 Open Access Article
Open Access ArticleEpitome of polyoxotungstate-coordinated lanthanide-based single-molecule magnets†
Pradip Kumar
Sahu‡
 ,
Sandhya
Kapurwan‡
,
Sandhya
Kapurwan‡
 and
Sanjit
Konar
and
Sanjit
Konar
 *
*
Department of Chemistry, IISER Bhopal, Bhauri, Bhopal by-pass road, Bhopal, Madhya Pradesh-462066, India. E-mail: skonar@iiserb.ac.in
First published on 24th March 2025
Abstract
Single-molecule magnets (SMMs) have garnered significant interest due to their possible use in high-density data storage devices and quantum computing. SMMs based on lanthanide-encapsulated polyoxotungstate (POT) have emerged due to their stability under ambient conditions and potential application in magnetic devices. The POTs can stabilize various anisotropic lanthanide ions (Ln(III)) with a range of strong to moderate ligand fields. The systematic combination of Ln(III) ions with POTs leads to significant perturbation in the electronic structure of the Ln(III) ion, which notably influences their magnetic properties. The magnetic properties of Ln(III)-POT clusters are highly dependent on the first coordination geometry and ligand field strength around the Ln(III) ions. Moreover, the bulky and diamagnetic POTs efficiently minimize the intermolecular magnetic interactions. As a result, the under-barrier magnetic relaxation is suppressed, and magnetic performance is enhanced. Over the years, a diverse array of Ln-POT SMMs have enriched the literature containing vacant POTs as building blocks (such as Keggin, Lindqvist, Wells–Dawson, and Preyssler types). In this review, we have discussed and summarized the effects of structural and bonding diversities of POTs on the SMM behaviour of Ln-POT clusters. This review aims to provide future direction and exploration of the challenging and compelling field of POT-based SMMs.
Introduction
Single-molecule magnets (SMMs) are paramagnetic metal complexes with the ability to block magnetization below a certain temperature (TB), allowing them to retain magnetization for long periods and slowly demagnetize in the absence of a magnetic field.1–4 They have potential applications in quantum computing, molecular spintronics, and high-density data storage, where each molecule can store magnetic information.5–8 The lanthanide (Ln(III)) complexes are the most advantageous system due to their huge unquenched orbital angular momentum and inherent magnetic anisotropy, which is a crucial criterion for achieving high-performance SMMs.9–12 By modulating the coordination geometry and symmetry around the metal center with the aid of an appropriate ligand field, the magnetic anisotropy of SMMs can be regulated.13–20 However, to achieve the practical usefulness of SMM-based technology, the SMM should possess a high energy barrier for slow relaxation of magnetization (Ueff) and high blocking temperature (TB), where Ueff refers to the effective energy barrier required for the conversion of a single-molecule magnet (SMM) into a paramagnetic state. This requires a substantially large anisotropy around the Ln(III) ion. Therefore, the presence of a strong axial coordination environment is the foremost requirement.21–23 Several strategies have been employed to develop high-performance lanthanide-based single-molecule magnets, focusing on crystal field (CF) symmetry, predominant bonding interactions, organometallic frameworks, and strong magnetic coupling.24–34 Along this line, many dysprosium metallocene cations were reported with TB near the boiling point of liquid nitrogen (77 K).23 Despite these advances, these SMMs are highly reactive to air and moisture. Thus, these molecules must be handled under an inert atmosphere. On the other hand, SMMs based on lanthanide-encapsulated polyoxometalates (Ln-POMs) offer a promising alternative due to their easy synthesis and exceptional stability under ambient conditions. Polyoxometalates (POMs) are anionic oxo-clusters of early transition metals in their high oxidation states, which provide aesthetical structural features as well as strong to moderate ligand field strength towards the metal ions.35–39 They are effective multidentate building blocks for creating cluster complexes based on transition and lanthanide metals because of their strong negative charge and numerous oxo-donor sites.40–47 POMs provide highly symmetric coordination sites toward Ln(III) ions while maintaining effective magnetic isolation and stability of Ln-POM clusters in both the solution and the solid state. The magnetic behaviour of Ln-POMs can be finely tuned, resulting in a wide range of magnetic phenomena, such as molecular spin frustration, electron delocalization-related properties, and SMMs.48–54SMMs based on POMs can be classified into three families based on encapsulated metal ions: (a) POMs encapsulating transition metal ions,55–60 (b) POMs encapsulating lanthanide ions,61–64 and (c) POMs incorporating a mixed-valence POM cluster.65–71 The beneficial properties of POMs have prompted great interest in the scientific community, leading to exponential growth in the number of publications over the last decade. POM clusters have been mainly reported with first-row transition metals (V, W, and Mo).35,36,52 Among those POMs, polyoxotungstate (POT)-based metal ion encapsulated clusters mainly enrich the literature.72–76 However, it should be noted that the formation of a POT cluster is not controllable during reaction. Recently, a few groups demonstrated the formation of various types of POMs using robotic machine learning methods.77–81 In parallel, the inherent emerging nature of this lanthanide POM research field is expanding to new avenues, from materials design towards catalysis, to novel device fabrication, and to Qubits.82–87
This feature article provides a comprehensive overview of Ln(III) encapsulated polyoxotungstate-based molecules, focusing on their structural diversities. We categorized Ln-POTs based on their POM building units (Schemes 1, 2 and Table S1, ESI†). The bonding motif of these POM building units plays a crucial role in modulating the geometry around the first coordination sphere of the metal ions. This, in turn, influences the magnetic properties of Ln-POMs by altering the electronic behavior of the Ln(III) ions. In this feature article, we explore the coordinating geometry-dependent magnetic modulation phenomenon and highlight how structural variation affects the magnetic properties of these molecules.
 | ||
| Scheme 2 Diverse binding motifs of different polyoxotungstates (POTs) with Ln(III) ions. Color code: light blue octahedron {WO6}, blue sphere Ln(III), red O, pink P/Si. | ||
Types of POMs and their synthetic strategies
The protonation of an oxometallate ion under controlled conditions (such as pH, concentration, and temperature) initiates the polycondensation of MO42− units, forming complex polyanions known as polyoxometalates (POMs).88–91 POMs are categorized into isopolyanions (IPAs) and heteropolyanions (HPAs).| n[MO4]2− + 2m[H]+ ↔ [MnO(4n−m)](2n–m)− + mH2O |
| 12[WO4]2− + [HPO4]2− + 23[H]+ → [PW12O40]3− + 12H2O |
Among all the POM clusters, tungsten-based POMs are robust, and this fact has been exploited to develop tungsten-based Keggin and Dawson anions with vacancies known as lacunary polyoxotungstates (Scheme 1). These lacunary POMs are produced by controlled treatment of polyoxotungstate species with a base, resulting in the removal of one or two MO units from fully occupied POMs like [XW12VIO40]n−, leading to mono, di, and tri-lacunary forms such as [XM11O39]n−, [XM10O36]n−, and [XM9O34]n−. The incorporation of heteroatoms with stereoactive lone pairs of electrons further enhances their versatility. The lone pair in the precursor molecule plays a crucial role in directing the self-assembly process, facilitating the formation of larger and more complex structures upon reaction with electrophiles. The coordination of Ln(III) cations with POM units to prepare Ln-POMs can be achieved through two main methods: one-pot synthesis and the building block method.
Significance of POMs as a ligand
In the literature, Ln-SMMs are mainly reported with organic ligands. POMs as an inorganic ligand towards Ln(III) ions are less explored due to their uncontrolled synthetic pathways. However, POMs offer significant advantages over organic ligands. This includes-(a) Lacunary POMs can act as a multidentate ligand with the Ln(III) ion localized in the lacuna site (Scheme 2). Furthermore, the lacuna sites can be modified by synthetic modification, which provides various kinds of binding affinity towards the Ln(III) ion through oxo bridging sites.
(b) POMs are larger in size compared to organic ligands. They are rigid and diamagnetic also. This suppresses the intermolecular magnetic interactions in the crystal lattice.
(c) Moreover, it is possible to create clusters with a greater number of nuclei by manipulating specific chemical factors such as stoichiometry and pH. This property enables the control of both the number of nuclei and the specific type of magnetic interactions between various ions, thereby achieving a chosen spin ground state.
Types of POT building units used for SMMs
 | ||
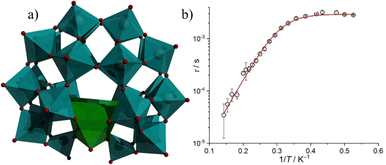
| Fig. 1 (a) The molecular structure of [ErW10O36]9−, (b) the symmetry around the Er(III) ion, and (c) out-of-phase magnetic susceptibility plot of the complex. Color code: cyan octahedron {WO6}, red O, violet Er. Figure reprinted (adapted) with permission from ref. 92. Copyright (2008) American Chemical Society. | ||
 | ||
| Fig. 2 The molecular structure of the [Dy30Co8Ge12W108O408(OH)42(OH2)30]56− molecule (a), and the temperature-dependent in-phase (b) and out-of-phase (c) magnetic susceptibility plot at zero DC field of the complex. Color code: cyan octahedron {WO6}, green polyhedron Dy unit, blue polyhedron Co unit. Hydrogen atoms are omitted for clarity. Figures adapted from ref. 95, with permission from Wiley, copyright 2015. | ||
 | ||
| Fig. 3 The reaction conditions for the formation of [K⊂{(AsW9O33)Dy(H2O)2}6]35− (b) and [Cs⊂{Tb4(H2O)8(α-AsW9O33)4}]23− (c) from [As2W19O67(H2O)]14− (a) and their corresponding magnetic relaxation plot (d) and frequency-dependent out-of-phase magnetic susceptibility plot (e). Color code: cyan octahedron {WO6}, green Dy, red O, yellow K, violet Cs, pink As. Hydrogen atoms are omitted for clarity. Figures reprinted (adapted) with permission from ref. 97 and 101. Copyright (2022) American Chemical Society, and (2017) Royal Society of Chemistry, respectively. | ||
Previous studies have demonstrated that bridging structures between two metal cations in silicodecatungstate can be transformed through chemical stimuli.107–110 This ability of POM ligands opens up the possibility of reversibly controlling SMM behavior. In this regard, Mizuno and co-workers reported reversible switching of SMM behavior by structural transformation of a dinuclear Dy-core in heteropolyoxotungstate [{Dy(H2O)2(CH3COCH3)}2(γ-SiW10O36)2]10− (complex 1) to [Dy2(μ2-OH)2(γ-SiW10O36)2]12− (complex 2) upon addition of tetra-n-butyl ammonium hydroxide (TBAOH), which promotes hydrolysis of the H2O ligand (Fig. 4a and b).107 The AC susceptibility data for complex 2 indicate slow magnetic relaxation behavior with an effective energy barrier of 65.7 K, whereas complex 1 hardly shows SMM behavior (Fig. 4a and b). This change is attributed to the alteration in coordination geometry, which reduces repulsive contacts between ligands and the f-orbital electron density of dysprosium cations, thereby increasing the magnetic anisotropy of the Dy(III) ion.
 | ||
| Fig. 4 The molecular structure and out-of-phase magnetic susceptibility of [{Dy(H2O)2(CH3COCH3)}2(γ-SiW10O36)2]10− (a) to [Dy2(μ2-OH)2(γ-SiW10O36)2]12− (b) with their reaction conditions for reversible transformation. Color code: cyan octahedron {WO6}, green Dy, red O, grey C. Hydrogen atoms are omitted for clarity. Pictures reproduced from ref. 107 with permission from the Royal Society of Chemistry. | ||
Furthermore, the effect of the ionic radius of the Ln(III) center adjacent to the Dy(III) ion in the dinuclear core of complex 2 was investigated by synthesizing hetero-dinuclear lanthanide-containing POTs [{Ln(μ2-OH)2Ln′}(γ-SiW10O36)2]12− (LnLn′; Ln = Dy; Ln′ = Dy, Eu, Yb, Lu) through stepwise incorporation of Ln(III) ions into vacant sites of lacunary POMs.108 AC susceptibility measurements showed that all complexes exhibit slow relaxation of magnetization, with the magnetization barrier increasing in the order: DyLu < DyYb < DyDy < DyEu. This is due to the elongation of the bond distance of bridging oxygen and DyLn′, which enhances the magnetic anisotropy of the Dy(III) ion and the energy barrier for magnetization reversal. The [GeW10O38]10− building unit has also been utilized to synthesize large clusters, such as [(H2O)6⊂Dy12(H2O)24(GeW10O38)6]36− and [(GeW10O38)6(Dy14(H2O)W8(OH)O30)]43−, where it coordinates through different vacant sites.109,110 However, the frequency-dependent AC signals are too weak for a meaningful fitting analysis. This is due to the diverse and complex coordination geometries of the crystallographically independent Dy(III) ions, which result in multiple magnetization relaxation processes.
 | ||
| Fig. 5 The molecular structure of [(PW11O39)2Dy2X2(H2O)2]10− [X = OH (a), F (c)] and the frequency-dependent out-of-phase magnetic susceptibility plots of the respective complexes (b and d). Color code: cyan octahedron {WO6}, green Dy, red O, deep green F. Hydrogen atoms are omitted for clarity. Figures reprinted (adapted) with permission from ref. 116. Copyright (2019) American Chemical Society. | ||
Highnuclearity {Wn}-unit based SMMs
Magnetic polyoxometalates (POMs) can be categorized into two main families. The first consists of lacunary POMs, such as Keggin and Dawson types that encapsulate metal ions connected through oxo bridges, forming magnetic clusters with variable nuclearity and high symmetries. The second family includes larger POM clusters that encapsulate lanthanide ions, resulting in complexes where 4f-magnetic ions interact with the crystal field generated by the POM ligands. This interaction leads to various symmetries such as D2d, D3h, and C∞, etc. Beyond the lacunary POM building blocks, these larger POM clusters offer significant advantages, including internal cavities that accommodate a range of metal cations, a high oxygen-rich surface, and exceptional stability across a broad pH range.117–125 In 2012, Coronado and co-workers reported an interesting example, [LnW30O110]12− (Ln3+ = Dy, Ho), where {W30} polyoxometalates act as a rigid ligand and are used for the design of metal complexes with very unusual C5 axial symmetry.126 Structural analysis revealed that Ln(III) ions occupy two identical coordination sites, leading to a mono-capped pentagonal antiprism geometry (Fig. 6a). Despite low symmetry around the Ln(III) centers, the SMM behavior was observed for both Dy and Ho derivatives with energy barrier Ueff/kB = 24 K and Ueff/kB ≈ 0.8 K, along with hysteresis at low temperatures (Fig. 6b). More recently, P. Kögerler and coworkers reported solution-stable polyanions [Na{Ln(H2O)}{WO(H2O)}P4W26O98]12− (Ln = Tb, Dy, Er, Ho, Tm, Yb), where Ln(III) is in a rare trigonal prismatic O6 environment (Fig. 7a).127 Structural analysis shows that in complex {P4W26}, two identical {P2W12} units connected through a μ2-bridge by four oxygen atoms, which further coordinated with two WVI ions through the open end of each {P2W12} building block and the central cavity of the {P4W26} entity is occupied by one W(VI) and Ln(III) ion (Ln = Sm–Lu). The Ln(III) ion exhibits nearly C2h-symmetric trigonal prismatic coordination symmetry. The AC magnetic susceptibility measurements reveal field-induced (1000 Oe applied DC field) SMM behaviour of the Dy-analog with an energy barrier of 26 K (Fig. 7b). Furthermore, the impact of O–H vibration on magnetic relaxation was determined by an exchange of coordinated H2O to Dy(III) by D2O. The deuterated compound shows a 20% larger Ueff than the hydrated compound, which implies that the relaxation of the Ln(III) center is moderately affected by the vibrational mode of the water ligand. | ||
| Fig. 6 The molecular structure (a) and magnetic hysteresis plot (b) of [LnW30O110]12−. Color code: cyan octahedron {WO6}, green Dy. Figure reprinted (adapted) with permission from ref. 126. Copyright (2012) American Chemical Society. | ||
 | ||
| Fig. 7 The molecular structure (a) and magnetic relaxation plot (b) of [{Dy(H2O)}{WO(H2O)}P4W26O98]13−. Color code: cyan octahedron {WO6}, green polyhedra Dy unit. Hydrogen atoms are omitted for clarity. Figures adapted from ref. 127, with permission from Wiley, copyright 2021. | ||
Organic–inorganic POT-based SMMs
Organic–inorganic hybrids have garnered significant attention and made rapid strides in recent years by integrating unique properties of individual components into unified systems. Various types of polynuclear polyoxometalates (POMs), particularly dysprosium-based single-molecule magnets (Dy-SMMs), have emerged as high-performance examples.128 These include organic–inorganic hybrid Ln-POMs incorporating carboxylates, organophosphates, amino acids, and other ligands, showcasing exceptional SMM behavior.Organic–inorganic hybrid Ln-POMs can be divided into three broad families. The first class includes POMs encapsulating multiple Ln(III) ions connected by organic acids, ligands, or other POMs to form magnetic clusters with variable nuclearity and high symmetry.129–133 A notable breakthrough in this field was achieved by C. Boskovic and co-workers through the use of POMs combined with organic acids as ligands. They reported a family of POT-ligated tetranuclear complexes, [Ln4As5W40O144(H2O)10(L)2]21− (L = glycine acid), where the organic acid bridges two {As2W19O68} building units.134 Those compounds were the first example of POM-supported polynuclear lanthanide-based SMMs, showcasing the potential of such hybrid materials. Later J. Wang and coworkers reported two tartaric acid bridged Ln-POT molecules, [{Dy(C4H2O6)(α-PW11O39)}2]9−, where two mono-Dy substituted Keggin units were bridged by two organic tartaric acid ligands, resulting in eight coordinate highly distorted square antiprism geometries.135 The frequency dependence of the ac-susceptibility suggested a typical single-molecule magnet (SMM) behavior with an energy barrier of 20 K. Furthermore, M.-L. Tong and coworkers reported [{(As-W9O33)3Dy2(H2O)4W4O9(H2O)}2(NH2(CH2PO3)2)]33−, utilizing organo-phosphonate ligands (Fig. 8a).136 Structural analysis revealed two identical subunits connected by {NH2(CH2PO3)2} ligands, with each phosphate group binding one W(VI) ion and one Dy(III) ion via P–O bonds. The magnetic measurements show nice frequency and temperature dependence of out-of-phase (χ′′) ac susceptibility under zero field, indicating the slow relaxation of magnetization behavior. The linear fit of the Arrhenius equation results in an energy barrier to the spin reversal, Ueff = 99 K, and relaxation time, τ0 = 1.16 × 10−6 s (Fig. 8b). Additionally, the variable-temperature magnetization measurements show hysteresis up to 8 K. Nano-sized Dy-POM clusters, [Dy6(L)4(H2O)23(ampH2)(PW11O39)2] (complex 3) (ampH2 = (aminomethyl)phosphonic acid) (pH = 2.9) and [Dy9(CO3)3(L)2(H2O)12(PW10O37)6]35− (complex 4) {L = (amino methyl)phosphonic acid (ampH2)} (pH = 7.4) were reported by controlling the pH of the reaction mixture.137 The structural analysis of complex 3 shows a hexanuclear Dy(III) core, sandwiched between two [PW11O39]7− units, which are oriented at 180° to each other where the geometry around the Dy(III) centers is square anti-prismatic. On the other hand, complex 4 consists of six [PW10O37]9− building blocks assembled around the [Dy9(CO3)3(ampH)2(H2O)12]19+ ({Dy9}) cluster core, which further consists of three {Dy3} units capped around two ampH− ligands where Dy(III) ions display distorted square antiprism geometry and capped trigonal prismatic geometry. The ac measurement reveals that only complex 4 shows zero field SMM behavior and magnetic hysteresis at 2 K.
 | ||
| Fig. 8 The molecular structure (a) and magnetic relaxation plot (b) of [{(As-W9O33)3Dy2(H2O)4W4O9(H2O)}2(NH2(CH2PO3)2)]33−. Color code: cyan octahedron {WO6}, green polyhedral Dy unit, red O, blue N, pink P, grey C, white H. Hydrogen atoms are omitted for clarity. Figure reprinted (adapted) with permission from ref. 136. Copyright (2017) American Chemical Society. | ||
Recently, M.-L. Tong and co-workers reported two Dy(III)–SMM complexes: [Dy(OPPh3)4(H2O)3][PW12O40] (complex 5) and [{Dy(OPPh3)3(H2O)3}{PW12O40}] (complex 6), utilizing the saturated Keggin-type polyoxoanion [PW12O40]3−.138 In these complexes, the DyIII sites exhibit pseudo-pentagonal bipyramidal (PBP) symmetry with axial anisotropy provided by the strong axial Ph3PO ligand. Magnetic studies reveal field-induced SMM behavior in complex 5, while complex 6 shows better SMM behavior, with an energy barrier of 310(7) K at zero field. Magneto-structural analysis shows that the enhancement in SMM behavior in complex 6 arises from replacing equatorial Ph3PO with POM, which strengthens axial anisotropy. This provides a novel strategy for improving SMM behavior in lanthanide-based systems.
As double-decker-type complexes have shown great potential toward high-performance SMMs by harnessing single-ion anisotropy,139–141 P. Kogerler and coworkers synthesized a Dy-double-decker complex, [DyIIIPc(PW11O39)]6− which comprises both Pc2− and a mono lacunary α-Keggin [PW11O39]7− ligand (Fig. 9a).142 Structural analysis shows that the Dy(III) ion coordinated to four oxygens of the lacunary site of [PW11O39]7− and the Pc2− unit results in a distorted antiprismatic N4O4 geometry. Furthermore, the frequency and temperature dependence of the ac susceptibility data show the presence of slow magnetic relaxation with the spin reversal barrier of Ueff = 47.48 K and relaxation time of τ0 = 1.08 × 10−7 s (Fig. 9b). In the realm of organic–inorganic hybrid polyoxometalates (POMs), a second family features heterometallic 3d–4f compounds aimed at enhancing magnetic interactions and spin ground states.130 A significant example was reported by Mizuno and co-workers, featuring 3d–3d′–4f heterotrimetallic heptanuclear clusters [(A-α-SiW9O34)2FeMn4O2{Ln(acac)2}2] (Ln = Gd3+, Dy3+, Lu3+).98 This involves the sequential introduction of metal cations into a trivacant lacunary POM [A-α-SiW9O34]6−, resulting in two [Ln(L)2]+ units at the ends of pentanuclear clusters [FeMn4O18(OH)2]23− and [FeCu4O18(OH)2]27−. The addition of a third 4f ion significantly enhances magnetic interactions between Mn3+−Mn3+ (J2 = −9.51 cm−1 for FeMn4Gd2; J2 = −7.10 cm−1 for FeMn4Lu2). The AC magnetic susceptibility studies indicated that only the FeMn4Lu2 derivative exhibited typical single-molecule magnet (SMM) behavior with an effective energy barrier (Ueff) of 19.7 K. In 2019, S.-T. Zhang and co-workers reported novel Cr–Ln heterometallic clusters with the formula [Cr4Ln4(μ4-O)4(μ3-O)4(C8H4O4)4-(H2O)10](H6SiW12O40)-Cl2 featuring prismatic [Cr4Ln4] cores stabilized by organic ligands and inorganic POM species.143 The magnetic investigation reveals the coexistence of ferromagnetic and antiferromagnetic couplings within the Cr4Dy4 core. The complexes did not show well-defined out-of-phase magnetic susceptibility (χ′′) maxima, making it difficult to extract the Ueff value. Furthermore, the same group reported Fe–Dy heterometallic clusters, such as the “8”-shaped polyanion [Fe(L)Dy(H2O)2Fe2(B-α-GeW9O34)(GeW7O29)]2 (L = trans-1,2-cyclohexanediamine), where the organic ligand coordinates to Fe3+.144 Magnetic analysis revealed frequency-dependent in-phase  signals and significant out-of-phase
signals and significant out-of-phase  signals indicative of potential single-molecule magnet (SMM) behavior.
signals indicative of potential single-molecule magnet (SMM) behavior.
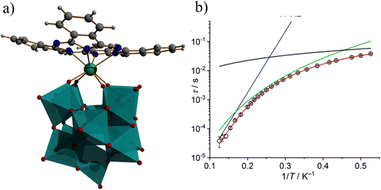 | ||
| Fig. 9 The molecular structure (a), and the magnetic relaxation plot (b) of [DyIIIPc(PW11O39)]6−. Color code: cyan octahedron {WO6}, green Dy, grey C, blue N, white H. Picture reproduced from ref. 142 with permission from the Royal Society of Chemistry. | ||
In 2023, T. Minato and coworkers reported a cationic glue strategy to form large-sized multinuclear metal encapsulated clusters using SiW9 building units.145 This work demonstrated the formation of the Ln2Mn2(FeMn4)2 cluster via the intermediate FeMn4 cluster (Fig. 10a). The multinuclear clusters {(FeMn4)Mn2Lu2(FeMn4)} (IILu) showed intramolecular ferromagnetic interaction, but these clusters did not show any significant AC magnetic susceptibility under different frequencies and temperatures (Fig. 10b). To reduce the ferromagnetic interaction, the acetate ligands were removed by the addition of acid in acetonitrile solution and formed a Lu2Mn2(FeMn4)2 cluster (IIILu). The octahedral distortion parameter decreases around one of the Mn centers due to the removal of acetate ligands. The cluster shows significant frequency and temperature-dependent AC magnetic susceptibility enhancement with an energy barrier of 29.1 K at zero external DC field (Fig. 10c). Finally, a third family of interest involves the functionalization of polyoxotungstates (POTs) by incorporating organic groups, such as organophosphates (RPVO32−). Various homometallic organophosphate-functionalized lacunary POTs have been reported, including Keggin-type structures like [A-α-XW9(RPO)2]5 (X = PV, AsV), as well as Wells–Dawson-type complexes such as [α2-P2W17(RPO)2]6− and [P4W24O92(C6H5PO)2]16−.146–149 In these compounds, the organophosphate PV centers bind to two oxygen atoms of the POT, one of which is a terminal oxygen atom, while the organic moiety (R) adopts a tetrahedral geometry. This integration combines the inherent properties of the polyanions with the reactive, chiral, and photosensitive characteristics of the organic moieties, resulting in versatile organic–inorganic hybrids. However, comprehensive studies integrating magnetism are currently lacking. One notable example by P. Kogerler and coworkers explored the reactivity of a phenyl phosphonate-decorated mono lacunary Wells–Dawson cluster [a2-PV2 W17VIO61(C6H5PVO)2]6 ({a2-P2W17(PhP)2}) with magnetically anisotropic dysprosium(III) ions.150 The resulting polyanion [{(H2O)7Dy2III (a2, a2′-P2W16O60)(PhPO)2}2]8− comprises dimers of bis-lacunary Wells–Dawson-type units {a2,a2’-P2W16O60} functionalized with both phenyl phosphonate and heterometal (Dy(III)) centers (Fig. 11a and b). Magnetic ac susceptibility data (under an optimal 600 Oe DC field) reveal two distinct slow relaxation processes in different temperature intervals (low-temperature range with Ueff = 17.3 (±2.2) K, and higher temperature range with Ueff = 12 (±1.2) K) (Fig. 11c).
 | ||
| Fig. 10 The molecular structure of IIILu (a), the frequency-dependent out-of-phase magnetic susceptibility plots of IILu (b) and IIILu (c). Color code: cyan octahedron {WO6}, yellow polyhedron Dy unit, blue polyhedron Mn unit, green Fe unit, red O, and grey C. Hydrogen atoms are omitted for clarity. Figures adapted from ref. 145, with permission from Wiley, copyright 2023. | ||
 | ||
| Fig. 11 The structure of the building unit, [a2-P2W17(PhPO)2]6− (a), the molecular structure of [{(H2O)7Dy2 (a2, a2′-P2W16O60)(PhPO)2}2]8− (b), and magnetic relaxation plot of the complex (c). Color code: cyan octahedron {WO6}, green polyhedral Dy, red O, pink P, grey C, white H. Pictures reproduced from ref. 150 with permission from the Royal Society of Chemistry. | ||
Dual heteroatom POT-based SMMs
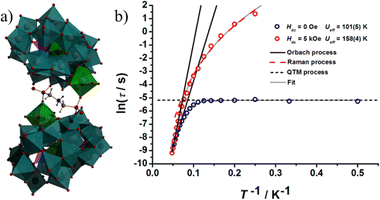
Heteropolyoxotungstates (HPOTs) represent a promising subclass within the polyoxometalates (POMs) family, valued for their unique electronic properties and spatial geometry. The inclusion of heteroatoms (HAs) in POM building blocks significantly impacts the structural formation and intrinsic properties of these materials.35,118 In 2018, Cronin and co-workers advanced polyoxometalate chemistry by reporting cross-shaped clusters with different heteroatoms, laying a foundation for the design of new nanoscale molecular architectures.118 Most lanthanide-containing heteropolyoxometalates (Ln-HPOMs) incorporate a single type of heteroatom. In contrast, those containing two types of heteroatoms are rare due to the differing reactivity and coordination abilities of HAs, making their synthesis challenging. The first dual-HA template HPOT with lanthanides, [Ln3Ni9II(μ3-OH)9(SbW9O33)2(PW9O34)3(CH3COO)3]30− was reported by Kong and co-workers, which included both [SbIIIW9O33]9− and [PVW9O34]9− in the same polyanion.151 More recently, Zhao and co-workers presented the cluster {[Ln4(H2O)18W7O15(H2MA)4][α-SbW9O33]2[HPSbW15O54]2}20−, featuring both Keggin [α-SbW9O33]9− and Dawson [HPSbW15O54]11− units.152 These examples illustrate the potential of utilizing mixed heteroatom templates to design diverse Ln-containing polyoxometalates. In this context, our group has reported two Dy(III)-confined polyoxotungstate complexes [Dy2(H2O)7(W4O9)(HPSeW15O54)(α-SeW9O33)2]14− (complex 7) and {[Dy2(H2O)13W14O40]2[α-SeW9O33]4[HPSeW15O54]2}34− (complex 8) (Fig. 12a and c) using [HPO3]2− and [SeO3]2− in the presence of Dy(III) ions.50 Both complexes exhibit the simultaneous presence of trivacant Keggin [α-SeW9O33]8− and Dawson [HPSeW15O54]10− building blocks, incorporating P(III) and Se(IV) heteroatoms. By varying the reaction medium pH and equivalent ratios of the reactant while using the same reactants, we obtained different structural assemblies. Complex 7 consists of a fused structure where two trivacant Keggin [α-SeW9O33]8− and Dawson [HPSeW15O54]12− units encapsulate a [Dy2(H2O)(W4O9)]12+ cluster. In contrast, complex 8 comprises four trivacant Keggin and two Dawson units, encapsulating a small cluster of isopolyanions [Dy4(H2O)20W14O59]44−. Magnetic studies indicate that complex 7 shows slow relaxation of magnetization, with a phenomenological energy barrier of Ueff = 13.58 K without an external magnetic field (Fig. 12b), which increases to Ueff = 24.57 K under a 500 Oe dc field. In contrast, complex 8 favors quantum tunneling of magnetization (QTM) in the absence of an external field but demonstrates field-induced slow relaxation of magnetization (1500 Oe applied DC field) with an energy barrier of Ueff = 11.11 K (Fig. 12d). The observed differences in energy barriers can be attributed to the symmetries and arrangements of the Dy ions within the clusters. The presence of a high symmetry Dy(III) ion and a slightly tilted anisotropy axis results in the SMM behavior of complex 7. Although the Dy(III) ions in complex 8 exhibit higher symmetries, their zig-zag arrangement within the cluster reduces the overall anisotropy, thus favoring field-induced single-molecule magnet (SMM) behavior. This marks the first mixed heteroatom-based Dy(III)-substituted polyoxotungstate complex exhibiting single-molecule magnet (SMM) behavior. | ||
| Fig. 12 The molecular structures of [Dy2(H2O)7(W4O9)(HPSeW15O54)(α-SeW9O33)2]14− (a) and {[Dy2(H2O)13W14O40]2[α-SeW9O33]4[HPSeW15O54]2}34− (c). Cole–Cole plot of [Dy2(H2O)7(W4O9)(HPSeW15O54)(α-SeW9O33)2]14− (b) and {[Dy2(H2O)13W14O40]2[α-SeW9O33]4[HPSeW15O54]2}34− (d). Color code: cyan, yellow and blue octahedron {WO6}, green Dy, red O, pink P, yellow Se. Figure reprinted (adapted) with permission from ref. 50. Copyright (2024) American Chemical Society. | ||
Conclusions and future outlook
The increasing fascination with Ln-based POMs undoubtedly stems from the compositional variability of these compounds, which exhibit distinctive chemical and physical properties arising from the synergistic interplay between the POM coordinative structures and Ln(III) constituents. This review succinctly outlines the preparation methods for different types of POTs and provides a thorough summary of how the magnetic properties of these Ln-based POTs are influenced by the coordination symmetry surrounding the Ln-ions.The synthesis of POT-based Ln-clusters has been challenging due to the easy hydrolysis of Ln(III) ions and the strong reactivity between Ln and POTs, leading to precipitation and difficult crystallization, as well as coordination competition between different metal ions and POTs, especially for high-nuclearity clusters. To overcome these challenges, a mixed strategy integrating inorganic clusters with organic ligands, anionic template methods, and a slow-release strategy of lanthanide ions may be the most effective approach for the synthesis of more POT-based lanthanide oxo clusters (LnOCs).
Nevertheless, as a crucial aspect of magnetism, it is essential to investigate methods for enhancing the thermal energy barrier and the magnetic blocking temperature of LnOCs, which continues to present a formidable challenge. One method to achieve a significant thermal energy barrier in LnOCs involves adjusting the geometry of Ln(III) ions to enhance their axial symmetry. This can be accomplished through the utilization of a monovacant POT unit in conjunction with a moderately flexible electron donor organic ligand. In addition to this, organic–inorganic LnOCs, featuring Ln(III) ions, can impart magnetic properties alongside luminescent characteristics. Furthermore, POMs can contribute redox and catalytic properties,153,154 and organic co-ligands can add chirality and solubility. Thus, LnOCs offer the potential to combine multiple functionalities within a single molecule.
Future research could also focus on the selective combination of different heteroatoms to construct unusual POM building blocks and enable the design of multi-component lanthanide-based POM derivatives. Combining all component properties in lanthanide-based POMs and studying their effects on magnetic behavior will open new avenues for applications in molecular magnetism.
There is still a long way to go before we can design and manufacture practical Ln-based POMs with unique features, but we should be able to do so in the future and see them used in more research fields.
Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
PKS and SK thank UGC for the SRF fellowship. SK thanks the IISER Bhopal and MoE STARS grant (project no. MoE/STARS/STARS-2/2023-0301) for the financial support.References
- R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CAS.
- A. Zabala-Lekuona, J. M. Seco and E. Colacio, Coord. Chem. Rev., 2021, 441, 213984 CAS.
- S. Goswami, A. K. Mondal and S. Konar, Inorg. Chem. Front., 2015, 2, 687–712 RSC.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CAS.
- M. Mannini, F. Pineider, P. Sainctavit, C. Danieli, E. Otero, C. Sciancalepore, A. M. Talarico, M.-A. Arrio, A. Cornia, D. Gatteschi and R. Sessoli, Nat. Mater., 2009, 8, 194–197 CAS.
- M. A. Hay and C. Boskovic, Chem. – Eur. J., 2021, 27, 3608–3637 CAS.
- S. Thiele, F. Balestro, R. Ballou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Science, 2014, 344, 1135–1138 CAS.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CAS.
- R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328–2341 CAS.
- Y.-S. Meng, S.-D. Jiang, B.-W. Wang and S. Gao, Acc. Chem. Res., 2016, 49, 2381–2389 CAS.
- H.-D. Li, S.-G. Wu and M.-L. Tong, Chem. Commun., 2023, 59, 6159–6170 RSC.
- J.-L. Liu, Y.-C. Chen and M.-L. Tong, Chem. Soc. Rev., 2018, 47, 2431–2453 RSC.
- S. G. McAdams, A.-M. Ariciu, A. K. Kostopoulos, J. P. S. Walsh and F. Tuna, Coord. Chem. Rev., 2017, 346, 216–239 CrossRef CAS.
- P. Kumar Sahu, A. Mondal and S. Konar, Chem. – Eur. J., 2023, 29, e202203664 CrossRef CAS PubMed.
- M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574–7594 CrossRef CAS PubMed.
- P. Kumar Sahu, R. Kharel, S. Shome, S. Goswami and S. Konar, Coord. Chem. Rev., 2023, 475, 214871 CrossRef CAS.
- Y.-F. Deng, T. Han, B. Yin and Y.-Z. Zheng, Inorg. Chem. Front., 2017, 4, 1141–1148 RSC.
- A. B. Canaj, S. Dey, O. Céspedes, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2020, 56, 1533–1536 RSC.
- P. K. Sahu and S. Konar, Inorg. Chim. Acta, 2024, 572, 122255 CrossRef CAS.
- S. T. Liddle and J. V. Slageren, Chem. Soc. Rev., 2015, 44, 6655–6669 CAS.
- P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728–1763 CAS.
- Z. Zhu and J. Tang, Chem. Soc. Rev., 2022, 51, 9469–9481 CAS.
- Z. Zhu and J. Tang, Nat. Sci. Rev., 2022, 9, nwac194 CrossRef PubMed.
- A. Mondal, M. Raizada, P. K. Sahu and S. Konar, Inorg. Chem. Front., 2021, 8, 4625–4633 CAS.
- E. Lowe, C. Wilson, A. B. Canaj and M. Murrie, Dalton Trans., 2025, 54, 477–481 CAS.
- A. Mondal and S. Konar, Chem. – Eur. J., 2021, 27, 3449–3456 CAS.
- A. Mondal and S. Konar, Dalton Trans., 2021, 50, 13666–13670 CAS.
- S. P. Bera, A. Mondal and S. Konar, Inorg. Chem. Front., 2020, 7, 3352–3363 RSC.
- S. P. Bera, A. Mondal and S. Konar, Inorg. Chem. Front., 2020, 7, 3352–3363 RSC.
- Y.-C. Chen and M.-L. Tong, Chem. Sci., 2022, 13, 8716–8726 RSC.
- A. Mondal and S. Konar, Dalton Trans., 2022, 51, 1464–1473 CAS.
- P. Kumar Sahu and S. Konar, Chem. – Eur. J., 2024, 30, e202402439 CAS.
- A. Mondal and S. Konar, Dalton Trans., 2022, 51, 1464–1473 RSC.
- Q. Zheng, M. Kupper, W. Xuan, H. Oki, R. Tsunashima, D.-L. Long and L. Cronin, J. Am. Chem. Soc., 2019, 141, 13479–13486 CAS.
- A. Paul, S. D. Adhikary, S. Kapurwan and S. Konar, J. Mater. Chem. A, 2022, 10, 13152–13169 Search PubMed.
- L. Cronin and A. Müller, Chem. Soc. Rev., 2012, 41, 7333–7334 CAS.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 Search PubMed.
- E. Coronado and C. J. Gómez-García, Chem. Rev., 1998, 98, 273–296 CAS.
- P. Ma, R. Wan, Y. Wang, F. Hu, D. Zhang, J. Niu and J. Wang, Inorg. Chem., 2016, 55, 918–924 Search PubMed.
- A. H. Ismail, B. S. Bassil, G. H. Yassin, B. Keita and U. Kortz, Chem. – Eur. J., 2012, 18, 6163–6166 CAS.
- J. Goura, B. S. Bassil, X. Ma, A. Rajan, E. Moreno-Pineda, J. Schnack, M. Ibrahim, A. K. Powell, M. Ruben, J. Wang, L. Ruhlmann and U. Kortz, Chem. – Eur. J., 2021, 27, 15081–15085 Search PubMed.
- K. Fukaya and T. Yamase, Angew. Chem., Int. Ed., 2003, 42, 654–658 CrossRef CAS PubMed.
- J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Chem. Soc. Rev., 2012, 41, 7464–7478 RSC.
- Z. Li, X.-X. Li, T. Yang, Z.-W. Cai and S.-T. Zheng, Angew. Chem., Int. Ed., 2017, 56, 2664–2669 CrossRef CAS.
- P. K. Sahu, A. Mondal and S. Konar, Dalton Trans., 2021, 50, 3825–3831 RSC.
- W.-C. Chen, C. Qin, X.-L. Wang, Y.-G. Li, H.-Y. Zang, K.-Z. Shao, Z.-M. Su and E.-B. Wang, Dalton Trans., 2015, 44, 11290–11293 RSC.
- M. J. Martínez-Pérez, S. Cardona-Serra, C. Schlegel, F. Moro, P. J. Alonso, H. Prima-García, J. M. Clemente-Juan, M. Evangelisti, A. Gaita-Ariño, J. Sesé, J. van Slageren, E. Coronado and F. Luis, Phys. Rev. Lett., 2012, 108, 247213 CrossRef.
- M. Shiddiq, D. Komijani, Y. Duan, A. Gaita-Ariño, E. Coronado and S. Hill, Nature, 2016, 531, 348–351 CrossRef CAS PubMed.
- S. Kapurwan, P. K. Sahu and S. Konar, Inorg. Chem., 2024, 63, 4492–4501 CrossRef CAS PubMed.
- S. Takahashi, I. S. Tupitsyn, J. van Tol, C. C. Beedle, D. N. Hendrickson and P. C. E. Stamp, Nature, 2011, 476, 76–79 CAS.
- J. M. Clemente-Juan and E. Coronado, Coord. Chem. Rev., 1999, 193–195, 361–394 CAS.
- U. Kortz, A. Müller, J. van Slageren, J. Schnack, N. S. Dalal and M. Dressel, Coord. Chem. Rev., 2009, 253, 2315–2327 CAS.
- P. Kögerler, B. Tsukerblat and A. Müller, Dalton Trans., 2010, 39, 21–36 Search PubMed.
- C. Ritchie, A. Ferguson, H. Nojiri, H. N. Miras, Y.-F. Song, D.-L. Long, E. Burkholder, M. Murrie, P. Kögerler, E. K. Brechin and L. Cronin, Angew. Chem., Int. Ed., 2008, 47, 5609–5612 CAS.
- J.-D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekallé, J. Marrot, F. Sécheresse, E. Rivière, G. Rogez and W. Wernsdorfer, Angew. Chem., Int. Ed., 2009, 48, 3077–3081 CAS.
- Z.-M. Zhang, S. Yao, Y.-G. Li, H.-H. Wu, Y.-H. Wang, M. Rouzières, R. Clérac, Z.-M. Su and E.-B. Wang, Chem. Commun., 2013, 49, 2515–2517 RSC.
- M. Ibrahim, Y. Lan, B. S. Bassil, Y. Xiang, A. Suchopar, A. K. Powell and U. Kortz, Angew. Chem., Int. Ed., 2011, 50, 4708–4711 CrossRef CAS.
- J. J. Baldoví, S. Cardona-Serra, A. Gaita-Ariño and E. Coronado, Adv. Inorg. Chem., 2017, 69, 213–249 CrossRef.
- K. Y. Monakhov, M. Moors and P. Kögerler, Adv. Inorg. Chem., 2017, 69, 251–286 CAS.
- C. Ritchie, M. Speldrich, R. W. Gable, L. Sorace, P. Kögerler and C. Boskovic, Inorg. Chem., 2011, 50, 7004–7014 CAS.
- S. Ghosh, S. Datta, L. Friend, S. Cardona-Serra, A. Gaita-Ariño, E. Coronado and S. Hill, Dalton Trans., 2012, 41, 13697–13704 CAS.
- M. Vonci, M. J. Giansiracusa, W. Van den Heuvel, R. W. Gable, B. Moubaraki, K. S. Murray, D. Yu, R. A. Mole, A. Soncini and C. Boskovic, Inorg. Chem., 2017, 56, 378–394 CAS.
- Z.-X. Yang, X.-W. Liang, D. Lin, Q. Zheng and Y. Huo, Inorg. Chem., 2023, 62, 1466–1475 CAS.
- N. V. Izarova, N. Vankova, A. Banerjee, G. B. Jameson, T. Heine, F. Schinle, O. Hampe and U. Kortz, Angew. Chem., Int. Ed., 2010, 49, 7807–7811 CAS.
- P. Hermosilla-Ibáñez, P. E. Car, A. Vega, J. Costamagna, F. Caruso, J.-Y. Pivan, E. L. Fur, E. Spodine and D. Venegas-Yazigi, Cryst. Eng. Commun., 2012, 14, 5604–5612 RSC.
- S. Cardona-Serra, J. M. Clemente-Juan, A. Gaita-Ariño, N. Suaud, O. Svoboda and E. Coronado, Chem. Commun., 2013, 49, 9621–9623 RSC.
- S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, N. Suaud, O. Svoboda, R. Bastardis, N. Guihéry and J. J. Palacios, Chem. – Eur. J., 2015, 21, 763–769 CAS.
- A. Palii, S. Aldoshin, B. Tsukerblat, J. J. Borràs-Almenar, J. M. Clemente-Juan, S. Cardona-Serra and E. Coronado, Inorg. Chem., 2017, 56, 9547–9554 CAS.
- M. Nicolaou, M. G. Papanikolaou, A. C. Tsipis, T. A. Kabanos, A. D. Keramidas, S. Sproules and H. N. Miras, Chem. – Eur. J., 2018, 24, 3836–3845 CAS.
- P. Wu, Y. Wang, W. Chen, X. Hu, B. Huang and Z. Xiao, Inorg. Chem., 2021, 60, 4347–4351 CAS.
- C. M. Granadeiro, D. Julião, S. O. Ribeiro, L. Cunha-Silva and S. S. Balula, Coord. Chem. Rev., 2023, 476, 214914 CAS.
- Z.-X. Yang, F. Gong, D. Lin and Y. Huo, Coord. Chem. Rev., 2023, 492, 215205 CAS.
- J.-W. Zhao, Y.-Z. Li, L.-J. Chen and G.-Y. Yang, Chem. Commun., 2016, 52, 4418–4445 CAS.
- A. Bijelic, M. Aureliano and A. Rompel, Chem. Commun., 2018, 54, 1153–1169 CAS.
- S.-R. Li, W.-D. Liu, L.-S. Long, L.-S. Zheng and X.-J. Kong, Polyoxometalates, 2023, 2, 9140022 Search PubMed.
- D. Lockey, C. Mathis, H. N. Miras and L. Cronin, Matter, 2022, 5, 302–313 CrossRef CAS.
- N. L. Bell, M. Kupper and L. Cronin, Chem. Mater., 2021, 33, 7263–7271 CrossRef CAS.
- M. T.-K. Ng, N. L. Bell, D.-L. Long and L. Cronin, J. Am. Chem. Soc., 2021, 143, 20059–20063 CAS.
- S. She, N. L. Bell, D. Zheng, J. S. Mathieson, M. D. Castro, D.-L. Long, J. Koehnke and L. Cronin, Chem, 2022, 8, 2734–2748 CAS.
- T. Minato, D. Salley, N. Mizuno, K. Yamaguchi, L. Cronin and K. Suzuki, J. Am. Chem. Soc., 2021, 143, 12809–12816 CAS.
- Y. Chen, F. Li, S. Li, L. Zhang and M. Sun, Inorg. Chem. Commun., 2022, 135, 109084 CAS.
- Y. Zhou, G. Chen, Z. Long and J. Wang, RSC Adv., 2014, 4, 42092–42113 CAS.
- D. Wang, L. Liu, J. Jiang, L. Chen and J. Zhao, Nanoscale, 2020, 12, 5705–5718 CAS.
- B. Huang, D.-H. Yang and B.-H. Han, J. Mater. Chem. A, 2020, 8, 4593–4628 RSC.
- N. I. Gumerova and A. Rompel, Nat. Rev. Chem., 2018, 2, 0112 CrossRef CAS.
- J. Lehmann, A. Gaita-Ari
![[n with combining macron]](https://www.rsc.org/images/entities/char_006e_0304.gif) o, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312–317 CrossRef CAS.
o, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312–317 CrossRef CAS. - D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CAS.
- M. Ammam, J. Mater. Chem. A, 2013, 1, 6291–6312 CAS.
- Y. Gao, M. Choudhari, G. K. Such and C. Ritchie, Chem. Sci., 2022, 13, 2510–2527 CAS.
- N. I. Gumerova and A. Rompel, Sci. Adv., 2023, 9, eadi0814 CAS.
- M. A. AlDamen, J. M. Clemente-Juan, E. Coronado, C. Martí-Gastaldo and A. Gaita-Ariño, J. Am. Chem. Soc., 2008, 130, 8874–8875 CAS.
- O. Y. Mariichak, S. Kaabel, Y. A. Karpichev, G. M. Rozantsev, S. V. Radio, C. Pichon, H. Bolvin and J.-P. Sutter, Magnetochemistry, 2020, 6, 53 CAS.
- J. Flores Gonzalez, V. Montigaud, V. Dorcet, K. Bernot, B. Le Guennic, F. Pointillart and O. Cador, Chem. – Eur. J., 2021, 27, 10160–10168 CAS.
- M. Ibrahim, V. Mereacre, N. Leblanc, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem. Int. Ed., 2015, 54, 15574–15578 CAS.
- J. Xiong, Z.-X. Yang, P. Ma, D. Lin, Q. Zheng and Y. Huo, Inorg. Chem., 2021, 60, 7519–7526 CrossRef CAS PubMed.
- S. Kapurwan, A. Mondal, P. K. Sahu and S. Konar, Inorg. Chem., 2022, 61, 17459–17468 CrossRef CAS PubMed.
- R. Sato, K. Suzuki, T. Minato, K. Yamaguchi and N. Mizuno, Inorg. Chem., 2016, 55, 2023–2029 CrossRef CAS PubMed.
- S. Kapurwan, P. K. Sahu and S. Konar, Cryst. Growth Des., 2024, 24, 6300–6310 CAS.
- E. Tanuhadi, E. Al-Sayed, G. Novitchi, A. Roller, G. Giester and A. Rompel, Inorg. Chem., 2020, 59, 8461–8467 CAS.
- Y. Huo, Y.-C. Chen, J.-L. Liu, J.-H. Jia, W.-B. Chen, S.-G. Wu and M.-L. Tong, Dalton Trans., 2017, 46, 16796–16801 CAS.
- S. Kapurwan, P. K. Sahu, M. Raizada, R. Kharel and S. Konar, Dalton Trans., 2023, 52, 9377–9388 Search PubMed.
- Y. Nakagawa, K. Uehara and N. Mizuno, Inorg. Chem., 2005, 44, 9068–9075 CAS.
- K. Uehara, T. Taketsugu, K. Yonehara and N. Mizuno, Inorg. Chem., 2013, 52, 1133–1140 CAS.
- L.-X. Shi, W.-F. Zhao, X. Xu, J. Tang and C.-D. Wu, Inorg. Chem., 2011, 50, 12387–12389 CAS.
- E. Ruiz-Bilbao, A. Iturrospe, S. Reinoso, B. Artetxe, G. Beobide, L. San Felices, L. Lezama, J. M. Gutiérrez-Zorrilla, S. Darwish, D. Sensharma and M. J. Zaworotko, Angew. Chem., Int. Ed., 2023, 62, e202307436 Search PubMed.
- K. Suzuki, R. Sato and N. Mizuno, Chem. Sci., 2013, 4, 596–600 Search PubMed.
- R. Sato, K. Suzuki, M. Sugawa and N. Mizuno, Chem. – Eur. J., 2013, 19, 12982–12990 CAS.
- K.-Y. Wang, B. S. Bassil, Z. Lin, I. Römer, S. Vanhaecht, T. N. Parac-Vogt, C. Sáenz de Pipaón, J. R. Galán-Mascarós, L. Fan, J. Cao and U. Kortz, Chem. – Eur. J., 2015, 21, 18168–18176 CrossRef CAS.
- Y.-J. Wang, S.-Y. Wu, Y.-Q. Sun, X.-X. Li and S.-T. Zheng, Chem. Commun., 2019, 55, 2857–2860 RSC.
- L.-Z. Han, C.-Q. Jiao, W.-C. Chen, K.-Z. Shao, L.-Y. Jin and Z.-M. Su, Dalton Trans., 2021, 50, 11535–11541 RSC.
- P. Mialane, L. Lisnard, A. Mallard, J. Marrot, E. Antic-Fidancev, P. Aschehoug, D. Vivien and F. Sécheresse, Inorg. Chem., 2003, 42, 2102–2108 CrossRef CAS.
- M. A. AlDamen, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, C. Martí-Gastaldo, F. Luis and O. Montero, Inorg. Chem., 2009, 48, 3467–3479 CAS.
- A. S. Mougharbel, S. Bhattacharya, B. S. Bassil, A. Rubab, J. van Leusen, P. Kögerler, J. Wojciechowski and U. Kortz, Inorg. Chem., 2020, 59, 4340–4348 CrossRef CAS PubMed.
- M. Ibrahim, I. M. Mbomekallé, P. de Oliveira, A. Baksi, A. B. Carter, Y. Peng, T. Bergfeldt, S. Malik and C. E. Anson, ACS Omega, 2019, 4, 21873–21882 CAS.
- Y. Huo, Y.-C. Chen, S.-G. Wu, J.-L. Liu, J.-H. Jia, W.-B. Chen, B.-L. Wang, Y.-Q. Zhang and M.-L. Tong, Inorg. Chem., 2019, 58, 1301–1308 CAS.
- M. H. Alizadeh, S. P. Harmalker, Y. Jeannin, J. Martin-Frere and M. T. Pope, J. Am. Chem. Soc., 1985, 107, 2662–2669 CAS.
- Q. Zheng, L. Vilà-Nadal, Z. Lang, J.-J. Chen, D.-L. Long, J. S. Mathieson, J. M. Poblet and L. Cronin, J. Am. Chem. Soc., 2018, 140, 2595–2601 CAS.
- J. A. Fernández, X. López, C. Bo, C. de Graaf, E. J. Baerends and J. M. Poblet, J. Am. Chem. Soc., 2007, 129, 12244–12253 Search PubMed.
- S. G. Mitchell, T. Boyd, H. N. Miras, D.-L. Long and L. Cronin, Inorg. Chem., 2011, 50, 136–143 CAS.
- C.-H. Zhan, R. S. Winter, Q. Zheng, J. Yan, J. M. Cameron, D.-L. Long and L. Cronin, Angew. Chem., Int. Ed., 2015, 54, 14308–14312 CrossRef CAS.
- M. Stuckart, N. V. Izarova, M. Glöβ, J. Klose, C. Schmitz-Antoniak, P. Kögerler, B. Kersting and K. Y. Monakhov, Inorg. Chem., 2021, 60, 8437–8441 CrossRef CAS PubMed.
- T. Iftikhar, N. V. Izarova, J. van Leusen and P. Kögerler, Chem. – Eur. J., 2021, 27, 8500–8508 CrossRef CAS PubMed.
- M. Zhu, T. Iwano, M. Tan, D. Akutsu, S. Uchida, G. Chen and X. Fang, Angew. Chem., Int. Ed., 2022, 61, e202200666 CrossRef CAS PubMed.
- Y.-L. Wu, X.-X. Li, Y.-J. Qi, H. Yu, L. Jin and S.-T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572–8576 CrossRef CAS.
- S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, A. Camón, M. Evangelisti, F. Luis, M. J. Martínez-Pérez and J. Sesé, J. Am. Chem. Soc., 2012, 134, 14982–14990 Search PubMed.
- T. Iftikhar, N. V. Izarova, J. van Leusen and P. Kögerler, Chem. – Eur. J., 2021, 27, 13376–13383 CrossRef CAS.
- W. Guan, G. Wang, B. Li and L. Wu, Coord. Chem. Rev., 2023, 481, 215039 CrossRef CAS.
- W. Cañón-Mancisidor, G. Paredes-Castillo, P. Hermosilla-Ibáñez, D. Venegas-Yazigi, O. Cador, B. Le Guennic and F. Pointillart, Eur. J. Inorg. Chem., 2021, 4596–4609 CrossRef.
- S. Reinoso, Dalton Trans., 2011, 40, 6610–6615 RSC.
- J. Wang, W. Shi, S. Li, Q. Mao, P. Ma, J. Niu and J. Wang, Dalton Trans., 2018, 47, 7949–7955 RSC.
- H. An, Z. Han and T. Xu, Inorg. Chem., 2010, 49, 11403–11414 CrossRef CAS.
- X.-M. Luo, N.-F. Li, Z.-B. Hu, J.-P. Cao, C.-H. Cui, Q.-F. Lin and Y. Xu, Inorg. Chem., 2019, 58, 2463–2470 CAS.
- C. Ritchie, M. Speldrich, R. W. Gable, L. Sorace, P. Kögerler and C. Boskovic, Inorg. Chem., 2011, 50, 7004–7014 CrossRef CAS PubMed.
- P. Ma, F. Hu, R. Wan, Y. Huo, D. Zhang, J. Niu and J. Wang, J. Mater. Chem. C, 2016, 4, 5424–5433 RSC.
- Y. Huo, R. Wan, P. Ma, J. Liu, Y. Chen, D. Li, J. Niu, J. Wang and M.-L. Tong, Inorg. Chem., 2017, 56, 12687–12691 CAS.
- Y. Huo, Y.-C. Chen, S.-G. Wu, J.-H. Jia, W.-B. Chen, J.-L. Liu and M.-L. Tong, Inorg. Chem., 2018, 57, 6773–6777 CAS.
- H. Kong, Z.-Y. Ruan, Y.-C. Chen, W. Deng, P.-Y. Liao, S.-G. Wu and M.-L. Tong, Inorg. Chem., 2024, 63, 15964–15972 CAS.
- I. Werner, J. Griebel, A. Masip-Sánchez, X. López, K. Załęski, P. Kozłowski, A. Kahnt, M. Boerner, Z. Warneke, J. Warneke and K. Y. Monakhov, Inorg. Chem., 2023, 62, 3761–3775 CAS.
- Y. Jiao, S. Sanz, N. V. Izarova, J. van Leusen, S. Sarwar, S. J. Dalgarno, E. K. Brechin and P. Kögerler, Dalton Trans., 2022, 51, 5409–5413 CAS.
- E. Ruiz-Bilbao, M. Pardo-Almanza, I. Oyarzabal, B. Artetxe, L. S. Felices, J. A. García, J. M. Seco, E. Colacio, L. Lezama and J. M. Gutiérrez-Zorrilla, Inorg. Chem., 2022, 61, 2428–2443 CAS.
- S. Sarwar, S. Sanz, J. van Leusen, G. S. Nichol, E. K. Brechin and P. Kögerler, Dalton Trans., 2020, 49, 16638–16642 CAS.
- Y.-N. Gu, D. Zhao, H. Yu, R. Ge, Z. Li, C.-B. Tian, X.-X. Li, Y.-Q. Sun and S.-T. Zheng, RSC Adv., 2019, 9, 13543–13549 CAS.
- S.-Y. Wu, Y.-J. Wang, J.-X. Jing, X.-X. Li, Y.-Q. Sun and S.-T. Zheng, Inorg. Chem. Front., 2020, 7, 498–504 RSC.
- T. Minato and M. Sadakane, Angew. Chem. Int. Ed., 2023, 62, e202309469 CrossRef CAS.
- X. Yi, N. V. Izarova and P. Kögerler, Chem. Commun., 2018, 54, 2216–2219 RSC.
- A. V. Anyushin, A. Kondinski and T. N. Parac-Vogt, Chem. Soc. Rev., 2020, 49, 382–432 CAS.
- E. Hampson, J. M. Cameron, S. Amin, J. Kyo, J. A. Watts, H. Oshio and G. N. Newton, Angew. Chem., Int. Ed., 2019, 58, 18281–18285 CAS.
- J. M. Cameron, S. Fujimoto, K. Kastner, R.-J. Wei, D. Robinson, V. Sans, G. N. Newton and H. H. Oshio, Chem. – Eur. J., 2017, 23, 47–50 CAS.
- W. Wang, N. V. Izarova, J. van Leusen and P. Kögerler, Chem. Commun., 2020, 56, 14857–14860 Search PubMed.
- J. Cai, X.-Y. Zheng, J. Xie, Z.-H. Yan, X.-J. Kong, Y.-P. Ren, L.-S. Long and L.-S. Zheng, Inorg. Chem., 2017, 56, 8439–8445 CAS.
- S. Xie, D. Wang, Z. Wang, J. Liu, L. Chen and J. Zhao, Inorg. Chem. Front., 2022, 9, 350–362 RSC.
- H. Kong, J.-Y. Wang, J.-C. Liu, L. Zhang, P.-Y. Liao, Y.-Q. Qi, Z. Liu, S.-G. Wu and M.-L. Tong, Angew. Chem. Int. Ed., 2025, e202422557 Search PubMed.
- D.-H. Li, X.-Y. Zhang, J.-Q. Lv, P.-W. Cai, Y.-Q. Sun, C. Sun and S.-T. Zheng, Angew. Chem. Int. Ed., 2023, 62, e202312706 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc00732a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |




