Laser synthesis of amorphous CoSx nanospheres for efficient hydrogen evolution and nitrogen reduction reactions†
Lili
Zhao‡
a,
Bin
Chang‡
 ad,
Tianjiao
Dong‡
a,
Haifeng
Yuan
a,
Yue
Li
a,
Zhenfei
Tang
a,
Zhen
Liu
a,
Hong
Liu
ad,
Tianjiao
Dong‡
a,
Haifeng
Yuan
a,
Yue
Li
a,
Zhenfei
Tang
a,
Zhen
Liu
a,
Hong
Liu
 ab,
Xiaoli
Zhang
ab,
Xiaoli
Zhang
 c and
Weijia
Zhou
c and
Weijia
Zhou
 *a
*a
aInstitute for Advanced Interdisciplinary Research (iAIR), School of Chemistry and Chemical Engineering, University of Jinan, Jinan, 250022, P. R. China. E-mail: ifc_zhouwj@ujn.edu.cn
bState Key Laboratory of Crystal Materials, Shandong University, Jinan, 250100, P. R. China
cSchool of Materials Science and Engineering, Zhengzhou University, Zhengzhou, 450001, P. R. China
dState Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, 350116, P. R. China
First published on 9th July 2022
Abstract
Transition metal sulfides (TMSs) have been demonstrated to be excellent electrode materials for various applications of electrochemical energy conversion and storage. However, the synthesis of TMSs through conventional approaches commonly suffers from toxic or environmentally unfriendly reagents as well as time consuming procedures, high thermal power and energy loss. Herein, CoSx nanospheres with uniform distribution and strong adhesion on carbon fiber cloths (CoSx/CC-L) are synthesized via the confined laser temperature field under a H2S atmosphere under normal temperature and pressure conditions. It is demonstrated that the high pressure field induced by a laser yields amorphous CoSx containing numerous S vacancies, which could provide more active sites for enhancing the hydrogen evolution reaction (HER) and nitrogen reduction reaction (NRR). The as-prepared CoSx/CC-L exhibits a low overpotential of ∼87 mV at 10 mA cm−2 for the HER in 0.5 M H2SO4 aqueous electrolyte and maintains a stable catalytic performance for 15 h with a high current density of 650 mA cm−2. In addition, a high ammonia (NH3) production rate (12.2 μg h−1 cmcat−2) and Faraday efficiency (10.1%) of CoSx/CC-L at −0.2 V vs. RHE in a neutral 0.05 M Na2SO4 electrolyte were obtained. The good proton activation and nitrogen adsorption abilities arising from the sulfur vacancies contribute to the active nitrogen association/hydrogenation process during the NRR. The laser synthesis provides an alternative process to produce efficient amorphous catalysts, which are expected to be promising HER and NRR electrocatalysts for energy conversion applications.
Introduction
The production of renewable hydrogen (H2) fuel from water splitting and NH3 from the nitrogen reduction reaction is attractive as a promising and vibrant strategy for green energy storage.1,2 The HER for electrochemical water splitting and the NRR for electrochemical nitrogen fixation are of great significance, which are highly dependent on electrocatalytic materials with excellent catalytic activity.3–5 Currently, precious metals are the most excellent electrocatalysts for electrochemical ammonia and hydrogen production, especially palladium-based nanomaterials for the NRR6 and platinum-based nanomaterials for the HER7 due to their moderate electronic structure to adsorb intermediates. However, the high cost and insufficient reserves of precious metals have severely restricted their practical applications. The development of low-cost, earth-abundant and non-precious electrocatalysts is significant for achieving large-scale hydrogen and ammonia production. TMSs have been widely employed as non-precious electrocatalysts for efficient water splitting and nitrogen reduction reactions. In the past decade, a variety of approaches have been performed for the synthesis of TMSs, including the hydrothermal method,8 the coprecipitation method,9 the sol–gel method10 and the high-temperature calcination process.11 Nevertheless, the strategies through these conventional approaches commonly suffer from toxic or environmentally unfriendly reagents and some issues of high thermal power, long synthesis time and energy loss. Thus, new technologies for large-scale production of TMSs with specific structures are necessary.As an alternative candidate, laser synthesis has been reported to produce diverse nanomaterials with advantages of being cost-effective, time-saving, non-contact, maskless and environmentally friendly. The laser processing for material synthesis is realized by the photothermal reaction through an irradiated laser generating a confined temperature field at the desired position. Tour et al.12 used a laser direct-write process to fabricate the HER and oxygen evolution reaction (OER) electrocatalytic electrodes on opposite faces of a commercial polyimide (PI) sheet for overall water splitting, where the impregnation of a PI film in a Pt ion solution and subsequent laser-induced graphene (LIG) forming process were utilized and efficient HER and OER activities were demonstrated. It was noteworthy that these strategies for laser synthesis of electrocatalysts were primarily performed to obtain oxides,13 carbides14 and LIG-based materials.15,16 For laser-induced TMSs, laser synthesis in liquid was the most common method.17,18 For instance, through nanosecond pulsed laser processing of an aqueous precursor solution, nickel sulfide with excellent electrocatalytic HER activity (−159 mV vs. RHE at 10 A g−1) and long-term durability were achieved.19 However, the TMSs synthesized by this method were primarily in powder-like form and the three-dimensional electrode with a large size was difficult to achieve. Therefore, how to expand the variety of catalysts and fabricate three-dimensional electrodes without a binder by laser synthesis will take more effort.
In this work, we demonstrated a facile approach toward the fabrication of CoSx nanospheres anchored on carbon fiber cloths (CoSx/CC-L) by using laser ablation of a Co-MOF grown on carbon fiber cloths (Co-MOF/CC) under a H2S atmosphere. Herein, H2S as the gas sulfur source reacted with Co-MOF/CC under the confined temperature field generated by a laser, which was a safe and green method without high temperature and high pressure. In particular, the CoSx/CC-L with a large size of 10 cm × 10 cm was prepared within a short time of 16 min, which was directly used as a multifunctional electrode for the HER in acidic electrolyte and NRR in neutral electrolyte. As the HER electrode, CoSx/CC-L exhibited a low overpotential (∼87 mV at 10 mA cm−2) in 0.5 M H2SO4 aqueous electrolyte. Meanwhile, owing to its excellent proton activation ability and amorphous structure, the CoSx/CC-L sample also exhibited a high rate of NH3 production of 12.2 μg h−1 cmcat−2 and Faraday efficiency of 10.1% at −0.2 V vs. RHE for the NRR. The presence of S vacancies in amorphous CoSx/CC-L due to the high pressure field induced by laser processing provided more active sites and then enhanced the HER and NRR activities.
Results and discussion
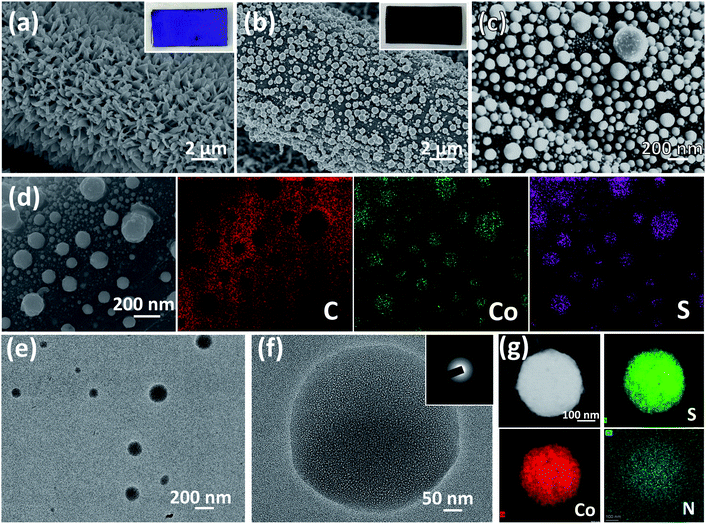
The schematic illustration of the fabrication process of CoSx/CC-L is shown in Scheme 1. It was noteworthy that the as-prepared CoSx/CC-L possessed good flexibility (Fig. S2†), and could be easily fabricated in a large scale, such as 10 cm × 10 cm (Fig. S3†) within 16 min, which was attributed to the time-saving advantage of the laser processing technique. The rapid processing and normal temperature conditions (Fig. S4†) were beneficial in reducing the emission of H2S gas. The Co-MOF (ZIF-67) was first grown on the CC by immersing the CC into Co2+ and 2-methylimidazole mixed solution (Scheme 1a). As shown in Fig. 1a and S5,† the field emission scanning electron microscopy (SEM) images of Co-MOF/CC displayed uniform and compact Co-MOF nanosheets grown on the CC. X-ray diffraction (XRD) patterns confirmed the formation of ZIF-67 (ref. 20) (Fig. S6a†). In addition, the other peaks at approximately 25.3° and 43.3° corresponded to (002) and (100) facets of graphited carbon (JCPDS no. 41-1487). The laser synthesis of CoSx/CC-L under a H2S atmosphere was performed in a chamber with a light transmittable quartz window and valves for the inlet and outlet of gas (Scheme 1b). Before the laser irradiation, the chamber was bubbled with H2S/Ar to remove the air. After pulsed laser irradiation, the sulfuration reaction between H2S and the Co-MOF was carried out. Because of the high and confined power generated by the laser, the Co-MOF sputtered and reacted with H2S to be deposited onto the surface of CC (Scheme 1c). As observed in the SEM images shown in Fig. 1b and c, the CoSx nanospheres with a diameter of 100–300 nm were evenly anchored on the surface of CC. Due to the decomposition of the Co-MOF grown on CC, a vast number of holes were formed on the surface of the CC (Fig. S7†), which was favorable for the anchoring of CoSx nanospheres on CC with strong adhesion. The energy dispersive X-ray spectroscopy (EDS) mapping results revealed that a large number of CoSx nanospheres are anchored on the surface of CC and the uniform distribution of Co and S elements of CoSx nanospheres can be clearly identified (Fig. 1d). A low EDS signal for the N element was also detected (Fig. S6b and S8†). However, the corresponding XRD pattern for CoSx/CC-L did not exhibit apparent diffraction peaks of CoS2, except for those of the carbon substrate and MOF-derived C11H10 (Fig. S6a†), verifying the formation of amorphous CoSx. Additionally, the transmission electron microscopy (TEM) images (Fig. 1e and f) showed that no lattice fringes were observed, and a typical halo around the bright spot in the center of amorphous materials was detected from the selected area electron diffraction pattern (SAED) (inset of Fig. 1f). These results further demonstrated that the as-obtained CoSx possessed an amorphous nature. The elemental mapping results (Fig. 1g) showed that the S and Co elements were uniformly distributed in a single CoSx nanosphere and the molar ratio of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S was about 1
S was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15, which was obtained from the EDS results during TEM measurement and was determined according to the spectral peak area and correction factor. The relatively weak N element signal was also detected in the whole region including CoSx nanospheres and the surrounding carbon fiber, implying that N-doped carbon and N-doped CoSx were formed, consistent with the XPS results (Fig. S9c†).
1.15, which was obtained from the EDS results during TEM measurement and was determined according to the spectral peak area and correction factor. The relatively weak N element signal was also detected in the whole region including CoSx nanospheres and the surrounding carbon fiber, implying that N-doped carbon and N-doped CoSx were formed, consistent with the XPS results (Fig. S9c†).
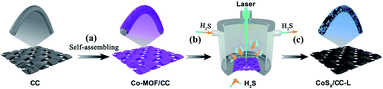 | ||
| Scheme 1 Schematic illustration of the fabrication process of CoSx/CC-L using a laser combined with a H2S atmosphere (b and c) from Co-MOF/CC (a). | ||
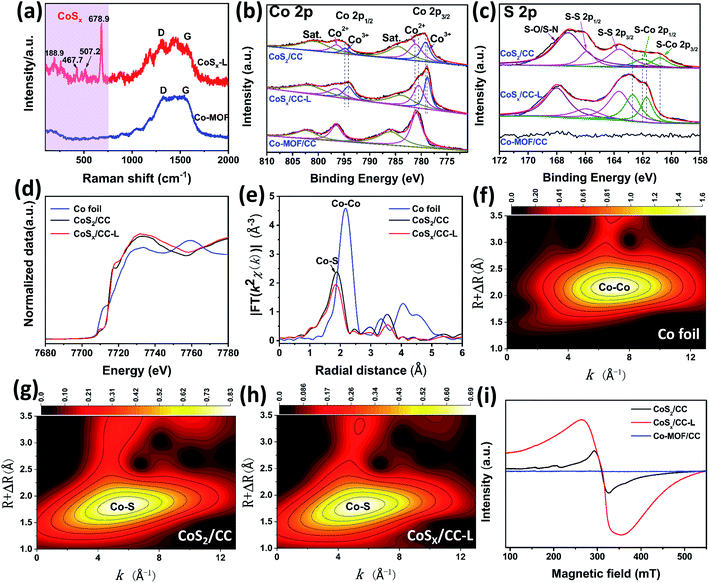
In order to further confirm the chemical composition of the as-prepared CoSx/CC-L, Raman spectra and X-ray photoelectron spectroscopy (XPS) spectra were evaluated. Raman spectra of CoSx/CC-L (Fig. 2a) showed that the peaks located at 188.9, 467.7, 507.2, and 678.9 cm−1 could be assigned to the characteristic peaks of CoSx.21,22 Two additional peaks appearing at 1335 cm−1 and 1590 cm−1 could be indexed to defective carbon (D band) and sp2-graphitic carbon (G band), respectively. Thus, the above results could verify the formation of CoSx from the Co-MOF on CC. As depicted by the survey XPS spectra in Fig. S9a,† Co, S, C, N and O were detected in CoSx/CC-L and CoS2/CC. For the Co-MOF/CC, no S element appeared. Fig. 2b shows the high-resolution Co 2p spectra of the three samples. The Co 2p spectrum of Co-MOF/CC is composed of only Co2+ 2p3/2 and 2p1/2 at 781.1 eV and 796.6 eV, respectively, and the corresponding satellite peaks. For CoSx/CC-L, three strong peaks were deconvoluted for each Co 2p3/2 and Co 2p1/2: the peaks centered at 778.8 and 794.2 eV were ascribed to Co3+ and those at 780.7 and 796.6 eV to Co2+, while the corresponding satellite peaks appeared at 784.1 and 801.9 eV. The results suggested that mixed Co2+ and Co3+ oxidation states were formed in CoSx/CC-L.23,24 With regard to the S 2p high-resolution spectrum of CoSx/CC-L (Fig. 2c), the characteristic peaks at 161.7 eV, 162.7 eV, 163.6 eV, 166.2 eV, and 168.0 eV are attributed to S–Co 2p3/2, S–Co 2p1/2, S–S 2p3/2, S–S 2p1/2 and S–O/S–N bonding,25,26 respectively. The coexistence of Co and S elements confirmed the formation of CoSx in the CoSx/CC-L sample.
For comparison, crystallographic cobalt sulfide on CC was synthesized by calcination of Co-MOF/CC under a H2S atmosphere at 600 °C, and the corresponding XRD pattern and SEM images of the as-obtained CoS2/CC are shown in Fig. S10 and S11.† The as-obtained CoS2/CC showed well-defined peaks corresponding to (111), (200), (210), (211), (220), (311), (222), (023) and (321) planes of CoS2 (PDF 70-2865), which demonstrated that crystalline cobalt sulfide was obtained (Fig. S10†). In addition, the SEM image in Fig. S11† showed that the obtained CoS2 on CC had irregular morphology, which also confirmed the advantage of laser synthesis. Furthermore, the XPS results (Fig. 2b, c and S9†) revealed a negative shift of Co 2p and positive shift of S–Co for CoSx/CC-L compared with CoS2/CC, which verified the higher density of Co electronic states in CoSx/CC-L. This suggested that there were more S vacancies in CoSx/CC-L due to the high pressure field induced by laser processing, which was consistent with the XRD result (Fig. S6a and S10†). The high-resolution spectrum of C 1s for CoSx/CC-L could be divided into four peaks after peak de-convolution (Fig. S9b†), corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–S/C–N, C–C/C
O, C–S/C–N, C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–Co bonding at 287.1 eV, 285.4 eV, 284.5 eV and 281.9 eV,26 respectively. This result suggested that CoSx nanospheres anchored on the surface of CC with a strong bonding interaction, which was beneficial to the fast electron transfer from CC to CoSx nanospheres. The high-resolution spectrum of N 1s for CoSx/CC-L could be divided into two main peaks corresponding to the pyridinic and graphitic nitrogen (Fig. S9c†). Combined with the EDS results for the N element, it was confirmed that nitrogen mainly existed in the form of a small amount of dopant. The high-resolution spectrum of O 1s for CoSx/CC-L showed that the lattice oxygen (OL) peak at 529.6 eV attributed to O–Co and adsorbed oxygen (OAd) peak at 531.2 eV attributed to the absorbed oxygen at the S vacancy site were observed, which came from surface oxidation of CoSx. The laser preparation process resulted in less surface oxidation of CoSx compared with that of CoS2 (Fig. S9d†).
C and C–Co bonding at 287.1 eV, 285.4 eV, 284.5 eV and 281.9 eV,26 respectively. This result suggested that CoSx nanospheres anchored on the surface of CC with a strong bonding interaction, which was beneficial to the fast electron transfer from CC to CoSx nanospheres. The high-resolution spectrum of N 1s for CoSx/CC-L could be divided into two main peaks corresponding to the pyridinic and graphitic nitrogen (Fig. S9c†). Combined with the EDS results for the N element, it was confirmed that nitrogen mainly existed in the form of a small amount of dopant. The high-resolution spectrum of O 1s for CoSx/CC-L showed that the lattice oxygen (OL) peak at 529.6 eV attributed to O–Co and adsorbed oxygen (OAd) peak at 531.2 eV attributed to the absorbed oxygen at the S vacancy site were observed, which came from surface oxidation of CoSx. The laser preparation process resulted in less surface oxidation of CoSx compared with that of CoS2 (Fig. S9d†).
For further gaining more accurate structural information about the coordination environments of cobalt atoms in CoSx/CC-L, X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy were performed for CoSx/CC-L, CoS2/CC and Co foil. As shown in Fig. 2d, the Co K-edge XANES spectrum of CoSx/CC-L exhibited a 1s → 3d pre-edge peak at 7710.1 eV. Additionally, the highly similar spectrum variation of the Co K-edge XANES of CoSx/CC-L and CoS2/CC indicated that they possessed a similar cobalt coordination environment. The Co EXAFS data was further checked to achieve the quantitative information about bond lengths, coordination numbers and structural disorder. As shown in Fig. 2e, the Fourier transform (FT) of the k2-weighted Co EXAFS spectrum of CoSx/CC-L gave the Co–S coordination information while no Co–Co coordination was detected. Furthermore, the Co K-edge WT-EXAFS of these three samples (Fig. 2f–h) showed that the peak signal for CoSx/CC-L was highly similar to that for CoS2/CC, indicating the existence of a Co–S signal in CoSx/CC-L. The Co–S distance at 1.86 Å (without phase correction) was resolved from the FT analysis. It was noteworthy that the fitting coordination number of Co–S in CoSx/CC-L was slightly lower than that in CoS2/CC (Table S1†), possibly because of the amorphous nature of CoSx in CoSx/CC-L that creates numerous S vacancies from laser processing. These above results further demonstrated the formation of amorphous CoSx.
Since the elemental mapping of CoSx nanoparticles in Fig. 1g implied the molar ratio of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S was about 1
S was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15, the sulfur vacancies should be present. Therefore, the electron paramagnetic resonance (EPR) spectra of Co-MOF/CC, CoSx/CC-L and CoS2/CC were applied to verify the existence of S vacancies in CoSx/CC-L. As shown in Fig. 2i, CoSx/CC-L exhibited the EPR signals at g = 2.001, which was attributed to the trapped electrons from the S vacancies.27 The Co-MOF/CC sample exhibited a very low signal at the same position, demonstrating that S vacancies have been formed in CoSx/CC-L. In addition, the CoSx/CC-L presented a much stronger EPR signal than CoS2/CC, confirming the existence of more sulfur vacancies in CoSx/CC-L, induced by the high pressure field during laser processing, which could provide more active sites and thus enhance the HER and NRR activities.28
1.15, the sulfur vacancies should be present. Therefore, the electron paramagnetic resonance (EPR) spectra of Co-MOF/CC, CoSx/CC-L and CoS2/CC were applied to verify the existence of S vacancies in CoSx/CC-L. As shown in Fig. 2i, CoSx/CC-L exhibited the EPR signals at g = 2.001, which was attributed to the trapped electrons from the S vacancies.27 The Co-MOF/CC sample exhibited a very low signal at the same position, demonstrating that S vacancies have been formed in CoSx/CC-L. In addition, the CoSx/CC-L presented a much stronger EPR signal than CoS2/CC, confirming the existence of more sulfur vacancies in CoSx/CC-L, induced by the high pressure field during laser processing, which could provide more active sites and thus enhance the HER and NRR activities.28
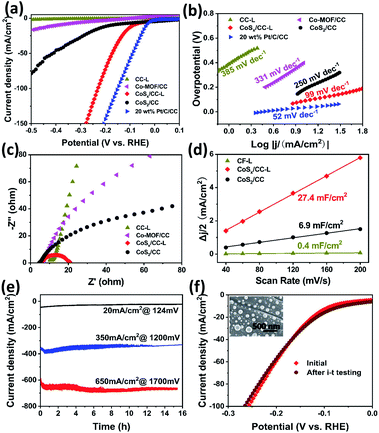
The HER performance of the CoSx/CC-L and control samples of CC-L (SEM image in Fig. S12†), Co-MOF/CC and CoS2/CC, was measured in a standard three-electrode configuration with IR-compensation to exclude the influence of the series resistance (Rs) arising from the solution, electrochemical workstation and electrodes in 0.5 M H2SO4 aqueous electrolyte. As shown in Fig. 3a, the CC processed by the laser under a H2S atmosphere (CC-L) displayed almost no HER performance. CoSx/CC-L (with an overpotential of ∼87 mV at 10 mA cm−2) exhibited a much better HER performance than CC-L and Co-MOF/CC (∼396 mV at 10 mA cm−2), indicating that the formed CoSx nanospheres provided the active species for the HER. This could also be confirmed by the fact that H2 bubbles were mainly generated on the area where the laser has processed (Video S1†). In addition, it is noteworthy that, although with the same composition phase as that of cobalt sulfide, the current density of CoSx/CC-L was much higher than that of CoS2/CC (with an overpotential of ∼178 mV at 10 mA cm−2), implying that the abundant sulfur vacancies in CoSx/CC-L were beneficial to enhance the HER performance, which is in agreement with the XPS and EPR spectra (Fig. 2). However, the overpotential value (∼87 mV) required to obtain a current density of 10 mA cm−2 was still poorer than that of commercial 20 wt% Pt/C (∼35 mV). For driving a current density of 100 mA cm−2, CoSx/CC-L and 20 wt% Pt/C required an overpotential of 229 mV and 150 mV, respectively. Through comparison with the other cobalt sulfide-based electrocatalysts, the HER activity of CoSx/CC-L with a low overpotential of 87 mV to achieve −10 mA cm−2 in 0.5 M H2SO4 was better than or comparable to the leading cobalt sulfide-based HER catalysts, such as cobalt sulfide/reduced graphene oxide/carbon nanotubes (CoS2/RGO–CNTs) (142 mV),29 CoS2 nanowires (145 mV),30 Co0.9S0.58P0.42 (139 mV),31 surface selenized meso-CoS2 (110 mV),32 Co9S8@MoSx (98 mV),33 Co9S8-NDCL (96 mV),34 Mo2N/CoS2 (85 mV),35 Zn0.30Co2.70S4 (80 mV),36 CoS2@MoS2/RGO (98 mV),37 Co9S8/MoS2 (97 mV)38 and Co3S4@MoS2 (210 mV)39 (Table S2†). For further studying the influence of N-doping on the achieved HER activity of CoSx/CC-L, we prepared control samples by laser processing Co-MOF/CC using the mixed gases of H2S and NH3 with their different ratios (10% NH3/H2S, 20% NH3/H2S and 50% NH3/H2S), to obtain different N doped CoSx/CC-L samples (denoted as CoSx/CC–10% NH3-L, CoSx/CC–20% NH3-L and CoSx/CC–50% NH3-L). In addition, bare carbon cloth was processed using a laser under an NH3 atmosphere to obtain the N-doped carbon cloth (CC–NH3-L). The corresponding HER polarization curves of CC–NH3-L, CoSx/CC-L, CoSx/CC–10% NH3-L, CoSx/CC–20% NH3-L and CoSx/CC–50% NH3-L in 0.5 M H2SO4 are shown in Fig. S13.† The CC–NH3-L displayed almost no HER activity, implying that the N-doped carbon did not contribute to the electrocatalytic performance in this work. Furthermore, CoSx/CC-L exhibited a much better HER performance than CoSx/CC–10% NH3-L, CoSx/CC–20% NH3-L and CoSx/CC–50% NH3-L, suggesting that the increase in the nitrogen content decreased rather than enhancing the performance. This proves that the excellent HER activity of CoSx/CC-L is solely attributed to the formed CoSx spheres. The CoSx/CC-L with different laser powers (6 W, 12 W and 18 W) were also synthesized, which were denoted as CoSx/CC-L-6 W, CoSx/CC-L-12 W and CoSx/CC-L-18 W, respectively. As shown in Fig. S14,† the HER activity slightly increased with the increase of laser powers.
The Tafel slope revealed the inherent reaction dynamics of the HER (Fig. 3b). The iR-corrected Tafel slope value of 20 wt% Pt/C was 52 mV dec−1. The corresponding Tafel slope value of 99 mV dec−1 for CoSx/CC-L implied that the HER on CoSx/CC-L proceeded through a Volmer–Heyrovsky mechanism and the electrochemical desorption process was the rate-limiting step. In addition, this Tafel slope was much smaller than those of CoS2/CC (250 mV dec−1), Co-MOF/CC (331 mV dec−1) and CC-L (385 mV dec−1), demonstrating a more efficient HER performance of CoSx/CC-L. This is attributable to the laser-induced sulfur vacancies in CoSx/CC-L, which facilitated the adsorption and reduction of H3O+ to form the Hads intermediate, thus speeding up the Volmer reaction. Electrochemical impedance spectroscopy (EIS) was also performed and the Nyquist plots are shown in Fig. 3c and S15† to investigate the electrocatalytic kinetics of the HER. As is well known, the lower charge transfer resistance (Rct) obtained from the semicircle in the low frequency zone corresponds to the faster reaction rate. As shown in Fig. 3c, the Rct of CoSx/CC-L was the smallest at an overpotential of 200 mV, indicating that CoSx/CC-L had a faster electron transfer rate at the electrocatalyst/electrolyte interface and was more active. In addition, the decreased Rct values of CoSx/CC-L with progressive overpotentials from 100 mV to 200 mV in Fig. S15† also confirmed the advantageous HER kinetics towards the electrolyte interface. The double-layer capacitance (Cdl) was measured to evaluate the electrochemically active surface area (ECSA). As shown in Fig. 3d and S16,† the ECSA of CoSx/CC-L (27.4 mF cm−2) was much larger than those of CoS2/CC (6.9 mF cm−2) and CC-L (0.4 mF cm−2), which was attributed to the high specific surface area of the formed CoSx nanospheres and was beneficial to the exposure of more available active sites for the HER and diffusion of electrocatalytic active species.
For electrocatalytic HER, long-term catalytic stability is an important parameter for the possible practical application, especially at a large current density. Here, the catalytic durability of CoSx/CC-L was assessed using current–time (i–t) plots (Fig. 3e) and polarization curves before and after i–t testing (Fig. 3f) in 0.5 M H2SO4 electrolyte. The negligible attenuations could be observed for continuous current–time plots at different overpotentials of ca. 124 mV, 1200 mV and 1700 mV with a current density of 20 mA cm−2, 350 mA cm−2 and 650 mA cm−2, respectively. With the almost identical polarization curves of CoSx/CC-L before and after the i–t test (Fig. 3f) and no observed significant changes in the morphology (inset of Fig. 3f), as well as the remaining amorphous structure after HER measurements (Fig. S17†), the good long-term catalytic and structure stability for the HER could be verified.
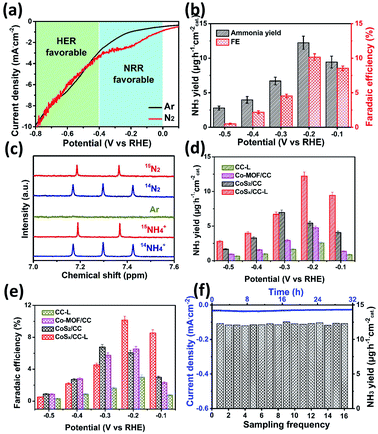
The electrochemical reduction of N2 to NH3 provides an effective and sustainable strategy to store and carry hydrogen to produce on-demand fertilizers.40 In addition, the synthesis of NH3 is a process of active nitrogen association with active hydrogen, thus a certain proton activation ability is needed and this has been demonstrated in the HER performance for CoSx/CC-L (Fig. 3). Considering that the enriched active hydrogen would directly result in hydrogen production under acidic conditions and thus reduce the selectivity of the NRR, we applied a lower cathodic overpotential and engineered the CoSx/CC-L sample in a neutral electrolyte (0.05 M Na2SO4) to minimize the HER and increase the efficiency of the NRR. The best NH3 yield and faradaic efficiency were achieved for the samples synthesized with a laser power of 18 W (Fig. S18†). Therefore we chose CoSx/CC-L-18 W as the target sample. As shown by polarization curves (LSV) in Fig. 4a, the current density in a potential range of 0 V to −0.4 V vs. RHE of CoSx/CC-L under a N2 atmosphere was increased apparently in contrast to that under an Ar atmosphere, suggesting that this region was NRR favorable. When the polarization potential increased to be more negative than −0.4 V vs. RHE, the same current density for N2 and Ar atmospheres demonstrated that this region was HER favorable. Then NRR characterization studies using CoSx/CC-L as cathodic catalysts were performed under ambient conditions with continuous N2 bubbling. After the corresponding i–t curves measurements at different potentials of −0.5 V to −0.1 V vs. RHE (Fig. S19†), the generated NH3 was detected from the UV-vis absorption spectra by using the indophenol blue method (Fig. S20†), and the obtained NH3 yields and FEs are displayed in Fig. 4b. As shown, the highest average NH3 yield and the corresponding faradaic efficiency of CoSx/CC-L were achieved at −0.2 V vs. RHE, reaching ∼12.2 μg h−1 cmcat−2 and 10.1%, respectively. At the more negative potentials, the NH3 yields and FEs decreased gradually due to the competitive HER on the cathode.
The 15N isotopic labeling experiment (Fig. 4c) was further used to confirm that the N source of NH3 resulted from supplied N2. The 1H NMR spectra of commercial 14NH4Cl and 15NH4Cl samples were collected as standard spectra and the internal standard method and 1H NMR spectra with different reactive times were utilized to verify the authenticity of the data (Fig. S21†). As shown, the 1H NMR spectra for the electrolytes after NRR at −0.2 V showed a 14N triple peak signal of 14NH4+ when using 14N2 as the feeding gas. On the other hand, when using 15N2 as the feeding gas, a 15N doublet (I = 1/2) in the range of 7.2–7.4 ppm without obvious peaks of the 14N triplet was detected. The peak positions for both the triplet and doublet closely matched the reference substances 14NH4Cl and 15NH4Cl, demonstrating the successful production of ammonia during the NRR process and the detected NH3 completely derived from the supplied N2.
In order to demonstrate the effects of S vacancies on the NRR performance, the NRR performance analyses of CoSx/CC-L and control samples CC-L, Co-MOF/CC and CoS2/CC in neutral electrolyte at different potentials were carried out (Fig. S22†). As shown in Fig. 4d and e, the CoSx/CC-L and CoS2/CC possessed higher NH3 yields and faradaic efficiency than Co-MOF/CC and CC-L, suggesting the vital role of CoSx in catalytic NRR. In addition, compared with the optimal NH3 yield and the faradaic efficiency (∼7.0 μg h−1 cmcat−2 and 6.8%) of CoS2/CC at −0.3 V vs. RHE, the CoSx/CC-L displayed higher electrocatalytic NRR activities of ∼12.2 μg h−1 cmcat−2 and 10.1% at a lower polarization potential of −0.2 V vs. RHE. This is most likely related to the S vacancies produced by laser processing, which could provide more adsorption sites for N2. S vacancies as the electron-rich sites led to efficient N2 adsorption by Co ions, which was beneficial for subsequent electrocatalytic N2 activation.41,42 For studying the influence of N-doping on the achieved NRR activity of CoSx/CC-L, the NH3 yields of CoSx/CC-L, CoSx/CC–10% NH3-L, CoSx/CC–20% NH3-L and CoSx/CC–50% NH3-L were determined and compared (Fig. S23†). As shown, increasing the N-doping level did not enhance the NH3 yields, and the optimal NRR performance was still 12.2 μg h−1 cmcat−2 for the CoSx/CC-L sample at −0.2 V. As a consequence, the HER and NRR results for different N-doped samples indicated that N doping may affect the electrocatalytic performance, but HER and NRR activities were mainly derived from amorphous CoSx in CoSx/CC-L. For comparison, the performance of the CoSx/CC-L was better than or comparable to most of the previously reported NRR catalysts43–54 under neutral conditions, as illustrated in Table S3.† In addition, the synthesis of sulfide by a laser combined with a H2S atmosphere is a universal method. The CuSx/CC-L and FeSx/CC-L were prepared by the laser processing of the Cu-MOF and Fe-MOF under a H2S atmosphere and their NRR performance was measured (Fig. S24†). As shown, the optimal NH3 yields for FeSx/CC-L and CuSx/CC-L were 15.5 μg h−1 cmcat−2 at −0.3 V vs. RHE and 13.6 μg h−1 cmcat−2 at −0.5 V vs. RHE, respectively. The excellent NNR activity demonstrated the advantages of laser processing as a universal method for the synthesis of amorphous sulfides.
The NRR stability of CoSx/CC-L was evaluated using a chronoamperometry test at −0.2 V vs. RHE comprising sixteen cycles (each for 2 h of electrolysis) (Fig. 4f). As seen, no significant NH3 yield attenuation occurred during the sixteen consecutive N2 reduction cycles, verifying the excellent NRR catalytic stability for CoSx/CC-L. No significant changes to the spherical morphology could be observed (Fig. S25†), confirming the structural integrity of CoSx/CC-L. Meanwhile, the Faraday efficiency during the whole cycling process was calculated to be about 11.57%.
Conclusions
A facile approach toward the fabrication of amorphous CoSx nanospheres anchored on carbon fiber cloths (CoSx/CC-L) with uniform distribution and strong adhesion via the confined temperature field generated by a laser under a H2S atmosphere was demonstrated, which was carried out within a short time and at normal temperature. The obtained CoSx/CC-L was directly applied as an efficient and multifunctional electrode for electrocatalytic HER and electrocatalytic NRR. The amorphous nature of CoSx nanospheres played a vital role in enhancing the HER and NRR activities. As a HER electrocatalyst, CoSx/CC-L exhibited a low overpotential (∼87 mV at 10 mA cm−2) in 0.5 M H2SO4 aqueous electrolyte. As a NRR electrocatalyst, the CoSx/CC-L exhibited a high rate of NH3 production of 12.2 μg h−1 cmcat−2 and Faraday efficiency of 10.1% at −0.2 V vs. RHE. The laser-produced amorphous CoSx/CC-L catalyst was expected to be a promising HER and NRR electrocatalyst for large-scale application in energy conversion and storage fields.Author contributions
Lili Zhao: data curation, formal analysis, investigation, writing the original draft, funding acquisition. Bin Chang: methodology, supervision, validation. Tianjiao Dong: visualization, supervision, validation. Haifeng Yuan: formal analysis, supervision. Yue Li: software, validation. Zhenfei Tang: supervision, validation. Zhen Liu: supervision, validation. Hong Liu: project administration, resources, supervision, validation. Xiaoli Zhang: supervision, validation. Weijia Zhou: conceptualization, funding acquisition, project administration, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Taishan Scholar Project of Shandong Province (tsqn201812083), the Natural Science Foundation of Shandong Province (ZR2019YQ20, ZR2019BB001, ZR2021JQ15, and ZR2021QE011), the Innovative Team Project of Jinan (2021GXRC019), the National Natural Science Foundation of China (51902132, 51972147, and 52022037), the China Postdoctoral Science Foundation (2021M701402), the Postdoctoral Innovative Talents Support Program of Shandong Province (SDBX2020009) and the Open Project Program of the State Key Laboratory of Photocatalysis on Energy and Environment (SKLPEE-KF202110).Notes and references
- H. Wu, H. N. Alshareef and T. Zhu, InfoMat, 2019, 1(3), 417–425 CrossRef CAS.
- L. Du, L. Xing, G. Zhang, X. Liu, D. Rawach and S. Sun, SusMat, 2021, 1(2), 150–173 CrossRef.
- Q. Zhang and J. Guan, Adv. Funct. Mater., 2020, 30(31), 2000768 CrossRef CAS.
- P. Zhou, G. Zhai, X. Lv, Y. Liu, Z. Wang, P. Wang, Z. Zheng, H. Cheng, Y. Dai and B. Huang, Appl. Catal., B, 2021, 283, 119590 CrossRef CAS.
- F. Yu, L. Wang, Q. Xing, D. Wang, X. Jiang, G. Li, A. Zheng, F. Ai and J.-P. Zou, Chin. Chem. Lett., 2020, 31(6), 1648–1653 CrossRef CAS.
- H. Liu, L. Syama, L. Zhang, C. Lee, C. Liu, Z. Dai and Q. Yan, SusMat, 2021, 1(4), 482–505 CrossRef.
- L. Zhang, K. Doyle-Davis and X. Sun, Energy Environ. Sci., 2019, 12(2), 492–517 RSC.
- L. Zhao, J. Jia, Z. Yang, J. Yu, A. Wang, Y. Sang, W. Zhou and H. Liu, Appl. Catal., B, 2017, 210, 290–296 CrossRef CAS.
- H.-F. Wang, C. Tang, B. Wang, B.-Q. Li and Q. Zhang, Adv. Mater., 2017, 29(35), 1702327 CrossRef PubMed.
- T. Chen, Y. Ma, Q. Guo, M. Yang and H. Xia, J. Mater. Chem. A, 2017, 5(7), 3179–3185 RSC.
- L. Zhao, T. Dong, J. Du, H. Liu, H. Yuan, Y. Wang, J. Jia, H. Liu and W. Zhou, Sol. RRL, 2021, 5(2), 2000415 CrossRef CAS.
- J. Zhang, C. Zhang, J. Sha, H. Fei, Y. Li and J. M. Tour, ACS Appl. Mater. Interfaces, 2017, 9(32), 26840–26847 CrossRef CAS PubMed.
- G. Ou, P. Fan, H. Zhang, K. Huang, C. Yang, W. Yu, H. Wei, M. Zhong, H. Wu and Y. Li, Nano Energy, 2017, 35, 207–214 CrossRef CAS.
- X. Zang, C. Shen, Y. Chu, B. Li, M. Wei, J. Zhong, M. Sanghadasa and L. Lin, Adv. Mater., 2018, 30(26), 1800062 CrossRef PubMed.
- C. Thamaraiselvan, J. Wang, D. K. James, P. Narkhede, S. P. Singh, D. Jassby, J. M. Tour and C. J. Arnusch, Mater. Today, 2020, 34, 115–131 CrossRef CAS.
- R. Ye, D. K. James and J. M. Tour, Adv. Mater., 2019, 31(1), 1803621 CrossRef PubMed.
- A. M. Mostafa, E. A. Mwafy and M. S. Hasanin, Opt. Laser Technol., 2020, 121, 105824 CrossRef CAS.
- H. Zeng, X.-W. Du, S. C. Singh, S. A. Kulinich, S. Yang, J. He and W. Cai, Adv. Funct. Mater., 2012, 22(7), 1333–1353 CrossRef CAS.
- T.-F. Hung, Z.-W. Yin, S. B. Betzler, W. Zheng, J. Yang and H. Zheng, Chem. Eng. J., 2019, 367, 115–122 CrossRef CAS.
- Y. Pan, K. Sun, S. Liu, X. Cao, K. Wu, W.-C. Cheong, Z. Chen, Y. Wang, Y. Li, Y. Liu, D. Wang, Q. Peng, C. Chen and Y. Li, J. Am. Chem. Soc., 2018, 140(7), 2610–2618 CrossRef CAS PubMed.
- Q. Lu, J. Yu, X. Zou, K. Liao, P. Tan, W. Zhou, M. Ni and Z. Shao, Adv. Funct. Mater., 2019, 29(38), 1904481 CrossRef.
- Y. Xiao, J.-Y. Hwang, I. Belharouak and Y.-K. Sun, Nano Energy, 2017, 32, 320–328 CrossRef CAS.
- L. Yang, L. Zhang, G. Xu, X. Ma, W. Wang, H. Song and D. Jia, ACS Sustainable Chem. Eng., 2018, 6(10), 12961–12968 CrossRef CAS.
- T. Liu, P. Li, N. Yao, T. Kong, G. Cheng, S. Chen and W. Luo, Adv. Mater., 2019, 31(21), 1806672 CrossRef PubMed.
- T. Yang, D. Yang, Y. Liu, J. Liu, Y. Chen, L. Bao, X. Lu, Q. Xiong, H. Qin, Z. Ji, C. D. Ling and R. Zheng, Electrochim. Acta, 2018, 290, 193–202 CrossRef CAS.
- X. Liu, C. Hao, L. He, C. Yang, Y. Chen, C. Jiang and R. Yu, Nano Res., 2018, 11(8), 4169–4182 CrossRef CAS.
- L. Pan, S. Sun, Y. Chen, P. Wang, J. Wang, X. Zhang, J.-J. Zou and Z. L. Wang, Adv. Energy Mater., 2020, 10(15), 2000214 CrossRef CAS.
- B. Jin, X. Ye, H. Zhong, F. Jin and Y. H. Hu, Chin. Chem. Lett., 2022, 33(2), 812–816 CrossRef CAS.
- S. Peng, L. Li, X. Han, W. Sun, M. Srinivasan, S. G. Mhaisalkar, F. Cheng, Q. Yan, J. Chen and S. Ramakrishna, Angew. Chem., Int. Ed., 2014, 53(46), 12594–12599 CAS.
- M. S. Faber, R. Dziedzic, M. A. Lukowski, N. S. Kaiser, Q. Ding and S. Jin, J. Am. Chem. Soc., 2014, 136(28), 10053–10061 CrossRef CAS PubMed.
- Z. Dai, H. Geng, J. Wang, Y. Luo, B. Li, Y. Zong, J. Yang, Y. Guo, Y. Zheng, X. Wang and Q. Yan, ACS Nano, 2017, 11(11), 11031–11040 CrossRef CAS PubMed.
- B. Dutta, Y. Wu, J. Chen, J. Wang, J. He, M. Sharafeldin, P. Kerns, L. Jin, A. M. Dongare, J. Rusling and S. L. Suib, ACS Catal., 2019, 9(1), 456–465 CrossRef CAS.
- X. Zhou, X. Yang, M. N. Hedhili, H. Li, S. Min, J. Ming, K.-W. Huang, W. Zhang and L.-J. Li, Nano Energy, 2017, 32, 470–478 CrossRef CAS.
- J. Mujtaba, L. He, H. Zhu, Z. Xiao, G. Huang, A. A. Solovev and Y. Mei, ACS Appl. Nano Mater., 2021, 4(2), 1776–1785 CrossRef CAS.
- Y. Zang, B. Yang, A. Li, C. Liao, G. Chen, M. Liu, X. Liu, R. Ma and N. Zhang, ACS Appl. Mater. Interfaces, 2021, 13(35), 41573–41583 CrossRef CAS PubMed.
- Z.-F. Huang, J. Song, K. Li, M. Tahir, Y.-T. Wang, L. Pan, L. Wang, X. Zhang and J.-J. Zou, J. Am. Chem. Soc., 2016, 138(4), 1359–1365 CrossRef CAS PubMed.
- Y. Guo, L. Gan, C. Shang, E. Wang and J. Wang, Adv. Funct. Mater., 2017, 1602699 CrossRef.
- H. Zhu, G. Gao, M. Du, J. Zhou, K. Wang, W. Wu, X. Chen, Y. Li, P. Ma, W. Dong, F. Duan, M. Chen, G. Wu, J. Wu, H. Yang and S. Guo, Adv. Mater., 2018, 30, 1707301 CrossRef PubMed.
- Y. Guo, J. Tang, H. Qian, Z. Wang and Y. Yamauchi, Chem. Mater., 2017, 29, 5566–5573 CrossRef CAS.
- N. C. Kani, A. Prajapati, B. A. Collins, J. D. Goodpaster and M. R. Singh, ACS Catal., 2020, 10(24), 14592–14603 CrossRef CAS.
- X. Zi, J. Wan, X. Yang, W. Tian, H. Zhang and Y. Wang, Appl. Catal., B, 2021, 286, 119870 CrossRef CAS.
- X. Kong, H.-Q. Peng, S. Bu, Q. Gao, T. Jiao, J. Cheng, B. Liu, G. Hong, C.-S. Lee and W. Zhang, J. Mater. Chem. A, 2020, 8(16), 7457–7473 RSC.
- M. Arif, G. Yasin, L. Luo, W. Ye, M. A. Mushtaq, X. Fang, X. Xiang, S. Ji and D. Yan, Appl. Catal., B, 2020, 265, 118559 CrossRef CAS.
- Y. Tian, B. Chang, G. Wang, L. Li, L. Gong, B. Wang, R. Yuan and W. Zhou, J. Mater. Chem. A, 2022, 10(6), 2800–2806 RSC.
- J. Zhou, X. Liu, X. Xu, X. Sun, D. Wu, H. Ma, X. Ren, Q. Wei and H. Ju, ACS Sustainable Chem. Eng., 2021, 9(40), 13399–13405 CrossRef CAS.
- B. Chang, L. Deng, S. Wang, D. Shi, Z. Ai, H. Jiang, Y. Shao, L. Zhang, J. Shen, Y. Wu and X. Hao, J. Mater. Chem. A, 2020, 8(1), 91–96 RSC.
- Y. Ma, T. Yang, H. Zou, W. Zang, Z. Kou, L. Mao, Y. Feng, L. Shen, S. J. Pennycook, L. Duan, X. Li and J. Wang, Adv. Mater., 2020, 32(33), 2002177 CrossRef CAS PubMed.
- Y. Guo, T. Wang, Q. Yang, X. Li, H. Li, Y. Wang, T. Jiao, Z. Huang, B. Dong, W. Zhang, J. Fan and C. Zhi, ACS Nano, 2020, 14(7), 9089–9097 CrossRef CAS PubMed.
- N. Cao, Z. Chen, K. Zang, J. Xu, J. Zhong, J. Luo, X. Xu and G. Zheng, Nat. Commun., 2019, 10(1), 2877 CrossRef.
- R. Zhang, J. Han, B. Zheng, X. Shi, A. M. Asiri and X. Sun, Inorg. Chem. Front., 2019, 6(2), 391–395 RSC.
- R. Zhang, X. Ren, X. Shi, F. Xie, B. Zheng, X. Guo and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10(34), 28251–28255 CrossRef CAS PubMed.
- L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, Z. Tian, A. M. Asiri, L. Chen, B. Tang and X. Sun, Adv. Mater., 2018, 30(28), 1800191 CrossRef PubMed.
- X. Li, L. Li, X. Ren, D. Wu, Y. Zhang, H. Ma, X. Sun, B. Du, Q. Wei and B. Li, Ind. Eng. Chem. Res., 2018, 57(49), 16622–16627 CrossRef CAS.
- L. Zhang, X. Ren, Y. Luo, X. Shi, A. M. Asiri, T. Li and X. Sun, Chem. Commun., 2018, 54(92), 12966–12969 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta01982e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |