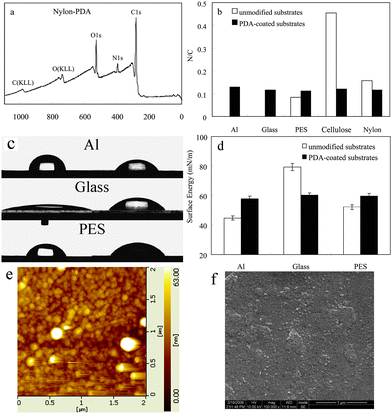 | ||
| Fig. 2 (a) XPS spectra of PDA-coated nylon; (b) the N/C molar ratio; (c) water contact angles of the PDA-coated substrates; (d) the surface energy of the PDA-coated substrates; (e) AFM and (f) FSEM images of the surface morphology of the PDA-coated glass. | ||
The surface morphology of the PDA coatings was investigated by AFM. Fig. 2e shows the image of the PDA-coated glass, which is in vivid contrast to the native glass surface (Fig. S5, ESI†). It indicated that the PDA-coated layer was composed of aggregated nanoparticles, which can be also seen clearly on the FSEM images (Fig. 2f and Fig. S5, ESI). The surface morphology of the PDA-coated Al and PES films (Fig. S5, ESI†) cannot be observed as clearly as PDA-coated glass by AFM, since the Al and PES substrates, which were more flexible than glass, were hard to be spread flat on the test board of the AFM instrument. However, obvious changes can be observed before and after coating the PDA by both AFM and FSEM (Fig. S6, ESI†). Furthermore, there were no evident differences between the oxidant-induced and pH-induced PDA coating from the SEM images (Fig. S6, ESI†). The little differences may be caused by the different reaction degrees of DA polymerization.
The wettability of the PDA coatings was investigated by the water contact angle measurement. The water contact angles of the PDA-coated substrates ranged from 50° to 60°, which approached to the theoretical value of pure PDA film, though the initial wetting behaviours of the substrates were significantly different (Fig. 2c). The surface energy data showed the similar results (Fig. 2d). These also demonstrated that PDA layers were coated onto the substrates successfully. For the PDA coated and uncoated glasses, advancing (θa) and receding (θr) contact angles with water are presented in Table S1.† The hysteresis (θa–θr) reflected not only the chemical heterogeneity but also the roughness (physical heterogeneity) of the surfaces.13 AFM results showed the roughness of PDA coated (Ra = 7.20 nm) and uncoated (Ra = 3.95 nm) glasses. The roughness of the glasses increased after coating PDA, which might be caused by the PDA nanoparticles aggregated randomly on the glass surface as described in the last paragraph. Both the roughness increase and the chemical composition change led to the hysteresis changed after coating PDA.
Furthermore, the use of ammonium persulfate is harmful to some proteins and metals such as copper.14 Sodium periodate and potassium chlorate mentioned above can also induce the polymerization reaction for the surface modification (data not shown). And we can infer reasonably that other oxidants such as hydrogen peroxide and oxidase can be also used.
Oxidant-induced PDA coatings also support the secondary immobilization reactions for the creation of functional organic ad-layers as the pH-induced PDA coatings.4a,9a,15 Bovine serum albumin (BSA), which could be used to improve the blood compatibility of materials,16 was used as a model functional molecule to be immobilized onto the PDA coated surfaces by the terminal amine and amine of lysine in BSA. From the analysis of XPS, it was found that the surface chemical composition altered obviously after the BSA immobilization on PDA (Fig. 3a). The peaks corresponded to the nitrogen and oxygen increased, while that to the carbon decreased (Table S2, ESI†). The N/C molar ratio for the PDA-coated Nylon was 0.117. It altered to 0.223 after the immobilization of BSA. AFM images also demonstrated these results as shown in Fig. 3b. The surface morphology of PDA-coated glass altered obviously after the BSA immobilization, and the roughness of the BSA immobilized glass increased to 11.34 nm.
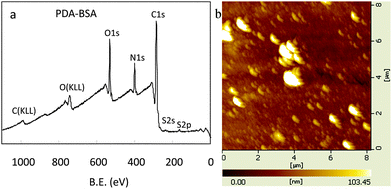 | ||
| Fig. 3 (a) XPS spectra of PDA-coated nylon after BSA immobilization. (b) AFM images of PDA-coated glass after BSA immobilization. | ||
In conclusion, we have presented a general protocol for surface modification by oxidant-induced dopamine coating, and this modification is material-independent and multifunctional. By oxidant-induced polymerization, polydopamine-coatings can be prepared in acidic, neutral and alkaline aqueous media; and many alkaline corrosive and pH sensitive materials can be modified by dopamine in an acidic or neutral aqueous media. By the addition of oxidant, the reaction speed of dopamine in alkaline aqueous media increased. The oxidant-induced method extended the application range of polydopamine-coatings.
This work was financially sponsored by the National Natural Science Foundation of China (No. 50972070), and Sichuan Youth Science and Technology Foundation (08ZQ026-038).
References
- F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37–54 RSC; L. A. Cao and M. Kruk, Polym. Chem., 2010, 1, 97–101 RSC; R. Langer, Science, 2001, 293, 58–59 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS; B. Yameen, M. Ali, M. Alvarez, R. Neumann, W. Ensinger, W. Knoll and O. Azzaroni, Polym. Chem., 2010, 1, 183–192 RSC; Langmuir–Blodgett Films, ed. G. Roberts, Plenum, New York, 1990 Search PubMed; S. R. Whaley, D. S. English, E. L. Hu, P. F. Barbara and A. M. Belcher, Nature, 2000, 405, 665–668 Search PubMed.
- M. Yu, J. Hwang and T. J. Deming, J. Am. Chem. Soc., 1999, 121, 5825–5826 CrossRef CAS; J. H. Waite and X. X. Qin, Biochemistry, 2001, 40, 2887–2893 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS; S. M. Kang, J. Rho, I. S. Choi, P. B. Messersmith and H. Lee, J. Am. Chem. Soc., 2009, 131, 13224–13225 CrossRef CAS.
- H. Lee, Y. Lee, A. R. Statz, J. Rho, T. G. Park and P. B. Messersmith, Adv. Mater., 2008, 20, 1619–1623 CrossRef CAS.
- F. Bernsmann, L. Richert, B. Senger, P. Lavalle, J. C. Voegel, P. Schaaf and V. Ball, Soft Matter, 2008, 4, 1621–1624 RSC.
- Y. H. Lee, H. Lee, Y. B. Kim, J. Y. Kim, T. Hyeon, H. Park, P. B. Messersmith and T. G. Park, Adv. Mater., 2008, 20, 4154–4157 CAS; F. Bernsmann, A. Ponche, C. Ringwald, J. Hemmerle, J. Raya, B. Bechinger, J. C. Voegel, P. Schaaf and V. Ball, J. Phys. Chem. C, 2009, 113, 8234–8242 CrossRef CAS; J. K. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132–2139 CrossRef CAS.
- B. Yu, D. A. Wang, Q. Ye, F. Zhou and W. M. Liu, Chem. Commun., 2009, 6789–6791 RSC.
- H. Lee, J. Rho and P. B. Messersmith, Adv. Mater., 2009, 21, 431–434 CrossRef CAS; T. A. Morris, A. W. Peterson and M. J. Tarlov, Anal. Chem., 2009, 81, 5413–5420 CrossRef CAS.
- D. Ruan, L. N. Zhang, Y. Mao, M. Zeng and X. B. Li, J. Membr. Sci., 2004, 241, 265–274 CrossRef CAS; S. Harrisson, F. Ercole and B. W. Muir, Polym. Chem., 2010, 1, 326–332 RSC; Q. Wei, J. Li, B. S. Qian, B. H. Fang and C. S. Zhao, J. Membr. Sci., 2009, 337, 266–273 CrossRef CAS.
- B. P. Lee, J. L. Dalsin and P. B. Messersmith, Biomacromolecules, 2002, 3, 1038–1047 CrossRef CAS; M. C. van der Leeden, Langmuir, 2005, 21, 11373–11379 CrossRef CAS.
- R. Z. Ouyang, J. P. Lei and H. X. Ju, Chem. Commun., 2008, 5761–5763 RSC.
- Ph. Game, D. Sage and J. P. Chapel, Macromolecules, 2002, 35, 917–923 CrossRef CAS.
- G. C. Jo, G. S. Chae, Y. S. Hwang, O. N. Kwon, K. M. Lee, K. J. Baek and T. H. Rhee, US patent, 2005, 6881679; S. K. Bae, H. K. Kim, Y. B. Lee, X. F. Xu, J. S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. Song, Y. J. Kim, K. S. Kim, B. Özyilmaz, J. H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574–578 Search PubMed.
- J. H. Waite, Nat. Mater., 2008, 7, 8–9 CrossRef CAS.
- Z. B. Liu, X. P. Deng, M. Wang, J. X. Chen, A. M. Zhang, Z. W. Gu and C. S. Zhao, J. Biomater. Sci., Polym. Ed., 2009, 20, 377–397 CrossRef CAS; B. H. Fang, Q. Y. Ling, W. F. Zhao, Y. L. Ma, P. L. Bai, Q. Wei, H. F. Li and C. S. Zhao, J. Membr. Sci., 2009, 329, 46–55 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Related reagents, characterization of the dopamine polymerization by UV-vis, polydopamine coating, BSA immobilization on the polydopamine layer, the colour change of DA with the running time, UV-vis spectra of PDA induced by sodium periodate and potassium chlorate, XPS, water contact angle, AFM and SEM characterization of PDA-coated substrates, XPS and AFM characterization of BSA immobilization. See DOI: 10.1039/c0py00215a |
| This journal is © The Royal Society of Chemistry 2010 |
