DOI:
10.1039/D5SC00059A
(Review Article)
Chem. Sci., 2025,
16, 6598-6619
Sustainable and cost-effective electrode manufacturing for advanced lithium batteries: the roll-to-roll dry coating process
Received
4th January 2025
, Accepted 21st March 2025
First published on 28th March 2025
Abstract
The transition to electric vehicles motivated by global carbon neutrality targets has intensified the demand for lithium-ion batteries (LIBs) with high energy density. While the innovation of cathode/anode active materials has reached a plateau, development of thick electrodes has emerged as a critical breakthrough to achieving high-energy-density LIBs. However, the conventional wet coating process has intrinsic limitations, such as binder migration during the solvent drying process, which becomes increasingly problematic with thick electrodes. To address these challenges, dry coating processes have been actively explored in three main forms: electrostatic spraying, hot pressing with thermoplastic polymers, and roll-to-roll dry coating utilizing the polytetrafluoroethylene binder. This review highlights the roll-to-roll dry coating process, a scalable and industrially viable approach, by introducing its underlying mechanisms, latest developments, and applications in all-solid-state batteries and lithium–sulfur batteries. By combining technical advancements with manufacturing scalability, the roll-to-roll dry coating process demonstrates significant potential to enable next-generation battery systems.
 Joonhyeok Park | Joonhyeok Park earned a BS degree from the Department of Materials Science and Engineering at Hanyang University, South Korea, in 2019. His current research focuses on interfacial engineering for all-solid-state batteries, and battery manufacturing.. |
 Jiwoon Kim | Jiwoon Kim earned a BS degree from the Department of Energy Engineering at Hanyang University, South Korea, in 2020. His research interest is focused on the synthesis of nanomaterials for energy device applications and battery manufacturing. |
 Jaeik Kim | Jaeik Kim earned a BS degree from the Department of Energy Engineering at Hanyang University, South Korea, in 2019. His current research focuses on all-solid-state batteries, anode-less systems, Li metal anodes, and interfacial engineering. |
 Minsung Kim | Minsung Kim earned a BS degree from the Department of Energy Engineering at Hanyang University, South Korea, in 2021. His research interest is focused on the synthesis of nanomaterials for energy device applications and battery manufacturing. |
 Taeseup Song | Taeseup Song received his PhD degree from the Department of Materials Science and Engineering at Hanyang University, South Korea, in 2012. He is currently an associate professor in the Department of Energy Engineering at Hanyang University. His research interest is mainly focused on the synthesis of nanostructured materials for energy device applications. |
 Ungyu Paik | Ungyu Paik is a distinguished professor in the Department of Energy Engineering at Hanyang University, South Korea. He received his PhD degree from the Department of Ceramic Engineering at Clemson University in 1991. His research interest is the synthesis and engineering of nanomaterials for applications in energy devices. |
1. Introduction
1.1 Demand for sustainable and cost-effective electrode manufacturing for high-energy-density lithium batteries
As the global drive for carbon neutrality has accelerated the transition from internal combustion engine vehicles to electric vehicles (EVs), the demand for medium- and large-scale high-energy-density lithium-ion-batteries (LIBs), essential for long driving ranges and fast charging, has grown significantly.1–3 To meet these demands, nations and industries around the world have initiated projects, such as the U.S. Department of Energy's Battery 500, Japan's NEDO RISING II, and China's “Made in China 2025”, all targeting energy densities exceeding 500 W h kg−1 by 2030 (ref. 4–8) (Fig. 1a). Since Sony's commercialization of lithium–cobalt oxide and graphite-based LIBs in 1991, significant progress has been made in increasing the energy density of LIBs through material innovations.9,10 Advances such as high-nickel cathode materials, silicon-based anode materials, and high-voltage electrolytes have enabled LIBs to achieve energy densities of approximately 300 W h kg−1 for commercial applications and up to 350 W h kg−1 in the laboratory scale.11–15 However, without the introduction of innovative materials that can exhibit a higher capacity than the theoretical capacity of existing cathode and anode materials, further increases in the energy density of LIBs through material innovation alone are limited. The limitation of materials innovation has shifted research attention to electrode design, particularly to thick and dense electrodes with high mass loadings, which can be applied to various battery systems such as lithium metal batteries (LMBs), all-solid-state batteries (ASSBs), and lithium–sulfur (Li–S) batteries.16,17 Thick and dense electrodes present a promising pathway to achieving higher gravimetric and volumetric energy densities by reducing inactive components such as current collectors and separators.18–20 For instance, transitioning from conventional electrode thicknesses (∼25 μm) to thick electrodes (∼200 μm) can increase the fraction of the cell occupied by active materials, thereby enhancing energy densities by reducing the stack count required in a battery pack (Fig. 1b).
 |
| | Fig. 1 Demand for sustainable and cost-effective electrode manufacturing for high-energy-density lithium batteries: (a) the history and development of Li-ion batteries according to the worldwide nations and industry. Reproduced with permission from ref. 8. Copyright (2019) Elsevier B.V. (b) Schematic illustration of the advantages of thick electrodes in terms of energy density of lithium rechargeable batteries. | |
1.2 Comparison of dry and wet processes
Achieving high-energy-density LIBs with thick and dense electrodes poses significant challenges in the conventional wet coating process. The wet coating process, the industry standard for manufacturing electrodes, is hindered by several limitations that compromise both electrochemical performance and environmental/sustainability/economic.21,22 The critical issue of the wet coating process is binder migration during the solvent drying process, which induces an inhomogeneous microstructure in the electrode23–26 (Fig. 2a). During the solvent drying process, the binder migrates toward the top region of the electrode along with the solvent due to capillary forces. Then, the binder blocks the pores in the top region of the electrode while leaving behind large voids within the electrode or causing an uneven distribution of active materials. When the calendering process is applied to such an electrode, localized collisions between active material particles lead to cracks and the difference in composition of electrode components between the top and bottom regions of the electrode further contributes to the formation of an inhomogeneous microstructure. The inhomogeneity of the electrode microstructure becomes more pronounced as the electrode thickness increases because the increase in solvent drying time aggravates the binder migration. Accordingly, wet-processed thick electrodes exhibit significant differences in porosity and density within the electrode after the calendering process27,28 (Fig. 2b). The inhomogeneity of the electrode microstructure degrades lithium-ion transport kinetics, leading to an increase in ionic resistance and poor electrochemical properties such as cycling stability or rate capability.29–33 Furthermore, the reliance on organic solvents in the wet coating process, such as N-methyl-2-pyrrolidone (NMP), increases energy demand for solvent evaporation/recovery and generates hazardous waste, adding to the environmental and economic costs of the battery manufacturing process.34,35 In contrast, dry coating processes exclude these drawbacks by removing the solvent drying and recovery processes entirely.36 The dry coating processes effectively prevent binder migration caused during the solvent drying process, enabling the uniform distribution of active materials, conductive additives and binders within the electrode and resulting in improvements in the homogeneity of the electrode microstructure and electrochemical properties37,38 (Fig. 2c). Moreover, dry coating processes align with global sustainability goals by removing the use of toxic solvents, reducing energy consumption by approximately 46%, and lowering production costs by up to 19%.39,40 The advantages of the dry coating process over the wet coating process, as outlined in Table 1, establish the dry coating method as an economically viable and environmentally friendly manufacturing option for medium- and large-scale high-energy-density LIBs.
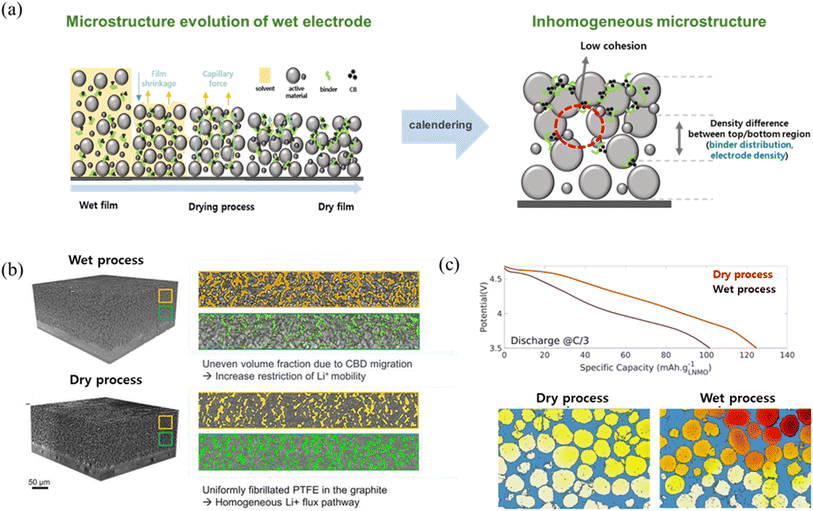 |
| | Fig. 2 Comparison of dry and wet processed electrodes: (a) schematic illustration of degradation of wet-processed thick electrodes during the solvent drying and lamination process by binder migration. (b) Comparison of the electrode microstructure and (c) electrochemical performance. Reproduced with permission from ref. 28 and 37. Copyright (2023) Elsevier B.V. and copyright (2023) RSC Publishing. | |
Table 1 Comparison between wet and dry coating processes
|
|
Wet coating process |
Dry coating process |
Reference |
| Environmental effect |
Using toxic solvents |
Eliminating toxic solvents |
41 and 42 |
| Excessive CO2 emissions |
| Process efficiency |
Complex process |
Simplified process (no drying/solvent recovery processes) |
43 and 44 |
| Cost |
High OPEX and CAPEX (using NMP solvent, drying infrastructure) |
Low OPEX and CAPEX (estimated reduction of the process and solvent) |
22, 45 and 46 |
| Microstructure |
Inhomogeneous microstructures attributed to the binder migration during the fabrication of thick electrodes |
Homogeneous microstructures associated with uniform binder distribution during the thick electrode fabrication |
26 and 47 |
| Areal capacity |
Limitations caused by binder migration (<7 mA h cm−2) |
Increase in thickness and density of electrodes fabricated by the dry process (≥5 mA h cm−2) |
48 and 49 |
This review also explores the dry coating processes in next-generation rechargeable LIB systems. In particular, the roll-to-roll dry coating process was highlighted among various dry coating processes with the future research directions to achieving high energy density LIBs that will support the transition to a sustainable energy future. By integrating material innovations with sustainable manufacturing processes, the battery industry can meet the growing demand for high-energy-density and environmentally friendly energy storage systems.
1.3 Introduction of various dry coating processes
Dry coating processes have emerged as transformative technologies in the realm of battery electrode manufacturing, offering a sustainable alternative to the conventional solvent-based wet coating process. These methods eliminate the need for solvents, thereby reducing environmental impacts, improving energy efficiency, and addressing the growing demand for global carbon neutrality. Among the various dry coating processes, electrostatic spray coating, hot pressing, and roll-to-roll dry coating stand out, each offering unique advantages and challenges to meet the specific requirements of electrode manufacturing.
1.3.1 Electrostatic spray coating.
Electrostatic spray coating utilizes high-voltage electric fields to precisely deposit a mixture of active materials, binders, and conductive additives onto a substrate50 (Fig. 3a). The process begins with the preparation of a homogeneous dry mixture, which is introduced through an electrostatic spray nozzle. The high voltage applied to the nozzle generates a fine mist of charged particles, which are directed toward the substrate due to electrostatic attraction. These charged particles adhere uniformly to the substrate's surface, forming a thin and uniform layer. After deposition, a thermal or mechanical stabilization step enhances the mechanical integrity and chemical stability of the deposited layer. The primary advantage of electrostatic spray coating lies in its precision and ability to produce highly uniform layers with precise control over thickness, which is critical for achieving consistent electrochemical performance.51–53 Additionally, the capability to apply extremely thin coatings makes this technique ideal for advanced battery designs that demand uniformity in layer thickness and material composition. Brandon et al. demonstrated the use of this method by preparing a dry mixture of the LiCoO2 active material, carbon black (CB) conductive additive, and PVdF binder using a high-energy mixer, which was deposited onto an aluminum current collector using electrostatic spray coating.45 The mechanical strength and electrochemical properties of the fabricated electrode were evaluated and compared with a wet-processed electrode of the same composition. The dry electrode exhibited superior performance, which is attributed to the homogeneous distribution of the binder achieved through the electrostatic spray coating process. Similarly, Mohanad et al. employed the electrostatic spray coating method to fabricate both positive and negative electrodes and compared their properties to those of wet-processed electrodes with the same composition.54 They reported that the dry electrodes showed improved properties resulting from the uniform distribution of binders and conductive additives on the surfaces of graphite and NCM811 particles. This uniform distribution also acted as a protective layer, minimizing side reactions with the electrolyte and promoting the formation of stable CEI and SEI layers on the active material surface. Consequently, the dry electrodes achieved high areal capacities (6 mA h cm−2) and demonstrated excellent electrochemical performance. Despite its advantages, the electrostatic spray coating method faces challenges for the commercial production of thick electrodes for medium-to-large secondary batteries, due to the reliance on high-voltage equipment with high complexity and increased operational costs. Additionally, the slow deposition rate limits its suitability for high-throughput manufacturing environments.55 These limitations currently restrict its application to small-scale production and specialized markets where precise control of the electrode architecture is essential.
 |
| | Fig. 3 Various dry coating processes: (a) schematic illustration of electrostatic spray drying, (b) hot pressing and (c) roll-to-roll dry coating processes. Reproduced with permission from ref. 50, 56 and 57. Copyright (2017) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright (2023) The Author(s) and copyright (2024) The Authors. Small Science published by Wiley-VCH GmbH. (d) The total number of papers on dry coating processes (electrostatic spray drying, hot pressing and roll-to-roll dry coating processes) and (e) papers on each dry coating process (2016–2024). | |
1.3.2 Hot pressing.
Hot pressing is a dry coating technology that combines high temperatures and pressures to compact a dry mixture into dense and mechanically durable electrode films58 (Fig. 3b). The process begins by preparing a uniform mixture of active materials, conductive additives, and binders, which is then placed into a mold or die. The mixture is exposed to elevated temperatures, typically ranging from 100 °C to 300 °C, and pressures exceeding several MPa. A key advantage of hot pressing is its ability to produce highly compact electrode structures with various thermoplastic binders and retain strong cohesion and mechanical stability with low content of binders.59,60 Ryu et al. utilized hot pressing to fabricate ultra-thick electrodes incorporating multi-walled carbon nanotubes (MWNTs) as conductive additives and polyvinylidene fluoride (PVDF) as a binder.56 Throughout the hot pressing, electrodes with an areal capacity of 17.6 mA h cm−2 and a thickness of 573 μm were developed, demonstrating its suitability for high-energy-density applications. Zhang et al. also developed a LiFePO4 cathode using lithium-ion-conductive and biodegradable poly(propylene carbonate) (PPC) as a dry binder. PPC, with its low glass transition temperature (Tg) of 36.5 °C, allowed the fabrication of the dry electrode in a rubbery state, providing strong mechanical properties to the LFP cathode.61 Additionally, PPC's high ionic conductivity reduced ionic resistance within the electrode compared to conventional dry electrodes, significantly improving its electrochemical performance. Despite its advantages, hot pressing encounters challenges when applied to the commercial-scale production of thick electrodes for medium-to-large secondary batteries. The need for high temperatures and pressures leads to significant energy consumption, increasing manufacturing costs. Additionally, the challenges in the continuous process of hot pressing limit its scalability, making it difficult to be applied in industrial-scale manufacturing.62
1.3.3 Roll-to-roll dry coating.
The roll-to-roll dry coating method is recognized as one of the most scalable and efficient technologies for large-scale electrode fabrication57 (Fig. 3c). This method involves preparing a dry mixture of active materials, conductive additives, and the polytetrafluoroethylene (PTFE) binder, which is then continuously fed into a roller or extrusion system, where the PTFE is fibrillized by external shear forces. The resulting electrode film is subsequently conveyed to downstream roller systems, enabling continuous production and making the roll-to-roll dry coating process particularly suitable for mass production, which helps to minimize manufacturing time and costs. The PTFE binder used in the roll-to-roll dry coating process forms a fibrous network within the electrode film, enhancing its mechanical strength and structural integrity.63–66 Due to these advantages, the roll-to-roll dry coating process has become a critical point of research in both industry and academia. For example, Tao et al. developed dry cathodes and anodes with an areal capacity of 6 mA h cm−2 using the roll-to-roll dry coating process and evaluated their electrochemical properties against wet-processed full cells. The dry electrodes showed superior electrochemical performances.67 Beyond LIB systems, research on the roll-to-roll dry coating process has expanded to next-generation batteries. Hippauf et al. reported NCM cathodes for ASSBs with an areal capacity of 6.5 mA h cm−2 using the PTFE binder in concentrations ranging from 0.1 to 1 wt%.68 Similarly, Hu et al. developed dry cathodes for lithium–sulfur batteries with high sulfur loadings (2 mg cm−2) using just 1 wt% PTFE binder.38 The roll-to-roll dry coating process has also been implemented in industrial-scale production. For instance, Tesla has adopted this method for manufacturing dry-coated anode films, demonstrating its feasibility for large-scale production of advanced battery components.69 However, challenges remain in further optimizing the roll-to-roll dry coating process. The fibrillization of the PTFE binder, which is crucial for improving mechanical and electrochemical properties, requires precise control. Furthermore, achieving the homogeneous distribution of active materials, conductive additives, and binders within the electrode remains an ongoing area of research.
Among the various dry coating techniques (electrostatic spray coating, hot pressing, and roll-to-roll dry coating), each offers distinct advantages and limitations, making them suitable for different applications. Electrostatic spray coating is suitable for precision and uniformity, making it well-suited for small-scale or research-focused applications. Hot pressing excels in producing dense and mechanically robust films, making it valuable for high-performance electrodes on a smaller scale. Roll-to-roll dry coating, in contrast, is the most promising technique for industrial-scale production due to its scalability, efficiency, and compatibility with high-throughput manufacturing. The roll-to-roll dry coating process has gained significant attention in recent years driven by the increasing demand for high-performance and cost-effective LIBs, demonstrated by the number of publications related to dry coating processes in lithium rechargeable batteries since the early 2010s (Fig. 3d and e). The combination of environmental sustainability and industrial scalability of the roll-to-roll dry coating process would position it as a cornerstone technology for next-generation battery production.
2. Roll-to-roll dry coating process
2.1 PTFE binder for the roll-to-roll dry coating process
The roll-to-roll dry coating process represents a novel technology in electrode manufacturing, particularly for producing thick electrodes essential for high-energy-density lithium rechargeable batteries. However, its successful application requires an understanding of the materials and processes involved, particularly the uniform dispersion of electrode components and the behavior of PTFE fibrillization. PTFE fibrillization plays a crucial role in determining the mechanical and electrochemical properties of dry electrodes, making it vital to explore how this material transforms into a fibrous structure under the shear forces applied during the process. PTFE has a unique molecular structure characterized by a linear carbon–carbon backbone with symmetrically arranged fluorine atoms that form a helical conformation around the backbone70,71 (Fig. 4a). Unlike many other polymers, PTFE can deform under shear forces even at room temperature due to the change in the crystalline structure of PTFE, which undergoes phase transitions at relatively low temperatures.72 At 19 °C, PTFE transitions from a low-temperature triclinic phase to an intermediate hexagonal phase, and above 30 °C, it further transforms into a pseudo-hexagonal phase73 (Fig. 4c). The phase transition significantly affects the material's viscoelastic properties. As the temperature increases, the storage modulus, which represents the material's ability to store elastic energy under deformation, decreases. This change is attributed to the structural transformation of PTFE's crystalline regions. In the hexagonal phase, fluorine atoms are symmetrically aligned around the carbon backbone, and the polymer chains are oriented along the c-axis. This alignment reduces structural density and weakens intermolecular interactions, making PTFE more susceptible to shear deformation along the c-axis71 (Fig. 4b). Accordingly, as the temperature increases, the elastic modulus of PTFE decreases, indicating a reduced ability to recover from deformation caused by shear forces74 (Fig. 4d). The temperature- and pressure-induced transformation of PTFE's crystal structure, coupled with its fibrillization under shear forces at elevated temperatures, is directly applicable to the roll-to-roll dry coating process75,76 (Fig. 4e). During the roll-to-roll dry coating process, PTFE undergoes deformation due to the shear forces exerted during the fibrillization process, which occurs above room temperature. A crucial point to note is that PTFE, a polymer with a low coefficient of friction, cannot easily undergo deformation independently. Therefore, an intermediary capable of transmitting external shear forces to the PTFE binder is required. In the electrode system, active materials or conductive additives, components of the electrode, can act as intermediaries to transfer external shear forces to the PTFE binder. These shear forces, transmitted through the interaction with active materials or conductive additives, cause the PTFE binder to deform and develop a fibrous network77 (Fig. 4f and g). The PTFE fibrous network is critical for enhancing mechanical strength and ensuring the homogeneity of the electrode, contributing to its overall electrochemical properties. Accordingly, the control of the fibrillization behavior of the PTFE binder is a key factor in the PTFE binder-based roll-to-roll dry coating process. While equipment parameters influence fibrillization behavior, as mentioned earlier, the interaction between the PTFE binder and other electrode components, such as conductive additives or active materials, is considered particularly crucial. Stronger interactions between the PTFE binder and these intermediaries are expected to facilitate the effective transmission of external shear forces to the PTFE binder, thereby enhancing the fibrillization of the PTFE binder. Despite its importance, fundamental research on the particle interaction-driven PTFE fibrillization process remains limited. Therefore, future studies are needed to explore this fundamental aspect and deepen the understanding of PTFE fibrillization behavior in the roll-to-roll dry coating process.
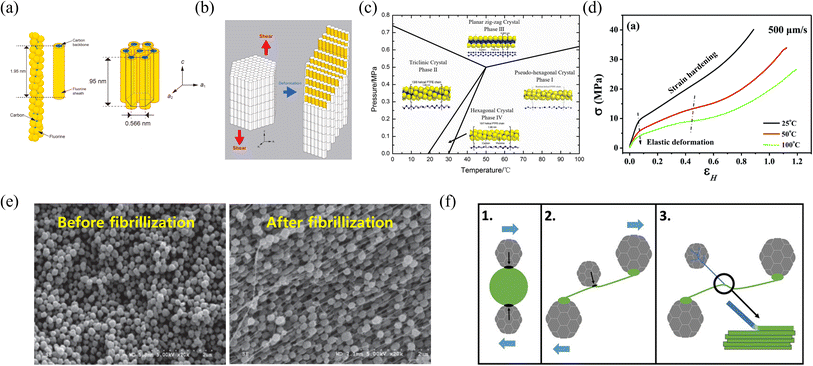 |
| | Fig. 4 PTFE binder for the roll-to-roll dry coating process: (a) The helical structure of an individual PTFE polymer chain in phase IV and (b) the hexagonal unit cell structure of phase IV PTFE. Reproduced with permission from ref. 71, copyright (2022) Elsevier Ltd. (c) Phase diagram of PTFE. Reproduced with permission from ref. 73, copyright (2020) Elsevier Ltd. (d) The true stress–strain curves of PTFE according to the temperature. Reproduced with permission from ref. 74, copyright (2021) RSC Publishing (e) SEM images of PTFE before and after the fibrillization process. Reproduced with permission from ref. 75, copyright (2017) Elsevier Ltd. (f) Schematic illustration and (g) SEM images of PTFE fibrillization in the roll-to-roll dry coating process. Reproduced with permission from ref. 77, copyright (2024) Matthews, Wheeler, Ramírez-González and Grant. | |
2.2 Main processes of the roll-to-roll dry coating process
The roll-to-roll dry coating process typically comprises three major processes: dry mixing, fibrillization, and free-standing film formation (Fig. 5). Each process plays a pivotal role in improving the mechanical and electrochemical properties of the electrode. The dry mixing process induces the uniform distribution of active materials, conductive additives, and binders. This uniformity is essential for superior electrochemical performance, as it is directly related to the ionic and electrical resistances of dry electrodes.78–80 The fibrillization process aims to promote PTFE fibrillization by applying shear forces, transforming the PTFE particle into fibrous structures. The enhancement of PTFE fibrillization improves the mechanical and binding properties of dry electrodes, promotes pore formation, and enables uniform dispersion of conductive additives, collectively reducing ionic and electrical resistances.81 The free-standing film formation process produces a mechanically robust free-standing electrode film, which is subsequently adhered to the current collector.
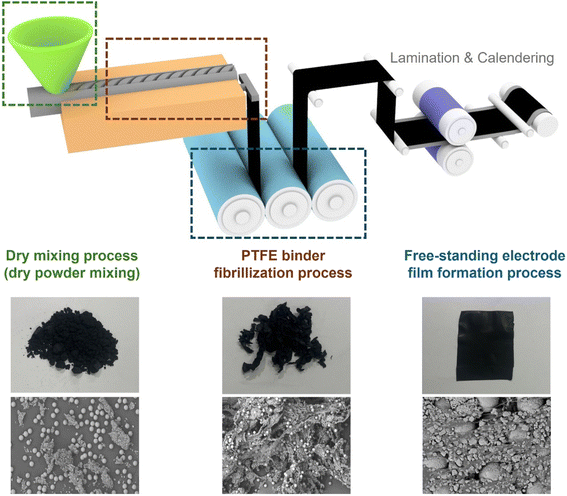 |
| | Fig. 5 Main processes of the roll-to-roll dry coating process. | |
To achieve high energy density lithium rechargeable batteries, the development of thick dry electrodes is required. However, as the electrode thickness increases, ionic and electronic resistances (Rion and Rct) within the electrode also increase.82Rion and Rct represent the resistances of lithium-ion transport through the pore channel within the electrode and lithium-ion reactions at the interface of active materials33 (Fig. 6a). As the electrode becomes thicker, the transport distance of lithium-ions from top to bottom regions increases, leading to an increase in Rion. This, in turn, reduces the lithium-ion concentration in the bottom region of the electrode, thereby increasing Rct (Fig. 6b and d). As a result, the overall increase of ionic resistance within the dry thick electrode led to degradation of rate capability (Fig. 6c). To alleviate these issues, it is necessary to minimize the lithium-ion transport distance within the dry electrode by improving the homogeneity of the electrode microstructure. Through the engineering of the three processes (dry mixing, fibrillization, and free-standing film formation), the homogeneity of the electrode microstructure could be achieved with proper pore size and distribution, improved PTFE fibrillization and crack-free cathode active materials, thereby improving the overall electrochemical properties of the dry electrode (Fig. 6e).
 |
| | Fig. 6 Relationship between ionic resistance and electrochemical performance of dry thick electrodes: (a) schematic illustration of ionic resistance (Rion and Rct). (b) Ionic resistance and (c) C-rate capability of dry electrodes depending on the areal capacity. (d) The schematic illustration of the issue of ultra-high thick electrodes and (e) requirement of main characteristics for dry electrodes with homogeneous microstructures. Reproduced with permission from ref. 83, copyright (2024) Wiley-VCH GmbH. | |
2.2.1 Dry mixing process.
Dry thick electrodes inherently have long pathways for lithium-ion and electron transport, which increases ionic and electrical resistance. To overcome these challenges, the dry mixing process should be designed to ensure a dry mixture in which the active materials, conductive additive and PTFE binder are uniformly dispersed. Accordingly, researchers have conducted extensive studies to optimize the dry mixing process. Tao et al. investigated the effects of blade speed and mixing time on the distribution of LiNi0.6Mn0.2Co0.2O2 (NMC), carbon black, and PTFE84 (Fig. 7a). Similarly, Shin et al. addressed the aggregation problem of carbon nanotubes (CNTs) during the dry mixing process, inducing the degradation of electrochemical properties of CNT applied dry electrodes85 (Fig. 7b). CNTs tend to clump together due to their high surface energy, leading to poor distribution within the dry electrode. To overcome this issue, CNT-coated LiNi0.8Co0.15Al0.05O2 (NCA) was introduced in the roll-to-roll dry coating process, where the wet coating method was applied to coat CNTs onto the surface of NCA particles, improving their dispersion and reducing the formation of aggregates. Throughout the modification of the surface of NCA, the electrical conductivity and mechanical properties of dry electrodes were improved, associated with uniform distribution of CNTs within the dry electrode. Controlling the PTFE distribution within the dry electrode has also been explored. Choi et al. took a different approach by modifying the surfaces of CB and PTFE with ionic surfactants86 (Fig. 7c). This modification increased electrostatic interactions between the CB and PTFE, resulting in a more uniform distribution of CB and PTFE. Lee et al. used cryogenic freezer milling to reduce the PTFE particle size, which allowed for uniform dispersion of PTFE within the electrode87 (Fig. 7d). This approach not only improved the uniformity of the binder but also reduced ionic resistance within the electrode. These studies above highlight the importance of addressing dispersion issues of active materials, conductive additives and the PTFE binder in the dry mixing process to provide the reliability of thick dry electrodes with superior electrochemical properties.
 |
| | Fig. 7 Engineering of the dry mixing process: (a) control of shear force by the dry mixing time. Reproduced with permission from ref. 84. Copyright (2023) Springer. (b) The schematic illustration of improvement of CNT dispersion in the dry electrode by wet coating of CNTs on NCA. Reproduced with permission from ref. 85. Copyright (2022) John Wiley & Sons Ltd. (c) The schematic illustration of surface modification of PTFE and carbon black for improvement of PTFE and CB dispersion. Reproduced with permission from ref. 86. Copyright (2023) Springer. (d) Controlling the size of PTFE to improve the dispersion of the PTFE binder. Reproduced with permission from ref. 87. Copyright (2023) Springer. | |
2.2.2 PTFE fibrillization process.
The fibrous network formed by PTFE fibrillization enhances the mechanical strength of the electrode film and uniformity of pore distribution within the dry electrode, closely related to the tortuosity, resulting in robust durability of the electrode film during cell manufacturing processes such as winding and lamination, as well as a homogeneous microstructure of the dry electrode with improved ionic resistance. Various approaches have been explored to enhance PTFE fibrillization. Oh et al. investigated the behavior of PTFE fibrillization as a function of the kneading time by measuring the applied torque during the process88 (Fig. 8a). They found that increases in the kneading time induced excessive fibrillization of PTFE, leading to the thin PTFE fiber and resulting in brittle characteristics of the dry electrode film. Similarly, Kim et al. observed the temperature dependence for the PTFE fibrillization behavior and suggested the temperature region that can retain the morphology of the PTFE fibrous network within the dry electrode83 (Fig. 8b). They reported that while higher temperatures facilitated fibrillization, excessive heating could cause the fibrous structures to aggregate, affecting the morphology of the PTFE film and reducing the tensile strength of the dry electrode film. Research on engineering the PTFE fibrillization process is not limited to cathode materials such as high nickel cathode materials. Studies on lithium–iron phosphate (LFP), a cathode material with low ionic conductivity, have shown that its small particle size increases the surface area and shear forces exerted on PTFE, leading to excessive fibrillization. This excessive fibrillization makes the electrode film brittle, lowering its mechanical properties. Wiegmann et al. addressed this issue by adjusting the screw configuration in extrusion equipment to control the number of fibrillization zones, thereby suppressing the excessive fibrillization and improving the mechanical properties of the electrode film89 (Fig. 8c). Beyond LIBs, fibrillization of PTFE has been investigated for the fabrication of solid-state electrolyte membranes. Lee et al. studied the effects of PTFE binder content, temperature and direction of the fibrillization process on the fibrous network morphology of PTFE in solid-state electrolyte membranes90 (Fig. 9a). Based on how the morphology of PTFE within solid-state electrolyte membranes influenced the electrochemical and mechanical stability of the ASSB, a durable and electrochemically stable bi-layer electrolyte membrane was developed. Furthermore, Yoon et al. conducted a study to examine the fibrillization behavior of PTFE as a function of molecular weight91 (Fig. 9b). They utilized XRD analysis to quantify fibrillization under varying shear forces and temperatures for PTFE with different molecular weights. Despite the significant advancements in understanding the PTFE fibrillization process and its influence on the electrochemical performance of dry electrodes, further fundamental research is necessary to fully understand the behavior of PTFE fibrillization during the roll-to-roll dry coating process. Such studies are essential to address remaining challenges and enable the successful commercialization of the roll-to-roll dry coating process, particularly in ensuring consistent fibrillization behavior and its correlation with the mechanical and electrochemical properties of dry electrodes.
 |
| | Fig. 8 Engineering of the PTFE fibrillization process (LIBs). Investigation of PTFE fibrillization behavior according to the (a) fibrillization process time, (b) fibrillization process temperature and (c) the number of kneading configurations. Reproduced with permission from ref. 83, 88 and 89. Copyright (2024) The Authors. Published by Elsevier B.V., copyright (2024) Wiley-VCH GmbH and copyright (2023) The Authors. Licensee MDPI, Basel, Switzerland. | |
 |
| | Fig. 9 Engineering of the PTFE fibrillization process (ASSBs). Investigation of PTFE fibrillization behavior in the solid electrolyte film according to (a) various process parameters and (b) the molecular weight of PTFE. Reproduced with permission from ref. 90 and 91. Copyright (2023) Wiley-VCH GmbH and copyright (2024) Wiley-VCH GmbH. | |
2.2.3 Free-standing electrode film formation process.
The free-standing electrode film was formed and laminated onto the current collector through the roll pressure to achieve the desired electrode thickness and density during the rolling and lamination process. During the rolling and lamination process, proper control of the rolling parameters is essential to prevent crack of active materials, ensure strong cohesion between electrode components, and improve adhesion between the electrode film and the current collector.83
The roll-to-roll dry coating process shows great potential for producing high-energy-density and cost-effective electrodes. However, its success depends on optimizing each process. While significant progress has been made in understanding and improving these processes, challenges remain. For example, PTFE fibrillization behavior, influenced by particle properties such as size, shape and surface characteristics, as well as processing conditions, requires further research for a comprehensive understanding. Accordingly, establishing a database of PTFE fibrillization behavior under various conditions could support the improvement of the roll-to-roll dry coating process, accelerating the commercialization of thick dry electrodes and their integration into next-generation battery technologies.
3. Applications for the roll-to-roll dry coating process
3.1 Lithium-ion batteries
The roll-to-roll dry coating process has been introduced to improve the performance of LIBs, yet it still faces several challenges, including issues related to ionic and electrical resistance, chemical stability, and mechanical stability. This section discusses the major issues and the solutions to address them.
3.1.1 Improvement of lithium-ion & electron resistance and chemical stability of dry electrodes.
Dry electrodes face significant technical challenges in terms of ionic and electrical resistance and chemical stability. In particular, the roll-to-roll dry coating process faces significant dispersion issues due to the absence of solvents, leading to non-uniform dispersion of conductive additives.84 This issue is particularly pronounced with conventional conductive additives such as CB and CNTs.92–95 When CB or CNTs are introduced into a cathode composite through physical mixing without solvents, the aggregation of conductive additives occurs, lowering electrical conductivity within the dry electrode and hindering the reaction of lithium ions with active materials. To address the issue of aggregation of conductive additives, studies have focused on the modification of their surface characteristics. For example, Kim et al. proposed CNTs treated with ozone (O3) surface treatment as a conductive additive for the roll-to-roll dry coating process to enhance the dispersion of CNTs within the dry electrode96 (Fig. 10a). The ozone treated CNTs have high affinity with NCM811, inducing the uniform dispersion of CNTs on the surface of NCM 811 and resulting in superior electrochemical properties. The morphology of active materials can also affect the ionic resistance of dry electrodes, associated with the different microstructures according to the morphology of active materials. Hwang et al. reported that the arrangement of graphite within the dry electrode, whether in-plane (parallel to the yz-plane) or through-plane (perpendicular to the yz-plane), depends largely on morphology of graphite, resulting in variations in porosity and tortuosity97 (Fig. 10b). Dry electrodes with fine spherical graphite (FSG) exhibited a higher degree of alignment along their longest principal axes parallel to the current collector compared to dry electrodes with small natural graphite (SNG). Moreover, dry electrodes with SNG exhibited a more pronounced pore gradient, resulting in lower tortuosity compared to dry electrodes with FSG and improved fast-charging capability. Furthermore, the different behaviors of electrical resistance within the dry electrode were reported depending on whether the active materials are single- or poly-crystalline. Tao et al. showed that dry electrodes with single-crystal active materials (SC DPE) exhibited more uniform distribution of CB compared to dry electrodes with polycrystalline active materials (PC DPE), resulting in improved electrical conductivity98 (Fig. 10c). It is attributed to the different conductive pathways where the conductive pathway in PC DPE was anisotropic along the electrode thickness direction, whereas the conductive pathways are more isotropic in SC DPE. In addition to studies on the ionic and electrical resistance of dry electrodes, studies have also been reported on the electrochemical stability of the PTFE binder. Tao et al. reported the side reaction of PTFE within the dry cathode during cycling by conducting cycle tests of dry electrodes with electrolytes containing either LiPF6 or LiClO4 (ref. 99) (Fig. 10d). By eliminating the fluorine source (LiPF6) in electrolyte and applying LiClO4 as a lithium salt, the decomposition behavior of PTFE was investigated, where the detection of LiF in cells with LiClO4 confirmed that PTFE undergoes side reactions during cycling.
 |
| | Fig. 10 Improvement of lithium ion/electron resistance and chemical stability of dry electrodes (LIBs): (a) ozonation of the SWCNT and its application to the dry cathode process. Reproduced with permission from ref. 96. Copyright (2023) American Chemical Society. (b) Pore structure of dry-processed SNG and FSG graphite electrodes. Reproduced with permission from ref. 97. Copyright (2024) The Author(s). Published by Elsevier B.V. (c) SEM images and linear curve fittings of Z′ versus ω−1/2 in the low-frequency region of SC and PC NMC. Reproduced with permission from ref. 98. Copyright (2024) Elsevier B.V. (d) Schematic illustration of CEI layers and TEM images of the LiPF6-cycled and LiClO4-cycled DPEs. Reproduced with permission from ref. 99. Copyright (2023) American Chemical Society. | |
3.1.2 Improvement of mechanical properties of dry electrodes.
Dry electrodes with insufficient fibrillization of PTFE exhibit poor mechanical properties and structural stability. To address this issue, extensive research has been conducted from both material and process perspectives to enhance the fibrillization of PTFE. However, significant limitations remain and accordingly, efforts have been made to improve the mechanical properties of dry electrodes by introducing additives beyond conventional materials. For example, He et al. developed a high-performance dry electrode (DP-F electrode) incorporating flour as a partial binder100 (Fig. 11a). The addition of 1 wt% flour (DP-1% F electrode) results in superior mechanical properties, excellent rate performance and high cycling stability. The DP-1% F electrode demonstrated a tensile strength of 1.12 MPa (high mechanical strength) and a minimum elastic modulus of 8.04 MPa (high flexibility), attributed to the cross-linking between proteins and starches in the flour. In the context of structural stability for dry electrodes, the calendering process poses challenges due to the high pressure required to densify thick, free-standing electrode films and increase electrode density. This high pressure often causes cracks in polycrystalline active materials, compromising the electrode's mechanical properties. To address this issue, Embleton et al. introduced a solvent-assisted binder dry coating process, which mitigates microstructural problems arising from such cracks101 (Fig. 11b). Using a low-boiling-point solvent such as ethanol (<3 wt%), this approach enhanced electrode processability and PTFE fibrillization, with the solvent removed through elevated roll press temperatures. In this way, small amounts of solvents (additives) have been utilized to enhance the mechanical properties of dry electrodes. However, to fully achieve the advantages of the dry coating process, such as low manufacturing cost and scalability, it is ultimately necessary to improve the mechanical properties and structural stability without relying on additives.
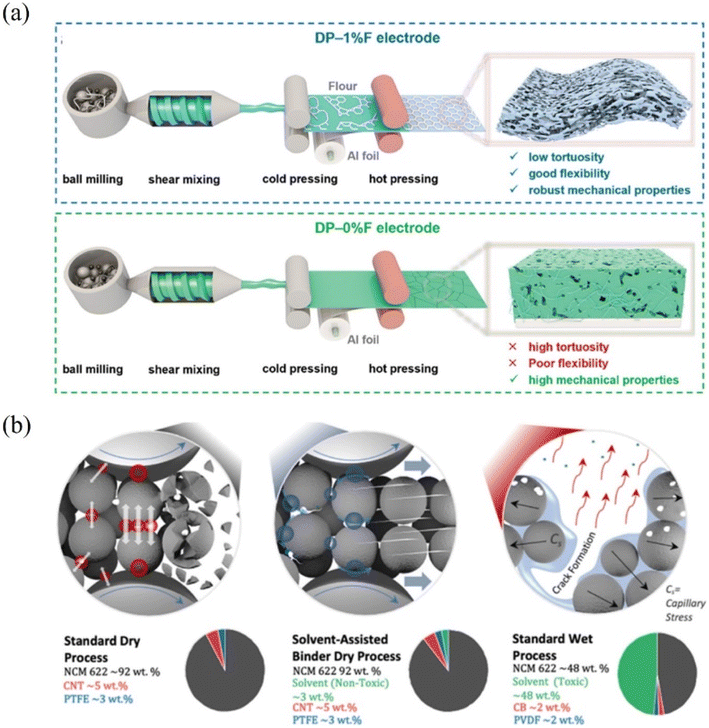 |
| | Fig. 11 Improvement of mechanical properties of dry electrodes (LIBs): (a) fabrication procedures of DP-1% F and DP-0% F electrodes. Reproduced with permission from ref. 100. Copyright (2024) Wiley-VCH GmbH. (b) Fracturing of active materials, effects of the solvent-assisted-binder (SaB) dry process, and capillary stresses in relation to mixture composition during dry processing. Reproduced with permission from ref. 101. Copyright (2023) The Authors. Published by Elsevier B.V. | |
3.2 Next-generation batteries
Next-generation batteries, such as ASSBs and Li–S batteries, have benefited significantly from the roll-to-roll dry coating process utilizing the PTFE binder, which has brought transformative advancements by addressing challenges in stability, energy density, and scalability. In particular, traditional ASSBs with wet coating processes suffer from undesirable side reactions between solvents used in wet coating processes and solid electrolytes. The roll-to-roll dry coating process eliminates the need for solvents, thereby preventing side reactions, while forming uniform and robust electrode and electrolyte films that improve structural and electrochemical stability.102–104 Additionally, binder migration induced by solvent evaporation during the wet coating process often results in electrode cracking for thick electrodes designed to achieve high energy density. The roll-to-roll dry coating process effectively addresses this issue, establishing itself as a key technology for the development of high-capacity next-generation batteries.
3.2.1 All-solid-state batteries (ASSBs).
All-solid-state batteries (ASSBs) have been extensively studied as next-generation battery technology due to their high energy density and exceptional safety. Sulfide-based solid electrolytes, which offer good malleability and high ionic conductivity at room temperature, are commonly employed.105 However, sulfide-based solid electrolytes present challenges in electrode fabrication due to their narrow electrochemical window and poor chemical compatibility with polar solvents.106 In particular, their poor chemical compatibility with polar solvents induces side reactions between the solid electrolyte and the solvent during electrode fabrication, limiting the choice of solvents in wet processing. To address these issues, a roll-to-roll dry coating process, which eliminates the use of solvents, was adopted. Nevertheless, the dry coating process also faces challenges, such as contact issues between the solid electrolyte and active materials, inhomogeneous electrode microstructures, and low ionic conductivity of the PTFE binder. Lee et al. systematically investigated the effect of shear force on coverage, the percentage of electrolyte-covered surface area of the active materials, by comparing wet and dry electrodes for ASSBs107 (Fig. 12a). Since the slowest lithium-ion movement in ASSBs is solid-state diffusion between active materials, coverage plays an important role in electrochemical performance such as rate performance. Dry electrodes exhibited significantly higher coverage compared to wet-processed electrodes, attributed to the deformation of the ductile sulfide solid electrolytes by shear forces, resulting in high ionic conductivity. However, the PTFE binder faces another challenge related to its intrinsic low ionic conductivity. To overcome the limitation of the PTFE binder with low ionic conductivity, Lee et al. developed a functional dry binder system by introducing the solvate ionic liquid (SIL)-infiltrated ethyl cellulose (EC) into the roll-to-roll dry coating system, which enabled the fabrication of functional dry electrodes (FDEs)108 (Fig. 12b). By incorporating the SIL-infiltrated EC into the dry electrode, enhanced charge-transfer kinetics and effective contact area among particles were achieved in ASSBs. Despite these efforts to enhance the ionic conductivity of ASSBs, residual voids within the dry electrode remain a challenge, preventing uniform pressure distribution during the calendering process. To suppress the residual voids within the dry electrode, a practically applicable dual-functional electrode additive was introduced. Kim et al. introduced dry electrodes with dense bimodal structures by using argyrodite solid electrolytes and lithium difluorophosphate (LiPO2F2) additives with different particle sizes109 (Fig. 12c). LiPO2F2 fills the interparticle voids within the dry electrode, ensuring uniform pressure distribution during the calendering process and reducing residual voids within the dry electrode. Accordingly, the addition of LiPO2F2 enables the fabrication of dry electrodes with high ionic and electronic conductivity. Despite these advantages of the roll-to-roll dry coating process in ASSBs, further research on functional binders, electrode additives, and manufacturing techniques will be essential for optimizing dry electrode fabrication and accelerating the commercialization of high-performance ASSBs.
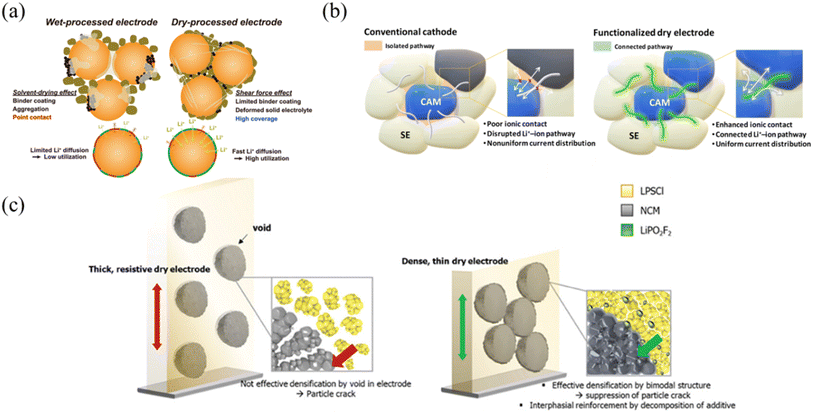 |
| | Fig. 12 Next-generation batteries (ASSBs): (a) disparity in electrode coverage and Li+ diffusion between the wet process and dry process. Reproduced with permission from ref. 107. Copyright (2024) The Author(s). (b) The conventional cathode with an ionically insulating PTFE binder and SIL-infiltrated EC. Reproduced with permission from ref. 108. Copyright (2024) RSC Publishing. (c) The role of LiPO2F2 as an additive in dry electrodes for ASSBs. Reproduced with permission from ref. 109. Copyright (2023) Wiley-VCH GmbH. | |
3.2.2 Lithium–sulfur (Li–S) batteries.
Lithium–sulfur (Li–S) batteries have garnered significant attention as promising next-generation batteries due to their high theoretical energy density (2600 W h kg−1) and high specific capacity (1672 mA h g−1). Additionally, sulfur (S) is earth-abundant, nontoxic, and low-cost, further enhancing its potential as a next-generation battery.110 To achieve high energy density in Li–S batteries, several factors must be considered, such as the electrolyte-to-sulfur ratio (E/S), negative-to-positive capacity ratio (N/P), and sulfur loading.111 Among these factors, high sulfur loading has recently attracted attention, as seen in LIBs and ASSBs. However, achieving high energy density through high sulfur loading has critical challenges such as cracks and delamination of thick electrodes, associated with solvent evaporation during the wet coating process, resulting in sluggish reaction kinetics.112 To address these issues, Sul et al. developed high-sulfur-loading electrodes (>7 mg cm−2) through the roll-to-roll dry coating process113 (Fig. 13a). Moreover, the Li–S batteries were integrated with ASSBs for high energy density lithium rechargeable batteries. Shen et al. reported that PTFE fibrillization of the PTFE binder facilitates the interweaving of Li6PS5Cl particles and FeS2 cathode active materials, resulting in the formation of flexible cathodes for all-solid-state-lithium–sulfur-batteries114 (Fig. 13b). Hu et al. also reported dry electrode technology for scalable and flexible high-energy sulfur cathodes in all-solid-state lithium–sulfur batteries115 (Fig. 13c). The fibrous network of the PTFE binder effectively enables the formation of lithium-ion and electron conductive networks within the dry sulfur cathode, leading to the improved electrochemical performance. The direct current (DC) and alternating current (AC) tests of electrodes demonstrated the improved ionic and electrical conductivity of dry sulfur cathodes in ASSBs due to the fibrous network of the PTFE binder. These studies demonstrate the potential of the roll-to-roll dry coating process in advancing the scalability and performance of Li–S batteries, paving the way for their practical implementation in next-generation energy storage systems.
 |
| | Fig. 13 Next-generation batteries (Li–S batteries): (a) dry electrode fabrication procedure for a sulfur cathode with high mass loading. Reproduced with permission from ref. 113. Copyright (2024) Wiley-VCH GmbH. (b) Schematic illustration of the preparation of the LPSCl electrolyte membrane and FeS2 cathode by the roll-to-roll dry coating process. Reproduced with permission from ref. 114. Copyright (2024) Elsevier Inc. (c) Schematic illustration and measurement of ionic/electrical conductivity of wet- and dry-processed sulfur cathodes. Reproduced with permission from ref. 115. Copyright (2022) Science Press. Published by Elsevier B.V. | |
4. Summary and outlook
4.1 Summary
Dry coating processes represent a paradigm shift for high energy density lithium rechargeable batteries with thick electrodes, offering a sustainable alternative to conventional wet coating methods. Among these, the roll-to-roll dry coating process emerges as the most promising technique for industrial-scale production, thanks to its scalability and production efficiency, which aligns with the growing demand for high-energy-density and cost-effective batteries. Despite its potential, significant challenges remain on the path to commercialization. Addressing these challenges necessitates advancements in the main processes of the roll-to-roll dry coating process, including the dry mixing process, PTFE binder fibrillization process, and free-standing electrode film formation process, and materials such as the PTFE binder, conductive additives, active materials, and additives to achieve the low resistance dry electrode with a homogeneous microstructure. The process and material advancements not only promise to overcome current barriers but also extend the applicability of the roll-to-roll dry coating process to next-generation batteries such as all-solid-state batteries (ASSBs) and lithium–sulfur (Li–S) batteries. This provides a versatile foundation for further research and development, paving the way for the commercialization of advanced battery systems. Ultimately, the roll-to-roll dry coating process is poised to become a cornerstone technology for fabricating high energy density lithium rechargeable batteries with cost efficiency and environmental sustainability.
4.2 Future work
As discussed, the roll-to-roll dry coating process utilizing the PTFE binder is advantageous for the commercialization of thick electrodes. While significant research has been conducted to achieve the commercialization of the roll-to-roll dry coating process, there remain areas requiring further study.116–118 First, the methodology for the analysis of the degree of PTFE fibrillization is required. Until now, several studies have analyzed the degree of PTFE fibrillization in dry electrodes by measuring the storage and elastic modulus of electrode films through DMA (Dynamic Mechanical Analysis) and crystallinity of PTFE within dry electrode films through DSC (Differential Scanning Calorimetry) or evaluating the mechanical properties using a UTM (Universal Testing Machine)71,83,119–123 (Fig. 14a and b). XRD (X-ray diffraction) measurement was also employed to analyze the degree of deformation of PTFE according to the intensity of specific peaks affected by deformation of PTFE71 (Fig. 14c). These methods allow for indirect comparison and evaluation of PTFE fibrillation within electrodes. However, for underlying understanding, more precise measurements for the analysis of the degree of PTFE fibrillization are necessary. Therefore, developing a methodology to quantitatively assess the degree of PTFE fibrillation in electrode films is an important area for future research. Second, the PTFE binder exhibits a lower Lowest Unoccupied Molecular Orbital (LUMO) level compared to polyvinylidene fluoride (PVDF), commonly used in wet coating processes, and solvents such as ethylene carbonate and ethyl methyl carbonate124 (Fig. 14d). This property allows PTFE to attract electrons more readily. As a result, when the PTFE binder is applied to anodes, it can react with lithium and electrons during lithiation (charging), leading to the reduction and side reaction of PTFE. The reduction of PTFE increases the irreversible capacity within the electrode, thereby resulting in degradation of reversible capacity.64,125 While several studies have proposed strategies to mitigate the reduction of the PTFE binder, such as coating of the polymer with high ionic conductivity on the surface of graphite active materials to block the electron path to the PTFE binder or modification of electrolyte additives to form the stable solid electrolyte interface (SEI), a complete solution has yet to be achieved and further development of strategies to address this challenge is needed.126,127 Third, the PTFE binder belongs to the category of per- and polyfluoroalkyl substances (PFAS), which may be subject to regulatory restrictions.128 PFAS regulations currently focus on limiting the use of materials where one or more carbon atoms are fully or partially fluorinated. Today, while the primary targets are low-molecular-weight PFAS compounds, such as PFOA and PFOS, which have direct environmental and health issues, high-molecular-weight substances such as PVDF and PTFE are not currently subject to direct regulation. However, given the uncertainty regarding how future PFAS regulations will evolve, it is essential to develop alternative binders capable of fibrillization to replace the PTFE binder. The alternatives could ensure compliance with future regulatory changes while retaining the advantageous properties of PTFE129 (Fig. 14e). While the PTFE binder holds significant potential for thick electrode manufacturing in the roll-to-roll dry coating process, overcoming challenges related to quantification of fibrillization, reduction issues, and regulatory compliance will be addressed carefully for its successful commercialization. Additionally, as mentioned earlier, due to the side reaction issues of the PTFE binder on the anode side, developing alternative binders suitable for dry anode applications is essential. Fourth, conductive additives are crucial for the enhancement of the electrical conductivity of electrodes. In conventional wet coating processes, a key challenge is the dispersion of conductive additives in the solvent. Poor dispersion can degrade the electrical conductivity of the electrode, necessitating the use of conductive additives with low specific surface area or a high concentration of dispersants to improve dispersion of conductive additives.130 However, the suppression of agglomeration of conductive additives in the solvent remains difficult, significantly limiting the selection of suitable conductive additives for the conventional wet coating process. In contrast, dry processes eliminate solvents, reducing dispersion-related constraints and broadening the range of applicable conductive additives (Fig. 14f). However, 1D and 2D conductive additives, such as CNTs and graphene, are still prone to agglomeration in the dry coating process. Fortunately, strong shear forces applied during the dry coating process can mitigate this issue, making agglomeration less problematic than in conventional wet coating processes. Consequently, exploring and developing conductive additives optimized for dry processes is essential. As various conductive additives are applicable in dry processes, it is crucial to investigate their impact on the fibrillization behavior of the PTFE binder, which significantly influences the mechanical properties and electrode microstructure. Moreover, understanding how different characteristics of conductive additives affect ionic and electrical resistances in dry electrodes is vital for developing optimized conductive additives for dry processing. Fifth, adhesion between the free-standing electrode film and the current collector in dry processes is primarily based on mechanical interlocking rather than chemical bonding, which often results in poor adhesion.131 To address this, advanced current collectors, such as etched or carbon-coated copper or aluminum foil, are commonly used to improve the adhesion56 (Fig. 14g). However, these current collectors significantly increase production costs. Therefore, developing cost-effective current collectors that retain strong adhesion with the free-standing electrode film is crucial, and investigating new surface treatment techniques or alternative coating materials that enhance mechanical interlocking while reducing costs will be essential for the advancement of the dry coating process. Focusing on achievements in conductive additives and current collectors for the dry coating process will drive innovation, improve electrochemical performances, and reduce the costs of dry electrode manufacturing, facilitating their broader industrial adoption. Addressing these issues through continued research and innovation will pave the way for advancing the roll-to-roll dry coating process and next-generation battery applications.
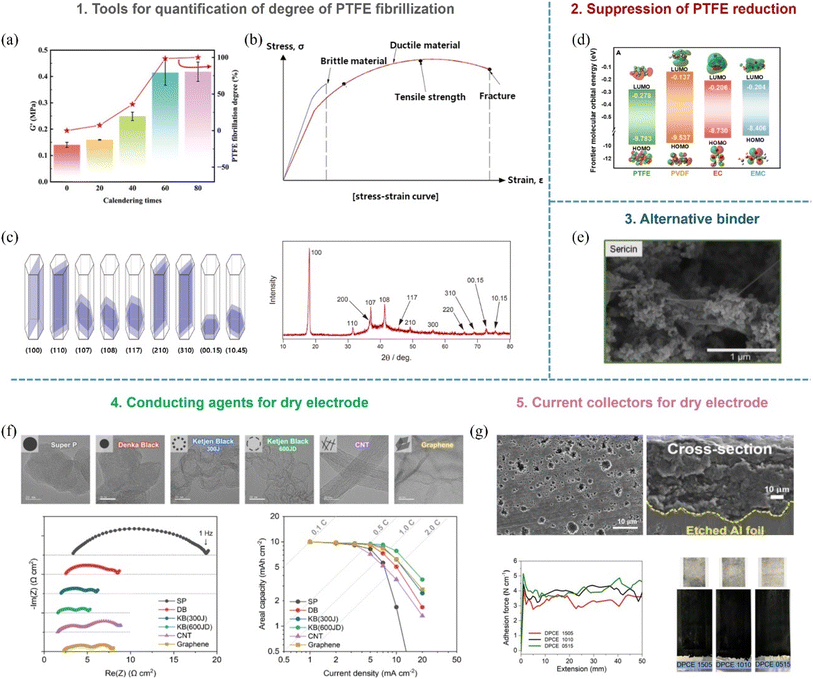 |
| | Fig. 14 Future work on the roll-to-roll dry coating process: (a) storage modulus from DMA measurement. Reproduced with permission from ref. 121. Copyright (2024) Wiley-VCH GmbH, (b) stress–strain curve from UTM measurement and (c) (107) and (108) planes from XRD measurement of the electrode film. Reproduced with permission from ref. 71. Copyright (2022) Elsevier Ltd. (d) The LUMO and HOMO energies of polymers and electrolyte solvent. Reproduced with permission from ref. 124. Copyright (2024) Elsevier Inc. (e) Development of sustainable binder (sericin) for replacing the PTFE binder as a dry coating process binder. Reproduced with permission from ref. 129. Copyright (2022) The Authors. ChemSusChem published by Wiley-VCH GmbH. (f) Application of conductive additives with various dimensions and specific surface areas for the roll-to-roll dry coating process. Reproduced with permission from ref. 130. Copyright (2025) RSC Publishing. (g) Application of etched Al current collectors to improve the adhesion between the dry-processed cathode and current collectors. Reproduced with permission from ref. 131. Copyright (2023) The Author(s). | |
Data availability
This review does not include any primary research results, software, or code, and no new data were created.
Author contributions
Joonhyeok Park and Jiwoon Kim: conceptualization, draft preparation, reviewing & editing, visualization. Jaeik Kim: visualization. Minsung Kim: reviewing. Taeseup Song and Ungyu Paik: funding, supervision, reviewing.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Human Resources Program in Energy Technology of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), which was granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20214000000520). This work was also supported by the Technology Innovation Program (or the Industrial Strategic Technology Development Program-Materials & Components Technology Development Program) (20024261, Development of thick film electrode and cell manufacturing technology for high-performance lithium iron phosphate battery with energy density of over 200 W h kg−1) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
References
- D. Meng, Z. Xue, G. Chen, D. Zhou, Y. S. He, Z. F. Ma, Y. Liu and L. Li, Multiscale correlative imaging reveals sequential and heterogeneous degradations in fast-charging batteries, Energy Environ. Sci., 2024, 17, 4658–4669 CAS.
- F. Degen and O. Kratzig, Future in Battery Production: An Extensive Benchmarking of Novel Production Technologies as Guidance for Decision Making in Engineering, IEEE Trans. Eng. Manag., 2024, 71, 1038–1056 Search PubMed.
- S. Hy, H. Liu, M. Zhang, D. Qian, B. J. Hwang and Y. S. Meng, Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries, Energy Environ. Sci., 2016, 9, 1931–1954 CAS.
- X. Hao, H. Wang, Y. Zheng, Y. Lin, S. Han, R. Zhong and J. Li, Toward Carbon Neutral Road Transport: Development Strategies and New R&D Organizational Paradigms, Automot. Innov., 2024, 7, 209–224 Search PubMed.
- C. Zhang, X. Zhao, R. Sacchi and F. You, Trade-off between critical metal requirement and transportation decarbonization in automotive electrification, Nat. Commun., 2023, 14, 1616 CAS.
- Y. Qiao, H. Deng, P. He and H. Zhou, A 500 Wh/kg Lithium-Metal Cell Based on Anionic Redox, Joule, 2020, 4, 1445–1458 CAS.
- C. Xu, Q. Dai, L. Gaines, M. Hu, A. Tukker and B. Steubing, Future material demand for automotive lithium-based batteries, Commun. Mater., 2020, 1, 99 CrossRef.
- Y. Lu, X. Rong, Y. S. Hu, L. Chen and H. Li, Research and development of advanced battery materials in China, Energy Storage Mater., 2019, 23, 144–153 CrossRef.
- S. Megahed and B. Scrosati, Lithium-ion rechargeable batteries, J. Power Sources, 1994, 51, 79–104 CrossRef CAS.
- G. E. Blomgren, The Development and Future of Lithium Ion Batteries, J. Electrochem. Soc., 2017, 164, A5019–A5025 CAS.
- Q. Tao, L. Wang, C. Shi, J. Li, G. Chen, Z. Xue, J. Wang, S. Wang and H. Jin, Understanding the Ni-rich layered structure materials for high-energy density lithium-ion batteries, Mater. Chem. Front., 2021, 5, 2607–2622 CAS.
- W. Li, B. Song and A. Manthiram, High-voltage positive electrode materials for lithium-ion batteries, Chem. Soc. Rev., 2017, 46, 3006–3059 CAS.
- M. C. Schulze, F. Urias, N. S. Dutta, Z. Huey, J. Coyle, G. Teeter, R. Doeren, B. J. T. de Villers, S. D. Han, N. R. Neale and G. M. Carroll, Control of nanoparticle dispersion, SEI composition, and electrode morphology enables long cycle life in high silicon content nanoparticle-based composite anodes for lithium-ion batterie, J. Mater. Chem. A, 2023, 11, 5257–5266 CAS.
- B. Zhu, X. Wang, P. Yao, J. Li and J. Zhu, Towards high energy density lithium battery anodes: silicon and lithium, Chem. Sci., 2019, 10, 7132–7148 CAS.
- J. Entwistle, A. Rennie and S. Patwardhan, A review of magnesiothermic reduction of silica to porous silicon for lithium-ion battery applications and beyond, J. Mater. Chem. A, 2018, 6, 18344–18356 CAS.
- Y. Kuang, C. Chen, D. Kirsch and L. Hu, Thick Electrode Batteries: Principles, Opportunities, and Challenges, Adv. Energy Mater., 2019, 9, 1901457 Search PubMed.
- M. Pei, H. Shi, F. Yao, S. Liang, Z. Xu, X. Pei, S. Wang and Y. Hu, 3D printing of advanced lithium batteries: a designing strategy of
electrode/electrolyte architectures, J. Mater. Chem. A, 2021, 9, 25237–25257 RSC.
- X. Liu, Y. Zeng, W. Yuan, G. Zhang, H. Zheng and Z. Chen, Advances in multi-scale design and fabrication processes for thick electrodes in lithium-ion batteries, Energy Rev., 2024, 3, 100066 CrossRef.
- X. Yang, K. Doyle-Davis, X. Gao and X. Sun, Recent progress and perspectives on designing high-performance thick electrodes for all-solid-state lithium batteries, eTransportation, 2022, 11, 100152 Search PubMed.
- Y. B. Sim, B. K. Park and K. J. Kim, Reasonable design of thick electrodes in lithium-ion batteries, Front. Batter. Electrochem., 2023, 2, 1272439 Search PubMed.
- D. Bresser, D. Buchholz, A. Moretti, A. Varzi and S. Passerini, Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers, Energy Environ. Sci., 2018, 11, 3096–3127 CAS.
- D. L. Wood, J. D. Quass, J. Li, S. Ahmed, D. Ventola and C. Daniel, Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP, Dry. Technol., 2018, 36, 234–244 CAS.
- Y. S. Zhang, N. E. Courtier, Z. Zhang, K. Liu, J. J. Bailey, A. M. Boyce, G. Richardson, P. R. Shearing, E. Kendrick and D. J. L. Brett, A Review of Lithium-Ion Battery Electrode Drying: Mechanisms and Metrology, Adv. Energy Mater., 2022, 12, 2102233 CAS.
- Y. S. Zhang, J. J. Bailey, Y. Sun, A. M. Boyce, W. Dawson, C. D. Reynolds, Z. Zhang, X. Lu, P. Grant, E. Kendrick, P. R. Shearing and D. J. L. Brett, Applications of advanced metrology for understanding the effects of drying temperature in the lithium-ion battery electrode manufacturing process, J. Mater. Chem. A, 2022, 10, 10593–10603 CAS.
- B. G. Westphal and A. Kwade, Critical electrode properties and drying conditions causing component segregation in graphitic anodes for lithium-ion batteries, J. Energy Storage, 2018, 18, 509–517 CrossRef.
- M. Nikpour, B. Liu, P. Minson, Z. Hillman, B. A. Mazzeo and D. R. Wheeler, Li-ion Electrode Microstructure Evolution during Drying and Calendering, Batteries, 2022, 8, 107 CrossRef CAS.
- C. Meyer, H. Bockholt, W. Haselrieder and A. Kwade, Characterization of the calendering process for compaction of electrodes for lithium-ion batteries, J. Mater. Process. Technol., 2017, 249, 172–178 CAS.
- Y. Suh, J. K. Koo, H. j. Im and Y. J. Kim, Astonishing performance improvements of dry-film graphite anode for reliable lithium-ion batteries, Chem. Eng. J., 2023, 476, 146299 CAS.
- R. Xiong, T. Zhang, T. Huang, M. Li, Y. Zhang and H. Zhou, Improvement of electrochemical homogeneity for lithium-ion batteries enabled by a conjoined-electrode structure, Appl. Energy, 2020, 270, 115109 CAS.
- K. Park, S. Myeong, D. Lee, H. E. Yoo, J. Kim, C. Kim, J. Kim, S. Sun, J. Kwon, S. C. Kim, K. Lee, C. W. Cho, U. Paik and T. Song, Improved Li-ion kinetics of the anode by kneading process of binder for lithium-ion batteries with high energy density, Electrochim. Acta, 2023, 464, 142900 CrossRef CAS.
- K. Park, H. E. Yoo, Y. Jung, M. Ryu, S. Myeong, D. Lee, S. C. Kim, C. Kim, J. Kim, J. Kwon, K. Lee, C. W. Cho, U. Paik and T. Song, Styrene-butadiene rubber patterned current collector for improved
Li-ion kinetics of the anode for high energy density lithium-ion batteries, J. Power Sources, 2023, 577, 233238 CrossRef CAS.
- K. Yang, J. Zhao, X. Du, X. Xie and H. Du, Microstructure evolution and mechanical analysis of lithium battery electrode in calendering deformation zone, Comput. Part. Mech., 2024, 11, 2791–2801 CrossRef.
- N. Ogihara, Y. Itou, T. Sasaki and Y. Takeuchi, Impedance spectroscopy characterization of porous electrodes under different electrode thickness using a symmetric cell for high-performance lithium-ion batterie, J. Phys. Chem. C, 2015, 119, 4612–4619 CrossRef CAS.
- R. Sliz, J. Valikangas, H. S. Santos, P. Vilmi, L. Rieppo, T. Hu, U. Lassi and T. Fabritius, Suitable Cathode NMP Replacement for Efficient Sustainable Printed Li-Ion Batteries, ACS Appl. Energy Mater., 2022, 5, 4047–4058 CrossRef CAS PubMed.
- M. Ahuis, A. Aluzoun, M. Keppeler, S. Melzig and A. Kwade, Direct recycling of lithium-ion battery production scrap – solvent-based recovery and reuse of anode and cathode coating materials, J. Power Sources, 2024, 593, 233995 CAS.
- Y. Lu, C. Z. Zhao, H. Yuan, J. K. Hu, J. Q. Huang and Q. Zhang, Dry electrode technology, the rising star in solid-state battery industrialization, Matter, 2022, 5, 876–898 CAS.
- W. Yao, M. Chouchane, W. Li, S. Bai, Z. Liu, L. Li, A. X. Chen, B. Sayahpour, R. Shimizu, G. Raghavendran, M. A. Schroeder, Y. T. Chen, D. H. S. Tan, B. Sreenarayanan, C. K. Waters, A. Sichler, B. Gould, D. J. Kountz, D. J. Lipomi, M. Zhang and Y. S. Meng, A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating, Energy Environ. Sci., 2023, 16, 1620–1630 CAS.
- J. K. Hu, H. Yuan, S. J. Yang, Y. Lu, S. Sun, J. Liu, Y. L. Liao, S. Li, C. Z. Zhao and J. Q. Huang, Dry electrode technology for scalable and flexible high-energy sulfur cathodes in all-solid-state lithium-sulfur batteries, J. Energy Chem., 2022, 71, 612–618 CAS.
- H. Oh, G. S. Kim, B. U. Hwang, J. Bang, J. Kim and K. M. Jeong, Development of a feasible and scalable manufacturing method for PTFE-based solvent-free lithium-ion battery electrodes, Chem. Eng. J., 2024, 491, 151957 CrossRef CAS.
- S. Ahmed, P. A. Nelson, K. G. Gallagher and D. W. Dees, Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing, J. Power Sources, 2016, 322, 169–178 CAS.
- M. Erakca, M. Baumann, W. Bauer, L. de Biasi, J. Hofmann, B. Bold and M. Weil, Energy flow analysis of laboratory scale lithium-ion battery cell production, iScience, 2021, 24, 102437 CAS.
- M. Zackrisson, L. Avellán and J. Orlenius, Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles-critical issues, J. Clean. Prod., 2010, 18, 1519–1529 CAS.
- S. N. Bryntesen, A. H. Strømman, I. Tolstorebrov, P. R. Shearing, J. J. Lamb and O. S. Burheim, Opportunities for the state-of-the-art production of lib electrodes—a review, Energies, 2021, 14, 1406 CAS.
- L. C. Chen, D. Liu, T. J. Liu, C. Tiu, C. R. Yang, W. B. Chu and C. C. Wan, Improvement of lithium-ion battery performance using a two-layered cathode by simultaneous slot-die coating, J. Energy Storage, 2016, 5, 156–162 Search PubMed.
- B. Ludwig, Z. Zheng, W. Shou, Y. Wang and H. Pan, Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries, Sci. Rep., 2016, 6, 23150 CAS.
- D. L. Wood, J. Li and C. Daniel, Prospects for reducing the processing cost of lithium ion batteries, J. Power Sources, 2015, 275, 234–242 CAS.
- M. Stein, A. Mistry and P. P. Mukherjee, Mechanistic Understanding of the Role of Evaporation in Electrode Processing, J. Electrochem. Soc., 2017, 164, A1616–A1627 CAS.
- C. d. la Torre-Gamarra, M. E. Sotomayor, J. Y. Sanchez, B. Levenfeld, A. Várez, B. Laïk and J. P. Pereira-Ramos, High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries, J. Power Sources, 2020, 458, 228033 Search PubMed.
- M. E. Sotomayor, C. d. la Torre-Gamarra, B. Levenfeld, J. Y. Sanchez, A. Varez, G. T. Kim, A. Varzi and S. Passerini, Ultra-thick battery electrodes for high gravimetric and volumetric energy density Li-ion batteries, J. Power Sources, 2019, 437, 226923 CAS.
- B. Ludwig, J. Liu, I. M. Chen, Y. Liu, W. Shou, Y. Wang and H. Pan, Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes, Adv. Mater. Interfaces, 2017, 4, 1700570 CrossRef.
- E. Zhen, J. Jiang, C. Lv, X. Huang, H. Xu, H. Dou and X. Zhang, Effects of binder content on low-cost solvent-free electrodes made by dry-spraying manufacturing for lithium-ion batteries, J. Power Sources, 2021, 515, 230644 CrossRef CAS.
- A. Yonaga, S. Kawauchi, Y. Mori, L. Xuanchen, S. Ishikawa, K. Nunoshita, G. Inoue and T. Matsunaga, Effects of dry powder mixing on electrochemical performance of lithium-ion battery electrode using solvent-free dry forming process, J. Power Sources, 2023, 581, 233466 CrossRef CAS.
- Y. Li, Y. Wu, Z. Wang, J. Xu, T. Ma, L. Chen, H. Li and F. Wu, Progress in solvent-free dry-film technology for batteries and supercapacitors, Mater. Today, 2022, 55, 92–109 CAS.
- M. Al-Shroofy, Q. Zhang, J. Xu, T. Chen, A. P. Kaur and Y. T. Cheng, Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries, J. Power Sources, 2017, 352, 187–193 CAS.
- K. Zhang, D. Li, X. Wang, J. Gao, H. Shen, H. Zhang, C. Rong and Z. Chen, Dry Electrode Processing Technology and Binders, Materials, 2024, 17, 2349 CAS.
- M. Ryu, Y. K. Hong, S. Y. Lee and J. H. Park, Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication, Nat. Commun., 2023, 14, 1316 CAS.
- K. Kwon, J. Kim, S. Han, J. Lee, H. Lee, J. Kwon, J. Lee, J. Seo, P. J. Kim, T. Song and J. Choi, Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries, Small Sci., 2024, 4, 2300302 CAS.
- G. Park, H. S. Kim and K. J. Lee, Solvent-Free Processed Cathode Slurry with Carbon Nanotube Conductors for Li-Ion Batteries, Nanomaterials, 2023, 13, 324 CrossRef CAS PubMed.
- D. J. Kirsch, S. D. Lacey, Y. Kuang, G. Pastel, H. Xie, J. W. Connell, Y. Lin and L. Hu, Scalable Dry Processing of Binder-Free Lithium-Ion Battery Electrodes Enabled by Holey Graphene, ACS Appl. Energy Mater., 2019, 2, 2990–2997 CrossRef CAS.
- W. Fu, Y. Wang, K. Kong, D. Kim, F. Wang and G. Yushin, Materials and Processing of Lithium-Ion Battery Cathodes, Nanoenergy Adv., 2023, 3, 138–154 CrossRef.
- Z. Zhang, D. Han, M. Xiao, S. Wang, Y. Feng, S. Huang and Y. Meng, New potential substitute of PVDF binder: poly(propylene carbonate) for solvent-free manufacturing high-loading cathodes of LiFePO4|Li batteries, Ionics, 2023, 29, 3895–3906 CAS.
- Y. Zhang, S. Lu, Z. Wang, V. Volkov, F. Lou and Z. Yu, Solvent-free lithium iron phosphate cathode fabrication with fibrillation of polytetrafluoroethylene, Electrochim. Acta, 2023, 456, 142469 CAS.
- S. Kim, S. Shin, D. S. Jung, J. Chun, Y. C. Kang and J. H. Kim, Scalable Dry Process for Fabricating a Na Superionic Conductor-Type Solid Electrolyte Sheet, ACS Appl. Mater. Interfaces, 2024, 16, 10307–10315 CAS.
- Y. Zhang, F. Huld, S. Lu, C. Jektvik, F. Lou and Z. Yu, Revisiting Polytetrafluorethylene Binder for Solvent-Free Lithium-Ion Battery Anode Fabrication, Batteries, 2022, 8, 57 CAS.
- X. Wang, S. Chen, K. Zhang, L. Huang, H. Shen, Z. Chen, C. Rong, G. Wang and Z. Jiang, A Polytetrafluoroethylene-Based Solvent-Free Procedure for the Manufacturing of Lithium-Ion Batteries, Materials, 2023, 16, 7232 CAS.
- Y. Zhang, S. Lu, F. Lou and Z. Yu, Solvent-free lithium iron phosphate cathode fabrication with fibrillation of polytetrafluoroethylene, Electrochim. Acta, 2023, 456, 142469 CrossRef CAS.
- R. Tao, B. Steinhoff, X. G. Sun, K. Sardo, B. Skelly, H. M. Meyer, C. Sawicki, G. Polizos, X. Lyu, Z. Du, J. Yang, K. Hong and J. Li, High-throughput and high-performance lithium-ion batteries via dry processing, Chem. Eng. J., 2023, 471, 144300 CrossRef CAS.
- F. Hippauf, B. Schumm, S. Doerfler, H. Althues, S. Fujiki, T. Shiratsushi, T. Tsujimura, Y. Aihara and S. Kaskel, Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach, Energy Storage Mater., 2019, 21, 390–398 Search PubMed.
- E. Pameté, J. G. A. Ruthes, M. Hermesdorf, A. Seltmann, D. J. Tarimo, D. Leistenschneider and V. Presser, Dry Electrode Processing for Free-Standing Supercapacitor Electrodes with Longer Life, Higher Volumetric Outputs, and Reduced Environmental Impact, Energy Environ. Mater., 2024, e12775 Search PubMed.
- R. C. Huber, E. B. Watkins, J. L. Jordan, D. M. Dattelbaum, E. N. Brown, B. D. Bartram and L. L. Gibson, Capturing Polymer Chain Compression and Shock Driven Decomposition of Polytetrafluoroethylene During Dynamic Shock Compression with In Situ X-Ray Diffraction, J. Dyn. Behav. Mater., 2023, 1–10 Search PubMed.
- K. Sato, Y. Tominaga, Y. Imai, T. Yoshiyama and Y. Aburatani, Deformation capability of poly(tetrafluoroethylene) materials: estimation with X-ray diffraction measurements, Polym. Test., 2022, 113, 107690 CAS.
- E. N. Brown and D. M. Dattelbaum, The role of crystalline phase on fracture and microstructure evolution of polytetrafluoroethylene (PTFE), Polymer, 2005, 46, 3056–3068 CAS.
- J. Wu, H. Wang, B. Feng, Y. Li, S. Wu, Q. Yin, Z. Yu and J. Huang, The effect of temperature-induced phase transition of PTFE on the dynamic mechanical behavior and impact-induced initiation characteristics of Al/PTFE, Polym. Test., 2020, 91, 106835 CAS.
- C. Luo, J. Pei, W. Zhuo, Y. Niu and G. Li, Phase transition behavior and deformation mechanism of polytetrafluoroethylene under stretching, RSC Adv., 2021, 11, 39813–39820 CAS.
- D. Vavlekas, L. Melo, M. Ansari, E. Grant, F. Fremy, J. L. McCoy and S. G. Hatzikiriakos, Role of PTFE paste fibrillation on Poisson's ratio, Polym. Test., 2017, 61, 65–73 CAS.
- H. A. Ardakani, E. M. Itsoulis and S. G. H. Atzikiriakos, Polytetrafluoroethylene Paste Extrusion: A Fibrillation Model and Its Relation to Mechanical Properties, Int. Polym. Process., 2013, 28, 306–313 CrossRef CAS.
- G. A. B. Matthews, S. Wheeler, J. Ramírez-González and P. S. Grant, Solvent-free NMC electrodes for Li-ion batteries: unravelling the microstructure and formation of the PTFE nano-fibril network, Front. Energy Res., 2023, 11, 1336344 CrossRef.
- K. Konda, S. B. Moodakare, P. L. Kumar, M. Battabyal, J. R. Seth, V. A. Juvekar and R. Gopalan, Comprehensive effort on electrode slurry preparation for better electrochemical performance of LiFePO4 battery, J. Power Sources, 2020, 480, 228837 CrossRef CAS.
- B. Akuzum, L. Agartan, J. Locco and E. C. Kumbur, Effects of particle dispersion and slurry preparation protocol on electrochemical performance of capacitive flowable electrodes, J. Appl. Electrochem., 2017, 47, 369–380 CAS.
- J. Choi, C. Lee, S. Park, T. J. Embleton, K. Ko, M. Jo, K. S. Saqib, J. Yun, M. Jo, Y. Son and P. Oh, Analysis of Electrochemical Performance with Dispersion Degree of CNTs in Electrode According to Ultrasonication Process and Slurry Viscosity for Lithium-Ion Battery, Nanomaterials, 2022, 12, 4271 CAS.
- M. Horst, F. Beverborg, L. Bahlmann, S. Schreiber, J. Gerk, P. Michalowski and A. Kwade, Effect of active material morphology on PTFE-fibrillation, powder characteristics and electrode properties in dry electrode coating processes, Powder Technol., 2025, 451, 120451 CAS.
- J. Wu, X. Zhang, Z. Ju, L. Wang, Z. Hui, K. Mayilvahanan, K. J. Takeuchi, A. C. Marschilok, A. C. West, E. S. Takeuchi and G. Yu, From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems, Adv. Mater., 2021, 33, 2101275 CAS.
- J. Kim, K. Park, M. Kim, H. Lee, J. Choi, H. B. Park, H. Kim, J. Jang, Y. H. Kim, T. Song and U. Paik, 10 mAh cm−2 Cathode by Roll-to-Roll Process for Low Cost and High Energy Density Li-Ion Batteries, Adv. Energy Mater., 2024, 14, 2303455 CAS.
- R. Tao, B. Steinhoff, C. H. Sawicki, J. Sharma, K. Sardo, A. Bishtawi, T. Gibbs and J. Li, Unraveling the impact of the degree of dry mixing on dry-processed lithium-ion battery electrodes, J. Power Sources, 2023, 580, 233379 CAS.
- J. Shin, J. H. Lee, J. K. Seo, W. T. A. Ran, S. M. Hwang and Y. J. Kim, Carbon nanotubes-coated Ni-rich cathodes for the green manufacturing process of lithium-ion batteries, Int. J. Energy Res., 2022, 46, 16061–16074 CAS.
- H. Choi, D. Moon, J. Sheem, J. K. Koo, S. Hong, S.-M. Oh and Y.-J. Kim, A Solvent-Free Process Enabled by Polytetrafluoroethlyene/Carbon Black Composites for Fabricating Electrodes for Lithium-Ion Batteries with a High Volumetric Energy, J. Electrochem. Soc., 2023, 170, 090511 CAS.
- K. Lee, Y. Jo, J. S. Nam, H. Yu and Y. J. Kim, Dry-film technology employing cryo-pulverized polytetrafluoroethylene binder for all-solid-state batteries, Chem. Eng. J., 2024, 487, 150221 CAS.
- H. Oh, G. S. Kim, B. U. Hwang, J. Bang, J. Kim and K. M. Jeong, Development of a feasible and scalable manufacturing method for PTFE-based solvent-free lithium-ion battery electrodes, Chem. Eng. J., 2024, 491, 151957 CrossRef CAS.
- E. Wiegmann, S. Fischer, M. Leeb and A. Kwade, Sustainable Lithium Ferro-Phosphate Cathode Manufacturing: A Semi-Dry Approach with Water-Based Processing and Polytetrafluorethylene Binders, Batteries, 2023, 9, 567 CrossRef CAS.
- D. J. Lee, J. Jang, J. P. Lee, J. Wu, Y. T. Chen, J. Holoubek, K. Yu, S. Y. Ham, Y. Jeon, T. H. Kim, J. B. Lee, M. S. Song, Y. S. Meng and Z. Chen, Physio-Electrochemically Durable Dry-Processed Solid-State Electrolyte Films for All-Solid-State Batteries, Adv. Funct. Mater., 2023, 33, 2301341 CrossRef CAS.
- S. Yoon, S. H. Kang, J. Choi, J. Y. Kim, Y. G. Lee, Y. S. Park and D. O. Shin, Regulating Entanglement Networks of Fibrillatable Binders for Sub-20-μm Thick, Robust, Dry-Processed Solid Electrolyte Membranes in All-Solid-State Batteries, Small, 2024, 2407882 CrossRef PubMed.
- J. Entwistle, R. Ge, K. Pardikar, R. Smith and D. Cumming, Carbon binder domain networks and electrical conductivity in lithium-ion battery electrodes: a critical review, Renew. Sustain. Energy Rev., 2022, 166, 112624 CAS.
- K. Konda, M. S. Jacob, J. R. Seth, V. A. Juvekar, R. Gopalan and S. B. Moodakare, Capacity degradation of lithium-ion cell: the role of free carbon black content in the slurry and drying induced cracks in LiFePO4 electrode, J. Energy Storage, 2023, 74, 109477 Search PubMed.
- M. Cao, L. Wang, Q. Zhang, H. Zhang, S. Zhong and J. Chen, Boosting the comprehensive behaviors of LiNi0.5Co0.2Mn0.3O2 lithium-ion batteries via CNTs/Super-P composite conductive agent, Mater. Today Commun., 2023, 36, 106677 CAS.
- I. A. Kinloch, J. Suhr, J. Lou, R. J. Young and P. M. Ajayan, Composites with carbon nanotubes and graphene: an outlook, Science, 2018, 362, 547–553 CAS.
- H. Kim, J. H. Lim, T. Lee, J. An, H. Kim, H. Song, H. Lee, J. W. Choi and J. H. Kang, Ozone-Treated Carbon Nanotube as a Conductive Agent for Dry-Processed Lithium-Ion Battery Cathode, ACS Energy Lett., 2023, 8, 3460–3466 CAS.
- J. H. Hwang, H. Yoo, S. Oh and H. Kim, Relationship between particle shape and fast-charging capability of a dry-processed graphite electrode in lithium-ion batteries, Electrochem. Commun., 2024, 165, 107761 CAS.
- R. Tao, B. Su, S. Thapa, K. Uzun, H. Alolaywi, X. Lyu, B. Steinhoff, K. Sardo, Z. Du, Y. T. Cheng, C. Yuan, K. Z. Pupek, G. Polizos and J. Li, Exploring the potential and impact of single-crystal active materials on dry-processed electrodes for high-performance lithium-ion batteries, Chem. Eng. J., 2024, 500, 157194 CAS.
- R. Tao, S. Tan, H. M. Meyer, X. G. Sun, B. Steinhoff, K. Sardo, A. Bishtawi, T. Gibbs and J. Li, Insights into the Chemistry of the Cathodic Electrolyte Interphase for PTFE-Based Dry-Processed Cathodes, ACS Appl. Mater. Interfaces, 2023, 15, 40488–40495 CAS.
- R. He, W. Zhong, C. Cai, S. Li, S. Cheng and J. Xie, Flour-Infused Dry Processed Electrode Enhancing Lithium-Ion Battery Performance, Adv. Energy Mater., 2024, 14, 2402109 CAS.
- T. J. Embleton, J. H. Choi, S. J. Won, J. Ali, K. S. Saqib, K. Ko, M. Jo, J. Hwang, J. Park, J. H. Lee, J. Kim, M. K. Kim, J. W. Jung, M. Park and P. Oh, High-energy density ultra-thick drying-free Ni-rich cathode electrodes for application in Lithium-ion batteries, Energy Storage Mater., 2024, 71, 103542 Search PubMed.
- J. Lee, T. Lee, K. Char, K. J. Kim and J. W. Choi, Issues and Advances in Scaling up Sulfide-Based All-Solid-State Batteries, Acc. Chem. Res., 2021, 54, 3390–3402 CAS.
- R. Shah, V. Mittal and A. M. Precilla, Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles, J, 2024, 7, 204–217 CAS.
- T. Famprikis, P. Canepa, J. A. Dawson, M. S. Islam and C. Masquelier, Fundamentals of inorganic solid-state electrolytes for batteries, Nat. Mater., 2019, 18, 1278–1291 CrossRef CAS PubMed.
- Y. Han, S. H. Jung, H. Kwak, S. Jun, H. H. Kwak, J. H. Lee, S. T. Hong and Y. S. Jung, Single- or Poly-Crystalline Ni-Rich Layered Cathode, Sulfide or Halide Solid Electrolyte: Which Will be the Winners for All-Solid-State Batteries?, Adv. Energy Mater., 2021, 11, 2100126 CrossRef CAS.
- H. Gamo, A. Nagai and A. Matsuda, The effect of solvent on reactivity of the Li2S–P2S5 system in liquid-phase synthesis of Li7P3S11 solid electrolyte, Sci. Rep., 2021, 11, 21097 CrossRef PubMed.
- D. Lee, Y. Shim, Y. Kim, G. Kwon, S. H. Choi, K. S. Kim and D. J. Yoo, Shear force effect of the dry process on cathode contact coverage in all-solid-state batteries, Nat. Commun., 2024, 15, 4763 CrossRef CAS PubMed.
- D. Lee and A. Manthiram, Boosting the electrochemical performance with functionalized dry electrodes for practical all-solid-state batteries, J. Mater. Chem. A, 2024, 12, 3323–3330 CAS.
- H. s. Kim, J. Y. Jung, K. S. Kim, C. Hwang, J. Yu, M. S. Park and W. Cho, Functionalized Electrode Additive for Simultaneously Reinforcing Chemo-Mechanical Properties of Millimeter-Thick Dry-Electrode for High-Energy All-Solid-State Batteries, Adv. Energy Mater., 2024, 14, 2303965 CAS.
- M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescu and G. J. Offer, Lithium sulfur batteries, a mechanistic review, Energy Environ. Sci., 2015, 8, 3477–3494 CAS.
- C. V. Amanchukwu, The Electrolyte Frontier: A Manifesto, Joule, 2020, 4, 281–285 Search PubMed.
- M. Wang, Z. Bai, T. Yang, C. Nie, X. Xu, Y. Wang, J. Yang, S. Dou and N. Wang, Advances in High Sulfur Loading Cathodes for Practical Lithium-Sulfur Batteries, Adv. Energy Mater., 2022, 12, 2201585 CAS.
- H. Sul, D. Lee and A. Manthiram, High-Loading Lithium-Sulfur Batteries with Solvent-Free Dry-Electrode Processing, Small, 2024, 20, 2400728 CAS.
- C. Shen, L. Hu, H. Tao, Y. Liu, Q. Li, W. Li, T. Ma, B. Zhao, J. Zhang and Y. Jiang, Dry-processed technology for flexible and high-performance FeS2-based all-solid-state lithium batteries at low stack pressure, J. Colloid Interface Sci., 2024, 666, 472–480 CAS.
- J. K. Hu, H. Yuan, S. J. Yang, Y. Lu, S. Sun, J. Liu, Y. L. Liao, S. Li, C. Z. Zhao and J. Q. Huang, Dry electrode technology for scalable and flexible high-energy sulfur cathodes in all-solid-state lithium-sulfur batteries, J. Energy Chem., 2022, 71, 612–618 CAS.
- J. Li, J. Fleetwood, W. B. Hawley and W. Kays, From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing, Chem. Rev., 2022, 122(1), 903–956 CAS.
- W. Jin, G. Song, J. K. Yoo, S. K. Jung, T. H. Kim and J. Kim, Advancements in Dry Electrode Technologies: Towards Sustainable and Efficient Battery Manufacturing, ChemElectroChem, 2024, 11(17), e202400288 CAS.
- R. Tao, Y. Gu, Z. Du, X. Lyu and J. Li, Advanced electrode processing for lithium-ion battery manufacturing, Nat. Rev. Clean Technol., 2025, 1, 116–131 Search PubMed.
- M. Pishvar, M. Amirkhosravi and I. Manas-Zloczower, Thermomechanical Performance of Thermoplastic Polyurethane-Poly(tetrafluoroethylene) Fibril Nanocomposites, ACS Appl. Polym. Mater., 2023, 5, 5342–5348 CAS.
- W. Fang, G. Liang, J. Li and S. Guo, Microporous formation and evolution mechanism of PTFE fibers/isotactic polypropylene membranes by interface separation, J. Membr. Sci., 2021, 631, 119333 CAS.
- C. Ma, W. Cai, Z. Zhu, Z. Ji, J. Yang, H. Li, G. Wen, Z. Zhao, X. Fu, W. Yang and Y. Wang, How Binder Nanofibration Affects the Active-Material Microenvironment in Battery Electrodes?, Adv. Funct. Mater., 2024, 2412193 Search PubMed.
- A. Zhang, J. Chai, C. Yang, J. Zhao, G. Zhao and G. Wang, Fibrosis mechanism, crystallization behavior and mechanical properties of in situ fibrillary PTFE reinforced PP composites, Mater. Des., 2021, 211, 110157 CrossRef CAS.
- Y. Qiao, A. Jalali, J. Yang, Y. Chen, S. Wang, Y. Jiang, J. Hou, J. Jiang, Q. Li and C. B. Park, Non-isothermal crystallization kinetics of polypropylene/polytetrafluoroethylene fibrillated composites, J. Mater. Sci., 2021, 56, 3562–3575 CrossRef CAS.
- Z. Wei, D. Kong, L. Quan, J. He, J. Liu, Z. Tang, S. Chen, Q. Cai, R. Zhang, H. Liu, K. Xu, L. Xing and W. Li, Removing electrochemical constraints on polytetrafluoroethylene as dry-process binder for high-loading graphite anodes, Joule, 2024, 8, 1350–1363 CrossRef CAS.
- G. Li, R. Xue and L. Chen, The influence of polytetrafluorethylene reduction on the capacity loss of the carbon anode for lithium ion batteries, Solid State Ionics, 1996, 90, 221–225 CrossRef CAS.
- T. Lee, J. An, W. J. Chung, H. Kim, Y. Cho, H. Song, H. Lee, J. H. Kang and J. W. Choi, Non-Electroconductive Polymer Coating on Graphite Mitigating Electrochemical Degradation of PTFE for a Dry-Processed Lithium-Ion Battery Anode, ACS Appl. Mater. Interfaces, 2024, 16, 8930–8938 CrossRef CAS.
- S. Han, E. H. Noh, S. Chae, K. Kwon, J. Lee, J. S. Woo, S. Park, J. W. Lee, P. J. Kim, T. Song, W. J. Kwak and J. Choi, Mitigating PTFE decomposition in ultra thick dry-processed anodes for high energy density lithium-ion batteries, J. Energy Storage, 2024, 96, 112693 CrossRef.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. Dewitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier and Z. Wang, An overview of the uses of per- and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22, 2345–2373 Search PubMed.
- F. Schmidt, S. Kirchhoff, K. Jägle, A. De, S. Ehrling, P. Härtel, S. Dörfler, T. Abendroth, B. Schumm, H. Althues and S. Kaskel, Sustainable Protein-Based Binder for Lithium-Sulfur Cathodes Processed by a Solvent-Free Dry-Coating Method, ChemSusChem, 2022, 15, e202201320 CAS.
- H. Oh, G. S. Kim, J. Bang, S. Kim and K. M. Jeong, Dry-processed thick electrode design with a porous conductive agent enabling 20 mA h cm−2 for high-energy-density lithium-ion batteries, Energy Environ. Sci., 2025, 18, 645–658 CAS.
- N. Y. Kim, J. H. Kim, H. Koo, J. Oh, J. H. Pang, K. D. Kang, S. S. Chae, J. Lim, K. W. Nam and S. Y. Lee, Material Challenges Facing Scalable Dry-Processable Battery Electrodes, ACS Energy Lett., 2024, 9(11), 5688–5703 CAS.
Footnote |
| † These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab and
Ungyu
Paik
*ab and
Ungyu
Paik
 *a
*a




















